Key Points
Question
What is the efficacy and safety of VP-102, a drug-device combination containing cantharidin, 0.7% (w/v), in individuals aged 2 years or older with molluscum contagiosum compared with vehicle?
Findings
In 2 identical phase 3 trials (Cantharidin Application in Molluscum Patients [CAMP-1 and CAMP-2]) with a total of 582 participants, topical application of VP-102 or vehicle every 21 days for a maximum of 4 treatments resulted in complete lesion clearance rates of 46.3% (CAMP-1) and 54.0% (CAMP-2) with VP-102 vs 18% (CAMP-1) and 13% (CAMP-2) with the vehicle. The most common adverse events were primarily mild to moderate and included application site vesicles, pain, and pruritus.
Meaning
The findings of these trials support the efficacy and safety of a proprietary cantharidin-based drug-device combination for treatment of molluscum contagiosum in children and adults.
Abstract
Importance
Molluscum contagiosum (MC) is a common viral skin infection that primarily affects children. Cantharidin, a topical vesicant, has a long history of use for MC in compounded formulations, but the safety and efficacy of doses, regimens, and application methods have not been demonstrated in large-scale trials.
Objective
To determine the safety and efficacy of VP-102, a drug-device combination containing cantharidin, 0.7% (w/v), compared with vehicle in individuals with MC.
Design, Setting, and Participants
Two phase 3, randomized, double-blind, vehicle-controlled trials of identical design (Cantharidin Application in Molluscum Patients [CAMP-1 and CAMP-2]) were conducted in 31 centers across the US. A total of 528 individuals aged 2 years or older with MC participated. CAMP-1 was conducted from March 21 to November 26, 2018, and CAMP-2 was conducted from February 14 to September 26, 2018.
Interventions
Participants were randomized (3:2) to topical application of VP-102 or vehicle to all treatable lesions every 21 days until complete lesion clearance or up to 4 treatments.
Main Outcomes and Measures
The primary efficacy outcome was the proportion of VP-102–treated participants achieving complete clearance of all MC lesions (baseline and new) compared with those who received the vehicle at the end-of-study visit on day 84. Intent-to-treat analysis was conducted for the efficacy population. Secondary efficacy outcomes included the proportion of participants achieving complete clearance of lesions at days 21, 42, and 63. Safety outcomes included assessment of adverse events, including expected local skin reactions.
Results
Of the 528 participants enrolled, 527 received treatment (CAMP-1, n = 265; CAMP-2, n = 262). A total of 267 of 527 participants (50.7%) were male; mean (SD) ages for CAMP-1 and CAMP-2 were 7.5 (5.3) years and 7.4 (8.0) years for the VP-102 groups and 6.3 (4.7) years and 7.3 (6.7) years for the vehicle groups. Treatment with VP-102 demonstrated superior efficacy to vehicle in the percentage of participants with complete clearance of MC lesions at the end of the study visit for CAMP-1 (VP-102: 46.3% vs vehicle: 17.9%; P < .001) and CAMP-2 (VP-102: 54.0% vs vehicle: 13.4%; P < .001). Adverse events were observed in 99% (CAMP-1) and 95% (CAMP-2) of VP-102–treated participants and 73% (CAMP-1) and 66% (CAMP-2) of vehicle-treated participants. The most common adverse events included application site vesicles, pain, pruritus, erythema, and scab. Most adverse events were mild or moderate in severity.
Conclusions and Relevance
In the 2 phase 3 trials reported herein, VP-102 was statistically significantly superior to vehicle in achieving complete clearance of MC lesions at the end of the study visit in both trials, with adverse events that were generally mild to moderate and confined to application sites. These findings show that VP-102 is potentially an effective and safe treatment for MC, a common skin condition with no US Food and Drug Administration–approved treatments.
Trial Registrations
ClinicalTrials.gov Identifiers: NCT03377790 and NCT03377803
These randomized clinical trials examine the efficacy and safety of VP-102, a drug-device combination containing cantharidin, 0.7% (w/v), in treatment of patients with molluscum contagiosum.
Introduction
Molluscum contagiosum (MC) is a common viral cutaneous infection that primarily affects children, immunocompromised patients, and sexually active adults.1 With a prevalence between 5.1% and 11.5% in children aged 0 to 16 years,2 MC is the third most common viral skin infection in children and 1 of the 5 most prevalent skin diseases worldwide.3
Although MC is self-limiting in immunocompetent individuals, the average duration of MC is often measured in years. The largest cohort study in 306 children younger than 15 years documented a mean of 13.3 months to resolution without intervention; 30% of the cases persisted at 1.5 years, and 13% persisted at 2 years.4
A survey of US physicians found that the treatment of MC varies widely.5 The lack of treatment guidelines and categorization of the infection as benign and self-limiting1,5,6,7 may lead physicians to wait for natural clearance.7 Molluscum contagiosum may be associated with pain, itching, eczema, and secondary bacterial infections. Reasons for treatment of MC include alleviating discomfort, reducing risk of autoinoculation and spread to others, preventing scarring, and eliminating social stigma.7,8,9
Although there is no treatment approved by the US Food and Drug Administration for MC, cantharidin, an extract from blister beetles,10 has been used to treat MC for more than 60 years.11 The exact mechanism of action of cantharidin in MC is unknown, although cantharidin causes degradation of desmosomes in the epidermis and blistering, which is hypothesized to promote shedding of infected keratinocytes.12,13 However, the ability of physicians to use cantharidin for the treatment of MC has been restricted owing to its limited availability, lack of formulation standardization, and variations in concentration, treatment durations, and application methods.14 Herein, we present the results of 2 large, vehicle-controlled phase 3 clinical trials (Cantharidin Application in Molluscum Patients [CAMP-1 and CAMP-2]) that evaluated the treatment of MC using VP-102, a proprietary drug-device combination containing cantharidin, 0.7% (w/v), in a shelf-stable formulation following a standardized treatment schedule.
