Interferon gamma (IFN-γ) release assays (IGRAs) are increasingly used to test for latent tuberculosis (TB) infection. Although highly specific, IGRAs have a relatively high false-negative rate in active TB patients. A more sensitive assay is needed. IFN-γ-induced protein 10 (IP-10) is an alternative biomarker with a 100-fold-higher expression level than IFN-γ, allowing for different analysis platforms, including molecular detection. The PCR technique is already an integrated tool in most TB laboratories and, thus, an obvious platform to turn to.
KEYWORDS: IP-10, diagnosis, mRNA, qPCR, tuberculosis
ABSTRACT
Interferon gamma (IFN-γ) release assays (IGRAs) are increasingly used to test for latent tuberculosis (TB) infection. Although highly specific, IGRAs have a relatively high false-negative rate in active TB patients. A more sensitive assay is needed. IFN-γ-induced protein 10 (IP-10) is an alternative biomarker with a 100-fold-higher expression level than IFN-γ, allowing for different analysis platforms, including molecular detection. The PCR technique is already an integrated tool in most TB laboratories and, thus, an obvious platform to turn to. In this case-control study, we investigated the diagnostic sensitivity and specificity of a molecular assay detecting IP-10 mRNA expression following antigen stimulation of a blood sample. We included 89 TB patients and 99 healthy controls. Blood was drawn in QuantiFeron-TB gold in-tube (QFT) assay tubes. Eight hours poststimulation, IP-10 mRNA expression was analyzed, and 20 h poststimulation, IP-10 and IFN-γ protein plasma levels were analyzed using an in-house IP-10 enzyme-linked immunosorbent assay (ELISA) and the official QFT ELISA, respectively. The IP-10 mRNA assay provided high specificity (98%), sensitivity (80%), and area under the concentration-time curve (AUC) (0.97); however, the QFT assay provided a higher overall diagnostic potential, with specificity of 100%, sensitivity of 90%, and AUC of 0.99. The IP-10 protein assay performed on par with the QFT assay, with specificity of 98%, sensitivity of 87%, and AUC of 0.98. We have provided proof of high technical performance of a molecular assay detecting IP-10 mRNA expression. As a diagnostic tool, this assay would gain from further optimization, especially on the kinetics of IP-10 mRNA expression.
INTRODUCTION
The 2016 United Nations Millennium Development goals boldly aim for a tuberculosis (TB)-free world by 2035. Similar optimistic targets were recently proposed by the World Health Organization in the End TB strategy (1). With 10 million new cases of TB annually, a surge in antibiotic resistance and an estimated 2 to 3 billion people already infected with Mycobacterium tuberculosis, reaching these optimistic goals will require innovation in treatment, vaccines, and diagnostics, as well as better use of the currently available tools (2–4). One such approach launched by the WHO was a policy change with a renewed focus on preventive treatment of latent M. tuberculosis infection in low-income and high-burden countries. Rational use of preventive treatment is an effective strategy to prevent progression to active TB in people at risk; however, the currently available tools have several limitations and new initiatives are needed (2).
Currently, preventive treatment is guided by tuberculin skin test (TST) and interferon gamma (IFN-γ) release assay (IGRA) testing. The TST is an in vivo diagnostic test that measures a delayed-type hypersensitivity response in the skin 48 to 72 h after intradermal injection of antigens specific for mycobacteria. A major limitation of TSTs is cross-reactivity to the Mycobacterium bovis BCG vaccine and environmental mycobacteria, which renders the test unspecific in many cases (5, 6). The IGRAs are in vitro diagnostic alternatives to TSTs. These tests are based on stimulation of peripheral T cells found in the blood with M. tuberculosis-specific antigens and do not deliver false-positive results after BCG vaccination or exposure to most environmental mycobacteria (7–9). Although highly specific, IGRAs have a relatively high false-negative rate in active TB patients (10–13). In terms of diagnostic performance, both IGRA and TST have reduced diagnostic sensitivity in young children and people with immunosuppression, e.g., due to HIV infection, corticosteroid treatment, and other immunosuppressant drugs, although IGRAs perform better than TST in immunosuppressed individuals (6, 14).
IFN-γ-induced protein 10 (IP-10) is an alternative biomarker to IFN-γ in the IGRA test format (15). IP-10 is expressed in concert with IFN-γ but at 100-fold-higher levels (16, 17). The relative abundance of IP-10 allows for innovation in detection methods, including lateral flow, dried blood spots, and molecular methods not available for IFN-γ (17, 18). In addition, IP-10 seems to improve diagnostic performance by improving the detection of infection in children, HIV-infected individuals, and rheumatic patients receiving immunosuppressant therapies (19–24).
We recently established proof-of-concept for a rapid molecular detection platform for IP-10 mRNA in the IGRA test format (25). This study aims to investigate the diagnostic sensitivity and specificity of this method in a case-control study conducted in a clinical routine laboratory. Due to the absence of a gold standard test for latent M. tuberculosis infection, we conducted a case-control study comparing TB patients with confirmed TB disease to controls from a setting with low endemicity who had no known exposure (26–28).
MATERIALS AND METHODS
Ethical statement.
The study was approved by the ethical review board of the Ethics Committee of the Hospital Germans Trias i Pujol and subsequently for all the Ethics Committees of all the health care centers participating (reference number CEI_PI-15-073) and by the ethical review board of the Capital Region of Copenhagen (reference number H-3-2012-008). Written consent was obtained from all patients and controls enrolled in this study.
Study population.
