This cohort study investigates the role of sarcopenia as a risk factor associated with morbidity after liver resection for malignant tumors.
Key Points
Question
Does sarcopenia alter the postoperative outcomes of patients undergoing liver resection for malignant tumors?
Findings
In this cohort study of 234 patients, sarcopenia was associated with a higher rate of 90-day morbidity, a longer hospital stay, and a higher readmission rate. Furthermore, sarcopenia was an independent risk factor associated with 90-day morbidity.
Meaning
Muscle mass and strength should be assessed before liver resection for malignant tumors to identify patients with sarcopenia to minimize the risk of morbidity.
Abstract
Importance
Previous retrospective studies have shown that sarcopenia substantially alters the postoperative and oncological outcomes after liver resection for malignant tumors. However, the evidence is limited to small retrospective studies with heterogeneous results and the lack of standardized measurements of sarcopenia.
Objective
To investigate the role of sarcopenia as a risk factor associated with 90-day morbidity after liver resection for malignant tumors.
Design, Setting, and Participants
This cohort study included 234 consecutive patients undergoing liver resection for malignant tumors at San Camillo Forlanini Hospital, Rome, Italy, between June 1, 2018, and December 15, 2019. Muscle mass and strength were assessed using the skeletal muscle index (SMI) on preoperative computed tomographic scans and the handgrip strength test, respectively. Patients were then divided into the following 4 groups: group A (normal muscle mass and strength), group B (reduced muscle strength), group C (reduced muscle mass), and group D (reduced muscle mass and strength).
Main Outcomes and Measures
The primary outcome of the study was 90-day morbidity. The following secondary outcomes were investigated: 90-day mortality, hospital stay, and readmission rate.
Results
Sixty-four major and 170 minor hepatectomies were performed in 234 patients (median age, 66.50 [interquartile range, 58.00-74.25] years; 158 men [67.5%]). The median SMI of the entire population was 46.22 (interquartile range, 38.60-58.20) cm/m2. The median handgrip strength was 30.80 (interquartile range, 22.30-36.90) kg. Patients in group D had a statistically significantly higher rate of 90-day morbidity than patients in the other groups (51.5% [35 of 68] vs 38.7% [29 of 75] in group C, 23.1% [3 of 13] in group B, and 6.4% [5 of 78] in group A; P < .001). Compared with patients in the other groups, those in group D had a longer hospital stay (10 days vs 8 days in group C, 9 days in group B, and 6 days in group A; P < .001), and more patients in this group were readmitted to the hospital (8.8% [6 of 68] vs 5.3% [4 of 75] in group C, 7.7% [1 of 13] in group B, and 0% [0 of 78] in group A; P = .02). Sarcopenia, portal hypertension, liver cirrhosis, and biliary reconstruction were independent risk factors associated with 90-day morbidity.
Conclusions and Relevance
Sarcopenia appears to be associated with adverse outcomes after liver resection for malignant tumors. Both muscle mass measurements on computed tomographic scans and muscle strength assessments with the handgrip strength test should be performed at the first clinical encounter to better classify patients and to minimize the risk of morbidity.
Introduction
Surgical treatment with negative margins is the foundation for most gastrointestinal malignant tumors because it offers long-term survival compared with locoregional or systemic therapies.1,2,3 In addition, abdominal surgery is associated with both postoperative mortality and morbidity depending on the condition of the patient and the type of intervention. Despite substantial improvements in perioperative management and surgical techniques, hepatobiliary surgery carries a high risk of morbidity and mortality, reaching rates of 20% to 50% as reported in the literature.4,5,6 Liver resections are considered major surgical procedures, frequently involving a large incision, extensive mobilization, long operative time, bleeding risk, and a delicate postoperative course. Furthermore, patients with malignant tumors often have impaired liver function because of cirrhosis, neoadjuvant chemotherapy, and cholestasis, increasing the chance of unexpected postoperative events.7,8 Therefore, it is necessary to accurately select patients scheduled for liver surgery to provide the best oncological treatment while minimizing the risk of morbidity.
Age, performance status, comorbidities, and body mass index (BMI) are well-known patient-related variables that substantially alter morbidity rates after liver surgery; however, variables with the potential to better estimate short-term outcomes after major hepatobiliary surgery have been described.9,10 Sarcopenia is the degenerative loss of muscle mass, strength, and function, and its prevalence among patients undergoing surgery for gastrointestinal cancer ranges between 30% and 74%.11 It has been associated with worse short-term and long-term outcomes after liver transplantation and after surgery across a wide range of cancers, such as colorectal, gastric, esophageal, pancreatic, and liver.11,12 It has been suggested that the presence of sarcopenia may better predict outcomes of liver surgery compared with the American Society of Anesthesiologists score, performance status, and BMI.13,14,15,16,17,18,19 Previous studies10,13,15,20 have highlighted that impaired muscle mass substantially alters the postoperative and oncological outcomes after surgery for hepatocellular carcinoma and colorectal liver metastases.
Although it has been previously reported that sarcopenia substantially alters oncological outcomes after liver resection for malignant tumors, the available small studies11,13 are limited by heterogeneity of results, a retrospective design, and the lack of standardized measurements of sarcopenia, thus restricting applicability in current clinical practice. Because of a lack of high-quality evidence, the objective of this prospective cohort study was to investigate the role of sarcopenia as a risk factor associated with outcomes after liver resection for malignant tumors.
Methods
Between June 1, 2018, and December 15, 2019, all consecutive patients undergoing liver resection for malignant tumors at San Camillo Forlanini Hospital, Rome, Italy, were prospectively enrolled in this study. Patient demographics, disease presentation, type of surgical approach and type of resection performed, intraoperative data (Pringle maneuver, blood loss, blood transfusions, and operative time), and postoperative outcomes (90-day mortality, 90-day morbidity, and hospital stay) were prospectively recorded. The following patients were excluded: (1) those with benign lesions, (2) those undergoing exploratory laparotomy or laparoscopy without liver resection, (3) those undergoing extrahepatic resection, and (4) those with no preoperative computed tomographic (CT) scan.
The primary outcome of this prospective cohort study was 90-day morbidity. As secondary outcomes, 90-day mortality, hospital stay, and readmission rate were investigated. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
The trial protocol (Supplement 1) conforms to the ethical guidelines of the Declaration of Helsinki35 and was approved by the local institutional review board (Comitato Etico Lazio 1 Azienda Ospedaliera San Camillo Forlanini, Rome, Italy). Every case was discussed in a multidisciplinary setting, and written informed consent was obtained from each patient.