Methods
Two randomized, double-blind, vehicle-controlled, identically designed phase 3 trials were conducted to evaluate VP-102 in MC at 31 unique centers across the US. Research clinical sites included pediatric (10/31 [32.3%]), dermatology (16/31 [51.6%]), and pediatric dermatology (4/31 [12.9%]) clinics and a research clinic (1/31 [3.2%]). A list of the investigators is available in the eAppendix of Supplement 1. CAMP-1 was conducted from March 21 to November 26, 2018, and CAMP-2 was conducted from February 14 to September 26, 2018. The trial protocol is available in Supplement 2.
The studies followed Good Clinical Practice and country-specific laws and regulations. Compliance also conformed with US federal regulatory codes, the Nuremberg Code, and the Declaration of Helsinki.15 Local institutional review boards or ethics committees at each trial center approved the protocol and consent form, oversaw trial conduct, and maintained documentation. Written informed consent was obtained from all adult participants and the parents or guardians of participants younger than 18 years as per the local state regulations. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline for randomized clinical trials.
Participants
Eligible participants were healthy adults and children aged 2 years or older with a clinical diagnosis of MC and treatable lesions, as deemed by the investigator, at screening. Individuals with active atopic dermatitis, nonmucosal genital-area lesions, and inflamed lesions were included. Previous treatment for MC was allowed, but participants could not have received treatment within 14 days before the first treatment visit. Individuals who were systemically immunosuppressed (eg, receiving treatments such as chemotherapy or other nontopical immunosuppressive agents), had conditions of immunosuppression (eg, HIV/AIDS), or had lesions within 10 mm of a mucosal area deemed nontreatable by the investigator at baseline were excluded. Most trial participants were younger than 11 years. Diagnosis of MC could have been completed before the study by a health care professional, with the patient referred into the study and then verified by an investigator before the study start, or the participant could have been diagnosed by the investigator and entered the study the same day. In accordance with clinical practice, MC diagnosis was made clinically, and no biopsy or laboratory assessments were required. Study sites were selected based on the investigators having appropriate clinical expertise to make the clinical diagnosis of MC, and site personnel were trained regarding the characteristics of MC, including the common appearance and location of lesions, as well as differential diagnoses that could be mistaken for MC.
Treatment
VP-102 is a proprietary drug-delivery device combination containing cantharidin, 0.7% (w/v), in a film-forming topical solution. The formulation also includes gentian violet, a surgical dye that facilitates the distinction between treated and untreated lesions during application, as well as denatonium benzoate, a bittering agent to deter potential oral ingestion.
The drug-device combination consists of a glass ampule containing 450 μL of solution within a single-use applicator. When the ampule is crushed, the solution is released to flow through a filter and into the tip of the applicator. VP-102 is stable at room temperature for storage and is manufactured under Good Manufacturing Practices, and the cantharidin used in VP-102 is more than 99% pure. The vehicle treatment was identical to VP-102 in all aspects (eg, applicator, smell, texture, color, appearance, and formulation) except for the lack of the active ingredient, cantharidin. Participants were required to be able to be treated with a maximum of 2 applicators of VP-102 or vehicle per treatment. The number of lesions per treatment was not limited in the trial.
Randomization and Trial Procedures
Randomization was conducted via a centralized, interactive web-response system accessible to all trial sites. Participants were randomly assigned in a 3:2 ratio to receive treatment with VP-102 or vehicle. This ratio was chosen to give participants a greater opportunity to receive active drug. The size of the trials was adjusted to give adequate power in clearance rates between groups, as detailed in the Statistical Analysis section. Siblings and household members enrolled in the trials were randomized to the same treatment group at the study level to maintain blinding of the treatment. Most participants in the trial did not have housemates. Investigators, participants, and caregivers were blinded to the treatment.
Following randomization, participants underwent a screening period of up to 14 days for dermatologic examination and washout of any previous MC treatments. On day 1 (treatment visit 1), participants received their first treatment application (VP-102 or vehicle) in the clinic by an investigator. Thereafter, treatment was applied in the clinic once every 21 ± 4 days until complete lesion clearance was achieved or a maximum of 4 applications was administered (day 1/visit 1, day 21/visit 2, day 42/visit 3, and day 63/visit 4). All lesions (ie, those present at baseline and any newly emergent lesions) were treated without occlusion at each visit. If, at any treatment visit, there were no lesions present, all study activities were conducted but no treatment was applied. Participants were required to attend all study days/visits.
Participants or their caregivers removed the study drug with soap and water 24 hours after treatment or earlier if significant pain, blistering, or other treatment-emergent adverse events (TEAEs) occurred. Treatment-emergent AEs were defined as AEs, including expected local skin reactions (LSRs), that occurred any time after the first treatment application; LSRs at the application site were expected due to the pharmacodynamic reaction of the skin to the active ingredient, cantharidin, a vesicant. All AEs were coded with Medical Dictionary for Regulatory Activities terms. An initial safety evaluation to assess LSRs was completed at a clinic visit 24 hours after the first application of the study drug (day 1/visit 1). Thereafter, evaluations of response to treatment were performed at each clinic visit and via phone at 24 hours, 7 days, and 14 days after each treatment visit to assess LSRs.
