This prespecified analysis of a randomized clinical trial assesses the incidence of migraine attacks at 6- and 12-month follow-up after transcatheter atrial septal defect closure.
Key Points
Question
What is the optimal duration of clopidogrel therapy after atrial septal defect (ASD) closure to reduce the incidence of new-onset migraine attacks?
Findings
In this prespecified analysis of a randomized clinical trial of 171 patients (27 [15.8%] with new-onset migraine attacks), the occurrence and severity of migraine attacks decreased over time with a low percentage of patients with migraine attacks at 6- to 12-month follow-up and only 1% of patients with new-onset migraine attacks after the cessation of clopidogrel at 3 months after ASD closure.
Meaning
The occurrence of new-onset migraine attacks after ASD closure is an early transient complication and resolves (within 6-12 months) in most patients.
Abstract
Importance
Adding clopidogrel to aspirin for 3 months after transcatheter atrial septal defect (ASD) closure results in a lower incidence of new-onset migraine attacks. However, the outcomes at 6- to 12-month follow-up (after clopidogrel cessation at 3 months) remain largely unknown.
Objective
To assess the incidence of migraine attacks at 6- and 12-month follow-up after transcatheter ASD closure.
Design, Setting, and Participants
This prespecified analysis of a randomized, double-blind clinical trial included patients with no prior history of migraine undergoing ASD closure from 6 university hospitals in Canada from December 2008 to November 2014. Patients were followed up at 3, 6, and 12 months, and a migraine headache questionnaire was administered at each time. Analysis began June 2019.
Interventions
Patients were randomized (1:1) to receive dual antiplatelet therapy (aspirin plus clopidogrel; n = 84) vs single antiplatelet therapy (aspirin plus placebo; n = 87) for 3 months following transcatheter ASD closure. After 3 months, only single antiplatelet therapy (aspirin) was pursued.
Main Outcomes and Measures
Incidence and severity of migraine attacks at 6- and 12-month follow-up.
Results
The mean (SD) age of the study population was 38 (12) years, with 106 women (62%). A total of 27 patients (15.8%) had new-onset migraine attacks within the 3 months following ASD closure (8 of 84 [9.5%] vs 19 of 87 [21.8%] in the initial clopidogrel and placebo groups, respectively; P = .03). After cessation of clopidogrel and aspirin monotherapy, the percentage of patients with migraine attacks decreased over time, with 8 (4.7%) and 4 patients (2.3%) continuing to have migraine attacks at 6 and 12 months, respectively (vs 3 months: P < .001). The severity of migraine attacks progressively decreased over time; no moderate or severe attacks occurred at 6 and 12 months (vs 3 months: P < .001). There were no differences between groups in the rate of migraine attacks at 6 months (initial clopidogrel group: 2 of 84 [2.4%]; initial placebo group: 6 of 87 [6.9%]; P = .28) and 12 months (initial clopidogrel group: 3 of 84 [3.6%]; initial placebo group: 1 of 87 [1.1%]; P = .36) after ASD closure. Only 2 patients (1.2%; 1 patient per group) presented with new-onset migraine attacks after 3 months.
Conclusions and Relevance
New-onset migraine attacks after ASD closure improved or resolved spontaneously within 6 to 12 months in most patients. No significant rebound effect was observed after clopidogrel cessation at 3 months. These results demonstrate a low rate of migraine events beyond 3 months following transcatheter ASD closure and support the early discontinuation of clopidogrel therapy if administered.
Trial Registration
ClinicalTrials.gov Identifier: NCT00799045
Introduction
Transcatheter atrial septal defect (ASD) closure has been well established as the treatment of choice for most patients with hemodynamically significant ASD.1 However, approximately 15% of patients present new-onset migraine headaches after the procedure, usually within the days and weeks following the procedure, leading to a significant burden of medical consultations in addition to missed work or school days and reduced productivity.2,3,4 The randomized, double-blinded Clopidogrel for the Prevention of New Onset Migraine Headache Following Transcatheter Closure of Atrial Septal Defects (CANOA) trial showed the efficacy of clopidogrel to reduce the incidence of migraine attacks within the 3 months following transcatheter ASD closure.5 However, few data exist on the late outcomes of patients with migraine headaches after ASD closure, and the possible effects of clopidogrel cessation remain unknown. Thus, the objective of our study was to determine the late (6- and 12-month) incidence and severity of migraine attacks (both new onset and recurrent) after transcatheter ASD closure and after clopidogrel cessation in patients included in the CANOA trial. All 6- and 12-month events were recorded after discontinuation of clopidogrel.
