To evaluate the associations of inflammatory factors and serological test results with complicated brucellosis, we recruited 285 patients with a diagnosis of brucellosis between May 2016 and September 2019. The patients were subsequently classified into two groups according to the presence of complications. We collected demographic and clinical information and routine laboratory test results in addition to anti-Brucella IgG and IgM levels. Anti-Brucella IgG and IgM were uniformly tested using enzyme-linked immunosorbent assays (ELISAs) in this study.
KEYWORDS: Brucella spp, IgG, complicated brucellosis, enzyme-linked immunosorbent assay
ABSTRACT
To evaluate the associations of inflammatory factors and serological test results with complicated brucellosis, we recruited 285 patients with a diagnosis of brucellosis between May 2016 and September 2019. The patients were subsequently classified into two groups according to the presence of complications. We collected demographic and clinical information and routine laboratory test results in addition to anti-Brucella IgG and IgM levels. Anti-Brucella IgG and IgM were uniformly tested using enzyme-linked immunosorbent assays (ELISAs) in this study. Among the 285 patients with brucellosis, 111 (38.95%) had complicated brucellosis. Osteoarthritis occurred more often in the subacute and chronic stages than in the acute stage (P = 0.002). Genital infection occurred more frequently in the acute stage than in the other stages (P = 0.023). Fever was not frequently observed in complicated cases (P < 0.001). The erythrocyte sedimentation rate (ESR) and the C-reactive protein (CRP) and anti-Brucella IgM and IgG levels were higher in complicated-brucellosis patients than in uncomplicated-brucellosis patients (P < 0.001). Anti-Brucella IgG, with an area under the curve of 0.885 (95% confidence interval [CI], 0.847 to 0.924), was the most robust indicator of complicated brucellosis. Positive culture, anti-Brucella IgM, the ESR, and CRP could be considered indicators, but their efficacy was weaker than that of IgG. In conclusion, a high ESR, high CRP, high anti-Brucella IgM and IgG levels, and positive culture were indicators of complicated brucellosis; among these, anti-Brucella IgG was the most robust biomarker.
INTRODUCTION
Brucellosis is the most common zoonosis worldwide, with 500,000 new human cases diagnosed each year (1). Human brucellosis is well controlled in developed countries, with only sporadic cases relating to travel (2). Brucellosis is endemic in most developing countries, including China (3). From 2007 to 2017, a total of 435,108 cases of human brucellosis were reported in mainland China, with an average of 3,626 cases per month (4). The heavy burden of brucellosis in China calls for effective approaches to prevent and control this disease.
The diagnosis and treatment of brucellosis is still challenging for clinicians, and recurrence is one of the most characteristic manifestations of human brucellosis (1, 5, 6). Human brucellosis is caused by Brucella spp., which are slow-growing, facultatively intracellular bacteria (7). Bacteria can invade multiple tissues and organs and often induce chronic infection and focal infection. As a result, brucellosis has a wide range of clinical manifestations, and physical manifestations are usually nonspecific (1, 8). Compared to patients with conventional brucellosis without complications, those with complications involving specific tissues and organs usually present with more-severe illness and require a longer course of medication; despite the prolonged medication course, these patients still have high brucellosis-related mortality. In addition, complications occurring at different sites require different antimicrobial agents, considering their pharmacokinetics and pharmacodynamics (9–11). Therefore, early identification of complications and the selection of appropriate medical and surgical treatments are essential to reduce treatment failure, disability, and mortality due to brucellosis.
Traditionally, the diagnosis of brucellosis-related complications was based on symptoms and signs of focal inflammation as well as laboratory and radiographic findings in involved organs (12). However, the symptoms and signs of focal brucellosis are usually insidious and unspecific, leading to underdiagnosis or misdiagnosis of this disease (13–15). To date, no single biomarker is available to detect the presence of brucellosis-related complications (16). The white blood cell (WBC) count, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), and procalcitonin (PCT) are parameters that can, to some extent, reflect the level of inflammation within the human body (17); however, these four inflammatory factors are nonspecific and can be elevated in a variety of diseases. These four markers can be used to evaluate the status of brucellosis in clinical settings. Nevertheless, their role in detecting brucellosis-related complications is underinvestigated. Detection of antibodies (including IgM and IgG) against cytosolic proteins or S-form lipopolysaccharide (S-LPS) of Brucella by an enzyme-linked immunosorbent assay (ELISA) has become an increasingly acceptable method for the prompt diagnosis of brucellosis (7, 18). Anti-Brucella antibodies are products of adaptive humoral immunity and are pathogen specific. Their concentrations may be associated with the presence of complicated brucellosis; however, this remains to be explored.