To reduce possible functional unblinding and bias resulting from the expected LSRs (eg, blistering and erythema), blinded assessors conducted lesion counts, while separate members of the research team conducted the safety assessments and evaluations of response to treatment. Each blinded assessor was a trained research team member who was unaware of a participant’s ongoing response to treatment. Lesion counts were performed by a blinded assessor at each treatment visit before treatment application. The purpose of the end of study (EOS) visit was to conduct a final evaluation of response to treatment and assess the complete clearance or number of MC lesions present.
Assessments and Outcomes
The primary efficacy end point was the proportion of VP-102–treated participants achieving complete clearance of all treatable (baseline and new) MC lesions relative to those who received vehicle at the day 84/EOS visit in the trials’ intent-to-treat populations (ie, all randomized participants). Secondary efficacy outcomes included the proportion of participants achieving complete clearance of all MC lesions at days 21, 42, and 63. The percentage change of lesions from baseline to each treatment visit was assessed as an exploratory end point.
Secondary objectives included assessments of safety and tolerability of VP-102 in the trials’ safety population (ie, participants who received ≥1 treatment application of VP-102 or vehicle). Safety assessments included monitoring of TEAEs and LSRs, physical examinations, and rates and types of concomitant medication. Tolerability was determined based on discontinuation rates due to AEs related to treatment.
Statistical Analysis
Each study planned to enroll 250 individuals (approximately 150 receiving VP-102 and 100 receiving vehicle) at up to 15 sites per study. Determination of the sample size per trial was based on a Pearson χ2 test with a 2-sided significance level of .05 to give greater than or equal to 95% power to detect treatment differences in the percentage of participants with complete clearance.
For the primary efficacy end point (complete clearance at day 84/EOS), participants who did not have an assessment of status of complete clearance of all treatable lesions at day 84/EOS were considered to have missing data for the primary end point. Participants with missing clearance data at day 84/EOS were considered as not having achieved complete clearance.
For all efficacy variables, data were summarized using descriptive statistics or counts and percentages for the intent-to-treat population. The primary end point and other binary end points (eg, clearance at days 21, 42, and 63) were tested with Pearson χ2 analysis. All statistical tests were 2-sided with a significance level of α = .05. A post hoc sensitivity analysis was completed on the primary end point using logistical regression with a covariate of baseline lesion count. Continuous end points (eg, percentage change in lesion count) were analyzed with an analysis of covariance model. Sequential testing of secondary end points was used to control the overall study-wide α level. Statistical analyses were performed with the SAS statistical software package, version 9.3 (SAS Institute Inc). Most households contained only 1 individual who participated in the study (86.5% in CAMP-1 and 77.1% in CAMP-2); therefore, no adjustment was made for a cluster effect.
Results
Participants
A total of 528 participants were enrolled in the 2 trials (Figure 1). One individual did not meet eligibility criteria after enrollment/randomization and was excluded from the study before treatment. In CAMP-1, 266 individuals enrolled, with 160 randomized to VP-102 and 106 randomized to vehicle. For CAMP-2, a total of 262 individuals enrolled, with 150 randomized to VP-102 and 112 randomized to vehicle. In CAMP-1, 93.8% of VP-102 and 94.3% of vehicle-treated participants completed the trial. In CAMP-2, completion rates were 92.7% of the VP-102 group and 96.4% of the vehicle group.