Sarcopenia and Biometric Parameters
In addition to the demographic characteristics, further biometric parameters were measured for each patient. Muscle mass was measured and calculated at the level of the third lumbar vertebra on the most recent CT scan (a maximum of 1 month from the date of the images and the date of surgery). At this level, skeletal muscles were identified and quantified by a Hounsfield unit interval of −29 to +150; measurements were calibrated with water and air at fixed intervals, excluding both vasculature and fatty infiltration. Cross-sectional areas (in centimeters squared) of skeletal muscles were measured by manual outlining on the CT images (Figure 1) and normalized for height to obtain the skeletal muscle index (SMI). SliceOmatic software, version 5.0 (TomoVision) was used for calculations, and 2 of us (G.B. and G.A.) independently scored each patient in a blinded fashion to obtain a mean SMI. A receiver operating characteristic curve using morbidity as a marker of sensitivity and specificity was used to identify the threshold for reduced muscle mass in the study population. Visceral adipose tissue was also assessed by manual outlining on the SliceOmatic software using a Hounsfield unit interval of −150 to −50. As a further quantitative analysis, tricipital skinfold and subscapular skinfold were measured at hospital admission using a skinfold caliper.21,22
Figure 1. Computed Tomographic Scans Showing Areas of Visceral Adipose Tissue and Skeletal Muscle Mass in Patients Without Sarcopenia and Patients With Sarcopenia.
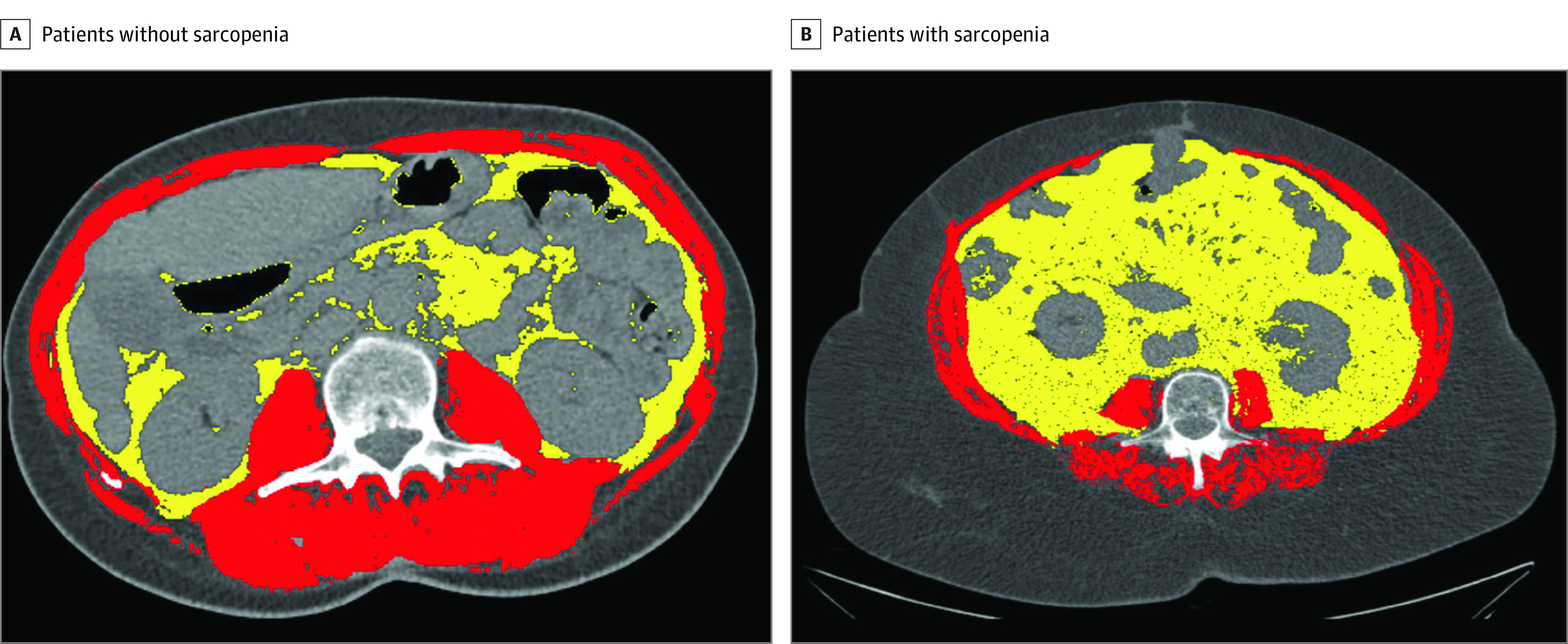
A and B, Yellow indicates visceral adipose tissue, and red indicates skeletal muscle mass.
To evaluate muscle strength, the handgrip strength test was performed in all patients the day before surgery using a dynamometer.23 Two measurements were taken from each hand, and the average of 4 values (in kilograms) was obtained. A cutoff of less than 30 kg for men and less than 20 kg for women was used to define patients with reduced muscle strength as reported elsewhere.24 In a further analysis, the gait speed test was performed (in meters per second) using a 10-m straight path.25
Patients were then divided into 4 groups based on the presence of sarcopenia using the SMI and results of the handgrip strength test. Group A had normal muscle mass and strength, group B had reduced muscle strength, group C had reduced muscle mass, and group D had reduced muscle mass and strength.
Definitions
Sarcopenia was defined by reduced muscle mass and strength as recommended recently by the European Working Group on Sarcopenia in Older People (EWGSOP).26 Portal hypertension was defined by the radiological presence of substantial splenomegaly, umbilical vein recanalization or portosystemic shunt, or preoperative platelet count less than 100 × 103/μL (to convert platelet count to ×109 per liter, multiply by 1.0).27 If the hepatic venous pressure gradient was available, a cutoff of 10 mm Hg was considered clinically significant portal hypertension.28 Patient comorbidities were graded using the Charlson Comorbidity Index score.29 Major liver resections were considered the resection of 3 or more segments.
Morbidity was graded according to the Clavien-Dindo classification30 and the comprehensive complication index.31 Postoperative ascites was considered a drainage output exceeding 10 mL/kg of preoperative body weight in 24 hours.32 Posthepatectomy liver failure and bile leakage were assessed according to definitions by the International Study Group of Liver Surgery.33,34
Perioperative Management of Patients
All patients with liver tumors referred to us are discussed in a multidisciplinary meeting involving hepatobiliary surgeons, hepatologists, medical oncologists, radiologists, and pathologists. Before surgery, comorbidities, liver function, bleeding, and thrombotic risk are assessed and managed accordingly. For major hepatectomies, CT scan volumetry is performed to assess the future liver remnant in an effort to estimate the risk of posthepatectomy liver failure. The future liver remnant volume percentage is calculated by dividing the future liver remnant volume (in milliliters) by the total functional liver volume. A cutoff of 20% for normal livers and a cutoff of 30% to 40% for postchemotherapy and cirrhotic livers are considered safe hepatectomies. All surgical procedures are performed under standard general anesthesia with invasive arterial and central venous pressure monitoring. During resection, fluid transfusion is restricted to obtain a central venous pressure of less than 5 mm Hg. Fluids are then restored at the end of the parenchymal transection. For both open and laparoscopic procedures, we apply a standardized fast-track protocol, including removal of the central line, nasogastric tube, and urinary catheter on postoperative day (POD) 1; early mobilization (POD 1); gradual diet advancement (full diet on POD 3-4); step-down switch to oral analgesia (POD 3); and early removal of drains (POD 3-4). Complete blood testing is performed on POD 1, 3, and 5. Patients are discharged home when adequate mobilization, toleration of a solid diet, and pain control with oral medication are achieved.