We carried out the current study to evaluate the relationship of inflammatory factors and serological test results with complicated brucellosis in order to identify a good biomarker for the detection of complications.
MATERIALS AND METHODS
The medical records of inpatients who were diagnosed with brucellosis between May 2016 and September 2019 were reviewed by two experienced infectious disease specialists. The diagnosis of brucellosis has been described in detail in previous studies (18). In brief, the diagnosis of brucellosis was based on the proper clinical context, including history (occupational exposure, consumption of raw dairy/meat products, or living in an area of endemicity), clinical presentation (fever, sweating, arthralgia, or hepatosplenomegaly), and laboratory results, as well as at least one of the following: a positive bacterial culture, a positive standard tube agglutination (STA) test result, or a positive ELISA result. The recruited patients were subsequently classified as having complicated brucellosis, defined as brucellosis with focal infection confirmed by laboratory findings and/or radiographic findings, or uncomplicated brucellosis without specific organ involvement. For example, complicated neurobrucellosis was confirmed if patients presented with symptoms and signs of encephalitis (fever, headache, neck stiffness, positive pathological reflexes, etc.) and/or had a positive Brucella culture, the presence of anti-Brucella antibodies, or a positive STA result from cerebrospinal fluid (CSF). Brucella-induced endocarditis was diagnosed when echocardiography confirmed valvular damage or vegetation, and infection by other pathogens was excluded. Respiratory involvement was considered when patients presented with symptoms or physical signs related to the respiratory system and/or had abnormal findings on radiologic images. Osteoarticular involvement was considered if some inflammatory signs (swelling, pain, functional disability, heat, or redness) occurred in any peripheral osteoarticular location and/or there was radiographic evidence of abnormalities. Orchitis and epididymitis were diagnosed by the presence of scrotal enlargement, swelling, pain, or tenderness and/or abnormal findings on ultrasound examination, not due to other causes. Pregnant patients, patients younger than 16 years old, and patients with immune-compromising conditions, such as tumor or anti-immune therapy, were excluded.
We collected the demographic and clinical information of brucellosis patients, including sex, age, exposure history, fever, WBC count, ESR, CRP, PCT, STA results, pathogen culture results, serum anti-Brucella IgG and IgM, and radiographic findings. We classified the patients into two groups according to the presence or absence of complicated or focal brucellosis as described above and compared the baseline and clinical features of the two groups. The stages of brucellosis were defined as follows: the acute stage, with a duration shorter than 8 weeks from symptom onset to admission; the subacute stage, with a duration ranging from 8 weeks to 24 weeks; and the chronic stage, with a duration longer than 24 weeks (12).
Laboratory tests other than anti-Brucella IgG and IgM tests were routinely performed in the hospital’s laboratory, and the results were collected retrospectively. Anti-Brucella IgG and IgM were uniformly tested using ELISAs by a specialized technician to ensure accuracy and reduce bias. A positive STA result was defined as a titer ≥1:100 with a minimum of 50% agglutination. A commercial ELISA kit was used to detect anti-Brucella IgG and IgM (IBL International GmbH, Germany). ELISA was performed following the manufacturer’s protocols, and the cutoff value for both positive IgM and IgG was ≥12 U/ml. In brief, for IgG detection, patient serum was first diluted in a 1:10 ratio, and then 100 μl of diluted serum was added to each well and incubated for 1 h. After that, surplus material was washed away using a balanced salt solution; an enzyme-conjugated reagent was added; and the sample was incubated for 30 min. After another round of washing, the substrate for the enzyme was added, and the sample was incubated for 20 min. Stop buffer was added, and the optical density (OD) was measured at 450 nm. The OD values of the controls were used to construct the standard curve. The OD values of the tested samples were calculated according to the standard curve. For the detection of IgM antibodies, the procedure was similar, with an extra step of preabsorption before the procedure.