For this study, we enrolled 95 TB patients through five different health care centers in Barcelona, Spain. We further enrolled 100 healthy controls with negative QuantiFeron-TB gold in-tube (QFT; Qiagen, Hilden, Germany) results at Statens Serum Institut in Denmark, of which only five had previous known exposure to M. tuberculosis. Such enrollment was done from February 2015 to March 2016.
Whole-blood stimulation.
Whole blood was drawn in the nil, TB antigen (Ag), and mitogen QFT tubes according to the manufacturer’s protocol. As the enrollment of the TB patients was performed at five different locations, the samples were transported to the main laboratory in a portable 37°C incubator. After arrival at the main laboratory, the tubes were transferred to a stationary 37°C incubator. Enrolment of healthy controls was performed at the site of the laboratory, so samples were placed directly in a stationary 37°C incubator. Whole blood was incubated for 20 h before plasma isolation. Plasma was stored at −20°C before analysis.
RNA extraction.
Based on our previous study (25), the optimal time for RNA extraction was considered to be 8 h after stimulation. Therefore, after 8 h of incubation, 250 μl whole blood was removed from each tube and total RNA was isolated using the HighPure RNA isolation kit (Roche, Schlieren, Switzerland) according to the manufacturers’ protocol. In brief, whole-blood cells were lysed and total RNA was column purified. The DNase step was included to limit contamination from DNA. Total RNA was eluted in 50 μl elution buffer and stored at −80°C. Due to the very small volume of blood extracted, it was not possible to do a contamination assessment (RNA/DNA) or a purity analysis. To limit sample variability, mRNA isolation was done from an aliquot of the same blood sample that would undergo a 20-h incubation period for protein detection.
Multiplex RT-qPCR.
RT-qPCR was performed with the extracted RNA as the template, using primers and hydrolysis probes specific for the IP-10 target gene (accession number NM_001565.3), with the β-actin gene (accession number NM_001101.3) as the reference gene, using the HawkZ05 fast one-step RT-PCR kit (Roche Custom Biotech, Mannheim, Germany) according to the manufacturer’s protocol. Briefly, 4 μl total RNA was used as the template in a total reaction mixture volume of 20 μl. The reaction mixture contained a final manganese acetate concentration of 1.5 mM. The primer and probe sequences and concentrations were as follows: IP-10 forward, 5′-TGT CCA CGT GTT GAG ATC ATT G-3′, and IP-10 reverse, 5′-GGC CTT CGA TTC TGG ATT CA-3′, 0.3 mM, 75 bp; IP-10 probe, FAM-5′-TAC AAT GAA AAA GAA GGG TGA GAA-39-MGB, 0.2 mM; β-actin forward, 5′-AGC CTC GCC TTT GCC GA-3′, and β-actin reverse, 5′-CTG GTG CCT GGG GCG-3′, 0.5 mM, 174 bp; and β-actin probe, HEX-5′-CCG CCG CCC GTC CAC ACC CGC C-3′-BHQ-1, 0.05 mM (FAM, 6-carboxyfluorescein; MGB, minor groove binder; HEX, 6-carboxy-2,4,4,5,7,7-hexachlorofluorescein; BHQ, black hole quencher).
The reverse transcription (RT)-qPCR parameters for all targets were 5 min at 55°C, 5 min at 60°C, and 5 min at 65°C for the reverse transcription step, followed by 45 cycles of 10 s at 94°C and 40 s at 56°C. The IP-10 and β-actin genes were analyzed in multiplex, and average quantification cycle (Cq) values were based on duplicate measurements. All samples were analyzed on a Roche LightCycler 96 with default software (Roche, Basel, Switzerland). Primer and probe concentration and temperature optimization were performed on a Roche LightCycler 96 (Roche, Basel, Switzerland) as previously described (25). The mRNA fold change was calculated using the ΔΔCq equation. All samples were measured on the same machine at one site to limit technical variability.
IFN-γ and IP-10 protein detection.
IP-10 protein levels were determined in plasma samples using an in-house IP-10 enzyme-linked immunosorbent assay (ELISA) in a 30-times dilution as described previously (22). IFN-γ levels were determined using the QuantiFeron-TB ELISA (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. Regarding the QFT, a result was considered positive when the amount of IFN-γ after specific stimulation (amount of IFN-γ released in the antigen tube after subtracting the levels present in the nil tube) was above 0.35 IU/ml. Indeterminate results were considered to be when the antigen response was negative and the amount of IFN-γ in the positive control was below 0.5 IU/ml or if the levels of IFN-γ were above 8.0 IU/ml in the negative control. In the case of IP-10 detection, positivity was considered to be when the amount of IP-10 after specific stimulation was higher than the cutoff value determined (see Results).
Statistics.
Differences in responses were compared using the Mann-Whitney t test and diagnostic accuracy using receiver operating characteristic (ROC) curves using GraphPad Prism 7 (GraphPad Software, Inc., La Jolla, CA, USA).
Data availability.
Due to participant privacy, the data collected for this study are available upon request to the corresponding author.
RESULTS
Study population.