Statistical Analysis
The distribution of variables was assessed using the Kolmogorov-Smirnov test and the Shapiro-Wilk test. Data are expressed as the mean (SD) for parametric continuous variables and as the median (interquartile range [IQR]) for nonparametric distribution of data. Categorical data are expressed as a number (percentage).
The χ2 test or Fisher exact test with Yates correction was used to compare differences in categorical variables when appropriate. Analysis of variance was used to compare continuous parametric variables, and the Kruskal-Wallis test was used for continuous nonparametric variables. Post hoc analysis was performed using Bonferroni adjustment in the case of statistically significant differences observed among multiple groups.
To estimate the probability of morbidity, multivariable logistic regression was performed that included statistically significant risk factors in the univariable analysis. Model selection was performed according to the lowest Akaike information criterion and bayesian information criterion. Statistical analysis was performed using SPSS software, version 20.0 (IBM Corp) for MacOSX. Two-sided P < .05 was considered statistically significant.
Results
In total, 288 patients were assessed for eligibility during the study period (Figure 2). Excluded were 28 patients with benign liver lesions and 2 with extrahepatic tumor spread. In addition, 7 patients were excluded because no preoperative CT scan was available, 8 patients because surgery was canceled after exploratory laparoscopy, and 9 patients because extrahepatic resection was carried out. In total, 234 patients underwent 64 major (27.4%) and 170 minor (72.6%) hepatectomies (median age, 66.50 [IQR, 58.00-74.25] years; 158 men [67.5%] and 76 women [32.5%]). The median BMI (calculated as weight in kilograms divided by height in meters squared) was 27.12 (IQR, 23.28-29.55), and the most common indication for surgery was hepatocellular carcinoma (101 [43.2%]) followed by colorectal liver metastases (96 [41.0%]). In total, 163 patients (69.7%) had comorbidities, 89 patients (38.0%) had liver cirrhosis, and 88 patients (37.6%) received a median of 8 (IQR, 6-11) cycles of neoadjuvant chemotherapy. At 90 days after hepatectomy, 5 of 234 patients (2.1%) had died, and 72 of 234 patients (30.8%) developed postoperative 90-day morbidity. The rate of major morbidity was 7.7% (18 of 234).
Figure 2. CONSORT Diagram of Patient Inclusion.
CT indicates computed tomographic.
Subgroup Analysis According to the Presence of Sarcopenia
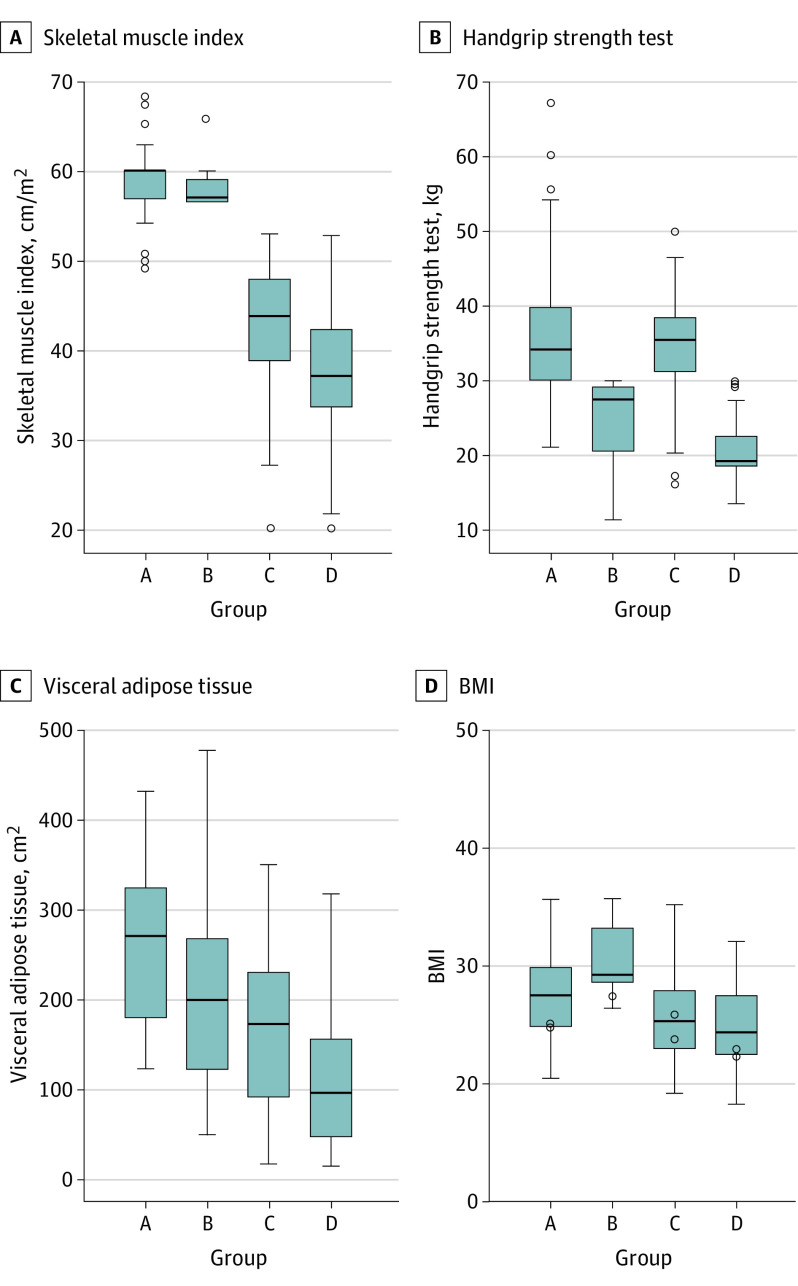
The median SMI of the entire population was 46.22 (IQR, 38.60-58.20) cm/m2. Based on the receiver operating characteristic curve analysis, the thresholds for defining patients with reduced muscle mass were 53.5 cm/m2 for men and 40.8 cm/m2 for women. The median handgrip strength of the entire population was 30.80 (IQR, 22.30-36.90) kg, with 39 of 158 men (24.7%) performing less than 30 kg and 41 of 76 women (53.9%) performing less than 20 kg. Based on the SMI and the handgrip strength test, patients were divided into the 4 groups (Table 1). Patients with reduced muscle mass and strength (group D) had a statistically significantly lower median SMI compared with patients in the other groups (37.14 [IQR, 33.06-42.47] vs 43.77 [IQR, 38.12-48.16] cm/m2 in group C, 57.07 [IQR, 52.51-59.46] cm/m2 in group B, and 60.00 [IQR, 56.92-60.00] cm/m2 in group A; P < .001), decreased visceral adipose tissue (93.15 [IQR, 44.00-154.20] vs 170.60 [IQR, 89.30-229.55] cm2 in group C, 198.50 [IQR, 19.60-270.60] cm2 in group B, and 269.50 [IQR, 164.52-323.90] cm2 in group A; P < .001), and weaker handgrip strength (19.20 [IQR, 18.20-22.40] vs 35.40 [IQR, 31.00-38.70] kg in group C, 27.30 [IQR, 18.95-29.10] kg in group B, and 34.00 [IQR, 30.00-39.77] kg in group A; P < .001) (Figure 3). No differences in BMI, tricipital skinfold, subscapular skinfold, and gait speed were observed among the groups.