Informed consent was obtained from each participant or their authorized relatives. This study was approved by the Ethics Committee of Qilu Hospital (document KYLL-2017-714).
The chi-square test or Fisher's exact test was used to compare the frequency data. Measurement data were analyzed by the Mann-Whitney U test. The Spearman correlation test was used to determine the correlation between two continuous variables. Receiver operating characteristic (ROC) curves were constructed for measurement data to determine the optimal cutoff values for diagnosing complicated brucellosis. Additionally, the sensitivity and specificity of each parameter in distinguishing complicated brucellosis from uncomplicated brucellosis were computed, and the area under the curve (AUC) was calculated. Continuous variables were converted into categorical variables according to cutoff values obtained from the ROC analysis. Binary logistic regression was employed to analyze the risk factors for complicated brucellosis. We used SPSS 22.0 (IBM Corp., Somers, NY, USA) for the statistical analyses, and statistical significance was defined as a two-tailed P value of <0.05.
All data included in this study are available upon reasonable request from the corresponding author.
RESULTS
A total of 285 brucellosis patients were enrolled in the current study, among whom 111 (38.95%) had complicated brucellosis. In addition, we found that five anatomical systems were affected by complications, and osteoarthritis was the most common focal complication. Detailed information about the kinds of complications and the proportions of patients experiencing those complications is listed in Table 1.
TABLE 1.
Organs involved in complicated brucellosis
| Complication | No. (%) of patients |
|---|---|
| Neurobrucellosis | 6 (5.41) |
| Osteoarthritis | 68 (61.26) |
| Spinal infection | 50 (45.05) |
| Sacroiliitis | 17 (15.32) |
| Hip synovitis | 1 (0.90) |
| Bronchitis and/or pneumonia | 17 (15.32) |
| Genital infection | 17 (15.32) |
| Endocarditis | 3 (2.70) |
Demographic and clinical information for the 285 brucellosis patients and comparisons between the complicated-brucellosis and uncomplicated-brucellosis subgroups are shown in Table 2. A total of 122 (42.81%) of 285 patients were female. The median age of the cohort was 53 years (range, 17 to 87 years), with a large proportion (68.07%) of patients younger than 60 years old. Upon admission, 142 (49.82%) patients presented with the acute stage, 90 (31.58%) patients presented with the subacute stage, and 53 (18.6%) patients presented with the chronic stage. Patients with complicated brucellosis were more likely to have subacute or chronic brucellosis than patients with uncomplicated brucellosis, but the difference was not statistically significant (P = 0.290). Among the complications involving different systems, osteoarthritis occurred more often in patients with the chronic stage than in patients with the acute stage (P < 0.001). In contrast, genital infection was more common in patients with the acute stage than in patients with the chronic stage (P = 0.023) (see Table S1 in the supplemental material). Among the 285 patients with brucellosis, 62 Brucella isolates were obtained, 1 of which was from cerebrospinal fluid, while the remaining positive specimens were from blood. The positivity rates for culture in the acute, subacute, and chronic phases were 35.92%, 11.11%, and 1.89%, respectively. ELISA demonstrated a high positivity rate of 98.95% (IgG and/or IgM positive). When IgG and IgM were analyzed separately, the positivity rates for IgM and IgG were 61.40% and 96.14%, respectively (Table S2). The correlation coefficients of stage with IgM and IgG were –0.352 (P <0.001) and 0.408 (P < 0.001), respectively.
TABLE 2.