We enrolled 95 patients with TB in Spain and 100 healthy controls in Denmark. Six patient samples were excluded from the final analysis, 4 due to unexplained differences in reference gene expression, 1 because the patient was included twice (only the first sample was included), and finally, 1 because the patient was not considered to have TB at final diagnosis. One control sample was excluded due to an unexplained difference in reference gene expression. This rendered a final cohort of 89 TB patients and 99 controls (Table 1). Table 1 shows the demographic and clinical information of the study participants. TB was confirmed by standard culture and/or PCR analysis in all but five participants, who were clinically diagnosed and had a relevant response to treatment (Table 1); three cases were negative in both culture and PCR, and two cases were negative in culture and had no PCR performed. In addition, one patient had a positive culture for Mycobacterium kansasii. All 6 patients’ samples were kept in the final analysis as all had a positive QFT test and M. tuberculosis and M. kansasii both contain ESAT-6 and CFP-10, the two major antigens in the QFT test. We found no difference in age between the two groups; however, the patient group was more ethnically diverse and included more males (72%) than the control group (30%). Among the patients, three (3%) were HIV seropositive and three (3%) were receiving immunosuppressive treatment or had other immune-suppressive comorbidities. In both groups, approximately half the enrolled participants were BCG vaccinated. Of the 89 TB patients, 81 (90%) tested QFT positive, whereas all controls tested negative.
TABLE 1.
Demographic and clinical data for the study participants included in the study analysis
| Parameter | Value [no. (%) or as indicated] for: |
|
|---|---|---|
| TB patients | Controls | |
| No. of participants | 89 | 99 |
| Age (mean yrs ± SD) | 40.6 ± 14.4 | 41.8 ± 10.7 |
| Male | 65 (72) | 30 (30) |
| Ethnicity | ||
| European | 46 (52) | 95 (96) |
| African | 16 (18) | 0 (0) |
| Asian | 15 (17) | 4 (4) |
| South America | 9 (10) | 0 (0) |
| Not announced | 3 (3) | 0 (0) |
| PCR/culture result | ||
| Positivea | 83 (93) | 0 (0) |
| Negative | 5 (6) | 0 (0) |
| Not done | 1 (1) | 99 (100) |
| In treatment | ||
| Yes | 77 (87) | 0 (0) |
| No | 12 (13) | 99 (100) |
| Not announced | 0 (0) | |
| Mean time of treatment (days) | 22.6 | |
| Previous TB infection | ||
| Yes | 12 (13) | 0 (0) |
| No | 64 (72) | 99 (100) |
| Unknown | 13 (15) | 0 (0) |
| Previous TB treatment | ||
| Yes | 8 (9) | 0 (0) |
| No | 54 (61) | 95 (96) |
| Unknown | 27 (30) | 4 (4) |
| BCG vaccinated | ||
| Yes | 35 (39) | 45 (45) |
| No | 27 (30) | 42 (42) |
| Unknown | 27 (30) | 12 (12) |
| HIV infected | ||
| Yes | 3 (3) | 0 (0) |
| No | 66 (74) | 96 (97) |
| Unknown | 20 (23) | 3 (3) |
| Other immunosuppression | ||
| Yes | 3 (3) | |
| No | 86 (97) | |
| Unknown | 0 (0) | |
| QFT result | ||
| Positive | 80 (90) | 0 (0) |
| Negative | 9 (10) | 99 (100) |
| Indeterminate | 0 (0) | 0 (0) |
| Close contact with TB patient | ||
| Yes | 8 (9) | 5 (5) |
| No | 73 (82) | 88 (89) |
| Unknown | 8 (9) | 6 (6) |
Considering at least one of the tests positive.
IFN-γ and IP-10 protein biomarker levels.
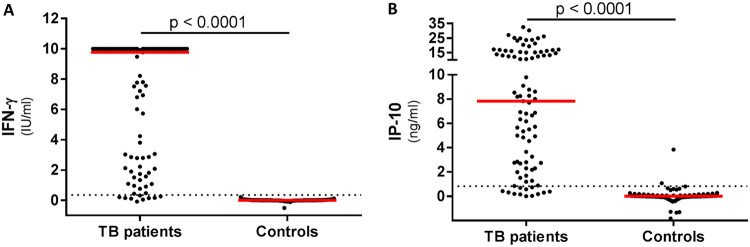
Antigen-specific IFN-γ and IP-10 protein levels were significantly higher in TB patients than in healthy controls (Fig. 1). Mitogen-specific release levels were similar for IP-10 between cases and controls. This comparison was not possible for QFT, as most participants had IFN-γ release at levels overshooting the upper limit of the assay (10 IU/ml) (Table 2 and Fig. S1A and B in the supplemental material). Background levels (nil) were significantly higher in TB patients than in controls for both IFN-γ and IP-10 (Table 2 and Fig. S1D and E).
FIG 1.
IFN-γ and IP-10 plasma release in TB patients and healthy controls. Whole-blood samples from 89 TB patients and 99 healthy controls were stimulated for 20 h in QFT tubes, and IFN-γ and IP-10 plasma levels were measured. (A) IFN-γ release was determined using the official QFT ELISA. Dotted line represents cutoff (0.35 IU/ml). (B) IP-10 plasma release was determined using an in-house IP-10 ELISA. Dotted line represents calculated cutoff (0.83 ng/ml). Data represented are background subtracted (antigen minus nil), and median values are indicated in red. Statistical analysis was done using the Mann-Whitney test.
TABLE 2.