Methods
Details of the trial rationale and design have been previously reported.5 The protocol and statistical analysis plan are available in Supplement 1. In brief, the study included patients 18 years and older with a clinical indication for transcatheter ASD closure from 6 university centers in Canada from December 2008 to November 2014. Institutional review board approval was obtained from all university centers. All patients provided written informed consent for trial participation. Patients with a history of migraine headaches, based on the International Headache Society criteria and subsequently evaluated by a neurologist,6 were excluded. Other exclusion criteria have already been reported.5 Patients were randomized (1:1) and blinded to treatment assignment before the ASD closure procedure to receive either aspirin (80 mg per day) plus placebo (initial placebo group) or aspirin (80 mg per day) plus clopidogrel (75 mg per day) (initial clopidogrel group). The treatment was initiated (without loading dose) within 24 hours prior to ASD closure and continued for 3 months thereafter. All procedures were performed with the Amplatzer Septal Occluder device (St Jude). After this period, the duration of aspirin treatment was left at the discretion of the investigator. The occurrence of headache episodes was assessed at 3-, 6-, and 12-month follow-up using a structured migraine headache questionnaire that included the Migraine Disability Assessment questionnaire (grade I [score of 0-5] indicates little or no disability; grade II [score, 6-10], mild disability; grade III [score, 11-20], moderate disability; and grade IV [score ≥21], severe disability). The questionnaires were provided by the study coordinator of each participating center. All questionnaires were evaluated by 2 neurologists (D.V. and A.M.) blinded to procedural details and treatment allocation. If headache episodes occurred, the diagnosis of migraine attacks was established according to the International Headache Society criteria and classified as with or without aura.6 The 3-month results have been previously published.5 We report here the extended 6- and 12-month follow-up (prespecified analysis). The primary outcome was the rate of migraine attacks at 6- and 12-month follow-up, and the secondary outcome was the severity of migraine headaches as evaluated by the Migraine Disability Assessment questionnaire. Data were analyzed using SAS statistical software version 9.4 (SAS Institute). Two-sided P values were significant at .05. Analysis began June 2019.
Results
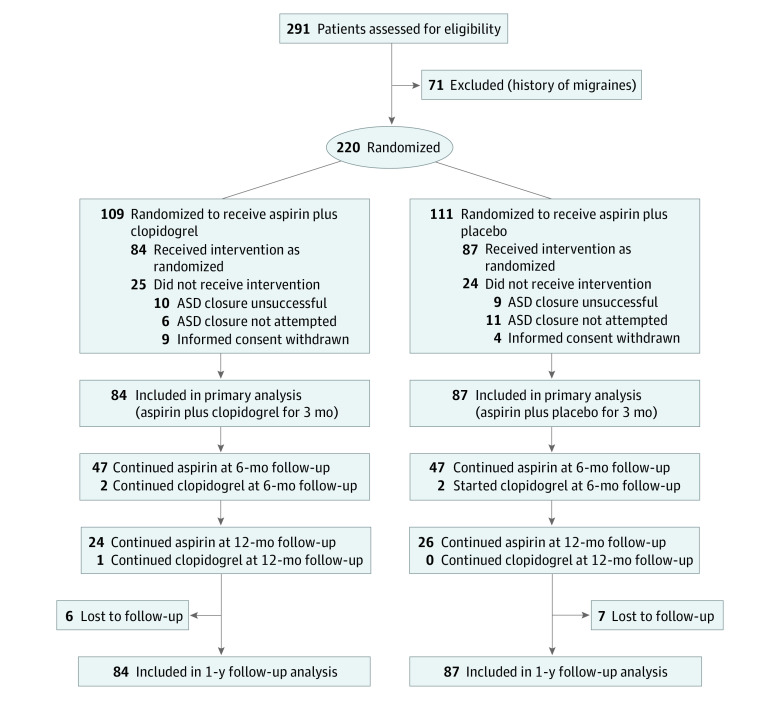
