Key Points
Question
What are the physiologic and clinical features of the Paralympic athlete's heart?
Findings
This cohort study included 252 consecutive Paralympic athletes who underwent cardiac evaluation. Cardiac remodeling in Paralympic athletes significantly differs, both in male and female athletes, depending on the type of injury and type of sport, with non–spinal cord injuries and endurance sports associated with the highest prevalence of training-associated electrocardiogram changes, the largest left ventricular cavity and mass, and the greatest peak oxygen uptake.
Meaning
This report provides characterization of the Paralympic athlete’s heart according to the type of lesion and type of sport, which may find immediate implementation in the setting of preparticipation cardiovascular evaluation of these athletes.
This cohort study characterizes the cardiovascular functioning of a group of Paralympic athletes by sex, type of disability (spinal cord vs non–spinal cord injury), and type of sport (endurance vs nonendurance).
Abstract
Importance
Paralympic medicine is a newly adopted term to describe the varied health care issues associated with athletes in the Paralympics. Scarce scientific data, however, are currently available describing the cardiac remodeling in Paralympic athletes.
Objective
To investigate the physiological and clinical characteristics of the Paralympic athlete's heart and derive the normative values.
Design, Setting, and Participants
This is a single-center study on a relatively large cohort of Paralympic athletes, conducted at the Italian Institute of Sport Medicine and Science. Paralympic athletes free of cardiac or systemic pathologic conditions other than their cause of disability were selected for participation in the Paralympic Games from January 2000 to June 2014. Athletes were arbitrarily classified for disability in 2 groups: those with spinal cord injuries (SCI) and those with non-SCI (NSCI). Data analysis occurred from March 2019 to June 2020.
Main Outcomes and Measures
The primary outcome was the difference in cardiac remodeling in Paralympic athletes according to disability type and sports discipline type. Athletes underwent cardiac evaluation, including 12-lead and exercise electrocardiograms, echocardiography, and cardiopulmonary exercise testing.
Results
Among 252 consecutive Paralympic athletes (median [interquartile range (IQR)] age, 34 [29-41] years; 188 men [74.6%]), 110 had SCI and 142 had NSCI. Those with SCI showed a higher prevalence of abnormal electrocardiogram findings than those with NSCI (13 of 110 [11.8%] vs 6 of 142 [4.2%]; P = .003), smaller left ventricular end-diastolic dimension (median [IQR], 48 [46-52] vs 51 [48-54] mm; P = .001) and left ventricular mass index (median [IQR], 80.6 [69-94] vs 91.3 [80-108] g/m2; P = .001), and lower peak oxygen uptake (VO2) (median [IQR], 27.1 [2-34] vs 38.5 [30-47] mL/min/kg; P = .001) in comparison with those with NSCI. Regarding sport discipline, endurance athletes had a larger left ventricular cavity (median [IQR], 52 [47-54] vs 49 [47-53] mm; P = .006) and higher peak VO2 (median [IQR], 46 [39-55] vs 30 [25-35] mL/min/kg; P = .001) than athletes in nonendurance sports.
Conclusions and Relevance
Cardiac remodeling in Paralympic athletes differed by disability and sport discipline. Having NSCI lesions and engaging in endurance sports were associated with the largest left ventricular cavity and left ventricular mass and highest VO2 peak. Having SCI lesions and engaging in nonendurance disciplines, on the contrary, were associated with the smallest left ventricular cavity and mass and lowest VO2 peak.
Introduction
Increasing attention over the last decades has been paid to individuals with physical and mental impairments, who represent a growing proportion of the active population in our society. Several legislative and societal initiatives have been promulgated to restore their capability to participate in daily work, leisure time, and sport activities.1 Consequently, involvement of individuals with different impairments in exercise programs and sporting events has grown exponentially in recent years, including in competitive athletics and participation in the Paralympic Games.2
The astonishing performances that Paralympic athletes can attain have also raised medical and scientific interest, with particular regard to their cardiovascular evaluation and medical care. Paralympic medicine is a newly adopted term to describe the varied health care issues associated with Paralympic athletes.3 However, scarce scientific data are currently available regarding the extent, characteristics, and clinical correlates of cardiac remodeling in Paralympic athletes.4,5,6,7,8,9 In the present study, therefore, we investigated cardiac remodeling, as defined by electrocardiographic, echocardiographic, and cardiopulmonary exercise testing, in a large cohort of Paralympic athletes, examined in the context of the preparticipation medical program implemented in our institute, the Institute of Sport Medicine and Science.
Methods
Study Population
The Institute of Sport Medicine and Science is responsible for the medical and performance evaluation of Italian athletes of elite rank before their participation in Olympic and Paralympic Games. The cardiovascular program that has been routinely implemented includes a history, a physical examination, 12-lead resting and adapted exercise electrocardiography, and echocardiography.10,11 The study design was evaluated and approved by the review board of the institute. All patients included in this study were fully informed of the types and nature of the evaluation and signed consent forms, pursuant to Italian law and the institute’s policy. All clinical data assembled from the study population are maintained in an institutional database.
For this study, we considered all Paralympic athletes selected for participation in the Paralympic Games, based on their performance and achievements, who were consecutively evaluated in our institution from January 2000 to June 2014. Participants were excluded in cases of cardiac abnormalities or technically inadequate or incomplete data. All included athletes were actively training and competing for a mean (SD) period of 3.2 (2.4) years before our evaluation and had achieved recognition in national and/or international events.
For the present analysis, Paralympic athletes were arbitrarily classified according to the disability in 2 major groups: (1) athletes with spinal cord injury (SCI; with the exclusion of individuals with quadriparesis), and (2) athletes with non–spinal cord injuries (NSCI), including those with amputation, postpoliomyelitis, cerebral palsy, and other different conditions (ie, les autres, a group that includes all those that do not fit into the aforementioned groups). With regard to sports, athletes were engaged in a variety of disciplines, which for the purpose of the present analysis were classified in 2 main groups: (1) endurance sports, including rowing, swimming, cycling, hand-bicycle, long-distance and marathon running, and cross-country skiing and (2) nonendurance sports, including (most commonly) fencing, basketball, alpine skiing, ice-sledge hockey, table tennis, and archery.
Physical Examination and 12-Lead Electrocardiogram
Physical examination and medical history were performed according to the recommendations of the International Olympic Committee.12 The 12-lead ECG was recorded with the athlete in the supine position during quiet respiration, at 25 mm/s, using a Cardioline ClickECG (Cardioline). The ECG patterns were analyzed according to the current international recommendations.13
Cardiopulmonary Exercise Testing
Exercise testing in athletes with SCI was performed through an arm-crank ergometer (Ergometrics and Ergoline 800; Ergoline GmbH).14 The test started with a 3-minute warm-up at 30 to 50 W (according to the athlete’s weight and practiced sport), with subsequent increments of 10 W/min until exhaustion, when the athlete was unable to maintain the power output despite constant encouragement, the heart reached 95% or more of the age-predicted value, or a plateau (or decline) in the oxygen uptake (VO2) was observed. In athletes with NSCI, exercise testing was performed on a bicycle-ergometer (Ergometrics ER 800S; Ergoline GmbH) according to the same protocol. Continuous ECG monitoring and recording (Delta 640 and Cube Stress Test; Cardioline) were effective during the exercise and subsequent 7-minute recovery period. Blood pressure was measured at baseline, peak exercise, and after 5 minutes of recovery.
During exercise, heart rate, pulmonary ventilation, VO2, and carbon dioxide production were measured using a breath-by-breath metabolimeter (Quark b2 and Quark CPET; COSMED). Peak VO2 was assessed in absolute (ie, L/min) and relative (ie, mL/min/kg) values.