Figure 1. CONSORT (Consolidated Standards of Reporting Trials) Flow Diagram of Participant Disposition in Cantharidin Application in Molluscum Patients (CAMP-1 and CAMP-2) Trials (Intent-to-Treat Population).
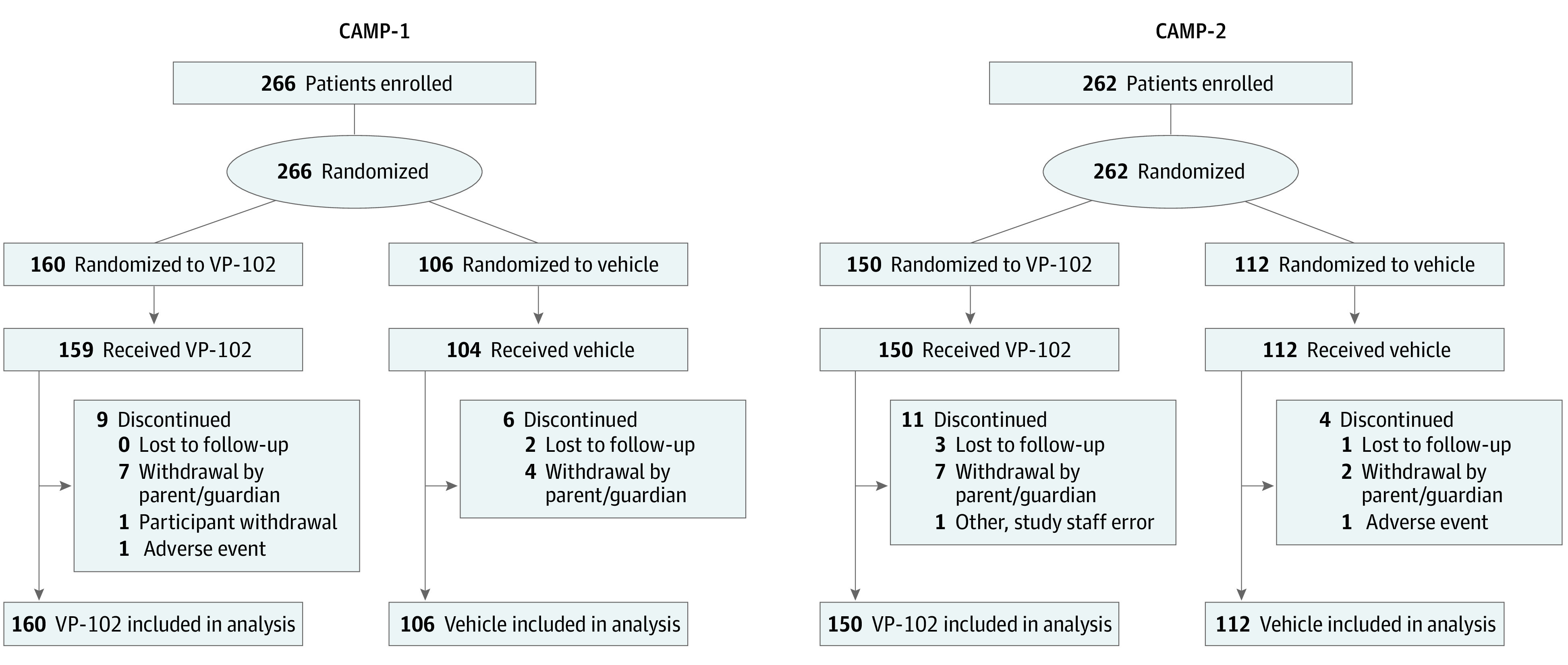
After randomization, one participant in the VP-102 group was found not to have met eligibility requirements and was not treated, although the participant was included in the intent-to-treat efficacy analyses. Two separate participants were not linked to housemates who were in the VP-102 group and were incorrectly randomized to the vehicle group. Both participants received treatment with VP-102 (consistent with their housemate’s group) and were included in safety analyses.
The demographic characteristics and MC histories at baseline did not differ significantly between treatment groups for all listed characteristics in CAMP-1 and CAMP-2 (Table 1). A total of 267 participants in the safety group (50.7%) were male and 260 participants (49.3%) were female. Most participants (471/527 [89.4%]) were aged 2 to 11 years (mean [SD] ages for CAMP-1 and CAMP-2 were 7.5 [5.3] years and 7.4 [8.0] years for the VP-102 groups and 6.3 [4.7] years and 7.3 [6.7] years for the vehicle groups). Time since diagnosis ranged from 1 to 1302 days across both studies (Table 1). The proportion of all participants who had previously received 1 or more treatments for MC was 30.6% (161/527). A history of atopic dermatitis was reported for 16.1% (85/527) of all participants, with 8.2% (43/527) having active atopic dermatitis as determined by concomitant medication use during the study. The mean (SD) lesion counts for CAMP-1 and CAMP-2 for the VP-102 groups were 21.9 (23.06; range, 1-107) and 18.7 (22.9; range, 1-184), and for the vehicle groups were 25.2 (25.0; range, 1-110) and 20.3 (19.3; range, 1-86).
Table 1. Baseline Demographic Characteristics and History of MC in CAMP-1 and CAMP-2 Participantsa.
| Characteristic | CAMP -1 | CAMP-2 | ||
|---|---|---|---|---|
| VP-102 (n = 161) | Vehicle (n = 104) | VP-102 (n = 150) | Vehicle (n = 112) | |
| Age, y | ||||
| Mean (SD) | 7.5 (5.3) | 6.3 (4.7) | 7.4 (8.0) | 7.3 (6.7) |
| Median (range) | 6.0 (2-41) | 5.0 (2-40) | 6.0 (2-60) | 6.0 (2-54) |
| Age group, No. (%), y | ||||
| ≥2-5 | 66 (41.0) | 53 (51.0) | 72 (48.0) | 52 (46.4) |
| ≥6-11 | 73 (45.3) | 41 (39.4) | 66 (44.0) | 48 (42.9) |
| ≥12-18 | 16 (9.9) | 9 (8.7) | 7 (4.7) | 8 (7.1) |
| ≥19 | 6 (3.7) | 1 (1.0) | 5 (3.3) | 4 (3.6) |
| Sex, No. (%) | ||||
| Female | 86 (53.4) | 59 (56.7) | 69 (46.0) | 46 (41.1) |
| Male | 75 (46.6) | 45 (43.3) | 81 (54.0) | 66 (58.9) |
| Race/ethnicity, No. (%) | ||||
| White | 135 (83.9) | 97 (93.3) | 142 (94.7) | 104 (92.9) |
| Black or African American | 11 (6.8) | 4 (3.8) | 3 (2.0) | 3 (2.7) |
| Asian | 4 (2.5) | 1 (1.0) | 2 (1.3) | 0 |
| American Indian/Alaskan Native | 0 | 0 | 0 | 1 (0.9) |
| Other | 11 (6.8) | 2 (1.9) | 3 (2.0) | 4 (3.6) |
| Baseline lesion count, No. | ||||
| Mean (SD) | 21.9 (23.6) | 25.2 (25.0) | 18.7 (22.9) | 20.3 (19.3) |
| Median (range) | 14.0 (1-107) | 18.0 (1-110) | 10.0 (1-184) | 14.0 (1-86) |
| Time since MC clinical diagnosis, d | ||||
| Mean (SD) | 128.0 (221.6) | 128.6 (206.6) | 118.3 (176.0) | 124.0 (193.1) |
| Median (range) | 26.0 (1-1247) | 32.0 (1-1302) | 28.0 (1-977) | 30.5 (1-957) |
| Age at diagnosis, y | ||||
| Mean (SD) | 7.1 (5.3) | 6.0 (4.7) | 7.1 (8.1) | 7.0 (6.8) |
| Median (range) | 6.0 (1-41) | 5.0 (1-40) | 5.0 (1-60) | 5.0 (1-54) |
| Previous treatment for MC, No. (%) | 42 (26.1) | 29 (27.9) | 48 (32.0) | 42 (37.5) |
| Atopic dermatitis, No. (%) | ||||
| Diagnosis | 31 (19.3) | 24 (23.1) | 19 (12.7) | 11 (9.8) |
| Activeb | 12 (7.5) | 13 (12.5) | 11 (7.3) | 7 (6.3) |
| Household information, No. (%)c | ||||
| Total households | 137 | 92 | 123 | 82 |
| 1 Member | 117 (85.4) | 81 (88.0) | 100 (81.3) | 58 (70.7) |
| 2 Members | 17 (12.4) | 9 (9.8) | 19 (15.4) | 19 (23.2) |
| 3 Members | 3 (2.2) | 1 (1.1) | 4 (3.3) | 4 (4.9) |
| 4 Members | 0 | 1 (1.1) | 0 | 1 (1.2) |
Abbreviations: CAMP, Cantharidin Application in Molluscum Patients; MC, molluscum contagiosum.