Demographic and clinical information on patients with brucellosis
| Parametera | Valueb
for patients with brucellosis |
P value | ||
|---|---|---|---|---|
| Total (n = 285) | Uncomplicated (n = 174) | Complicated (n = 111) | ||
| Female gender | 122 (42.81) | 73 (41.95) | 49 (44.14) | 0.716 |
| Age (yr) | 53 (17–87) | 52.5 (18–87) | 53 (17–86) | 0.867 |
| Age of: | ||||
| <60 yr | 194 (68.07) | 121 (69.54) | 73 (65.77) | 0.505 |
| ≥60 yr | 91 (31.93) | 53 (30.46) | 38 (34.23) | |
| Contact history | 214 (75.09) | 126 (72.41) | 88 (79.28) | 0.191 |
| Stage | 0.290 | |||
| Acute | 142 (49.82) | 93 (53.45) | 49 (44.14) | |
| Subacute | 90 (31.58) | 52 (29.89) | 38 (34.23) | |
| Chronic | 53 (18.60) | 29 (16.67) | 24 (21.62) | |
| Fever | 259 (90.88) | 169 (97.13) | 90 (81.08) | <0.001 |
| WBC (×109/liter) | 5.55 (1.85–43) | 5.54 (1.85–43) | 5.56 (2–9.9) | 0.741 |
| ESR (mm/h) | 41 (3–120) | 38 (8–120) | 48 (3–87) | <0.001 |
| C-reactive protein (mg/liter) | 21 (0.95–130.5) | 17 (0.95–130.5) | 29 (1.13–101.58) | <0.001 |
| Procalcitonin (ng/liter) | 0.238 (0.01–3.14) | 0.245 (0.011–0.85) | 0.234 (0.01–3.14) | 0.444 |
| STA | 186 (65.26) | 106 (60.92) | 80 (72.07) | 0.054 |
| ELISA result (U/ml) | ||||
| IgM | 17.54 (1.07–175.6) | 12.43 (1.07–175.6) | 23.85 (1.29–86.97) | <0.001 |
| IgG | 79.05 (1.26–700) | 47.05 (1.26–175.8) | 140.9 (32.62–700) | <0.001 |
| Positive culture | 62 (21.75) | 33 (18.97) | 29 (26.13) | 0.153 |
WBC, white blood cells; ESR, erythrocyte sedimentation rate; STA, standard tube agglutination test; ELISA, enzyme-linked immunosorbent assay.
Values are expressed as the number (percentage) of patients for categorical variables or as the median (range) for continuous variables.
In this study, five parameters that were significantly different between complicated and uncomplicated brucellosis were determined. Fever was observed less frequently in patients with complicated brucellosis than in those with uncomplicated brucellosis (P < 0.001). The ESR and the CRP and anti-Brucella IgM and IgG levels were higher in complicated-brucellosis patients than in uncomplicated-brucellosis patients (P < 0.001). In addition, STA positivity was more common in complicated-brucellosis patients than in uncomplicated-brucellosis patients, but the difference was not statistically significant (P = 0.054). Moreover, the positivity rate of Brucella culture was higher in complicated-brucellosis patients than in uncomplicated-brucellosis patients (26.13% versus 18.97%); however, the difference was not statistically significant (P = 0.153).
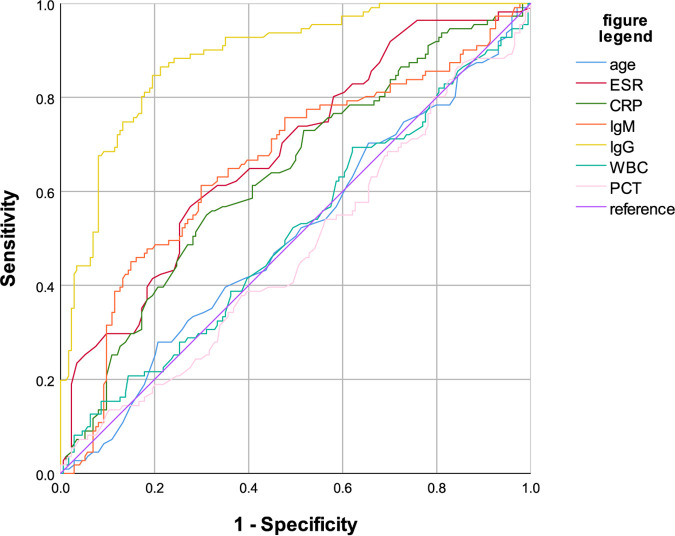
ROC curve analysis was performed (Fig. 1) to identify the optimal cutoff values of age, WBC count, ESR, CRP, PCT, and serum anti-Brucella IgG and IgM for detection of the occurrence of focal brucellosis. In addition, the AUCs of the ROC curves, the optimal cutoff values, and the sensitivity and specificity at the established cutoff values for each parameter are listed in Table 3. As Fig. 1 and Table 3 show, the concentration of serum anti-Brucella IgG, which had an AUC of 0.885 (95% confidence interval [95% CI], 0.847 to 0.924), was the most robust biomarker of complicated brucellosis, followed by the level of anti-Brucella IgM (AUC, 0.660 [95% CI, 0.593 to 0.727]). The AUCs of anti-Brucella IgG in the acute, subacute, and chronic stages were 0.858 (95% CI, 0.794 to 0.923), 0.921 (95% CI, 0.864 to 0.977), and 0.911 (95% CI, 0.837 to 0.985), respectively. The cutoff values of IgG, IgM, ESR, CRP, WBC, PCT, and age were 101.12 U/ml, 20.025 U/ml, 45.5 mm/h, 27 mg/liter, 5.325 × 109/liter, 0.4005 ng/liter, and 65.5 years, respectively. A logistic regression model indicated that patients with positive cultures had a higher risk of brucellosis complications than those with negative cultures (odds ratio [OR], 2.68 [95% CI, 1.05 to 6.86]); patients with an IgM level of >20.025 U/ml had a higher risk of brucellosis complications than those with an IgM level of ≤20.025 U/ml (OR, 4.37 [95% CI, 1.96 to 9.74]); patients with an IgG level of >101.12 U/ml had a higher risk of brucellosis complications than those with an IgG level of ≤101.12 U/ml (OR, 37.83 [95% CI, 14.90 to 96.04]); patients with an ESR of >45.5 mm/h had a higher risk of brucellosis complications than those with an ESR of ≤45.5 mm/h (OR, 6.51 [95% CI, 2.84 to 14.94]); and patients with a CRP level of >27 mg/liter had a higher risk of brucellosis complications than those with a CRP level of ≤27 mg/liter (OR, 2.68 [95% CI, 1.25 to 5.72]). Positive culture, the ESR, and CRP and IgM levels detected the occurrence of brucellosis complications, but their efficacy was weaker than that of the IgG level. PCT, WBC count, and age were not parameters indicative of complicated brucellosis. Logistic regression models including all statistically significant variables were constructed, and the results are shown in Table 4.
FIG 1.
Receiver operating characteristic curves for seven continuous parameters. WBC, white blood cell; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; PCT, procalcitonin.
TABLE 3.
Cutoff values, sensitivity, specificity, and area under the ROC curve of continuous data in predicting the presentation of complicated brucellosis
| Parametera | Cutoff value | Sensitivity | Specificity | AUC (95% CI) | P value |
|---|---|---|---|---|---|
| ELISA IgG | 101.12 U/ml | 0.865 | 0.787 | 0.885 (0.847–0.924) | <0.001 |
| ELISA IgM | 20.025 U/ml | 0.613 | 0.701 | 0.660 (0.593–0.727) | <0.001 |
| ESR | 45.5 mm/h | 0.568 | 0.724 | 0.681 (0.618–0.744) | <0.001 |
| C-reactive protein | 27 mg/liter | 0.550 | 0.690 | 0.636 (0.570–0.701) | <0.001 |
| WBC count | 5.325 × 109/liter | 0.694 | 0.379 | 0.512 (0.442–0.581) | 0.741 |
| Age | 65.5 yr | 0.279 | 0.793 | 0.506 (0.437–0.575) | 0.867 |
| Procalcitonin | 0.4005 ng/liter | 0.072 | 0.960 | 0.473 (0.404–0.542) | 0.444 |
ELISA, enzyme-linked immunosorbent assay; ESR, erythrocyte sedimentation rate; WBC, white blood cell.
TABLE 4.
Logistic regression analysis including statistically significant variables
| Itema | Exp(B) | 95%CI | P value |
|---|---|---|---|
| Fever | 0.152 | 0.043–0.536 | 0.003 |
| Culture (+) | 2.684 | 1.050–6.860 | 0.039 |
| ELISA IgM | 4.365 | 1.956–9.742 | 0.000 |
| ELISA IgG | 37.827 | 14.899–96.036 | 0.000 |
| ESR | 6.512 | 2.839–14.936 | 0.000 |
| C-reactive protein | 2.676 | 1.253–5.718 | 0.011 |
| Total | 0.050 | 0.000 |
ELISA, enzyme-linked immunosorbent assay; ESR, erythrocyte sedimentation rate.