Table 1. Baseline Characteristics of Patients According to Muscle Mass and Muscle Strength.
| Variable | Median (IQR) | P value | ||||
|---|---|---|---|---|---|---|
| Total (N = 234) | Group A: normal muscle mass and strength (n = 78) | Group B: reduced muscle strength (n = 13) | Group C: reduced muscle mass (n = 75) | Group D: reduced muscle mass and strength (n = 68) | ||
| Age, y | 66.50 (58.00-74.25) | 68.00 (59.75-74.00) | 66.00 (60.00-73.50) | 66.00 (60.00-74.00) | 68.00 (54.50-75.00) | .90 |
| Sex, No. | ||||||
| Female | 76 | 22 | 3 | 14 | 37 | <.001 |
| Male | 158 | 56 | 10 | 61 | 31 | |
| ASA score | 2.00 (2.00-2.00) | 2.00 (2.00-2.00) | 2.00 (2.00-2.00) | 2.00 (2.00-2.00) | 2.00 (2.00-2.00) | .84 |
| Performance statusa | 100 (100-100) | 100 (100-100) | 100 (100-100) | 100 (100-100) | 100 (100-100) | .09 |
| Comorbidities, No. (%) | 163 (69.7) | 56 (71.8) | 9 (69.2) | 57 (76.0) | 41 (60.3) | .22 |
| Charlson Comorbidity Index score | 4.00 (3.00-6.00) | 5.00 (3.00-6.00) | 3.00 (3.00-5.50) | 5.00 (3.00-6.00) | 3.00 (2.00-5.00) | .42 |
| BMI | 27.12 (23.28-29.55) | 27.10 (22.26-30.32) | 28.10 (22.70-31.75) | 26.34 (25.62-29.70) | 25.06 (23.40-31.18) | .11 |
| Skeletal muscle index, cm/m2 | 46.22 (38.60-58.20) | 60.00 (56.92-60.00) | 57.07 (52.51-59.46) | 43.77 (38.12-48.16) | 37.14 (33.06-42.47) | <.001 |
| Visceral adipose tissue, cm2 | 150.90 (71.37-224.17) | 269.50 (164.52-323.90) | 198.50 (19.60-270.60) | 170.60 (89.30-229.55) | 93.15 (44.00-154.20) | <.001 |
| Tricipital skinfold, mm | 16.00 (11.00-22.00) | 16.00 (10.00-23.00) | 15.00 (13.00-21.50) | 15.00 (12.00-20.00) | 16.00 (12.00-24.50) | .78 |
| Subscapular skinfold, mm | 17.00 (12.00-21.00) | 17.00 (17.00-22.00) | 17.00 (15.00-21.50) | 18.00 (12.00-21.00) | 15.50 (10.00-21.00) | .44 |
| Handgrip strength, kg | 30.80 (22.30-36.90) | 34.00 (30.00-39.77) | 27.30 (18.95-29.10) | 35.40 (31.00-38.70) | 19.20 (18.20-22.40) | <.001 |
| Gait speed, m/s | 1.00 (0.97-1.20) | 1.09 (1.00-1.20) | 1.09 (1.00-1.18) | 1.00 (0.90-1.12) | 1.00 (0.90-1.10) | .07 |
| Cause, No. (%) | ||||||
| Hepatocellular carcinoma | 101 (43.2) | 38 (48.7) | 7 (53.8) | 36 (48.0) | 20 (29.4) | .008 |
| Colorectal liver metastases | 96 (41.0) | 34 (43.6) | 1 (7.7) | 29 (38.7) | 32 (47.1) | |
| Cholangiocarcinoma | 30 (12.8) | 3 (3.8) | 4 (30.8) | 9 (12.0) | 14 (20.6) | |
| Gallbladder cancer | 4 (1.7) | 2 (2.6) | 1 (7.7) | 1 (1.3) | 0 | |
| Noncolorectal liver metastases | 3 (1.3) | 1 (1.3) | 0 | 0 | 2 (2.9) | |
| Portal hypertension, No. (%) | 23 (9.8) | 5 (6.4) | 2 (15.4) | 9 (12.0) | 7 (10.3) | .23 |
| Liver cirrhosis, No. (%) | 89 (38.0) | 25 (32.1) | 7 (53.8) | 33 (44.0) | 24 (35.3) | .27 |
| Child-Pugh score, No. (%) | ||||||
| A | 79 (33.8) | 22 (28.2) | 7 (53.8) | 28 (37.3) | 22 (32.4) | .42 |
| B | 4 (1.7) | 2 (2.6) | 0 | 2 (2.7) | 0 | |
| MELD score | 8.00 (7.00-9.00) | 8.00 (7.00-9.00) | 8.00 (6.50-9.00) | 8.00 (7.00-8.75) | 8.00 (5.50-9.00) | .88 |
| Neoadjuvant chemotherapy, No. (%) | 88 (37.6) | 31 (39.7) | 5 (38.5) | 23 (30.7) | 29 (42.6) | .48 |
| Preoperative hemoglobin, g/dL | 13.00 (12.00-14.20) | 12.90 (12.00-14.00) | 15.00 (12.70-15.50) | 13.50 (12.00-14.70) | 12.25 (10.52-13.50) | .001 |
| Preoperative creatinine, mg/dL | 0.90 (0.80-1.00) | 0.90 (0.80-1.00) | 1.10 (0.90-1.25) | 0.80 (0.80-1.00) | 0.89 (0.70-1.00) | .009 |
| Preoperative albumin, g/dL | 4.10 (3.70-4.10) | 3.90 (3.40-4.23) | 4.30 (4.10-4.50) | 4.10 (3.70-4.40) | 4.37 (3.92-4.65) | .04 |
| Preoperative AST level, U/L | 30.00 (23.00-50.00) | 33.50 (23.75-51.00) | 34.00 (30.00-69.00) | 29.50 (23.00-56.00) | 26.00 (21.50-35.00) | .62 |
| Preoperative ALT level, U/L | 28.00 (19.00-45.25) | 31.50 (19.00-42.00) | 36.00 (26.50-72.00) | 28.00 (18.75-55.25) | 39.65 (25.59-53.71) | .12 |
| Preoperative GGT level, U/L | 53.00 (26.50-154.00) | 50.00 (25.00-123.00) | 131.00 (47.00-248.50) | 50.00 (27.75-149.00) | 24.00 (17.00-38.00) | .07 |
| Preoperative bilirubin level, mg/dL | 0.90 (0.60-1.10) | 0.95 (0.60-1.17) | 1.00 (0.90-1.10) | 0.80 (0.60-1.20) | 0.60 (0.50-0.90) | .57 |
| Preoperative INR | 1.10 (1.02-1.18) | 1.10 (1.00-1.20) | 1.10 (1.02-1.17) | 1.08 (1.03-1.15) | 1.10 (1.01-1.17) | .65 |
| Preoperative platelet count, ×103/μL | 188 (134-248) | 184 (123-244) | 153 (122-211) | 177 (132-243) | 205 (160-276) | .02 |
| Lesion size, mm | 35.00 (25.00-60.00) | 30.00 (20.00-60.00) | 41.00 (24.25-60.00) | 31.50 (26.75-47.00) | 46.00 (30.00-67.50) | .04 |
| No. of lesions | 1.00 (1.00-2.00) | 1.00 (1.00-3.00) | 1.00 (1.00-1.00) | 1.00 (1.00-2.00) | 1.00 (100-2.00) | .15 |
Abbreviations: ALT, alanine aminotransferase; ASA, American Society of Anesthesiologists; AST, aspartate aminotransferase; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); GGT, γ-glutamyltransferase; INR, international normalized ratio; IQR, interquartile range; MELD, model for end-stage liver disease.