Echocardiography
Two-dimensional and Doppler echocardiography were performed with Philips Sonos ie33 and Epic 7 (Philips Medical Systems). Measurements of end-diastolic and end-systolic left ventricular (LV) cavity dimensions and anterior ventricular septal and posterior free-wall thicknesses were obtained as recommended by the American Society of Echocardiography.15 Each LV mass was calculated and indexed to body surface area. Ejection fractions were assessed by the modified Simpson rule. Parameters of LV filling and relaxation were obtained with pulsed Doppler and tissue Doppler imaging, as previously described.16
Statistical Analysis
Data are expressed as medians (IQRs). Comparisons between categorical and continuous variables were evaluated with χ2 tests and Mann-Whitney tests, as appropriate. A 2-tailed P value <.05 was considered statistically significant. Stepwise regression and analysis of variance analysis were used to assess the association of continuous variables (age, body surface area, heart rate, blood pressure, and LV mass) and categorical variables (type of disability and sports) with LV mass and peak oxygen uptake. Statistical analysis was performed using SPSS version 22 (IBM) from March 2019 to June 2020.
Results
Demographic and Clinical Characteristics
Of the 296 participants, 44 were eventually excluded because of cardiac abnormalities (n = 24) or technically inadequate or incomplete data (n = 20). Therefore, the final study group included 252 athletes who were free of any cardiac or systemic pathologic conditions (other than the condition causing their impairment and disability). There were 110 athletes with SCI (43.7%) and 142 athletes with NSCI (56.3%); 76 (30.2%) were in endurance sports, and 176 (69.8%) were in nonendurance sports. The athletes’ median (IQR) age was 34 (29-41) years, and 188 were men (74.6%). The median (IQR) body surface area was 1.8 (1.7-1.9) m2. Both systolic (median [IQR], 120 [112-125] mm Hg) and diastolic (median [IQR], 80 [70-80] mm Hg) blood pressure values were within normal ranges.
With regard to sex, men had a larger body size than women (mean [SD], 1.89 [0.18] m2 vs 1.60 [0.20] m2; P < .001) and relatively higher systolic (mean [SD], 120 [11] vs 115 [9] mm Hg) and diastolic (80 [7] vs 70 [8] mm Hg; P < .001) blood pressures values. Resting heart rates were similar in men and women.
With regard to the type of disability (SCI vs NSCI), no differences were observed for sex distribution, age, and body size among the 2 groups. Resting heart rate was higher in male athletes with SCI (median [IQR], 68 [61-75] bpm) than male athletes with NSCI (median [IQR], 62 [53-70] bpm; P = .003), while blood pressure was comparable among the 2 groups in both sexes (Table 1).
Table 1. Demographic, Electrocardiographic, Echocardiographic, and Functional Characteristics of Male and Female Paralympic Athletes With Respect to Type of Disability.
| Characteristic | Median (interquartile range) | |||||
|---|---|---|---|---|---|---|
| Male athletes (n = 188) | Female athletes (n = 64) | |||||
| With NSCI (n = 106) | With SCI (n = 82) | P value | With NSCI (n = 36) | With SCI (n = 28) | P value | |
| Age, y | 33.0 (29.5-40.1) | 34.0 (30.0-39.2) | .56 | 35.5 (23.5-42.0) | 34.5 (28-41.7) | .90 |
| Body surface area, m2 | 1.84 (1.7-1.9) | 1.9 (1.8-2.0) | .52 | 1.6 (1.5-1.7) | 1.6 (1.5-1.7) | .69 |
| Blood pressure, mm Hg | ||||||
| Systolic | 120 (120-130) | 120 (120-130) | .93 | 113 (105-120) | 115 (110-120) | .48 |
| Diastolic | 80 (75-80) | 80 (75-85) | .21 | 70 (70-80) | 70 (70-80) | .83 |
| Heart rate, bpm | 62 (53-70) | 68 (61-75) | .003 | 66 (57-72) | 68 (63-77) | .14 |
| Sinus bradycardia (heart rate <60 bpm), No. (%) | 42 (39.6) | 17 (20.7) | .007 | 11 (30.6) | 6 (21.4) | .42 |
| Electrocardiogram findings, No. (%) | ||||||
| Training associated | 42 (39.6) | 17 (20.7) | .007 | 12 (33.3) | 6 (21.4) | .17 |
| Borderline | 4 (3.8) | 2 (2.4) | .70 | 1 (2.8) | 0 | .38 |
| Abnormal | 5 (4.7) | 6 (7.3) | .54 | 1 (2.8) | 7 (25) | .008 |
| LV end-diastolic diameter, mm | 53 (50-55) | 50 (47-52) | <.001 | 47 (44-49) | 45 (42-48) | .06 |
| LV end-systolic diameter, mm | 33 (30-35) | 31 (28-33) | .01 | 29 (26-31) | 28 (23-32) | .19 |
| Septal LV thickness, mm | 9 (9-10) | 9 (9-10) | .27 | 9 (8-9) | 8 (8-9) | .07 |
| LV posterior wall, mm | 9 (9-10) | 9 (9-10) | .007 | 8 (7-9) | 8.0 (8-9) | .77 |
| Relative wall thickness ratio | 0.36 (0.34-0.38) | 0.37 (0.34-0.38) | .22 | 0.36 (0.33-0.37) | 0.36 (0.34-0.38) | .36 |
| LV mass index, g/m2 | 95.9 (86.7-111.4) | 84.2 (73.5-99.4) | <.001 | 80.3 (71.8-89.5) | 71.2 (60.9-84.0) | .03 |
| LV mass, g | 179.5 (158.2-205.6) | 153.4 (136.6-184.2) | <.001 | 129.0 (108.5-152.7) | 115.1 (95.3-142.0) | .08 |
| Left atrium, mm | 36.0 (33.3-38.1) | 35.0 (31.0-37.0) | .11 | 33.3 (30.0-35.7) | 31.5 (30.0-34.7) | .52 |
| Aortic root, mm | 32 (30-34) | 32 (30-34) | .73 | 28 (26-31) | 27 (26-29) | .24 |
| Pulsed wave Doppler | ||||||
| E wave, cm/s | 78 (66-89) | 70 (63-81) | .006 | 86 (76-97) | 79 (73-91) | .21 |
| A wave, cm/s | 53.1 (47.0-62.5) | 52.5 (46.7-61.2) | .89 | 56.0 (45.3-65.0) | 55.0 (46.0-66.2) | .84 |
| E:A ratio | 1.4 (1.2-1.7) | 1.3 (1.1-1.5) | .03 | 1.6 (1.3-1.9) | 1.4 (1.2-1.8) | .40 |
| Tissue Doppler imaging, cm/s | ||||||
| E wave | 11.5 (10.3-13.7) | 11.5 (9.3-14.0) | .34 | 11.9 (10.3-13.6) | 12.3 (10.9-13.9) | .52 |
| A wave | 9.9 (8.0-10.7) | 9.30 (8.1-10.3) | .66 | 8.5 (7.7-9.6) | 9.0 (8.1-11.4) | .45 |
| S wave | 9.1 (8.2-10.3) | 8.8 (7.8-10.4) | .35 | 9.0 (7.7-9.6) | 8.5 (7.8-9.1) | .22 |
| E:E' ratio | 8.3 (6.8-10.9) | 7.55 (6.5-10.2) | .26 | 9.5 (7.2-12.7) | 8.1 (6.2-10.2) | .11 |
| Ejection fraction, % | 64 (60-68) | 65 (60-70) | .76 | 67 (63.2-69.7) | 67 (59-70) | .67 |
| Maximum workload, W | 159.5 (133.7-233.2) | 107.0 (78.8-200.0) | <.001 | 107.0 (78.8-200.0) | 70.1 (55.3-92.0) | .001 |
| Maximum workload index, W/kg | 2.3 (1.9-3.1) | 1.8 (1.4-3.9) | <.001 | 1.8 (1.4-3.9) | 1.2 (1.0-1.7) | .02 |
| Peak VO2, L/min × kg | 39.5 (32.9-49.3) | 31.2 (23.8-42.1) | <.001 | 31.2 (23.8-42.1) | 23.7 (20.3-33.3) | .001 |
| Peak heart rate, bpm | 177.0 (161.7-184.0) | 175.0 (161.5-184.2) | .90 | 175.0 (161.5-184.2) | 174.2 (157.0-179.2) | .55 |
| Peak blood pressure | ||||||
| Systolic, mm Hg | 170 (155-185) | 165 (150-180) | .01 | 165 (150-180) | 170 (150-170) | .12 |
| Diastolic, mm Hg | 80 (70-80) | 80 (70-85) | .10 | 80 (70-85) | 70 (70-80) | .03 |
Abbreviations: A max, late (atrial) diastolic peak-flow velocity; A wave, late diastolic relaxation peak velocity; E max, early diastolic peak flow velocity; E:A ratio, ratio of the early to late diastolic peak flow velocities; E wave, early diastolic relaxation peak velocity; LV, left ventricular; NSCI, non–spinal cord injury; SCI, spinal cord injury; S wave, systolic peak velocity.