Safety population.
Active atopic dermatitis was determined by use of concomitant medications during the study, including topical calcineurin inhibitors, topical corticosteroids, and topical phosphodiesterase-4 inhibitors.
Percentage by total number of households; all members with MC in the household who were part of the trial are included.
Outcomes
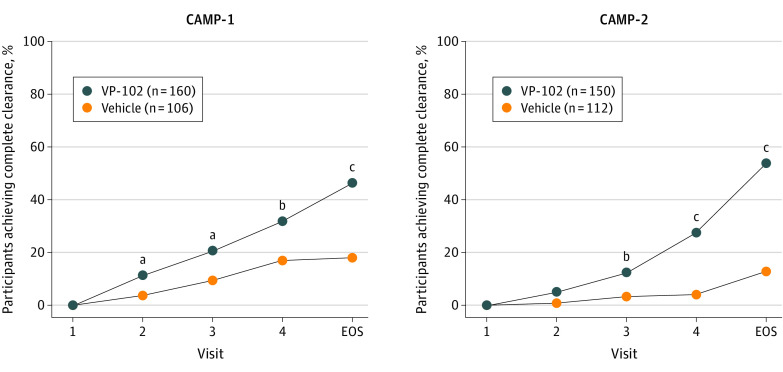
For the primary efficacy outcome, VP-102 demonstrated statistically superior efficacy compared with vehicle in the percentage of participants achieving complete clearance of all (baseline and new) treatable MC lesions on day 84/EOS (Figure 2). In CAMP-1, the percentage of participants with complete clearance at day 84 was 46.3% for VP-102 vs 17.9% for vehicle (P < .001); in CAMP-2, the percentages were 54.0% for VP-102 vs 13.4% for vehicle (P < .001) (Figure 2). The post hoc sensitivity analyses findings using logistical regression with a covariate of baseline lesion count were consistent with the primary analysis findings (eTable in Supplement 1).
Figure 2. Percentage of Participants With Complete Clearance of Baseline and New Molluscum Contagiosum Lesions After Treatments With VP-102 or Vehicle (Statistical Comparisons at Day 21, 42, 63, or 84).
Complete clearance of all treatable lesions (baseline and new) at individual time points after treatment, treated as binary end points and tested with a Pearson χ2 test. All statistical tests were 2-sided with a significance level of α = .05. P values were completed for the intent-to-treat population at individual time points, where independent assessments were performed at days 21, 42, 63, and 84, and not meant to imply statistical significance between those time points. Individual lesions were not tracked, and total lesion counts included any treatable (baseline and new) lesions that remained on the day/visit after baseline. If participant lesions cleared before the end of study (EOS), they were required to stay clear until the EOS visit to be counted as completely clear. At the EOS visit, 46.3% of participants in the VP-102 group and 17.9% in the vehicle group were completely clear of all lesions for Cantharidin Application in Molluscum Patients (CAMP)-1 (P < .001). In CAMP-2, 54.0% of VP-102 participants vs 13.4% of the vehicle participants were completely clear at the EOS visit (P < .001). While the methods for the trials were identical, clinical sites and participants were not the same between trials, and thus there is natural variability in results for each group from one trial to the other.
aP < .05.
bP < .01.
cP < .001
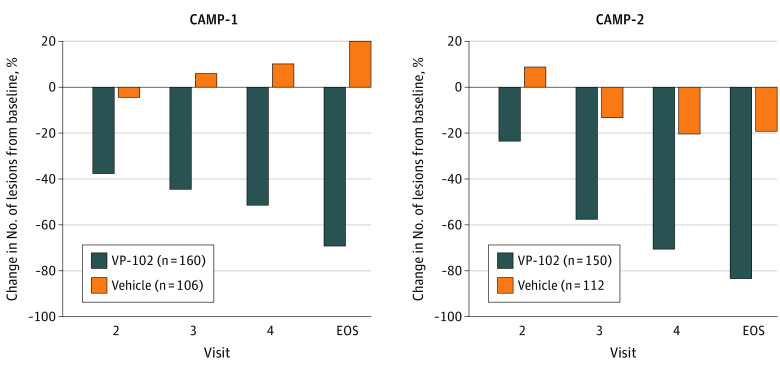
In the secondary outcomes, significant differences in favor of VP-102 in the percentage of participants achieving complete lesion clearance were observed after a single treatment at day 21/visit 2 for CAMP-1 (VP-102: 11.3% vs vehicle: 3.7%, P = .03) and for all subsequent time points for CAMP-1 (day 42, VP-102: 20.6% vs vehicle, 9.4%, P = .02; day 63: VP-102: 31.9% vs vehicle: 17%, P < .01) and CAMP-2 (day 42, VP-102: 12.7% vs vehicle: 3.6%, P < .01; day 63, VP-102: 28% vs vehicle: 4.5%, P < .001) (Figure 2). At day 84/EOS, VP-102–treated participants had experienced a mean percent decrease in lesions from baseline of 69% for CAMP-1 and 83% for CAMP-2 compared with a 20% increase and a 19% decrease in lesions from baseline for vehicle for each trial (P<.05 for both comparisons of VP-102 vs vehicle) (Figure 3).