The flow of patient participation, treatment allocation, and follow-up are shown in the Figure. A total of 171 patients were included in the primary analysis, with 84 (49.1%) and 87 patients (50.9%) in the initial clopidogrel and placebo groups, respectively. The main baseline and procedural characteristics of the study population are summarized in Table 1. The mean (SD) age of the patients was 49 (15) years, 106 (62%) were women, and all had successful transcatheter ASD closure. The clopidogrel or placebo treatment was ceased in all but 4 patients with clopidogrel (clopidogrel remained in 2 patients in the initial clopidogrel group and was started after 3 months in 2 patients in the placebo group without clearly stated reasons) after 3 months, and aspirin was maintained up to 6 months in 94 patients (55%; 47 of 84 [56%] and 47 of 87 [54%] in the initial placebo and clopidogrel groups, respectively; odds ratio [OR], 1.08; 95% CI, 0.59-1.96; P = .87) and up to 12 months in 50 patients (29%; 24 [28%] and 26 [31%] in the initial placebo and clopidogrel groups, respectively; OR, 0.84; 95% CI, 0.44-1.64; P = .73). Six patients (75%) and 1 patient (25%) with migraine attacks were taking aspirin at 6- and 12-month follow-up, respectively. At 6 months, 4 patients (50%) with migraine attacks received paracetamol, and 2 patients (50%) with migraine attacks received paracetamol (1 patient) and ibuprofen (1 patient) at 12 months. No patient received triptan therapy, and 2 patients had migraine preventive medication after 3 months (1 patient took amitriptyline and 1 patient took topiramate).
Figure. Flowchart of Recruitment, Treatment Allocation, and Follow-up of the Clopidogrel for the Prevention of New Onset Migraine Headache Following Transcatheter Closure of Atrial Septal Defects (CANOA) Study.
ASD indicates atrial septal defect.
Table 1. Baseline and Procedural Characteristics of the Study Population.
| Characteristic | Treatment group, No. (%) | |
|---|---|---|
| Aspirin + clopidogrel (n = 84) | Aspirin + placebo (n = 87) | |
| Age, mean (SD), y | 49 (16) | 48 (15) |
| Female | 48 (57.1) | 58 (66.7) |
| Smoking | 11 (12.9) | 11 (13.1) |
| Hypertension | 20 (23.8) | 24 (27.6) |
| Diabetes | 5 (5.9) | 8 (9.2) |
| New York Heart Association class | ||
| I | 68 (82.9) | 61 (73.5) |
| II | 12 (14.6) | 19 (22.9) |
| III or IV | 2 (2.4) | 3 (3.6) |
| Pulmonary pressure, mean (SD), mm Hg | 20.4 (5.6) | 22.4 (6.3) |
| Qp/Qs ratio, mean (SD) | 1.93 (0.84) | 1.89 (0.81) |
| Atrial septal aneurysm | 11 (15.5) | 11 (16.4) |
| ASD size, mean (SD), mm | ||
| Measured by TEE | 15.7 (5.7) | 16.9 (5.7) |
| Measured by balloon | 20.3 (6.3) | 21.7 (5.3) |
| Device size, median (IQR), mm | 22 (18-26) | 22 (19-28) |
| Hospitalization length, median (IQR), d | 1 (1-1) | 1 (1-1) |
| Residual shunt (hospital discharge) | 27 (32.1) | 26 (29.8) |
| Milda | 26 (30.9) | 25 (28.7) |
| Moderate to severeb | 1 (1.2) | 1 (1.1) |
| Residual shunt (3-mo follow-up) | 8 (12.7) | 9 (10.3) |
| Mild | 8 (12.7) | 8 (9.2) |
| Moderate to severe | 0 | 1 (1.1) |
Abbreviations: ASD, atrial septal defect; IQR, interquartile range; Qp/Qs, pulmonary flow/systemic flow ratio; TEE, transesophageal echocardiography.