With regard to the type of sport, no differences were observed for age, body size, or blood pressure between those involved in endurance vs nonendurance sports in both men and women. Resting heart rates were lower in male athletes in endurance sports than nonendurance sports (median [IQR], 67 [60-75] bpm vs 55 [51-63] bpm; P < .001; Table 2).
Table 2. Demographic, Electrocardiographic, Echocardiographic, and Functional Characteristics in Male and Female Paralympic Athletes With Respect to Type of Sport.
| Characteristic | Median (interquartile range) | |||||
|---|---|---|---|---|---|---|
| Male athletes (n = 188) | Female athletes (n = 64) | |||||
| Nonendurance (n = 136) | Endurance (n = 52) | P value | Nonendurance (n = 40) | Endurance (n = 24) | P value | |
| Age, y | 34 (30-39) | 34 (29-41) | .82 | 35 (28-43) | 33 (21-42) | .28 |
| Body surface area, m2 | 1.9 (1.8-2.0) | 1.8 (1.7-1.9) | .04 | 1.6 (1.5-1.7) | 1.6 (1.5-1.7) | .51 |
| Blood pressure, mm Hg | ||||||
| Systolic | 120 (120-130) | 120 (120-125) | .34 | 120 (110-125) | 110 (106-115) | .03 |
| Diastolic | 80 (75-85) | 80 (70-80) | .05 | 75 (70-80) | 70 (70-80) | .65 |
| Heart rate, bpm | 67 (60-75) | 55 (51-63) | <.001 | 69 (60-76) | 65 (56-68) | .08 |
| Sinus bradycardia (heart rate <60 bpm), No. (%) | 29 (21.3) | 30 (57.7) | <.001 | 10 (25) | 7 (29.2) | .77 |
| Electrocardiogram findings, No. (%) | ||||||
| Training associated | 29 (21.3) | 30 (57.7) | <.001 | 10 (25) | 8 (33.3) | .39 |
| Borderline | 2 (1.5) | 3 (5.8) | .05 | 0 | 1 (4.2) | .38 |
| Abnormal | 8 (5.9) | 3 (5.8) | >.99 | 7 (17.5) | 1 (4.2) | .24 |
| LV end-diastolic diameter, mm | 50.0 (48.1-53.0) | 53.5 (51.0-55.7) | <.001 | 45.0 (43.3-49.0) | 47.0 (44.5-49.0) | .17 |
| LV end-systolic diameter, mm | 32.0 (29.0-34.2) | 33.5 (30.0-36) | .001 | 28.5 (26.1-31.4) | 26.5 (25.0-31.4) | .45 |
| Septal LV thickness, mm | 9 (9-10) | 10 (9-11) | <.001 | 8 (8-9) | 8.0 (8.0-9.0) | .66 |
| LV posterior wall, mm | 9 (9.0-9.7) | 10 (9-10) | <.001 | 8 (7.2-8.7) | 8.0 (7.2-9.0) | .86 |
| Relative wall thickness ratio | 0.36 (0.34-0.38) | 0.37 (0.35-0.38) | .07 | 0.36 (0.33-0.38) | 0.35 (0.33-0.37) | .28 |
| LV mass index, g/m2 | 88.4 (75.6-97.4) | 109.3 (93.7-119.3) | <.001 | 72.0 (65.1-85.4) | 79.4 (71.5-89.3) | .16 |
| LV mass, g | 163.6 (139.9-188.3) | 199.3 (164.4-225.2) | <.001 | 121.8 (96.3-143.2) | 124.5 (108.5-146.8) | .57 |
| Left atrium, mm | 35.0 (32.1-37.4) | 36.1 (33.2-40.1) | .03 | 31.2 (29.2-34.7) | 33.2 (31.2-36.0) | .10 |
| Aortic root, mm | 32 (30-34) | 33 (31-36) | .03 | 28 (26-29) | 27 (25-30) | .68 |
| Pulsed-wave Doppler | ||||||
| E wave, cm/s | 74.1 (64.2-88.2) | 75.0 (65.1-86.0) | .95 | 83.0 (75.0-91.2) | 86.5 (73.7-105.2) | .11 |
| A wave, cm/s | 56.0 (48.3-64.0) | 50.1 (45.2-56.0) | .006 | 56.0 (45.7-66.2) | 64.5 (53.0-84.1) | .91 |
| E:A ratio | 1.36 (1.1-1.6) | 1.78 (1.4-2.1) | .02 | 1.36 (1.1-1.9) | 1.85 (1.6-2.3) | .14 |
| Tissue Doppler imaging, cm/s | ||||||
| E wave | 11.5 (9.5-13.8) | 11.5 (9.3-14.0) | .95 | 12.2 (10.7-13.8) | 12.3 (10.9-13.9) | .99 |
| A wave | 9.6 (8.3-10.6) | 9.3 (8.1-10.3) | .09 | 9.0 (8.1-11.3) | 9.0 (8.1-11.4) | .36 |
| S wave | 8.9 (7.8-10.3) | 8.8 (7.8-10.4) | .23 | 8.7 (7.8-9.2) | 8.5 (7.8-9.1) | .44 |
| E:E' ratio | 7.8 (6.5-10.1) | 7.55 (6.5-10.2) | .41 | 8.6 (8.1-11.3) | 8.1 (6.2-10.2) | .14 |
| Ejection fraction, % | 64 (60-70) | 65 (60-68) | .94 | 67 (63-70) | 66 (62-70) | .63 |
| Maximum workload, W | 127.5 (100.2-153.0) | 220.2 (166.1-300.0) | <.001 | 70.0 (56.5-82.4) | 151.0 (95.4-220.0) | <.001 |
| Maximum workload index, W/kg | 1.8 (1.4-2.1) | 3.0 (2.5-4.1) | <.001 | 1.2 (1.0-1.5) | 2.4 (1.7-4.1) | <.001 |
| Peak VO2, L/min × kg | 31.7 (25.4-36.1) | 49.0 (42.7-62.1) | <.001 | 23.6 (20.3-26.5) | 39.4 (31.8-48.7) | <.001 |
| Peak heart rate, bpm | 177 (164-184) | 179 (164-185) | .43 | 174 (157-182) | 174 (167-186) | .35 |
| Peak blood pressure, mm Hg | ||||||
| Systolic | 170 (150-174) | 180 (160-190) | .03 | 158.5 (150-170) | 170 (160-185) | .002 |
| Diastolic | 80 (70-80) | 80 (70-85) | .95 | 77 (70-80) | 75 (70-90) | .65 |
Abbreviations: A max, late (atrial) diastolic peak flow-velocity; A wave, late diastolic relaxation peak velocity; LV, left ventricular; E max, early diastolic peak flow velocity; E:A ratio, ratio of the early to late diastolic peak flow velocities; E wave, early diastolic relaxation peak velocity; S wave, systolic peak velocity.