SI conversion factors: To convert albumin level to grams per liter, multiply by 10; ALT level to microkatals per liter, multiply by 0.0167; AST level to microkatals per liter, multiply by 0.0167; bilirubin level to micromoles per liter, multiply by 17.104; creatinine level to micromoles per liter, multiply by 88.4; GGT level to microkatals per liter, multiply by 0.0167; hemoglobin level to grams per liter, multiply by 10.0; and platelet count to ×109/L, multiply by 1.0.
Eastern Cooperative Oncology Group score (range, 1 [low] to 100 [high]).
Figure 3. Skeletal Muscle Index, Handgrip Strength Test, Visceral Adipose Tissue, and BMI According to Muscle Mass and Muscle Strength.
A-D, The median (interquartile range) is shown. Group A is normal muscle mass and strength, group B is reduced muscle strength, group C is reduced muscle mass, and group D is reduced muscle mass and strength. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared).
Regarding postoperative results, patients with reduced muscle mass and strength (group D) had a statistically significantly higher rate of 90-day morbidity compared with patients in the other groups (51.5% [35 of 68] in group D vs 38.7% [29 of 75] in group C, 23.1% [3 of 13] in group B, and 6.4% [5 of 78] in group A; P < .001) and a higher median comprehensive complication index (60.45 [IQR, 20.90-82.00] vs 20.90 [IQR, 20.90-20.90] in group C, 20.90 [IQR, 20.90-33.75] in group B, and 2.90 [IQR, 2.90-2.90] in group A; P = .02) (Table 2). Although not statistically significant, the rate of major morbidity (Clavien-Dindo classification III-IV) was also higher in group D than in the other groups (17.6% [12 of 68] in group D vs 6.7% [5 of 75] in group C, 0% [0 of 13] in group B, and 1.3% [1 of 78] in group A; P = .30). In addition, the median hospital stay was longer in group D (10 [IQR, 7.00-15.00] days in group D vs 8 [IQR, 5.00-8.00] days in group C, 9 [IQR, 6.00-9.00] days in group B, and 6 [IQR, 5.00-7.50] days in group A; P < .001), and more patients in group D were readmitted to the hospital after discharge (8.8% [6 of 68] in group D vs 5.3% [4 of 75] in group C, 7.7% [1 of 13] in group B, and 0% [0 of 78] in group A; P = .02).
Table 2. Perioperative Results of Patients According to Muscle Mass and Muscle Strength.
| Variable | No. (%) | P value | ||||
|---|---|---|---|---|---|---|
| Total (N = 234) | Group A: normal muscle mass and strength (n = 78) | Group B: reduced muscle strength (n = 13) | Group C: reduced muscle mass (n = 75) | Group D: reduced muscle mass and strength (n = 68) | ||
| Surgical approach | ||||||
| Open | 182 (77.8) | 57 (73.1) | 10 (76.9) | 55 (73.3) | 60 (88.2) | .10 |
| Laparoscopic | 52 (22.2) | 21 (26.9) | 3 (23.1) | 20 (26.7) | 8 (11.8) | |
| Type of hepatectomy | ||||||
| Major | 64 (27.4) | 19 (24.4) | 4 (30.8) | 19 (25.3) | 22 (32.4) | .69 |
| Minor | 170 (72.6) | 59 (75.6) | 9 (69.2) | 56 (74.7) | 46 (67.6) | |
| Biliary reconstruction | 14 (6.0) | 4 (5.1) | 1 (7.7) | 4 (5.3) | 5 (7.4) | .92 |
| Pringle maneuver | 44 (18.8) | 20 (25.6) | 2 (15.4) | 11 (14.7) | 11 (16.2) | .30 |
| Blood loss, median (IQR), cm3 | 150.00 (100.00-250.00) | 12.00 (5.00-20.00) | 200.00 (150.00-400.00) | 100.00 (87.50-200.00) | 180.00 (100.00-300.00) | .09 |
| Blood transfusions | 5 (2.1) | 1 (1.3) | 0 | 0 | 4 (5.9) | .08 |
| Operative time, min | 180.00 (150.00-240.00) | 18.00 (14.00-236.00) | 200.00 (170.00-255.00) | 180.00 (137.50-217.50) | 220.00 (178.00-280.00) | .92 |
| 90-d Mortality | 5 (2.1) | 1 (1.3) | 1 (7.7) | 0 | 3 (4.4) | .13 |
| 90-d Morbidity | 72 (30.8) | 5 (6.4) | 3 (23.1) | 29 (38.7) | 35 (51.5) | <.001a |
| Clavien-Dindo classification | ||||||
| I-II | 54 (23.1) | 4 (5.1) | 3 (23.1) | 24 (32.0) | 23 (33.8) | .30 |
| III-V | 18 (7.7) | 1 (1.3) | 0 | 5 (6.7) | 12 (17.6) | |
| Comprehensive complication index, median (IQR) | 20.90 (20.90-33.70) | 2.90 (2.90-2.90) | 20.90 (20.90-33.75) | 20.90 (20.90-20.90) | 60.45 (20.90-82.00) | .02 |
| Hospital stay, median (IQR), d | 7.00 (5.00-10.00) | 6.00 (5.00-7.50) | 9.00 (6.00-9.00) | 8.00 (5.00-8.00) | 10.00 (7.00-15.00) | <.001 |
| Readmission rate | 11 (4.7) | 0 | 1 (7.7) | 4 (5.3) | 6 (8.8) | .02 |
Abbreviation: IQR, interquartile range.
P = .35 for group A vs group B, P < .001 for group A vs group C, P < .001 for group A vs group D, P > .99 for group B vs group C, P = .83 for group B vs group D, and P = .69 for group C vs group D.