DISCUSSION
Complicated brucellosis, also known as focal brucellosis, is a Brucella sp. infection in humans that causes damage to one or more organs or systems (9, 12, 15). Distinguishing focal brucellosis from focal infection induced by other pathogens is difficult, posing a challenge in the administration of effective medication (19, 20). Therefore, it is critical to understand the incidence and manifestations of complicated brucellosis and to identify biomarkers that can detect the occurrence of focal infection. The current study determined the incidence of complications of brucellosis and the differences in clinical features and laboratory findings between complicated and uncomplicated brucellosis. We demonstrated in this study that serum anti-Brucella IgG tested by ELISA could be an effective biomarker of focal brucellosis.
Previous studies have reported that osteoarthritis is one of the most common complications of brucellosis. The incidence of Brucella-induced arthritis ranged from 3% to 77% in different reports (9, 21). In our study, osteoarthritis was the most common complication of brucellosis, accounting for 61.26% of all complications. Spine involvement was the most frequent subtype of Brucella-induced osteoarthritis. Genitourinary involvement was also among the most common focal complications, with an incidence ranging from 1.1% to 25% (22). Complications involving other systems, such as the respiratory, cardiovascular, and neurological systems, were less common. The incidence of focal complications affecting these systems ranged from <1% to 5% (23–25). In summary, the incidence of brucellosis-associated complications in our study was in agreement with that in the existing literature.
The systemic clinical manifestations of complicated brucellosis were similar to those of uncomplicated brucellosis. According to the results of our study, a lower rate of fever was observed in the complicated- group than in the uncomplicated-brucellosis group, a result that was also validated by other researchers (12). In agreement with other reports, we also found that Brucella-associated osteoarthritis occurred more often in patients in the subacute or chronic stage than in patients in the acute stage (26). The differences in disease stages for other focal brucellosis complications could not be concluded due to low incidence rates. Nevertheless, the logistic regression analysis in our study found that fever and prolonged duration of illness might be negative factors for complicated brucellosis. We did not find significant differences in any other systemic clinical presentations between the complicated- and uncomplicated-brucellosis groups. Therefore, it is difficult to distinguish complicated brucellosis from uncomplicated brucellosis based on general symptoms alone.
Due to the difference in treatment regimens for complicated and uncomplicated brucellosis, it is of great importance to detect the occurrence of complications in brucellosis patients. Given the indistinguishable clinical features of focal and uncomplicated brucellosis, many researchers have explored the utility of inflammatory biomarkers and laboratory results in detecting the presence of complicated brucellosis.
Sen et al. found that the platelet-to-lymphocyte ratio (PLR) and ESR were significantly higher in complicated-brucellosis patients than in uncomplicated-brucellosis patients (P, 0.007 and <0.001, respectively) (16). They reported that the AUC for the PLR was 0.622 (95% CI, 0.538 to 0.707) for detecting complications of brucellosis (16). The neutrophil-to-lymphocyte ratio (NLR) and macrophage-to-lymphocyte ratio (MLR) were demonstrated by Balin et al. to be indicators of osteoarticular involvement (27). Nevertheless, Sen et al. concluded that the MLR and NLR were not valuable markers of complications in brucellosis patients when hematologic abnormalities were considered a complication. However, if only solid-organ involvement was regarded as a complication, and hematologic abnormalities were omitted, the ESR, mean platelet volume (MPV), NLR, PLR, and MLR all differed significantly between complicated- and uncomplicated-brucellosis patients (P, 0.001, 0.011, 0.001, 0.013, and 0.040, respectively). The AUC values for the NLR and MLR were 0.649 (95% CI, 0.570 to 0.728) and 0.589 (95% CI, 0.507 to 0.671), respectively (16). In the current study, we also found that the ESR and CRP were indicative of the presence of brucellosis complications, and their performance (AUC values, 0.681 [95% CI, 0.618 to 0.744] and 0.636 [95% CI, 0.570 to 0.701], respectively) was comparable to that in other studies. In addition, the ESR and CRP were correlated with an increase in anti-Brucella antibodies in serum (28). Other acute-stage response agents of inflammation, such as hepcidin or adenosine deaminase, might also be used as biomarkers to diagnose brucellosis, estimate the therapeutic efficacy of treatments, and predict the recurrence of brucellosis (29, 30). Several matrix metalloproteinase family members have been reported to be helpful in indicating osteoarticular involvement (31). Despite these findings, no effective biomarkers with ROC AUCs larger than 0.800 are currently available. Universal inflammatory biomarkers might not be appropriate indicators, since their levels can be influenced by autoimmune factors or immune-compromising diseases. Our study demonstrated that anti-Brucella IgG, with an AUC of 0.885 (95% CI, 0.847 to 0.924), is currently the most robust biomarker for detecting the presence of complicated brucellosis. It has been suggested that IgM be tested together with IgG to avoid false-negative results in brucellosis detection (7). IgG was more robust than IgM in detecting complications. In addition, IgG was relatively stable, with strong persistence in peripheral blood, and was easily detected. Therefore, we suggest that anti-Brucella IgG tested by ELISA should be applied in clinical settings not only for brucellosis diagnosis but also as a biomarker for complications.