Twelve-Lead Electrocardiography
The training-associated ECG changes13 were most of the ECG alterations, including sinus bradycardia (n = 62 [24.6%]), ST-segment elevation (n = 90 [35.7%]), increased QRS voltage suggestive of LV hypertrophy (n = 62 [24.6%]), incomplete right bundle branch block (n = 51 [20.2%]), J-wave pattern (early repolarization; n = 30 [11.9%]), and prolonged PR interval (>0.20 seconds; n = 29 [11.5%]). The training-associated ECG findings were more common in men with NSCI (42 [39.6%] of this group) vs men with SCI (17 [20.7%]; P = .007;Table 1) and male athletes competing in endurance disciplines (30 [57.7%] of this group) vs nonendurance disciplines (29 [21.3%]; P < .001; Table 2).
Abnormal ECG findings, not associated with training,13 were detected in 19 athletes (7.5% of the overall population). The most common were T-wave inversions (n = 15 [5.9%]), in anterior precordial leads (V1-V3) in 6 individuals and lateral or inferior-lateral leads in 9 individuals. Premature ventricular beats (≥2) were seen in the remaining 4 individuals (1.5%). These ECG abnormalities were more frequent in those with SCI than those with NSCI (13 of 110 [11.8%] vs 6 of 142 [4.2%]) and female compared with male athletes (8 of 64 women [12.5%] vs 11 of 188 men [5.9%]), because of the large prevalence of anterior T-wave inversions (Table 1).
Borderline ECG findings were seen in 7 individuals (2.8%) and included isolated left-axis deviation (n = 5), complete right bundle branch block (n = 1), and right-axis deviation (n = 1). These alterations showed no different distribution in association with the type of disability (Table 1) or sport (Table 2).
LV Dimensions and Mass
Distribution of LV dimensions in the overall Paralympic cohort is shown in Figure 1. The median (IQR) LV dimensions varied primarily according to sex, with men showing larger LV cavity dimensions (51 [48-54] vs 46 [43-49] mm; P < .001), LV wall thickness (9 [9-10] vs 8 [8-9] mm; P < .001), and normalized LV mass (92 [79-105] vs 77 [67-87] g/m2; P < .001) in comparison with women.
Figure 1. Distribution of Left Ventricular Cavity Dimension and Wall Thickness in the Overall Paralympic Athlete Population.
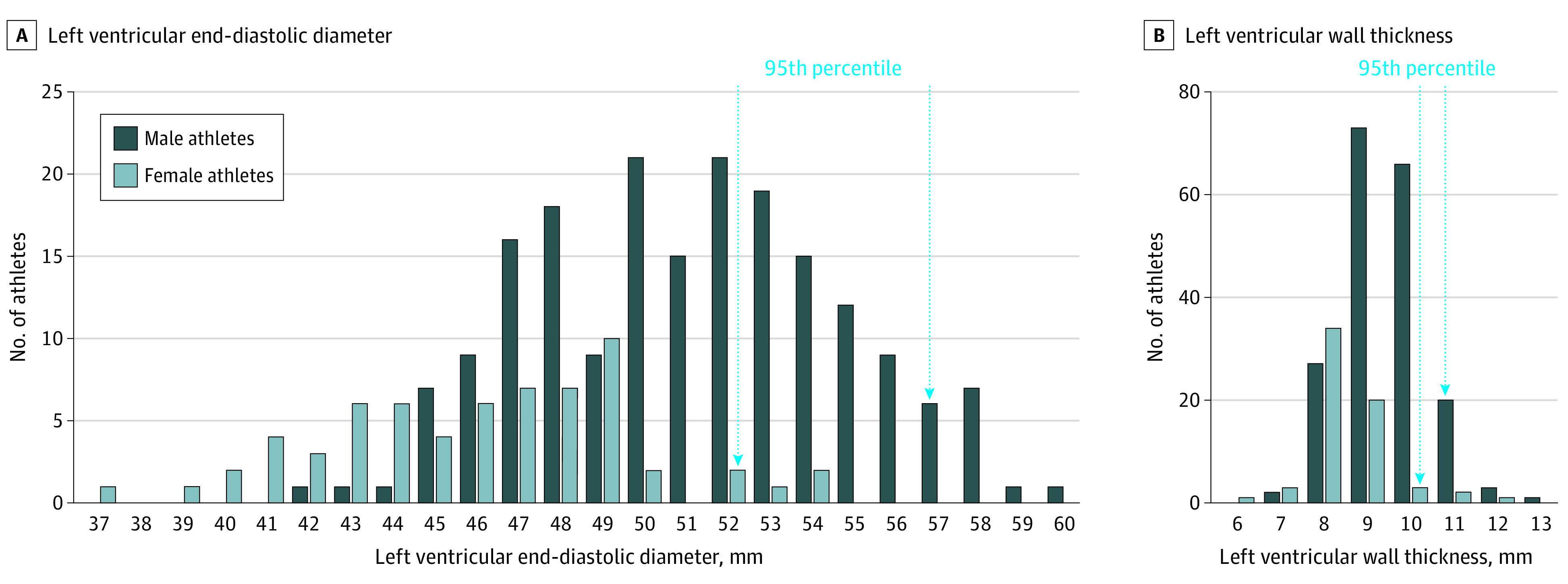
A, Left ventricular end-diastolic diameter. B, Left ventricular wall thickness. Both are shown in relation to sex. The 95th percentile values are indicated by the vertical arrows.
Major differences in LV dimensions and masses were observed in association with the type of disability. In the overall population, athletes with SCI had smaller LV end-diastolic dimension (48 [46-52] vs 51 [48-54] mm; P = .001) and LV mass index values (80.6 [69-94] vs 91.3 [80-108] g/m2; P = .001) in comparison with athletes with NSCI. Specifically, male athletes with SCI showed significantly smaller LV cavity size (median [IQR], 53 [50-55] vs 50 [47-52] mm; P = .001) and both absolute LV mass (median [IQR], 179.5 [158.2-205.6] g vs 153.4 [136.6-184.2] g; P < .001) and normalized LV mass (median [IQR], 95.9 [86.7-111.4] g/m2 vs 84.2 [73.5-99.4] g/m2; P < .001) compared with athletes with NSCI (Table 1). A similar pattern was observed among female athletes, with normalized LV mass reaching statistical significance (median [IQR], 80.3 [71.8-89.5] g/m2 vs 71.2 [60.9-84.0] g/m2; P = .03). No differences were observed for left atrium and aortic dimensions in both sexes.
With regard to the type of sports, athletes in endurance sports had larger LV cavities than athletes in nonendurance sports (median [IQR], 52.0 [47-54] mm vs 49.0 [47-53] mm; P = .006). Specifically, male athletes engaged in endurance disciplines showed larger LV cavities (median [IQR], 53.5 [51-55.7] mm vs 50 [48-53] mm; P = .001), greater wall thicknesses (median [IQR], 10 [9-11] mm vs 9 [9-10] mm; P = .001), and greater LV mass (median [IQR], 109 [93.7-119.3] g/m2 vs 88.4 [75.6-97] g/m2; P = .001) compared with athletes in nonendurance sports. Differences in LV dimensions were not significant among women. Moreover, no differences were observed for left atrial and aortic root dimensions in athletes of both sexes in endurance vs nonendurance sports (Table 2; Figure 2A).