Figure 3. Percentage Change From Baseline in Lesion Counts After Treatment With VP-102 or Vehicle (Statistical Comparisons at Day 21, 42, 63, or 84).
Lesion counts were taken at each time point/visit (baseline, day 21/visit 2, day 42/visit 3, day 63/visit 4, and day 84/the end of study (EOS) visit, intent-to-treat population). Lesion counts at each visit after baseline were compared with baseline to determine percentage change. Percentages were analyzed with an analysis of covariance model. VP-102 showed significant differences (P < .001) in percentage change in lesion count from baseline compared with vehicle at each time point/visit.
All safety analyses were conducted for the safety population. A total of 96.8% (301/311) of the VP-102 group and 58.8% (127/216) of the vehicle group experienced at least 1 TEAE considered to be related to the study drug. Most TEAEs were of mild or moderate severity and were primarily expected LSR TEAEs (Table 2). The most frequent TEAEs were application site vesicles, pain, pruritus, erythema, and scab.
Table 2. Incidence of TEAEs and TEAEs Leading to Discontinuation of Study Drug in CAMP-1 and CAMP-2.
| Variable | VP-102 | Vehicle | Discontinued treatment | |||||
|---|---|---|---|---|---|---|---|---|
| Mild | Moderate | Severe | Mild | Moderate | Severe | VP-102 | Vehicle | |
| CAMP-1 a | ||||||||
| Participants with ≥1 TEAE, No. (%) | 157 (97.5) | 105 (65.2) | 19 (11.8) | 66 (63.5) | 41 (39.4) | 1 (1.0) | 5 (3.1) | 0 |
| LSR TEAEs ≥5%, No. (%) | ||||||||
| Vesicles | 79 (49.1) | 70 (43.5) | 8 (5.0) | 25 (24.0) | 4 (3.8) | 0 | 4 (2.5) | 0 |
| Pruritus | 85 (52.8) | 18 (11.2) | 1 (0.6) | 33 (31.7) | 5 (4.8) | 0 | 0 | 0 |
| Pain | 58 (36.0) | 45 (28.0) | 7 (4.3) | 18 (17.3) | 2 (1.9) | 0 | 2 (1.2) | 0 |
| Discoloration | 48 (29.8) | 5 (3.1) | 1 (0.6) | 16 (15.4) | 2 (1.9) | 0 | 0 | 0 |
| Scab | 46 (28.6) | 16 (9.9) | 0 | 24 (23.1) | 1 (1.0) | 0 | 0 | 0 |
| Erythema | 32 (19.9) | 37 (23.0) | 0 | 21 (20.2) | 9 (8.7) | 0 | 0 | 0 |
| Dryness | 23 (14.3) | 1 (0.6) | 0 | 11 (10.6) | 0 | 0 | 0 | 0 |
| Edema | 15 (9.3) | 6 (3.7) | 0 | 4 (3.8) | 2 (1.9) | 0 | 0 | 0 |
| Erosion | 9 (5.6) | 1 (0.6) | 0 | 2 (1.9) | 0 | 0 | 0 | 0 |
| TEAE not at application site leading to study drug discontinuation, No. (%) | ||||||||
| Contact dermatitisb | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.6) | 0 |
| CAMP-2 c | ||||||||
| Participants with ≥1 TEAE, No. (%) | 141 (94.0) | 60 (40.0) | 4 (2.7) | 74 (66.1) | 18 (16.1) | 0 | 1 (0.7) | 1 (0.9) |
| LSR TEAEs ≥5%, No. (%) | ||||||||
| Vesicles | 108 (72.0) | 30 (20.0) | 3 (2.0) | 34 (30.4) | 0 | 0 | 1 (0.7) | 0 |
| Scab | 74 (49.3) | 11 (7.3) | 0 | 20 (17.9) | 2 (1.8) | 0 | 0 | 0 |
| Pain | 69 (46.0) | 14 (9.3) | 0 | 16 (14.3) | 0 | 0 | 1 (0.7) | 0 |
| Pruritus | 60 (40.0) | 5 (3.3) | 0 | 29 (25.9) | 8 (7.1) | 0 | 1 (0.7) | 0 |
| Erythema | 41 (27.3) | 28 (18.7) | 1 (0.7) | 22 (19.6) | 6 (5.4) | 0 | 0 | 0 |
| Discoloration | 39 (26.0) | 7 (4.7) | 0 | 9 (8.0) | 0 | 0 | 0 | 0 |
| Dryness | 35 (23.3) | 4 (2.7) | 0 | 19 (17.0) | 1 (0.9) | 0 | 0 | 0 |
| Erosion | 11 (7.3) | 1 (0.7) | 0 | 0 | 0 | 0 | 0 | 0 |
| TEAE not at application site leading to study drug discontinuation, No. (%) | ||||||||
| Gianotti Crosti syndromeb | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (0.9) |
Abbreviations: CAMP, Cantharidin Application in Molluscum Patients; LSR, local skin reaction; TEAEs, treatment-emergent adverse events.