Median values, interquartile ranges, and P values obtained by each test
| Test | Median value (interquartile range) for: |
P value | |
|---|---|---|---|
| TB patients (n = 89) | Controls (n = 99) | ||
| ELISA (QFT) (IU/ml) | |||
| Nila | 0.19 (0.15–0.31) | 0.03 (0.02–0.08) | <0.0001 |
| TB antigenb | 9.77 (2.08–10.00) | 0.00 (−0.01–0.01) | <0.0001 |
| Mitogenb | 10.00 (10.00–10.00) | 10.00 (10.00–10.00) | |
| ELISA (IP-10) (ng/ml) | |||
| Nila | 0.32 (0.16–0.70) | 0.12 (0.04–0.26) | <0.0001 |
| TB antigenb | 7.83 (2.69–15.20) | 0.00 (−0.04–0.06) | <0.0001 |
| Mitogenb | 9.71 (5.67–16.70) | 9.65 (7.78–12.96) | 0.646 |
| qPCR (IP-10) (fold change) | |||
| TB antigen | 76 (20–153) | 1.25 (0.76–1.95) | <0.0001 |
| Mitogen | 91 (37–230) | 54 (22.09–97.68) | 0.005 |
Unstimulated.
QFT TB antigen-/mitogen tube-stimulated with nil subtracted.
IP-10 mRNA expression.
IP-10 mRNA expression levels were determined in an aliquot of the same sample used for protein detection isolated after 8 h of incubation. As with the protein-based assays, we found a significant difference between TB patients and healthy controls (Fig. 2, Table 2, and Fig. S1C).
FIG 2.
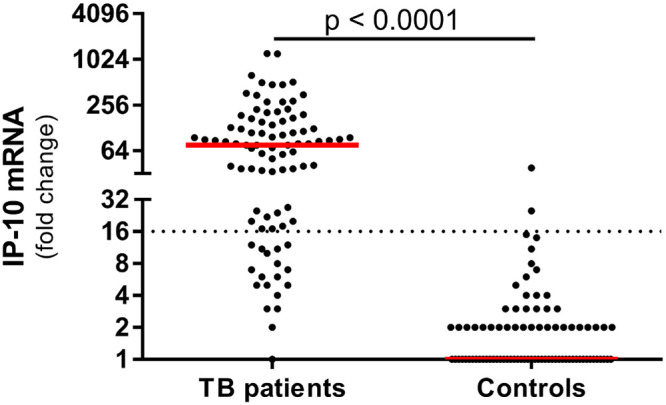
IP-10 mRNA expression in TB patients and healthy controls. Whole-blood samples from 89 TB patients and 99 healthy controls were stimulated for 8 h in QFT tubes, and total RNA was extracted. IP-10 mRNA expression was evaluated using our in-house RT-qPCR. Dotted line represents calculated cutoff (16-fold change). Median values are indicated in red. Statistical analysis was done using the Mann-Whitney test.
Technical performance of qPCR.
The repeatability of the quantitative PCR (qPCR) assay was very high, with standard deviations (SD) of <0.5 in more than 97% of duplicate measurements which fell within the linear range of the IP-10 and actin-β gene assays (Table S1 and Fig. S2A and B). The reproducibility of the assay is also very high, with comparable average PCR efficiencies for the target gene (IP-10 gene) and reference gene (β-actin gene) at 108% ± 4% (mean ± SD) and 99% ± 5% and with calibration curves (r2) of 1.00 and 0.99 across 42 individual runs of the 5-point standard curve (Table S1 and Fig. S2C and D).
Correlation between IFN-γ, IP-10 proteins, and IP-10 mRNA.
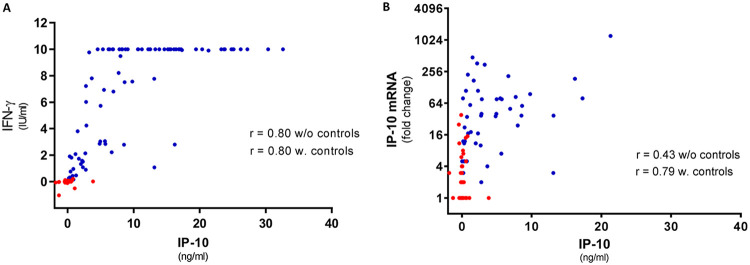
The correlation between the IFN-γ and IP-10 protein levels in the TB patients was good (Spearman’s r = 0.80). After omission of the control group (with almost all data points centered around 0 positively biasing the correlation), the correlation remained unaltered (r = 0.80) (Fig. 3A). The correlation between IP-10 mRNA and protein was poor in the patients (r = 0.43) but better when the controls were included (r = 0.79) (Fig. 3B).
FIG 3.
Correlation analysis between IFN-γ and IP-10 proteins and IP-10 protein and its mRNA. (A) Correlation between plasma IFN-γ and IP-10 protein levels. Data for TB patients and healthy controls are plotted together (n = 146). Due to the maximum limit of 10 IU/ml in the IFN-γ ELISA, all samples at this level (n = 42) have been omitted from the analysis; however, they are not omitted from the graph. (B) Correlation between plasma IP-10 protein and mRNA expression. Data for TB patients and healthy controls are plotted together (n = 188). Red dots represent healthy controls, and blue dots TB patients. Spearman correlation analysis was performed with and without data for healthy controls to disclose any bias due to the healthy control samples.
Diagnostic performance of IP-10 assays.