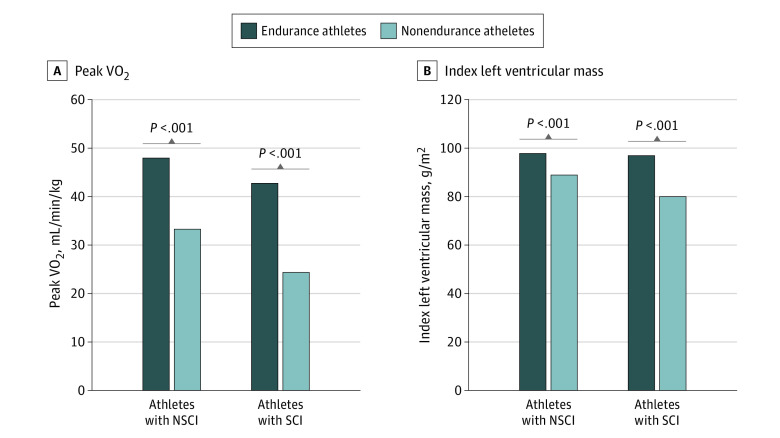
Figure 2. Distribution of Left Ventricular Mass Index and Peak Oxygen Uptake (VO2) in the Paralympic Athlete Population .
A, Left ventricular mass index; B, Peak VO2 in association with the type of disability and sport activity. NSCI indicates non–spinal cord injury; SCI, spinal cord injury.
When the type of sport was examined with regard to the type of disability, it was evident that within the nonendurance group, athletes with NSCI had larger LV cavity dimensions (median [IQR], 50 [47-53] mm vs 48 [46-51] mm; P = .001) and masses (median [IQR], 89 [77-97] g/m2 vs 80 [69-93] g/m2; P = .003) than athletes with SCI, as shown in Table 3 for men. On the other hand, among the athletes in endurance sports, the differences in LV dimensions between those with NSCI vs SCI were virtually absent or trivial. Similar findings were observed among the women athletes.
Table 3. Demographic, Electrocardiographic, Echocardiographic, and Functional Characteristics of Paralympic Male Athletes in Association With Type of Sport and Disability .
| Characteristic | Median (interquartile range) | |||||
|---|---|---|---|---|---|---|
| Nonendurance athletes (n = 135) | Endurance athletes (n = 52) | |||||
| NSCI (n = 66) | SCI (n = 69) | P value | NSCI (n = 39) | SCI (n = 13) | P value | |
| Age, y | 33 (30-39) | 35 (30-39) | .51 | 34 (29-41) | 34 (28-40) | .98 |
| Body surface area, m2 | 1.9 (1.8-2.0) | 1.9 (1.8-2.0) | .56 | 1.8 (1.7-2.0) | 1.8 (1.8-1.9) | .61 |
| Blood pressure, mm Hg | ||||||
| Systolic | 120 (120-130) | 120 (120-130) | .67 | 120 (120-130) | 120 (119-122) | .67 |
| Diastolic | 80 (75-85) | 80 (75-85) | .48 | 80 (75-80) | 80 (77-80) | .87 |
| Heart rate, bpm | 67 (58-74)a | 68 (63-75) | .23 | 55 (50-62)a | 59 (54-92) | .21 |
| Sinus bradycardia (heart rate <60 bpm), No. (%) | 18 (27)b | 11 (16)b | .15 | 24 (61)b | 6 (46)b | .35 |
| Electrocardiogram findings, No. (%) | ||||||
| Training associated | 18 (27)b | 11 (16)b | .15 | 24 (61)b | 6 (46)b | .35 |
| Borderline | 1 (1.5) | 1 (1.4) | >.99 | 3 (7.7) | 1 (7.7) | >.99 |
| Abnormal | 3 (4.5) | 5 (7.2) | .72 | 2 (5.1) | 1 (7.7) | >.99 |
| LV end-diastolic diameter, mm | 52 (49-54)c | 49 (47-52) | .002 | 54 (52-56)c | 53 (49-53) | .04 |
| LV end-systolic diameter, mm | 32 (29-34)d | 30 (28-33) | .17 | 34 (31-36)d | 32 (29-34) | .16 |
| Septal LV thickness, mm | 10 (9-10) | 9 (9-10) | .33 | 10 (10-11) | 10 (9-11) | .53 |
| LV Posterior wall, mm | 9 (9-10) | 9 (8-9) | .12 | 10 (9-10) | 10 (9-10) | .83 |
| Relative wall thickness ratio | 0.35 (0.34-0.38) | 0.37 (0.34-0.38) | .23 | 0.36 (0.35-0.39) | 0.38 (0.36-0.38) | .32 |
| LV mass index, g/m2 | 91 (81-99)a | 81 (73-94)e | .005 | 110 (95-121)a | 104 (84-113)e | .21 |
| LV mass, g | 169 (152-197)b | 153 (135-180) | .02 | 200 (169-227)b | 196 (152-200) | .22 |
| Left atrium, mm | 36 (33-37) | 34 (31-37) | .17 | 36 (33-40) | 36 (33-41) | .96 |
| Aortic root, mm | 32 (30-34) | 32 (30-34) | .57 | 33 (30-35) | 34 (32-36) | .52 |
| Pulsed-wave Doppler | ||||||
| E wave, cm/s | 79 (66-90) | 70 (64-81) | .01 | 76 (67-87) | 68 (63-83) | .20 |
| A wave, cm/s | 57 (48-65) | 54 (47-62) | .51 | 49 (45-57) | 50 (43-56) | .91 |
| E:A ratio | 1.4 (1.2-1.7) | 1.3 (1.1-1.5) | .15 | 1.5 (1.3-1.8) | 1.4 (1.3-1.6) | .32 |
| Tissue Doppler imaging, cm/s | ||||||
| E wave | 12.1 (10.5-13.7) | 10.5 (8.5-14.3) | .21 | 11 (10.1-13.3) | 12.2 (11.8-13.8) | .26 |
| A wave | 10.0 (8.5-11.3) | 9.3 (8.0-10.3) | .12 | 8.7 (7.6-10.3) | 9.5 (8.3-10.4) | .50 |
| S wave | 9.2 (8.2-10.4) | 8.6 (7.8-9.5) | .22 | 9.1 (8.2-10.0) | 9.5 (8.4-12.2) | .46 |
| E:E' ratio | 8.1 (6.1-10.1) | 7.5 (6.5-10.2) | .70 | 8.6 (7.2-11.3) | 7.7 (6.5-10.8) | .19 |
| Ejection fraction, % | 64 (60-69) | 64 (59-70) | .71 | 63 (59-68) | 66 (63-69) | .26 |
| Maximum workload, W | 145 (104-170)a | 112 (100-134)a | <.001 | 245 (167-320)a | 180 (154-212)a | .01 |
| Maximum workload index, W/kg | 1.97 (1.5-2.3)a | 1.6 (1.4-1.9)a | .005 | 3.4 (2.6-4.4)a | 2.8 (2.2-3.0)a | .12 |
| Peak VO2, L/min × Kg | 34.2 (30.0-40.4)a | 28.3 (23.8-33.3)a | <.001 | 52.2 (43.9-62.9)a | 44.9 (42.0-51.4)a | .05 |
| Peak heart rate, bpm | 176 (161-185) | 178 (165-184) | .73 | 179 (161-183) | 185 (173-189) | .44 |
| Peak blood pressure, mm Hg | ||||||
| Systolic | 170 (151-180)f | 167 (150-170) | .28 | 180 (170-200)f | 165 (152-177) | .02 |
| Diastolic | 80 (75-80) | 80 (70-80) | .08 | 80 (70-85) | 77 (70-80) | .65 |
Abbreviations: A max, late (atrial) diastolic peak flow-velocity; A wave, late diastolic relaxation peak velocity; E max, early diastolic peak flow velocity; E:A ratio, ratio of the early to late diastolic peak flow velocities; E wave, early diastolic relaxation peak velocity; LV, left ventricular; NSCI, non–spinal cord injury; SCI, spinal cord injury; S wave, systolic peak velocity.