Mild if color jet width (Doppler echocardiography) is 2 mm or less.
Moderate to severe if color jet width (Doppler echocardiography) is greater than 2 mm.
The incidence of migraine headaches over time is shown in Table 2. A total of 27 patients (15.8%) had the diagnosis of new-onset migraine attacks at 3-month follow-up (14 [52%] with aura), with a mean (SD) total number of migraine days of 15 (15), and 20 (74%) and 7 patients (26%) experiencing no/mild and moderate/severe disability during migraine attacks, respectively. There was a higher incidence of migraine headaches in the initial placebo group compared with the initial clopidogrel group at 3 months after ASD closure (placebo: 19 [21.8%]; clopidogrel: 8 [9.5%]; OR, 2.63; 95% CI, 1.26-6.66; P = .03). After discontinuation of clopidogrel, at 6-month follow-up, 6 patients (3.5%) continued to have recurrent migraine headaches, and 2 patients (1.2%) with no migraine headache at 3 months presented new-onset migraine attacks between 3 and 6 months, leading to a total of 8 patients (4.7%; 7 [87%] with aura) with migraine headaches at 6 months (vs 3 months: OR, 0.26; 95% CI, 0.13-0.52; P < .001). The mean (SD) total number of migraine days was 3 (2) (vs 3 months: least square means differences, 1.12; 95% CI, 0.11-2.12; P = .05), and no patient experienced moderate/severe disability (Migraine Disability Assessment score, I in all patients) (vs 3 months: P < .001). At 6-month follow-up, there were no differences between groups in the rate of migraine headaches (initial placebo group: 6 [6.9%]; initial clopidogrel group: 2 [2.4%]; OR, 3.12; 95% CI, 0.61-15.96; P = .28) and regarding new-onset migraine headaches after 3 months (initial placebo group: 1 [1.2%]; initial clopidogrel group: 1 [1.2%]; OR, 0.97; 95% CI, 0.06-15.86; P = .99). At 12-month follow-up, 4 patients (2.3%; 3 [75%] with aura) remained with recurrent migraine headaches (vs 3 months: OR, 0.14; 95% CI, 0.05-0.37; P < .001; vs 6 months: OR, 0.53; 95% CI, 0.22-1.27; P = .58), and no patient had new-onset migraine headaches after 6-month follow-up. The mean (SD) total number of migraine days was 2 (2) (vs 3 months: least square means differences, 1.76; 95% CI, 0.32-3.19; P < .001; vs 6 months: least square means differences, 1.37; 95% CI, −2.13 to 4.87; P = .46), and no patient experienced moderate/severe disability migraine attacks (vs 3 months: P < .001; vs 6 months: P = .99). The lack of differences in the incidence of migraine headaches between groups observed at 6 months was maintained at 12 months (initial placebo group: 1 [1.1%]; initial clopidogrel group: 3 [3.6%]; OR, 0.32; 95% CI, 0.03-3.11; P = .36).
Table 2. New-Onset, Recurrent, and Total Migraine Attacks at 3-, 6- and 12-Month Follow-up According to Treatment Allocation.
| Characteristic | No. (%) | P value | ||
|---|---|---|---|---|
| Overall (n = 171) | Treatment | |||
| Initial aspirin + clopidogrel group (n = 84) | Initial aspirin + placebo group (n = 87) | |||
| New-onset migraine attacks in the first 3 mo | 27 (15.8) | 8 (9.5) | 19 (21.8) | .03 |
| Continued migraine attacks, mo | ||||
| 3-6 | 6 (3.5) | 1 (1.2) | 5 (5.8) | .21 |
| 6-12 | 3 (1.8) | 2 (2.4) | 1 (1.2) | .62 |
| New-onset migraine attacks occurring after the first 3 mo | 2 (1.2) | 1 (1.2) | 1 (1.2) | >.99 |
| Total No. of patients experiencing migraine attacks at 6 mo | 8 (4.7)a | 2 (2.4) | 6 (6.9) | .28 |
| With aura | 7 (78) | 1 (50) | 6 (86) | .42 |
| Total No. of patients experiencing migraine attacks at 12 mo | 4 (2.3)b | 3 (3.6) | 1 (1.1) | .36 |
| With aura | 3 (75) | 2 (67) | 1 (100) | >.99 |
Migraine attacks at 6 months vs 3 months (P < .001).