Cutoffs for IP-10 assays were determined at the level which rendered a test with no more than 2% lower specificity than the QFT (Fig. S3). The IP-10 protein assay rendered an area under the concentration-time curve (AUC) at 0.98 and suggested a cutoff at 0.83 ng/ml with 97/99 (98%) specificity and 78/89 (87%) sensitivity. IP-10 ELISA results were considered indeterminate when the amount of IP-10 in the positive control after subtracting the nil was below 0.5 ng/ml. The cutoff for the mRNA-based IP-10 assay was best at 16-fold upregulation with an AUC at 0.97, rendering a 97/99 (98%) specificity and 72/89 (80%) sensitivity (Table 3 and Fig. S3). A reading of indeterminate for the IP-10 qPCR was not applied due to the assay setup. The concordance of the two protein assays was very good, with only 5/89 (6%) and 2/99 (2%) discordance for TB patients and controls, respectively (Table 4). The concordance between the QFT assay and the IP-10 mRNA assay was lower than for the protein assays, with 15/89 (17%) and 2/99 (2%) samples discordant for TB patients and controls, respectively. Finally, the discordance between the two IP-10 assays was also lower than for the two ELISAs, with 14/89 (16%) and 4/99 (4%) of the samples being discordant in TB patients and controls, respectively (Table 4). None of the assays had indeterminate results.
TABLE 3.
Results obtained by each test
| Parameter | Value [no. with result/total no. tested (%) or as indicated] for: |
||
|---|---|---|---|
| QFT ELISA | IP-10 ELISA | IP-10 qPCR | |
| Cutoff | 0.35 IU/ml | 0.83 ng/ml | 16-fold change |
| AUC | 0.99 | 0.98 | 0.97 |
| TB patients (n = 89) | |||
| Positive | 80/89 (90) | 77/89 (87) | 71/89 (80) |
| Negative | 9/89 (10) | 12/89 (13) | 18/89 (20) |
| Indeterminatea | 0/89 (0) | 0/89 (0) | |
| Controls (n = 99) | |||
| Positive | 0/99 (0) | 2/99 (2) | 2/99 (2) |
| Negative | 99/99 (100) | 97/99 (98) | 97/99 (98) |
| Indeterminate | 0/99 (0) | 0/99 (0) | |
Indeterminate for IP-10 ELISA was set to mitogen minus nil of <0.5 ng/ml. Indeterminate for IP-10 qPCR was not applied due to assay setup.
TABLE 4.
Two-by-two comparison of the results obtained by each test per group
| Test, groupa | Result | No. with indicated result by indicated test |
|
|---|---|---|---|
| Positive | Negative | ||
| QFT ELISA | IP-10 qPCR | ||
| TB patients | Positive | 68 | 12 |
| Negative | 3 | 6 | |
| Controls | Positive | 0 | 0 |
| Negative | 2 | 97 | |
| QFT ELISA | IP-10 ELISA | ||
| TB patients | Positive | 76 | 4 |
| Negative | 1 | 8 | |
| Controls | Positive | 0 | 0 |
| Negative | 2 | 97 | |
| IP-10 ELISA | IP-10 qPCR | ||
| TB patients | Positive | 67 | 10 |
| Negative | 4 | 8 | |
| Controls | Positive | 0 | 2 |
| Negative | 2 | 95 | |
TB patients, n = 89; healthy controls, n = 99.
DISCUSSION
In this study, we evaluated the diagnostic potential of a molecular IP-10 release assay for detection of infection with M. tuberculosis in a case-control study. The molecular assay was compared to an IP-10 protein-based IGRA and the QuantiFeron-TB (QFT) assay. We found that, at the protein level, the QFT and IP-10 release assays performed comparably; however, the sensitivity of the molecular assay was lower than that of the QFT.
IP-10 is emerging as a more versatile and potentially superior immunodiagnostic marker compared to IFN-γ, as demonstrated in multiple studies (16, 17, 22, 29). IP-10 is expressed in concert with IFN-γ when the specific T cells recognize M. tuberculosis-specific peptides presented on major histocompatibility complex (MHC) molecules on the surface of antigen-presenting cells (APCs). The mechanisms triggering IP-10 release from APCs involve cell surface receptor interactions at the immunological synapsis and detection of cytokines (IFN-γ, tumor necrosis factor [TNF], and others) from the adjacent T cells (17).
As expected from the immunological interdependence of IP-10 and IFN-γ, we found highly correlated releases at the protein level, also confirming findings in other studies (22). However, in our study, there was a lower sensitivity of the molecular assay than of immunological tests.
In a previous study, we have shown that after stimulation with TB antigenic peptides, the expression of mRNA is a relatively rapid event, with undetectable levels in the first couple of hours followed by an exponential increase to often several-hundredfold upregulation and a subsequent decline over the following hours. However, the expression peak time is not uniform but is highly dependent on the individual and the stimulant. We previously found that individual variability peaks at 6, 8, and 10 h poststimulation (25). Others have investigated the kinetics of mRNA expression of both IFN-γ and IP-10 upon stimulation with M. tuberculosis antigens and reached various conclusions. Some found that IFN-γ mRNA peaks around 6 h poststimulation, followed by a rapid degradation (25, 30), while others found no major difference in mRNA expression levels between 8 and 18 h poststimulation (31, 32). Kim et al. analyzed the IP-10 mRNA expression levels in cell pellets 24 h poststimulation with M. tuberculosis antigens and found a very high median response from active TB patients (33). However, given that IP-10 protein was upregulated in several of the samples with low mRNA signals, it seems likely that one of the causes of the underperformance of the molecular platform in this present study is that the 8-h time point selected for mRNA isolation was not the optimal time point for all patients.
This illustrates a major challenge in mRNA-based diagnostic tests. In contrast to protein-based cytokine release assays, where protein accumulates in the plasma, the mRNA increase is rapid and transient. Inhibition of the degradation of mRNA with measurement of an accumulated amount of mRNA would significantly improve the correlation between the protein and the mRNA levels and subsequently also lead to an overall higher diagnostic performance of the assay. However, the kinetics of the IP-10 mRNA release also suggests an opportunity to study whether mRNA expression responsiveness is associated with ongoing immune activation and risk of developing the disease, which could be explored for superior predictive performance.