Significance is P < .001 when comparing athletes with NSCI in nonendurance sports vs endurance sports or athletes with SCI in nonendurance vs endurance sports.
Significance is P = .001 when comparing athletes with SCI in nonendurance sports vs endurance sports or athletes with NSCI in nonendurance sports vs endurance sports.
Significance is P = .002 when comparing athletes with NSCI in nonendurance sports vs endurance sports.
Significance is P = .005 when comparing athletes with NSCI in nonendurance sports vs endurance sports.
Significance is P = .004 when comparing athletes with SCI in nonendurance sports vs endurance sports.
Significance is P = .03 when comparing athletes with NSCI in nonendurance sports vs endurance sports.
On individual analysis, LV cavity dimension exceeded the accepted normal limits (ie, 54 mm),17 ranging from 55 to 60 mm, in 36 athletes (14.3%). Most of these (n = 24) were men engaged in endurance disciplines (ie, cycling, swimming, marathon, and cross-country skiing). Left ventricular wall thickness did not exceed the accepted normal limits (ie, 12 mm),18 with the exception of 1 male cyclist. The left atrium was enlarged (ie, >40 mm)19 in 18 athletes (7.1%), 13 of whom were male athletes in endurance sports.
Systolic and Diastolic LV Function
Left ventricular functioning was normal (ejection fraction >54%)20 in all participants, without differences with regard to sex, type of disability, or sport (Tables 1, 2, and 3). Pulsed-wave Doppler studies showed a normal LV filling pattern, with the early to late diastolic peak velocities (E:A ratio) greater than 1 in 239 (94.8%). Analysis of the differences according to disability revealed that male athletes with NSCI showed higher E waves (median [IQR], 78 [66-89] cm/s vs 70 [63-81] cm/s; P = .006) and E:A ratios (median [IQR], 1.4 [1.2-1.7] vs 1.3 [1.1-1.5]; P = .03) compared with athletes with SCI (Table 1). With regard to types of sport, the male athletes in endurance sports had higher E:A ratios (median [IQR], 1.36 [1.1-1.6] vs 1.78 [1.4-2.1]; P = .02) and relatively lower A waves (median [IQR], 56 [48-64] cm/s vs 50 [45-56] cm/s; P = .006) compared with those in nonendurance sports (Table 2). Individual analysis of the 15 athletes showing an abnormal LV filling pattern revealed that most (n = 8) were older than 40 years.
The LV relaxation pattern (as assessed by tissue Doppler imaging) was normal in most athletes (230 [91.3%]), without differences with regard to sex, type of lesion, or sport (Table 2). Individual analysis of the 29 athletes with an abnormal LV relaxation pattern showed that most (n = 20) were 40 years or older.
Exercise Performance
Exercise performance differed according to type of disability. Specifically, the peak workload was higher in those with NSCI than those with SCI (male athletes: median [IQR], 159.5 [133.7-233.2] W vs 107 [78.8-200] W; P < .001; female athletes: median [IQR], 107 [78.8-200] W vs 70 [55-92] W; P = .001).
In the overall population, athletes with SCI had lower VO2 peak (median [IQR], 27.1 [2-34] mL/min/kg vs 38.5 [30-47] mL/min/kg; P < .001) in comparison with those with NSCI. Specifically, VO2 peak in male athletes (median [IQR], 39.5 [32.9-49.3] L/min × kg vs 31.2 [23.8-42.1] L/min × kg; P < .001) and female athletes (median [IQR], 31.2 [23.8-42.1] L/min × kg vs 23.7 [20.3-33.3] L/min × kg; P < .001) were significantly higher in athletes with NSCI compared with athletes with SCI (Table 1). The peak systolic and diastolic blood pressure, as well as maximum heart rate, however, were not different.
When examined with regard to sport, both male and female athletes competing in endurance disciplines attained higher peak workload (male athletes: median [IQR], 127.5 [100.2-153.0] W vs 220 [166-300] W; P < .001; female athletes: median [IQR], 70 [56.5-82] W vs 151 [95-220] W; P < .001). In the overall population, VO2 peak was higher in athletes in endurance sports compared with those in nonendurance sports (median [IQR], 45.6 [39-55] mL/min/kg vs 30.1 [25-35] mL/min/kg; P = .001). Specifically, VO2 peak was higher in both male athletes (median [IQR], 31.7 [25.4-36.1] L/min × kg vs 49.0 [42.7-62.1] L/min × kg; P < .001) and female athletes (median [IQR], 23.6 [20.3-26.5] L/min × kg vs 39.4 [31.8-48.7] L/min × kg; P < .001) compared with athletes competing in other disciplines. The peak diastolic blood pressure and maximum heart rate were not different (Table 2, Figure 2B).
Among male athletes engaged in nonendurance sports, athletes with NSCI had superior exercise performance and attained higher peak workload and VO2 peak compared with athletes with SCI (maximum workload: median [IQR], 145 [104-170] W vs 112 [100-134] W; P < .001; peak VO2: median [IQR], 34.2 [30-40] L/min × kg vs 28.3 [23.8-33.3] L/min × kg; P < .001). Among those participating in endurance sports, these differences became nonsignificant (ie, in index peak workload and VO2 peak) (Table 3).
Individual analysis revealed that VO2 peak exceeded 50 mL/kg/min in most male athletes in endurance sports (n = 33 [63.5%]). On the other hand, a markedly reduced VO2 peak (ie, ≤14 mL/kg/min)21 was recorded in a subset of 7 athletes (4%) competing in nonendurance disciplines (ie, shooting, curling, and archery).
Stepwise regression analysis showed that VO2 peak was largely associated with body surface area, LV mass, and peak workload (R2, 0.796; P < .001). Moreover, type of sport (F9,246 = 31.31; P < .001), and disability (F2,253 = 40.09; P < .001) were significantly associated with VO2 peak.
Discussion
Based on our analysis, we can describe the clinical characteristics of cardiac remodeling in Paralympic athletes (the Paralympic athlete’s heart) in several ways. This investigation was initiated to add novel scientific data regarding the characteristics of cardiac remodeling in Paralympic athletes, who represents an increasing proportion of the athletic population.
Electrocardiographic Features
The median resting heart rate was higher in athletes with SCI than NSCI, and this was likely associated with their smaller LV cavity (and stroke volume), as previously described.22 The other ECG patterns considered physiologic consequence of exercise training, including increased R-wave or S-wave voltages, ST-segment elevation, and incomplete right bundle branch block, were seen in about one-third of Paralympic athletes. This observation extends to the Paralympic athletes what has been previously reported in Olympic athletes who are able-bodied23,24 and supports the concept that individuals who are able to sustain higher training volume with larger muscle mass, such as the male athletes with NSCI in endurance sports, develop training-associated ECG changes more frequently.