Risk Factors Associated With Morbidity
According to the results of the multivariable logistic regression, the following factors were statistically significantly associated with an increased risk of postoperative 90-day morbidity in the univariable analysis: American Society of Anesthesiologists score, SMI, handgrip strength, sarcopenia, liver cirrhosis, preoperative portal hypertension, open surgery approach, major hepatectomy, biliary reconstruction, blood loss, operative time, and creatinine level (eTable in Supplement 2). In the multivariable analysis, sarcopenia (hazard ratio, 3.92; 95% CI, 1.96-7.80; P < .001), liver cirrhosis (hazard ratio, 3.63; 95% CI, 1.44-9.15; P = .006), portal hypertension (hazard ratio, 5.88; 95% CI, 1.83-18.89; P = .003), and biliary reconstruction (hazard ratio, 6.00; 95% CI, 1.60-22.43; P = .008) were independent risk factors associated with 90-day morbidity. Among 89 patients with liver cirrhosis (38.0%), those with reduced muscle mass and strength (group D) had a statistically significantly higher rate of postoperative 90-day morbidity (87.5% [21 of 24] in group D vs 72.7% [24 of 33] in group C, 28.6% [2 of 7] in group B, and 4.0% [1 of 25] in group A; P < .001).
Discussion
To our knowledge, this study is the first prospective cohort study reporting that patients with sarcopenia are at higher risk of developing postoperative 90-day morbidity after liver resection for malignant tumors. Patients with cancer are frequently older individuals with comorbidities, cachexia, and limited functional reserve. Furthermore, in the case of malignant tumors of the liver, patients frequently have signs of concomitant liver cirrhosis and may have undergone neoadjuvant chemotherapy. Almost 70% of patients in our study had comorbidities, 38.0% had liver cirrhosis, and 37.6% were referred after neoadjuvant chemotherapy. Therefore, alterations in general and liver-specific function increased surgical risk.
Liver resections are complex procedures burdened by the risk of postoperative morbidity. In our consecutive series of patients, the overall 90-day mortality and morbidity rates were 2.1% and 30.8%, respectively, in line with the available literature4,5,6 but still an issue to consider in hepatobiliary surgery. In this study, we observed that patients without sarcopenia had statistically significantly better outcomes than patients with sarcopenia. Because surgical morbidity is associated with longer hospitalizations and higher medical costs, it is important to identify patients undergoing hepatic resection who are at high risk for postoperative morbidity.15 Because many risk factors are not modifiable (eg, age, comorbidities, and type of surgery), our focus should be on factors that could be modified or ameliorated before surgery. The presence of sarcopenia is of great interest because it is associated with worse outcomes and may be addressed before surgery. In fact, numerous studies36,37,38,39,40,41,42 have focused on treating sarcopenia and the associated metabolic imbalances before surgery.
Published data also suggest that exercise and dietary supplementation might substantially improve muscle mass in patients with cancer (specifically, 30-40 minutes of exercise 3-4 times a week or 2000 extra steps daily, with the addition of late evening snacks containing branched-chain amino acids, for approximately 3 months).20,36,37 Such intervention seems promising because it could substantially alter both short-term and long-term outcomes after cancer surgery. However, delaying surgical intervention in patients with cancer may worsen prognosis and result in tumor progression. Among patients in whom surgery is scheduled after neoadjuvant chemotherapy (eg, for colorectal liver metastases or cholangiocarcinoma) or after locoregional treatment (eg, for hepatocellular carcinoma), exercise and dietary supplementation might be implemented before surgery. However, neoadjuvant chemotherapy is also associated with muscle mass loss and worsening body composition in patients with colorectal liver metastases.38
Recently, the EWGSOP recommended that the definition of sarcopenia should be multidimensional, reflecting both muscle mass and muscle strength.26 Although quantifying sarcopenia on a single CT scan slice is the most widely adopted method, this approach does not consider appendicular muscle mass or muscle strength. Mass is only 1 of the elements comprising sarcopenia: muscles might have reduced mass but may maintain substantial strength (and vice versa). Moreover, preoperative muscle weakness has been previously described as an important risk factor for adverse outcomes after surgery.39,40
Following the EWGSOP criteria,26 we assessed muscle mass, handgrip strength, and gait speed for each of our patients prospectively to better characterize sarcopenia in this study. There was limited variability in gait speed among our cohort, and patients with sarcopenia often had normal gait speed. Therefore, the gait speed test has low positive predictive value. In contrast, performance on the handgrip strength test was associated with all-cause mortality in a large study41 and showed high accuracy in identifying patients with reduced muscle mass and estimating morbidity after surgery in our cohort. Subdividing patients according to the EWGSOP definition of sarcopenia,26 those with sarcopenia defined by both muscle mass and muscle strength had worse outcomes than those with sarcopenia defined by 1 measurement. Patients with sarcopenia in our study had a 3.92 times increased risk of developing postoperative morbidity compared with patients without sarcopenia. Therefore, obtaining both muscle mass and muscle strength measurements enhances our ability to identify and classify patients with sarcopenia before surgery.
Muscle strength was easily and inexpensively measured herein using the handgrip strength test with a simple dynamometer. In this setting, tests investigating muscle function, including the handgrip strength test, the gait speed test, and the chair-stand test, are assessing limited muscle groups, mainly the appendicular systems, and their use may result in measurement variability and error. We do not recommend their use alone in diagnosing sarcopenia. In contrast, the SMI is a standardized method. It allows evaluation of the composition of core muscles and abdominal fat, resulting in a value that is normalized for height based on a validated method43 with little probability of misinterpretation. Using the SMI, muscle mass and muscle strength are complementary to obtain a precise and thorough diagnosis of sarcopenia. However, because advanced software (SliceOmatic, version 5.0; TomoVision) used to identify patients with reduced muscle mass is not available in every institution, the handgrip strength test could still be an effective tool to obtain a diagnosis of probable sarcopenia. Only 3 studies to date (2 on gastric cancer44,45 and 1 on colorectal cancer46) have tested the EWGSOP definition of sarcopenia.26 All 3 studies suggested an association of sarcopenia with postoperative outcomes, which was supported in our prospective study of liver resection for malignant tumors.
The incidence of sarcopenia varies among studies depending on the populations investigated and cutoff values used. Some researchers have recommended use of the original EWGSOP definition of sarcopenia26 (SMI <38.5 cm/m2 for women and <52.4 cm/m2 for men), as proposed by Prado et al.42 However, use of these cutoff levels may be limited because differences in races/ethnicities and populations may alter the classification of patients. Studies using predefined cutoff levels had an incidence of sarcopenia of approximately 53%, whereas studies using population-tailored cutoffs had an incidence of 35%.11
In our study, we identified a population-specific SMI threshold of 53.5 cm/m2 for men and 40.8 cm/m2 for women to define patients with reduced muscle mass. These cutoffs are similar to those reported in one of the first and most important Western studies on sarcopenia and cancer cachexia12 and are much higher than those reported in a recent article by researchers in Japan.20 Therefore, our results align with those reported in previous studies,11,20 emphasizing the need for population-specific data to correctly study and predict oncological outcomes and avoid patient misclassification. Notably, the amount of visceral adipose tissue decreased in parallel to the SMI in our cohort, whereas BMI remained comparable among groups. In addition to conventional patients with cachexia, normal-weight individuals or even overweight individuals with cancer were hospitalized at San Camillo Forlanini Hospital during the study period. This finding highlights the importance of biometric calculations based on preoperative diagnostic images, providing additional important insights and possibly improving patient selection.