Regarding HIV, three TB patients were HIV positive. HIV positivity is known to affect IGRA test results; however, all three had a positive QFT test response. One donor had a low QFT response and a negative IP-10 mRNA response, and another had a low QFT response with double-negative mRNA and IP-10 protein responses, indicating that the molecular test is to some extent affected by the HIV status.
Intra- and interassay variability of this molecular assay has been described previously, and this paper supports the high technical performance of the PCR now in a much larger cohort. The average PCR efficiency was >92% for both the target IP-10 gene and the reference β-actin gene. Furthermore, less than 2.5% of all measurements within the linear dynamic range had a standard deviation of >0.5 Cq values (Fig. S2 and Table S1). The technical performance of the assay validates the assay as suitable as a diagnostic tool. Adding to this, the platform itself is good for automation and is already integrated in most TB laboratories used as a diagnostic tool for active TB.
The main limitation of this study is the use of TB patients as the surrogate group of TB infection. Given that there is no gold standard test for latent M. tuberculosis infection, we chose to perform this first proof-of-concept of the molecular platform using a case-control study design including confirmed TB patients (microbiologically or by clinical diagnosis and treatment response in the case of five patients).
In conclusion, we compared a molecular IP-10 release assay to the QFT assay and a protein-based IP-10 release assay and found comparable levels of performance of the protein-based assays but with slightly lower sensitivity for the molecular assay. We suggest that the molecular platform underperforms due to the different mRNA expression kinetics between patients. To evaluate this platform further for TB infection diagnosis, a latent M. tuberculosis infection group should be included, apart from the healthy controls and active TB patients. Overall, we found high technical performance of the assay, making it suitable as a diagnostic tool. The rapid technological advances in hand-held and field-friendly PCR machinery and microfluidics suggest that faster, less expensive, and importantly, simpler test platforms could be developed for molecular detection of latent M. tuberculosis infection. Follow-up studies making head-to-head comparisons with other diagnostic tests, such as IGRAs, in the target population of individuals with latent M. tuberculosis infection are also needed.
Supplementary Material
ACKNOWLEDGMENTS
In Copenhagen, we thank our technician Katja Bøgebjerg Carlsen for invaluable help in obtaining donor material and also Sascha Wilk Michelsen for helping in recruitment of healthy controls. In Barcelona, we thank N. Saborit (Servei de Malalties Infeccioses, Hospital Universitari de la Vall d’Hebron, Barcelona, Spain); N. Forcada, M. Montes, J. Soteras, N. Altet, and J. Maldonado (Serveis Clínics, Unitat Clínica de Tractament Directament Observat de la Tuberculosi, Barcelona, Spain) for their technical assistance.
The study was supported by Statens Serum Institut and by grants from the Instituto de Salud Carlos III (grant numbers PI13/01546, PI16/01912, and PI18/00411), integrated in the Plan Nacional de I+D+I and cofunded by the ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER), and CERCA Program/Generalitat de Catalunya. J.D. is a researcher from the Miguel Servet program. M.R. receives funding from the European Commission H2020 program (grant number TBVAC2020 643381) and Research Council Norway (grant number GLOBVAC 248042/H10).
T.B. and M.R. designed the study and performed the analyses. In Copenhagen, T.B. headed and performed the recruitment and data acquisition of healthy controls in Copenhagen and wrote the first draft. L.L.H. assisted in recruitment. M.R. and P.A. supervised the project. In Barcelona, R.V.-H. headed and performed the recruitment and data acquisition of TB patient samples in Barcelona. J.D. supervised the project. E.G.-G. and I.L. assisted in recruitment and data acquisition. B.M.-M., M.L.D.S.-G., J.P.M., F.S., A.S.-M., J.R.-M., J.P., M.A.J., C.C., C.T., I.M.-P., Y.D.G.-D., J.S., A.C., and C.P. assisted in the recruitment of TB patients.
T.B., M.R., L.L.H., and P.A. are employed by Statens Serum Institut, a governmental nonprofit research organization that holds intellectual property rights on IP-10 for diagnostic purposes. M.R. is registered as inventor on said patents. All rights are assigned to SSI.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.World Health Organization. 2015. Gear up to end TB: introducing the end TB strategy. WHO, Geneva, Switzerland.
- 2.World Health Organization. 2019. WHO global tuberculosis report 2019. WHO, Geneva, Switzerland.