The ECG abnormalities that were not associated with training and potentially an expression of cardiac disease were preferentially observed among female athletes and athletes with SCI. The proportion of abnormal ECGs in the overall Paralympic athletes was not very different from what has been previously reported in athletes who are able-bodied,13,23,24,25 but most of these abnormalities were seen among athletes with SCI. Prevalence of abnormal ECGs, suggestive for cardiac diseases, in participants with SCI may be because of the greater prevalence of cardiovascular risk factors and cardiac or systemic comorbidities in these participants, as has been previously reported.26 The most frequent alterations were T-wave inversions (in the absence of overt cardiac disease): these athletes were allowed to pursue in competitions under a close follow-up program, because of the risk for future incident cardiac events.27
Echocardiographic Characteristics
The main finding of our study was that athletes with SCI participating in nonendurance sport showed the smallest LV cavity and LV mass, which likely explains their reduced physical performance. On the other hand, athletes with NSCI, being able to exercise with a larger muscle mass, have larger cardiac dimensions and attain higher physical performance. It was noteworthy, however, that within the SCI group, those engaged in an endurance sport showed a substantial cardiac remodeling, with increased LV cavity size and mass, similar to that seen in athletes with NSCI.
The small LV dimension in athletes with SCI is the consequence of several determinants. Spinal cord lesions disrupt descending autonomic pathways, inducing abnormalities in multiple organ systems, notably including cardiovascular function, and resulting in the loss of supraspinal regulation of sympathetic activity.28,29,30,31,32 As a consequence, the loss of adrenergic vasoconstriction below the level of lesion and reduction of the leg’s muscle pump during exercise cause a relative entrapment of peripheral venous blood below the level of the spinal cord lesion and, consequently, a reduced preload. The expected consequence is a small LV cavity, which in turn is associated with a low increase in cardiac output during exercise.8,29,30,31,32 This consideration is consistent with a previous experience showing a lower stroke volume during exercise in those with SCI compared with athletes with lower limb amputation or poliomyelitis22 and substantiated by recent observations in patients with SCI.33
The type of sport was a robust determinant for cardiac remodeling in Paralympic athletes, as previously described in athletes who are able-bodied.17,18 Specifically, endurance disciplines (ie, hand bike, cycling, rowing, cross-country skiing) were associated with significant cardiac remodeling, especially in athletes with SCI. This observation is consistent with our previous observation that athletes with SCI and endurance training can reach the same cardiac output during exercise as that achieved by athletes with NSCI.22 This observation suggests that the heart of individuals with SCI maintains the capability to undergo substantial morphologic and functional remodeling in response to intensive endurance training, as described in athletes who are able-bodied and engaged in endurance sports,18,19 and suggests that this type of sport may help them to offset the limitations inherent to the spinal cord lesion.
Of note, LV wall thickness remained within normal limits (ie, ≤12 mm) in all Paralympic athletes, regardless of the type of disability, type of sport, sex, and body size (Figure 1). Therefore, finding LV hypertrophy (ie, wall thickness ≥13 mm) in a Paralympic athlete should be viewed with suspicion and not as an expression of physiologic LV remodeling.
As previously reported in athletes who are able-bodied,16 the cardiac remodeling observed in Paralympic athletes does not convey LV dysfunctional changes. Also, the indexes of LV systolic and diastolic function remained within normal limits, regardless of the type and severity of disability or the sport discipline.
Functional Characteristics
Finally, the greatest cardiac remodeling observed in endurance athletes was associated with the highest degree of aerobic performance. Multivariate statistical analysis confirmed that a greater extent of remodeling (reflected by larger LV dimensions) and higher workload on cardiopulmonary exercise testing (seen in athletes in endurance sports) were associated with greater aerobic power (VO2 peak) (Figure 2, B). Our results are consistent with previous studies demonstrating highest VO2 peak and level of performance in athletes competing in endurance disciplines.14,32,33
Our data have limited confirmation in the scientific literature because of a paucity of investigations and/or limited sample of individuals included in previous analyses4,5,6,7,8,9 that were unable to exhaustively describe the cardiac remodeling in Paralympic athletes. Previous observations have reported conflicting results; differences in cardiac dimensions between Paralympic athletes and athletes with able bodies were even denied or minimized.7,9 Therefore, the novelty and value of the present investigation is the characterization of the range of physiologic cardiac remodeling in Paralympic athletes according to type of disability and sport participation, which may find immediate implementation in the setting of cardiovascular evaluation of these athletes.
At present, no specific recommendations for preparticipation cardiac screening of Paralympic athletes have been released from the sport governing bodies or scientific societies, unlike consensus statements addressing athletes who are able-bodied.12 We hope that this investigation may raise the awareness of the scientific and medical community on this unique athletic population, to provide appropriate and tailored recommendations for their safe sports participation.
Limitations
Several limitations are inherent to the study design, such as the long period of recruitment, because of the small number of Paralympic athletes entering our medical program each year. We also acknowledge that these small numbers limit the ability to define the normal values of cardiac remodeling for each type of sport and lesion. Also, the limited number of female athletes was likely responsible for the lack of statistical significance of the differences we observed in cardiac dimensions in association with the type of lesion and sport. Moreover, the classification we arbitrarily introduced (SCI vs NSCI) is grossly imperfect and does not appropriately consider the variety of lesions and functional impairments existing in the world of Paralympics. The same is true for the classification of sport (endurance vs nonendurance), which ignores the variety of hemodynamic and neurohormonal changes associated with different sport disciplines. Moreover, the lack of sedentary control participants with similar demographic and disability characteristics makes our data insufficient to precisely define the outer limits of cardiac remodeling. Finally, we excluded cardiac diseases by our diagnostic protocol, largely based on echocardiography. Therefore, we cannot exclude that certain cardiac abnormalities that might be detected by cardiac magnetic resonance, and genetic analyses might have been missed.
Conclusions
Cardiac remodeling occurs in Paralympic athletes in accordance to the type of disability and the participating sport discipline. Athletes with NSCI and those who compete in endurance sports showed the highest prevalence of training-associated ECG changes, the largest LV cavity dimensions and mass, and the greatest VO2 peak. In contrast, athletes with SCI and those involved in nonendurance sports demonstrate the least degree of cardiac remodeling, associated with relative reduction in VO2 peak.
References
- 1.Sherrill C; World Health Organization; the International Council of Sport Science and Physical Education Young people with disability in physical education/physical activity /sport in and out of schools: technical report form the World Health Organization. Accessed August 17, 2020. https://www.icsspe.org/sites/default/files/YOUNGPEOPLE.pdf.