Safety population: VP-102, n = 161; vehicle, n = 104.
Determined to not be related to the study drug.
Safety population: VP-102, n = 150; vehicle, n = 112.
VP-102 was well tolerated in both trials, as evidenced by the rate of discontinuation of treatment due to AEs in this largely pediatric population. Adverse events were observed in 99% (CAMP-1) and 95% (CAMP-2) of VP-102–treated patients and 73% (CAMP-1) and 66% (CAMP-2) of vehicle-treated participants. The trials were completed by 93.2% (289/310) of participants who received VP-102 and 95.4% (208/218) of those who received vehicle. The proportion of participants with at least 1 TEAE leading to study drug discontinuation was 1.9% (6/311) for the VP-102 group and 0.5% (1/216) for the vehicle group (Table 2). Of the 6 discontinuations in the VP-102 group, 5 participants discontinued treatment due to TEAEs at the application site, and 1 discontinued treatment due to contact dermatitis not at the application site. One participant in the vehicle group discontinued treatment after developing Gianotti Crosti syndrome, which was deemed to be unrelated to treatment.
No deaths occurred during the studies. One serious AE, appendicitis, was reported in a vehicle-treated participant and was considered to be unrelated to treatment. Adverse events potentially associated with infection were reported with a low frequency and were well balanced between the VP-102 and vehicle treatment groups. There was no evidence of TEAEs suggestive of systemic absorption of topically administered cantharidin, as all TEAEs considered to be related to the study drug were confined to the sites of application on the skin.
Discussion
In healthy individuals, the average number of MC lesions ranges between 10 and 20. Occasionally, lesions can present in the hundreds with widespread distribution; those with concomitant atopic dermatitis may have higher lesion counts, longer disease duration, or MC-triggered atopic dermatitis flares.1,16 Inflammatory reactions are common with MC infection, with one study reporting molluscum dermatitis in 38.5%, inflamed MC lesions in 22.3%, and a Gianotti Crosti syndrome–like reaction in 4.9% of children with MC infection. Atopic dermatitis is also associated with a higher incidence of molluscum dermatitis.16 It is therefore essential that physicians have a safe and effective way to treat patients with MC, including those with concomitant atopic dermatitis.17
Most of the data about cantharidin treatment in MC come from retrospective studies conducted with a compounded formulation. The results of these studies vary widely because of differences in cohort sizes, study durations, treatment regimens, and formulations of the compounded product.6,13,18,19 To our knowledge, there are only 2 published reports of prospective controlled trials. The first failed to find a significant difference between compounded cantharidin and vehicle,20 and the second reported superior efficacy compared with vehicle.12 The 2 studies faced similar limitations: compounded preparations, small cohorts, and differences in dosing, treatment durations, and methods of delivery. Our trials were designed to control for several of these variables by using VP-102, a commercially manufactured cantharidin formulation with a precision applicator in a large patient cohort and with a duration sufficient to demonstrate a response to treatment.
Treatment with VP-102 resulted in a statistically significantly higher percentage of participants with complete clearance of all baseline and new lesions at EOS in both trials compared with vehicle. The most commonly observed AEs were confined to treatment application sites, which is consistent with previous studies using cantharidin.13,18,20 The observed LSRs are well-known, reversible reactions related to the pharmacodynamic response of the skin to cantharidin, a vesicant. Local skin reactions were generally temporary and mild or moderate in intensity.12,13 Treatment was well tolerated as evidenced by a low discontinuation rate of medication due to AEs and lack of serious AEs in this largely pediatric study population.
While the effects of the excipients of the vehicle on MC are unknown, we believe that the vehicle did not have an effect on clearance rates given the short duration during which it was applied and that the clearance rates for vehicle were low and fit with expectations based on spontaneous clearance observed in large epidemiologic studies.2 The incidence of AEs in the vehicle group mirrors findings in patients with MC throughout the natural disease progression of lesions, including erythema, scabbing, pain, and pruritus.13,19 The vesiculation rate in the vehicle group replicates the findings in the placebo group of another study with compounded cantharidin and may be attributed to inflamed lesions or the flexible film having the appearance of a blister.20
Limitations
The trials had several limitations. Early recruitment numbers or reasons for disqualification for the study were not tracked at the study level. The primary end point was complete clearance of all baseline and new lesions and did not track the treatment of individual lesions, so it is not possible to determine the exact number of treatments needed to clear an individual lesion. Treatment with cantharidin is commonly continued until the skin is clear of MC lesions,21 but this study allowed a maximum of 4 treatments. Individuals with lesions within 10 mm of mucosal areas were included only if the investigator deemed them to be safe to treat, which could have potentially limited the enrollment of individuals with sexually transmitted disease. Individuals who were immunocompromised were excluded, and the studies enrolled a predominantly pediatric population, which may limit extrapolation of the results to adult patients. The study did not diagnose atopic dermatitis or track the location and severity of flares, so we are unable to make conclusions about the safety and efficacy of treatment with comorbid atopic dermatitis at or around the MC lesion sites. Further studies are required to determine safety and efficacy in these groups. Efficacy analyses did not adjust for clustering for siblings and households.