- 3.Knight GM, Griffiths UK, Sumner T, Laurence YV, Gheorghe A, Vassall A, Glaziou P, White RG. 2014. Impact and cost-effectiveness of new tuberculosis vaccines in low- and middle-income countries. Proc Natl Acad Sci U S A 111:15520–15525. doi: 10.1073/pnas.1404386111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houben R, Dodd PJ. 2016. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 13:e1002152. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang L, Turner M, Elwood R, Schulzer M, FitzGerald J. 2002. A meta-analysis of the effect of Bacille Calmette Guérin vaccination on tuberculin skin test measurements. Thorax 57:804–809. doi: 10.1136/thorax.57.9.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pai M, Denkinger CM, Kik SV, Rangaka MX, Zwerling A, Oxlade O, Metcalfe JZ, Cattamanchi A, Dowdy DW, Dheda K, Banaei N. 2014. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin Microbiol Rev 27:3–20. doi: 10.1128/CMR.00034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diel R, Goletti D, Ferrara G, Bothamley G, Cirillo D, Kampmann B, Lange C, Losi M, Markova R, Migliori GB, Nienhaus A, Ruhwald M, Wagner D, Zellweger JP, Huitric E, Sandgren A, Manissero D. 2011. Interferon-γ release assays for the diagnosis of latent Mycobacterium tuberculosis infection: a systematic review and meta-analysis. Eur Respir J 37:88–99. doi: 10.1183/09031936.00115110. [DOI] [PubMed] [Google Scholar]
- 8.Ewer K, Deeks J, Alvarez L, Bryant G, Waller S, Andersen P, Monk P, Lalvani A. 2003. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 361:1168–1173. doi: 10.1016/S0140-6736(03)12950-9. [DOI] [PubMed] [Google Scholar]
- 9.Latorre I, De Souza-Galvão M, Ruiz-Manzano J, Lacoma A, Prat C, Altet N, Ausina V, Domínguez J. 2010. Evaluating the non-tuberculous mycobacteria effect in the tuberculosis infection diagnosis. Eur Respir J 35:338–342. doi: 10.1183/09031936.00196608. [DOI] [PubMed] [Google Scholar]
- 10.de Visser V, Sotgiu G, Lange C, Aabye MG, Bakker M, Bartalesi F, Brat K, Chee CBE, Dheda K, Dominguez J, Eyuboglu F, Ghanem M, Goletti D, Dilektasli AG, Guglielmetti L, Koh W-J, Latorre I, Losi M, Polanova M, Ravn P, Ringshausen FC, Rumetshofer R, de Souza-Galvão ML, Thijsen S, Bothamley G, Bossink A, TBNET. 2015. False-negative interferon-γ release assay results in active tuberculosis: a TBNET study. Eur Respir J 45:279–283. doi: 10.1183/09031936.00120214. [DOI] [PubMed] [Google Scholar]
- 11.Santos JA, Duarte R, Nunes C. 13 December 2019. Host factors associated to false negative and indeterminate results in an interferon‐γ release assay in patients with active tuberculosis. Pulmonology doi: 10.1016/j.pulmoe.2019.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Sester M, Sotgiu G, Lange C, Giehl C, Girardi E, Migliori GB, Bossink A, Dheda K, Diel R, Dominguez J, Lipman M, Nemeth J, Ravn P, Winkler S, Huitric E, Sandgren A, Manissero D. 2011. Interferon-γ release assays for the diagnosis of active tuberculosis: a systematic review and meta-analysis. Eur Respir J 37:100–111. doi: 10.1183/09031936.00114810. [DOI] [PubMed] [Google Scholar]
- 13.Domínguez J, Ruiz-Manzano J, De Souza-Galvão M, Latorre I, Milà C, Blanco S, Jiménez MÁ, Prat C, Lacoma A, Altet N, Ausina V. 2008. Comparison of two commercially available gamma interferon blood tests for immunodiagnosis of tuberculosis. Clin Vaccine Immunol 15:168–171. doi: 10.1128/CVI.00364-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Domínguez J, Latorre I, Altet N, Mateo L, De Souza-Galvão M, Ruiz-Manzano J, Ausina V. 2009. IFN-gamma-release assays to diagnose TB infection in the immunocompromised individual. Expert Rev Respir Med 3:309–327. doi: 10.1586/ers.09.20. [DOI] [PubMed] [Google Scholar]
- 15.Ruhwald M, Dominguez J, Latorre I, Losi M, Richeldi L, Pasticci MB, Mazzolla R, Goletti D, Butera O, Bruchfeld J, Gaines H, Gerogianni I, Tuuminen T, Ferrara G, Eugen-Olsen J, Ravn P, TBNET. 2011. A multicentre evaluation of the accuracy and performance of IP-10 for the diagnosis of infection with M. tuberculosis. Tuberculosis 91:260–267. doi: 10.1016/j.tube.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Aabye MG, Ruhwald M, PrayGod G, Jeremiah K, Faurholt-Jepsen M, Faurholt-Jepsen D, Range N, Friis H, Changalucha J, Andersen AB, Ravn P. 2010. Potential of interferon-γ-inducible protein 10 in improving tuberculosis diagnosis in HIV-infected patients. Eur Respir J 36:1488–1490. doi: 10.1183/09031936.00039010. [DOI] [PubMed] [Google Scholar]
- 17.Chegou NN, Heyckendorf J, Walzl G, Lange C, Ruhwald M. 2014. Beyond the IFN- horizon: biomarkers for immunodiagnosis of infection with M. tuberculosis. Eur Respir J 43:1472–1486. doi: 10.1183/09031936.00151413. [DOI] [PubMed] [Google Scholar]