Migraine attacks at 12 months vs 3 months (P < .001).
Discussion
The pathophysiology linking transcatheter ASD closure and migraine attacks remains unclear. Nickel allergy, increased platelet aggregation, and the presence of microthrombus on the ASD closure device have been proposed as potential mechanisms.7,8 The positive effect of clopidogrel for reducing migraine headaches after ASD closure could be related to its antiplatelet properties leading to a reduction of platelet aggregation, which indeed would translate into a decrease in the release of substances such as serotonin or proinflammatory cytokines, linked to migraine attacks.9 Also, successful P2Y12 platelet inhibition (clopidogrel responders) has been associated with a reduction of migraine headache symptoms in some patients with patent foramen ovale, further supporting a platelet-based mechanism/trigger.10 The antioxidant, anti-inflammatory, and vasoprotective effects of clopidogrel could also play a role on the prevention of migraine attacks.11
The choice of 3 months of dual antiplatelet therapy after ASD closure was based on (1) preclinical studies showing complete endothelialization of interatrial devices within 3 months,12 also confirmed by studies on device nickel release7 and (2) limiting the potential bleeding complications associated with the prolonged use of dual antiplatelet therapy.13 However, some studies in humans have shown late (beyond 3 months) incomplete endothelization of ASD devices.14 Also, limited and controversial data from retrospective studies exist on the duration of new-onset migraine headaches after ASD or patent foramen ovale closure.2,15 Thus, the optimal duration of clopidogrel therapy following transcatheter ASD closure remains an unresolved issue. The present study showed that most migraine headaches after ASD closure had partially or completely resolved by 6 to 12 months after cessation of clopidogrel therapy, with only a minority of patients (2.3%, about 10% of the patients with migraine episodes within the first 3 months) with persistent migraine headaches at 12 months, all of them with no or mild disability features. Importantly, only 1% of patients had new-onset migraine attacks after clopidogrel cessation. Thus, these data support a limited duration (no longer than 3 months) of clopidogrel therapy following ASD closure. However, further studies with a longer follow-up are needed to further evaluate the outcomes of patients with persistent migraine headaches at 1 year after the procedure.
Limitations
There was no evaluation of platelet reactivity to determine a potential clopidogrel resistance in patients with remaining migraine attacks. Also, only 1 ASD closure device type was used in the study. Finally, there could be a potential effect of patients lost to follow-up (13 patients [7.6%] lost at 12-month follow-up) on the final results.
Conclusions
In conclusion, new-onset migraine headaches after ASD closure occurred early (within 3 months) and resolved or improved spontaneously within 6 to 12 months in most patients. Clopidogrel therapy for 3 months was effective at reducing the occurrence of migraine attacks, and no rebound effect was observed after clopidogrel cessation. The results of this study provide further reassurance for patients (usually young adults in working age) receiving transcatheter ASD closure by demonstrating a low rate of migraine events beyond 3 months following transcatheter ASD closure and supporting the early discontinuation of clopidogrel therapy if administered.