Conclusions
To date, the CAMP studies are the first large controlled trials of cantharidin with a consistent formulation, dosing schedule, and method of application for treatment of MC. These trials provide robust efficacy and safety data that support the use of VP-102 for the treatment of MC in participants aged 2 years and older.
eTable. Statistical Analyses of Percentage of Participants with Complete Clearance of all Baseline and New Lesions
eAppendix. Investigator List
eTable. Statistical Analyses of Percentage of Participants with Complete Clearance of all Baseline and New Lesions
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement
References
- 1.Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infect Dis. 2013;13(10):877-888. doi: 10.1016/S1473-3099(13)70109-9 [DOI] [PubMed] [Google Scholar]
- 2.Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Fam Pract. 2014;31(2):130-136. doi: 10.1093/fampra/cmt075 [DOI] [PubMed] [Google Scholar]
- 3.Shisler JL. Immune evasion strategies of molluscum contagiosum virus. Adv Virus Res. 2015;92:201-252. doi: 10.1016/bs.aivir.2014.11.004 [DOI] [PubMed] [Google Scholar]
- 4.Olsen JR, Gallacher J, Finlay AY, Piguet V, Francis NA. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infect Dis. 2015;15(2):190-195. doi: 10.1016/S1473-3099(14)71053-9 [DOI] [PubMed] [Google Scholar]
- 5.Hughes CM, Damon IK, Reynolds MG. Understanding US healthcare providers’ practices and experiences with molluscum contagiosum. PLoS One. 2013;8(10):e76948. doi: 10.1371/journal.pone.0076948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanna D, Hatami A, Powell J, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatr Dermatol. 2006;23(6):574-579. doi: 10.1111/j.1525-1470.2006.00313.x [DOI] [PubMed] [Google Scholar]
- 7.Silverberg N. Pediatric molluscum contagiosum: optimal treatment strategies. Paediatr Drugs. 2003;5(8):505-512. doi: 10.2165/00148581-200305080-00001 [DOI] [PubMed] [Google Scholar]
- 8.van der Wouden JC, van der Sande R, Kruithof EJ, Sollie A, van Suijlekom-Smit LW, Koning S. Interventions for cutaneous molluscum contagiosum. Cochrane Database Syst Rev. 2017;5:CD004767. doi: 10.1002/14651858.CD004767.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leung AK. The natural history of molluscum contagiosum in children. Lancet Infect Dis. 2015;15(2):136-137. doi: 10.1016/S1473-3099(14)71061-8 [DOI] [PubMed] [Google Scholar]
- 10.Wang GS. Medical uses of mylabris in ancient China and recent studies. J Ethnopharmacol. 1989;26(2):147-162. doi: 10.1016/0378-8741(89)90062-7 [DOI] [PubMed] [Google Scholar]
- 11.Forbat E, Al-Niaimi F, Ali FR. Molluscum contagiosum: review and update on management. Pediatr Dermatol. 2017;34(5):504-515. doi: 10.1111/pde.13228 [DOI] [PubMed] [Google Scholar]
- 12.Guzman AK, Schairer DO, Garelik JL, Cohen SR. Safety and efficacy of topical cantharidin for the treatment of pediatric molluscum contagiosum: a prospective, randomized, double-blind, placebo-controlled pilot trial. Int J Dermatol. 2018;57(8):1001-1006. doi: 10.1111/ijd.14079 [DOI] [PubMed] [Google Scholar]
- 13.Cathcart S, Coloe J, Morrell DS. Parental satisfaction, efficacy, and adverse events in 54 patients treated with cantharidin for molluscum contagiosum infection. Clin Pediatr (Phila). 2009;48(2):161-165. doi: 10.1177/0009922808326085 [DOI] [PubMed] [Google Scholar]
- 14.Agnetta V, Torres A, Desai SR, Hebert AA, Kircik LH. Compounding in dermatology update. J Drugs Dermatol. 2020;19(2):S18-S23. [Google Scholar]
- 15.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 16.Berger EM, Orlow SJ, Patel RR, Schaffer JV. Experience with molluscum contagiosum and associated inflammatory reactions in a pediatric dermatology practice: the bump that rashes. Arch Dermatol. 2012;148(11):1257-1264. doi: 10.1001/archdermatol.2012.2414 [DOI] [PubMed] [Google Scholar]
- 17.Kaufman WS, Ahn CS, Huang WW. Molluscum contagiosum in immunocompromised patients: AIDS presenting as molluscum contagiosum in a patient with psoriasis on biologic therapy. Cutis. 2018;101(2):136-140. [PubMed] [Google Scholar]
- 18.Moye VA, Cathcart S, Morrell DS. Safety of cantharidin: a retrospective review of cantharidin treatment in 405 children with molluscum contagiosum. Pediatr Dermatol. 2014;31(4):450-454. doi: 10.1111/pde.12276 [DOI] [PubMed] [Google Scholar]
- 19.Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. J Am Acad Dermatol. 2000;43(3):503-507. doi: 10.1067/mjd.2000.106370 [DOI] [PubMed] [Google Scholar]
- 20.Coloe Dosal J, Stewart PW, Lin JA, Williams CS, Morrell DS. Cantharidin for the treatment of molluscum contagiosum: a prospective, double-blinded, placebo-controlled trial. Pediatr Dermatol. 2014;31(4):440-449. doi: 10.1111/j.1525-1470.2012.01810.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatr Dermatol. 2009;26(4):405-408. doi: 10.1111/j.1525-1470.2008.00860.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Statistical Analyses of Percentage of Participants with Complete Clearance of all Baseline and New Lesions
eAppendix. Investigator List
eTable. Statistical Analyses of Percentage of Participants with Complete Clearance of all Baseline and New Lesions
Trial Protocol and Statistical Analysis Plan
Data Sharing Statement