- 18.Aabye MG, Latorre I, Diaz J, Maldonado J, Mialdea I, Eugen-Olsen J, Ravn P, Dominguez J, Ruhwald M. 2013. Dried plasma spots in the diagnosis of TB: IP-10 release assay on filter paper. Eur Respir J 42:495–503. doi: 10.1183/09031936.00129412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yassin M, Petrucci R, Garie K, Harper G, Teshome A, Arbide I, Asnake G, Ahmed H, Mammo T, Yesuf K, Cuevas LE. 2013. Added value of TST, IGRAS and IP-10 to identify children with TB infection. Eur Respir J 41:644–648. doi: 10.1183/09031936.00012212. [DOI] [PubMed] [Google Scholar]
- 20.Kabeer BS, Sikhamani R, Raja A. 2010. Comparison of interferon gamma and interferon gamma-inducible protein-10 secretion in HIV-tuberculosis patients. AIDS 24:323–325. doi: 10.1097/QAD.0b013e328334895e. [DOI] [PubMed] [Google Scholar]
- 21.Syed Ahamed Kabeer B, Sikhamani R, Raja A. 2011. Comparison of interferon gamma-inducible protein-10 and interferon gamma-based QuantiFERON TB gold assays with tuberculin skin test in HIV-infected subjects. Diagn Microbiol Infect Dis 71:236–243. doi: 10.1016/j.diagmicrobio.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aabye MG, Eugen-Olsen J, Werlinrud AM, Holm LL, Tuuminen T, Ravn P, Ruhwald M. 2012. A simple method to quantitate IP-10 in dried blood and plasma spots. PLoS One 7:e39228. doi: 10.1371/journal.pone.0039228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latorre I, Díaz J, Mialdea I, Serra-Vidal M, Altet N, Prat C, Díez N, Escribano A, Casas I, Rodrigo C, Ausina V, Ruhwald M, Domínguez J. 2014. IP-10 is an accurate biomarker for the diagnosis of tuberculosis in children. J Infect 69:590–599. doi: 10.1016/j.jinf.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 24.Villar-Hernández R, Latorre I, Mínguez S, Díaz J, García-García E, Muriel-Moreno B, Lacoma A, Prat C, Olivé A, Ruhwald M, Mateo L, Domínguez J. 2017. Use of IFN-γ and IP-10 detection in the diagnosis of latent tuberculosis infection in patients with inflammatory rheumatic diseases. J Infect 75:315–325. doi: 10.1016/j.jinf.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Blauenfeldt T, Heyckendorf J, Graff Jensen S, Lange C, Drabe C, Hermansen TS, de Thurah L, Lillebaek T, Eugen-Olsen J, Seersholm N, Hoff S, Bonde J, Ruhwald M. 2014. Development of a one-step probe based molecular assay for rapid immunodiagnosis of infection with M. tuberculosis using dried blood spots. PLoS One 9:e105628. doi: 10.1371/journal.pone.0105628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mori T, Sakatani M, Yamagishi F, Takashima T, Kawabe Y, Nagao K, Shigeto E, Harada N, Mitarai S, Okada M, Suzuki K, Inoue Y, Tsuyuguchi K, Sasaki Y, Mazurek GH, Tsuyuguchi I. 2004. Specific detection of tuberculosis infection. Am J Respir Crit Care Med 170:59–64. doi: 10.1164/rccm.200402-179OC. [DOI] [PubMed] [Google Scholar]
- 27.Zhou X-H, Obuchowski NA, McClish DK. 2002. Statistical methods in diagnostic medicine, 2nd ed John Wiley and Sons, Hoboken, NJ. doi: 10.1002/9780470317082. [DOI] [Google Scholar]
- 28.Yi L, Sasaki Y, Nagai H, Ishikawa S, Takamori M, Sakashita K, Saito T, Fukushima K, Igarashi Y, Aono A, Chikamatsu K, Yamada H, Takaki A, Mori T, Mitarai S. 2016. Evaluation of QuantiFERON-TB gold plus for detection of Mycobacterium tuberculosis infection in Japan. Sci Rep 6:30617. doi: 10.1038/srep30617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruhwald M, Bodmer T, Maier C, Jepsen M, Haaland MB, Eugen-Olsen J, Ravn P, TBNET. 2008. Evaluating the potential of IP-10 and MCP-2 as biomarkers for the diagnosis of tuberculosis. Eur Respir J 32:1607–1615. doi: 10.1183/09031936.00055508. [DOI] [PubMed] [Google Scholar]
- 30.Kim S, Kim YK, Lee H, Cho J-E, Kim HY, Uh Y, Kim YM, Kim H, Cho S-N, Jeon B-Y, Lee H. 2013. Interferon gamma mRNA quantitative real-time polymerase chain reaction for the diagnosis of latent tuberculosis: a novel interferon gamma release assay. Diagn Microbiol Infect Dis 75:68–72. doi: 10.1016/j.diagmicrobio.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 31.Bibova I, Linhartova I, Stanek O, Rusnakova V, Kubista M, Suchanek M, Vasakova M, Sebo P. 2012. Detection of immune cell response to M. tuberculosis-specific antigens by quantitative polymerase chain reaction. Diagn Microbiol Infect Dis 72:68–78. doi: 10.1016/j.diagmicrobio.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 32.Kasprowicz VO, Mitchell JE, Chetty S, Govender P, Huang K-HG, Fletcher HA, Webster DP, Brown S, Kasmar A, Millington K, Day CL, Mkhwanazi N, McClurg C, Chonco F, Lalvani A, Walker BD, Ndung’u T, Klenerman P. 2011. A molecular assay for sensitive detection of pathogen-specific T-cells. PLoS One 6:e20606. doi: 10.1371/journal.pone.0020606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S, Lee H, Kim H, Kim Y, Cho J-E, Jin H, Kim DY, Ha S-J, Kang YA, Cho S-N, Lee H. 2015. Diagnostic performance of a cytokine and IFN-γ–induced chemokine mRNA assay after Mycobacterium tuberculosis-specific antigen stimulation in whole blood from infected individuals. J Mol Diagnostics 17:90–99. doi: 10.1016/j.jmoldx.2014.08.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to participant privacy, the data collected for this study are available upon request to the corresponding author.