Strengths and Limitations
This investigation is the first prospective cohort study to date to evaluate the association of sarcopenia with outcomes after liver resection for malignant tumors. Both muscle mass and strength measures were gathered prospectively in an effort to reduce selection bias, which has not been done previously, to our knowledge. The sample size was assessed a priori and was reached in 18 months because the study was conducted in a high-volume center performing more than 250 liver resections per year. This strength avoids the limitations of previous retrospective studies11 that evaluated sarcopenia over 10 to 15 years, which may have resulted in selection bias and failure to control for recent advancements in chemotherapies, surgical techniques, and patient management.
This study has limitations. We included both patients with liver cirrhosis and patients without liver cirrhosis. Although these groups were well balanced, different grades of liver cirrhosis and varied degrees of portal hypertension might have introduced selection bias and impacted our outcomes.
Conclusions
Sarcopenia is associated with postoperative results after liver resection for both primary and secondary hepatic malignant tumors. When evaluating patients with sarcopenia, our data from a single center suggest that both muscle mass measurements on preoperative CT scans and muscle strength assessments with the handgrip strength test should be obtained at the first clinical encounter to better classify patients and to minimize the risk of morbidity. In addition, the inclusion of sarcopenia evaluation in routine clinical practice may be beneficial to address patient informed consent and to correct adverse preoperative conditions if possible. Future research is needed on the observed association between sarcopenia modification and postoperative outcomes.
Trial Protocol
eTable. Logistic Regression for Predictive Factors of Morbidity
References
- 1.Cohen DJ, Leichman L. Controversies in the treatment of local and locally advanced gastric and esophageal cancers. J Clin Oncol. 2015;33(16):1754-1759. doi: 10.1200/JCO.2014.59.7765 [DOI] [PubMed] [Google Scholar]
- 2.Heestand GM, Murphy JD, Lowy AM. Approach to patients with pancreatic cancer without detectable metastases. J Clin Oncol. 2015;33(16):1770-1778. doi: 10.1200/JCO.2014.59.7930 [DOI] [PubMed] [Google Scholar]
- 3.Knox JJ, Cleary SP, Dawson LA. Localized and systemic approaches to treating hepatocellular carcinoma. J Clin Oncol. 2015;33(16):1835-1844. doi: 10.1200/JCO.2014.60.1153 [DOI] [PubMed] [Google Scholar]
- 4.Belghiti J, Hiramatsu K, Benoist S, Massault P, Sauvanet A, Farges O. Seven hundred forty-seven hepatectomies in the 1990s: an update to evaluate the actual risk of liver resection. J Am Coll Surg. 2000;191(1):38-46. doi: 10.1016/S1072-7515(00)00261-1 [DOI] [PubMed] [Google Scholar]
- 5.Kamiyama T, Nakanishi K, Yokoo H, et al. . Perioperative management of hepatic resection toward zero mortality and morbidity: analysis of 793 consecutive cases in a single institution. J Am Coll Surg. 2010;211(4):443-449. doi: 10.1016/j.jamcollsurg.2010.06.005 [DOI] [PubMed] [Google Scholar]
- 6.Virani S, Michaelson JS, Hutter MM, et al. . Morbidity and mortality after liver resection: results of the Patient Safety in Surgery Study. J Am Coll Surg. 2007;204(6):1284-1292. doi: 10.1016/j.jamcollsurg.2007.02.067 [DOI] [PubMed] [Google Scholar]
- 7.Bhangui P, Laurent A, Amathieu R, Azoulay D. Assessment of risk for non-hepatic surgery in cirrhotic patients. J Hepatol. 2012;57(4):874-884. doi: 10.1016/j.jhep.2012.03.037 [DOI] [PubMed] [Google Scholar]
- 8.Nicoll A. Surgical risk in patients with cirrhosis. J Gastroenterol Hepatol. 2012;27(10):1569-1575. doi: 10.1111/j.1440-1746.2012.07205.x [DOI] [PubMed] [Google Scholar]
- 9.Nikfarjam M, Shereef S, Kimchi ET, et al. . Survival outcomes of patients with colorectal liver metastases following hepatic resection or ablation in the era of effective chemotherapy. Ann Surg Oncol. 2009;16(7):1860-1867. doi: 10.1245/s10434-008-0225-3 [DOI] [PubMed] [Google Scholar]
- 10.Pathak S, Tang JM, Terlizzo M, Poston GJ, Malik HZ. Hepatic steatosis, body mass index and long term outcome in patients undergoing hepatectomy for colorectal liver metastases. Eur J Surg Oncol. 2010;36(1):52-57. doi: 10.1016/j.ejso.2009.09.004 [DOI] [PubMed] [Google Scholar]
- 11.Simonsen C, de Heer P, Bjerre ED, et al. . Sarcopenia and postoperative complication risk in gastrointestinal surgical oncology: a meta-analysis. Ann Surg. 2018;268(1):58-69. doi: 10.1097/SLA.0000000000002679 [DOI] [PubMed] [Google Scholar]
- 12.Martin L, Birdsell L, Macdonald N, et al. . Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31(12):1539-1547. doi: 10.1200/JCO.2012.45.2722 [DOI] [PubMed] [Google Scholar]
- 13.Zhang G, Meng S, Li R, Ye J, Zhao L. Clinical significance of sarcopenia in the treatment of patients with primary hepatic malignancies, a systematic review and meta-analysis. Oncotarget. 2017;8(60):102474-102485. doi: 10.18632/oncotarget.19687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lodewick TM, van Nijnatten TJ, van Dam RM, et al. . Are sarcopenia, obesity and sarcopenic obesity predictive of outcome in patients with colorectal liver metastases? HPB (Oxford). 2015;17(5):438-446. doi: 10.1111/hpb.12373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng PD, van Vledder MG, Tsai S, et al. . Sarcopenia negatively impacts short-term outcomes in patients undergoing hepatic resection for colorectal liver metastasis. HPB (Oxford). 2011;13(7):439-446. doi: 10.1111/j.1477-2574.2011.00301.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nam NH, Kaido T, Uemoto S. Assessment and significance of sarcopenia in liver transplantation. Clin Transplant. 2019;33(12). doi: 10.1111/ctr.13741 [DOI] [PubMed] [Google Scholar]
- 17.Coelen RJ, van Gulik TM. Preoperative sarcopenia negatively impacts postoperative outcomes following major hepatectomy with extrahepatic bile duct resection. World J Surg. 2015;39(9):2368-2369. doi: 10.1007/s00268-015-3053-1 [DOI] [PubMed] [Google Scholar]
- 18.Pinto Dos Santos D, Kloeckner R, Koch S, et al. . Sarcopenia as prognostic factor for survival after orthotopic liver transplantation. Eur J Gastroenterol Hepatol. 2020;32(5):626-634. doi: 10.1097/MEG.0000000000001552 [DOI] [PubMed] [Google Scholar]