Trial Protocol
Data Sharing Statement
References
- 1.Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K; Amplatzer Investigators . Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39(11):1836-1844. doi: 10.1016/S0735-1097(02)01862-4 [DOI] [PubMed] [Google Scholar]
- 2.Rodés-Cabau J, Mineau S, Marrero A, et al. Incidence, timing, and predictive factors of new-onset migraine headache attack after transcatheter closure of atrial septal defect or patent foramen ovale. Am J Cardiol. 2008;101(5):688-692. doi: 10.1016/j.amjcard.2007.10.034 [DOI] [PubMed] [Google Scholar]
- 3.Mortelmans K, Post M, Thijs V, Herroelen L, Budts W. The influence of percutaneous atrial septal defect closure on the occurrence of migraine. Eur Heart J. 2005;26(15):1533-1537. doi: 10.1093/eurheartj/ehi170 [DOI] [PubMed] [Google Scholar]
- 4.Kato Y, Kobayashi T, Ishido H, Hayashi T, Furuya D, Tanahashi N. Migraine attacks after transcatheter closure of atrial septal defect. Cephalalgia. 2013;33(15):1229-1237. doi: 10.1177/0333102413490350 [DOI] [PubMed] [Google Scholar]
- 5.Rodés-Cabau J, Horlick E, Ibrahim R, et al. Effect of clopidogrel and aspirin vs aspirin alone on migraine headaches after transcatheter atrial septal defect closure: the CANOA Randomized Clinical Trial. JAMA. 2015;314(20):2147-2154. doi: 10.1001/jama.2015.13919 [DOI] [PubMed] [Google Scholar]
- 6.Headache Classification Subcommittee of the International Headache Society . The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24 suppl 1:9-160. doi: 10.1111/j.1468-2982.2003.00824.x [DOI] [PubMed] [Google Scholar]
- 7.Burian M, Neumann T, Weber M, et al. Nickel release, a possible indicator for the duration of antiplatelet treatment, from a nickel cardiac device in vivo: a study in patients with atrial septal defects implanted with an Amplatzer occluder. Int J Clin Pharmacol Ther. 2006;44(3):107-112. doi: 10.5414/CPP44107 [DOI] [PubMed] [Google Scholar]
- 8.Pan G, Xie Z-F, Zhang Y, Long S-C, Xu X-P, Zhang Z-W. Platelet activation through the efficacy of aspirin in congenital heart disease patients undergoing transcatheter closure of atrial septal defects or ventricular septal defects. Genet Test Mol Biomarkers. 2014;18(12):832-838. doi: 10.1089/gtmb.2014.0206 [DOI] [PubMed] [Google Scholar]
- 9.Leger CS, DeSouza JFX. Migraine modulation and debut after percutaneous atrial septal defect closure: a review. Front Neurol. 2017;8:68. doi: 10.3389/fneur.2017.00068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sommer RJ, Nazif T, Privitera L, Robbins BT. Retrospective review of thienopyridine therapy in migraineurs with patent foramen ovale. Neurology. 2018;91(22):1002-1009. doi: 10.1212/WNL.0000000000006572 [DOI] [PubMed] [Google Scholar]
- 11.Heitzer T, Rudolph V, Schwedhelm E, et al. Clopidogrel improves systemic endothelial nitric oxide bioavailability in patients with coronary artery disease: evidence for antioxidant and antiinflammatory effects. Arterioscler Thromb Vasc Biol. 2006;26(7):1648-1652. doi: 10.1161/01.ATV.0000225288.74170.dc [DOI] [PubMed] [Google Scholar]
- 12.Krizanic F, Sigler M, Figulla HR. Transvenous closure of patent foramen ovale: preliminary results with a new self-expanding nitinol wire mesh in a Swine model. Cardiol Res Pract. 2009;2009:943453. doi: 10.4061/2009/943453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palmerini T, Della Riva D, Benedetto U, et al. Three, six, or twelve months of dual antiplatelet therapy after DES implantation in patients with or without acute coronary syndromes: an individual patient data pairwise and network meta-analysis of six randomized trials and 11 473 patients. Eur Heart J. 2017;38(14):1034-1043. doi: 10.1093/eurheartj/ehw627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanabe Y, Sato Y, Izumo M, et al. Endothelialization of an amplatzer septal occluder device 6 months post implantation: is this enough time? an in vivo angioscopic assessment. J Invasive Cardiol. 2019;31(2):E44. [PubMed] [Google Scholar]
- 15.Voet A, Luermans JGLM, Thijs V, et al. New-onset and persistent migraine early after percutaneous atrial septal defect closure disappear at follow-up. Acta Clin Belg. 2008;63(4):262-268. doi: 10.1179/acb.2008.048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Data Sharing Statement