- 2.Legg D. Paralympic games: history and legacy of a global movement. Phys Med Rehabil Clin N Am. 2018;29(2):417-425. doi: 10.1016/j.pmr.2018.01.008 [DOI] [PubMed] [Google Scholar]
- 3.Webborn N, Van de Vliet P. Paralympic medicine. Lancet. 2012;380(9836):65-71. doi: 10.1016/S0140-6736(12)60831-9 [DOI] [PubMed] [Google Scholar]
- 4.Huonker M, Schmid A, Sorichter S, Schmidt-Trucksäb A, Mrosek P, Keul J. Cardiovascular differences between sedentary and wheelchair-trained subjects with paraplegia. Med Sci Sports Exerc. 1998;30(4):609-613. doi: 10.1097/00005768-199804000-00020 [DOI] [PubMed] [Google Scholar]
- 5.Price DT, Davidoff R, Balady GJ. Comparison of cardiovascular adaptations to long-term arm and leg exercise in wheelchair athletes versus long-distance runners. Am J Cardiol. 2000;85(8):996-1001. doi: 10.1016/S0002-9149(99)00917-0 [DOI] [PubMed] [Google Scholar]
- 6.Gates PE, Campbell IG, George KP. Absence of training-specific cardiac adaptation in paraplegic athletes. Med Sci Sports Exerc. 2002;34(11):1699-1704. doi: 10.1097/00005768-200211000-00002 [DOI] [PubMed] [Google Scholar]
- 7.Goosey-Tolfrey V, Castle P, Webborn N, Abel T. Aerobic capacity and peak power output of elite quadriplegic games players. Br J Sports Med. 2006;40(8):684-687. doi: 10.1136/bjsm.2006.026815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oliveira JA, Salvetti XM, Lira EB, Mello MT, Silva AC, Luna B. Athlete’s heart, oxygen uptake and morphologic findings in paralympic athletes. Int J Cardiol. 2007;121(1):100-101. doi: 10.1016/j.ijcard.2006.08.044 [DOI] [PubMed] [Google Scholar]
- 9.DE Rossi G, Matos-Souza JR, Costa E Silva AD, et al. Physical activity and improved diastolic function in spinal cord-injured subjects. Med Sci Sports Exerc. 2014;46(5):887-892. doi: 10.1249/MSS.0000000000000187 [DOI] [PubMed] [Google Scholar]
- 10.Pelliccia A, Maron BJ. Preparticipation cardiovascular evaluation of the competitive athlete: perspectives from the 30-year Italian experience. Am J Cardiol. 1995;75(12):827-829. doi: 10.1016/S0002-9149(99)80421-4 [DOI] [PubMed] [Google Scholar]
- 11.Pelliccia A, Adami PE, Quattrini F, et al. Are Olympic athletes free from cardiovascular diseases? systematic investigation in 2352 participants from Athens 2004 to Sochi 2014. Br J Sports Med. 2017;51(4):238-243. doi: 10.1136/bjsports-2016-096961 [DOI] [PubMed] [Google Scholar]
- 12.Ljungqvist A, Jenoure P, Engebretsen L, et al. The International Olympic Committee (IOC) consensus statement on periodic health evaluation of elite athletes March 2009. Br J Sports Med. 2009;43(9):631-643. doi: 10.1136/bjsm.2009.064394 [DOI] [PubMed] [Google Scholar]
- 13.Sharma S, Drezner JA, Baggish A, et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J. 2018;39(16):1466-1480. doi: 10.1093/eurheartj/ehw631 [DOI] [PubMed] [Google Scholar]
- 14.Bernardi M, Carucci S, Faiola F, et al. Physical fitness evaluation of paralympic winter sports sitting athletes. Clin J Sport Med. 2012;22(1):26-30. doi: 10.1097/JSM.0b013e31824237b5 [DOI] [PubMed] [Google Scholar]
- 15.Lang RM, Bierig M, Devereux RB, et al. ; American Society of Echocardiography’s Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology . Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79-108. doi: 10.1016/j.euje.2005.12.014 [DOI] [PubMed] [Google Scholar]
- 16.Caselli S, Di Paolo FM, Pisicchio C, Pandian NG, Pelliccia A. Patterns of left ventricular diastolic function in Olympic athletes. J Am Soc Echocardiogr. 2015;28(2):236-244. doi: 10.1016/j.echo.2014.09.013 [DOI] [PubMed] [Google Scholar]
- 17.Pelliccia A, Culasso F, Di Paolo FM, Maron BJ. Physiologic left ventricular cavity dilatation in elite athletes. Ann Intern Med. 1999;130(1):23-31. doi: 10.7326/0003-4819-130-1-199901050-00005 [DOI] [PubMed] [Google Scholar]
- 18.Pelliccia A, Maron BJ, Spataro A, Proschan MA, Spirito P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N Engl J Med. 1991;324(5):295-301. doi: 10.1056/NEJM199101313240504 [DOI] [PubMed] [Google Scholar]
- 19.Pelliccia A, Maron BJ, Di Paolo FM, et al. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J Am Coll Cardiol. 2005;46(4):690-696. doi: 10.1016/j.jacc.2005.04.052 [DOI] [PubMed] [Google Scholar]
- 20.Caselli S, Di Paolo FM, Pisicchio C, et al. Three-dimensional echocardiographic characterization of left ventricular remodeling in Olympic athletes. Am J Cardiol. 2011;108(1):141-147. doi: 10.1016/j.amjcard.2011.02.350 [DOI] [PubMed] [Google Scholar]
- 21.Balady GJ, Arena R, Sietsema K, et al. ; American Heart Association Exercise, Cardiac Rehabilitation, and Prevention Committee of the Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Interdisciplinary Council on Quality of Care and Outcomes Research . Clinician’s guide to cardiopulmonary exercise testing in adults: a scientific statement from the American Heart Association. Circulation. 2010;122(2):191-225. doi: 10.1161/CIR.0b013e3181e52e69 [DOI] [PubMed] [Google Scholar]
- 22.Bernardi M, Guerra E, Rodio A, et al. Assessment of exercise stroke volume and its prediction from oxygen pulse in Paralympic athletes with locomotor impairments: cardiac long-term adaptations are possible. Front Physiol. 2020;10:1451. doi: 10.3389/fphys.2019.01451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelliccia A, Maron BJ, Culasso F, et al. Clinical significance of abnormal electrocardiographic patterns in trained athletes. Circulation. 2000;102(3):278-284. doi: 10.1161/01.CIR.102.3.278 [DOI] [PubMed] [Google Scholar]
- 24.Sheikh N, Papadakis M, Ghani S, et al. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation. 2014;129(16):1637-1649. doi: 10.1161/CIRCULATIONAHA.113.006179 [DOI] [PubMed] [Google Scholar]
- 25.Papadakis M, Basavarajaiah S, Rawlins J, et al. Prevalence and significance of T-wave inversions in predominantly Caucasian adolescent athletes. Eur Heart J. 2009;30(14):1728-1735. doi: 10.1093/eurheartj/ehp164 [DOI] [PubMed] [Google Scholar]
- 26.Pelliccia A, Quattrini FM, Squeo MR, et al. Cardiovascular diseases in Paralympic athletes. Br J Sports Med. 2016;50(17):1075-1080. doi: 10.1136/bjsports-2015-095867 [DOI] [PubMed] [Google Scholar]
- 27.Pelliccia A, Di Paolo FM, Quattrini FM, et al. Outcomes in athletes with marked ECG repolarization abnormalities. N Engl J Med. 2008;358(2):152-161. doi: 10.1056/NEJMoa060781 [DOI] [PubMed] [Google Scholar]
- 28.Hou S, Rabchevsky AG. Autonomic consequences of spinal cord injury. Compr Physiol. 2014;4(4):1419-1453. doi: 10.1002/cphy.c130045 [DOI] [PubMed] [Google Scholar]
- 29.Partida E, Mironets E, Hou S, Tom VJ. Cardiovascular dysfunction following spinal cord injury. Neural Regen Res. 2016;11(2):189-194. doi: 10.4103/1673-5374.177707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Popa C, Popa F, Grigorean VT, et al. Vascular dysfunctions following spinal cord injury. J Med Life. 2010;3(3):275-285. [PMC free article] [PubMed] [Google Scholar]
- 31.Williams AM, Gee CM, Voss C, West CR. Cardiac consequences of spinal cord injury: systematic review and meta-analysis. Heart. 2019;105(3):217-225. doi: 10.1136/heartjnl-2018-313585 [DOI] [PubMed] [Google Scholar]
- 32.Bernardi M, Guerra E, Di Giacinto B, Di Cesare A, Castellano V, Bhambhani Y. Field evaluation of paralympic athletes in selected sports: implications for training. Med Sci Sports Exerc. 2010;42(6):1200-1208. doi: 10.1249/MSS.0b013e3181c67d82] [DOI] [PubMed] [Google Scholar]
- 33.Baumgart JK, Brurok B, Sandbakk Ø. Peak oxygen uptake in Paralympic sitting sports: a systematic literature review, meta- and pooled-data analysis. PLoS One. 2018;13(2):e0192903. doi: 10.1371/journal.pone.0192903 [DOI] [PMC free article] [PubMed] [Google Scholar]