- 19.Harimoto N, Hoshino H, Muranushi R, et al. . Skeletal muscle volume and intramuscular adipose tissue are prognostic predictors of postoperative complications after hepatic resection. Anticancer Res. 2018;38(8):4933-4939. doi: 10.21873/anticanres.12810 [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi A, Kaido T, Hamaguchi Y, et al. . Impact of sarcopenic obesity on outcomes in patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg. 2019;269(5):924-931. doi: 10.1097/SLA.0000000000002555 [DOI] [PubMed] [Google Scholar]
- 21.Chumlea WC, Roche AF. Ultrasonic and skinfold caliper measures of subcutaneous adipose tissue thickness in elderly men and women. Am J Phys Anthropol. 1986;71(3):351-357. doi: 10.1002/ajpa.1330710310 [DOI] [PubMed] [Google Scholar]
- 22.Santos LAA, Lima TB, Ietsugu MDV, Nunes HRC, Qi X, Romeiro FG. Anthropometric measures associated with sarcopenia in outpatients with liver cirrhosis. Nutr Diet. 2019;76(5):613-619. doi: 10.1111/1747-0080.12523 [DOI] [PubMed] [Google Scholar]
- 23.Abe T, Counts BR, Barnett BE, Dankel SJ, Lee K, Loenneke JP. Associations between handgrip strength and ultrasound-measured muscle thickness of the hand and forearm in young men and women. Ultrasound Med Biol. 2015;41(8):2125-2130. doi: 10.1016/j.ultrasmedbio.2015.04.004 [DOI] [PubMed] [Google Scholar]
- 24.Lauretani F, Russo CR, Bandinelli S, et al. . Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol (1985). 2003;95(5):1851-1860. doi: 10.1152/japplphysiol.00246.2003 [DOI] [PubMed] [Google Scholar]
- 25.Kulkarni SS, Chen H, Josbeno DA, et al. . Gait speed and grip strength are associated with dropping out of the liver transplant waiting list. Transplant Proc. 2019;51(3):794-797. doi: 10.1016/j.transproceed.2019.01.030 [DOI] [PubMed] [Google Scholar]
- 26.Cruz-Jentoft AJ, Bahat G, Bauer J, et al. ; Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2), and the Extended Group for EWGSOP2 . Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(4):601. doi: 10.1093/ageing/afz046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santambrogio R, Kluger MD, Costa M, et al. . Hepatic resection for hepatocellular carcinoma in patients with Child-Pugh’s A cirrhosis: is clinical evidence of portal hypertension a contraindication? HPB (Oxford). 2013;15(1):78-84. doi: 10.1111/j.1477-2574.2012.00594.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bosch J, García-Pagán JC. Complications of cirrhosis, I: portal hypertension. J Hepatol. 2000;32(1)(suppl):141-156. doi: 10.1016/S0168-8278(00)80422-5 [DOI] [PubMed] [Google Scholar]
- 29.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 30.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1-7. doi: 10.1097/SLA.0b013e318296c732 [DOI] [PubMed] [Google Scholar]
- 32.Ishizawa T, Hasegawa K, Kokudo N, et al. . Risk factors and management of ascites after liver resection to treat hepatocellular carcinoma. Arch Surg. 2009;144(1):46-51. doi: 10.1001/archsurg.2008.511 [DOI] [PubMed] [Google Scholar]
- 33.Rahbari NN, Garden OJ, Padbury R, et al. . Posthepatectomy liver failure: a definition and grading by the International Study Group of Liver Surgery (ISGLS). Surgery. 2011;149(5):713-724. doi: 10.1016/j.surg.2010.10.001 [DOI] [PubMed] [Google Scholar]
- 34.Koch M, Garden OJ, Padbury R, et al. . Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. 2011;149(5):680-688. doi: 10.1016/j.surg.2010.12.002 [DOI] [PubMed] [Google Scholar]
- 35.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 36.Horowitz M, Neeman E, Sharon E, Ben-Eliyahu S. Exploiting the critical perioperative period to improve long-term cancer outcomes. Nat Rev Clin Oncol. 2015;12(4):213-226. doi: 10.1038/nrclinonc.2014.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morley JE, Argiles JM, Evans WJ, et al. ; Society for Sarcopenia, Cachexia, and Wasting Disease . Nutritional recommendations for the management of sarcopenia. J Am Med Dir Assoc. 2010;11(6):391-396. doi: 10.1016/j.jamda.2010.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eriksson S, Nilsson JH, Strandberg Holka P, Eberhard J, Keussen I, Sturesson C. The impact of neoadjuvant chemotherapy on skeletal muscle depletion and preoperative sarcopenia in patients with resectable colorectal liver metastases. HPB (Oxford). 2017;19(4):331-337. doi: 10.1016/j.hpb.2016.11.009 [DOI] [PubMed] [Google Scholar]
- 39.Sultan P, Hamilton MA, Ackland GL. Preoperative muscle weakness as defined by handgrip strength and postoperative outcomes: a systematic review. BMC Anesthesiol. 2012;12:1. doi: 10.1186/1471-2253-12-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verweij NM, Schiphorst AH, Pronk A, van den Bos F, Hamaker ME. Physical performance measures for predicting outcome in cancer patients: a systematic review. Acta Oncol. 2016;55(12):1386-1391. doi: 10.1080/0284186X.2016.1219047 [DOI] [PubMed] [Google Scholar]
- 41.Leong DP, Teo KK, Rangarajan S, et al. ; Prospective Urban Rural Epidemiology (PURE) Study Investigators . Prognostic value of grip strength: findings from the Prospective Urban Rural Epidemiology (PURE) study. Lancet. 2015;386(9990):266-273. doi: 10.1016/S0140-6736(14)62000-6 [DOI] [PubMed] [Google Scholar]
- 42.Prado CM, Lieffers JR, McCargar LJ, et al. . Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629-635. doi: 10.1016/S1470-2045(08)70153-0 [DOI] [PubMed] [Google Scholar]
- 43.Seabolt LA, Welch EB, Silver HJ. Imaging methods for analyzing body composition in human obesity and cardiometabolic disease. Ann N Y Acad Sci. 2015;1353:41-59. doi: 10.1111/nyas.12842 [DOI] [PubMed] [Google Scholar]
- 44.Fukuda Y, Yamamoto K, Hirao M, et al. . Sarcopenia is associated with severe postoperative complications in elderly gastric cancer patients undergoing gastrectomy. Gastric Cancer. 2016;19(3):986-993. doi: 10.1007/s10120-015-0546-4 [DOI] [PubMed] [Google Scholar]
- 45.Huang DD, Zhou CJ, Wang SL, et al. . Impact of different sarcopenia stages on the postoperative outcomes after radical gastrectomy for gastric cancer. Surgery. 2017;161(3):680-693. doi: 10.1016/j.surg.2016.08.030 [DOI] [PubMed] [Google Scholar]
- 46.Huang DD, Wang SL, Zhuang CL, et al. . Sarcopenia, as defined by low muscle mass, strength and physical performance, predicts complications after surgery for colorectal cancer. Colorectal Dis. 2015;17(11):O256-O264. doi: 10.1111/codi.13067 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable. Logistic Regression for Predictive Factors of Morbidity