The current study validated the role of anti-Brucella antibodies in detecting the presence of focal brucellosis. However, there were several limitations to our study. First, the present study was retrospective, and long-term follow-up information was not obtained. Therefore, the association of anti-Brucella IgG/IgM with treatment efficacy was not clarified. Second, this study was carried out in a single center; thus, the results should be generalized with caution. Third, the sample size of this study was small. Despite these limitations, the present study could aid in the diagnosis of complicated brucellosis by clinical practitioners in areas of endemicity. Large-cohort and multicenter studies with long-term follow-up periods are needed to comprehensively investigate the presentation, diagnosis. and management of complicated brucellosis.
Supplementary Material
ACKNOWLEDGMENTS
We thank the participants for their contributions to this study. We appreciate the job of all other coworkers in our department in contributing to this study.
We declare no competing interests.
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Franco MP, Mulder M, Gilman RH, Smits HL. 2007. Human brucellosis. Lancet Infect Dis 7:775–786. doi: 10.1016/S1473-3099(07)70286-4. [DOI] [PubMed] [Google Scholar]
- 2.Gerada A, Beeching NJ. 2016. Brucellosis and travel. Travel Med Infect Dis 14:180–181. doi: 10.1016/j.tmaid.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Chen Z, Zhang W, Ke Y, Wang Y, Tian B, Wang D, Cui B, Zou W, Li S, Huang L, Song H. 2013. High-risk regions of human brucellosis in China: implications for prevention and early diagnosis of travel-related infections. Clin Infect Dis 57:330–332. doi: 10.1093/cid/cit251. [DOI] [PubMed] [Google Scholar]
- 4.Guan P, Wu W, Huang D. 2018. Trends of reported human brucellosis cases in mainland China from 2007 to 2017: an exponential smoothing time series analysis. Environ Health Prev Med 23:23. doi: 10.1186/s12199-018-0712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ariza J, Corredoira J, Pallares R, Viladrich PF, Rufi G, Pujol M, Gudiol F. 1995. Characteristics of and risk factors for relapse of brucellosis in humans. Clin Infect Dis 20:1241–1249. doi: 10.1093/clinids/20.5.1241. [DOI] [PubMed] [Google Scholar]
- 6.Ariza J, Gudiol F, Pallares R, Viladrich PF, Rufi G, Corredoira J, Miravitlles MR. 1992. Treatment of human brucellosis with doxycycline plus rifampin or doxycycline plus streptomycin. A randomized, double-blind study. Ann Intern Med 117:25–30. doi: 10.7326/0003-4819-117-1-25. [DOI] [PubMed] [Google Scholar]
- 7.Yagupsky P, Morata P, Colmenero JD. 2019. Laboratory diagnosis of human brucellosis. Clin Microbiol Rev 33:e00073-19. doi: 10.1128/CMR.00073-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pappas G, Akritidis N, Bosilkovski M, Tsianos E. 2005. Brucellosis. N Engl J Med 352:2325–2336. doi: 10.1056/NEJMra050570. [DOI] [PubMed] [Google Scholar]
- 9.Buzgan T, Karahocagil MK, Irmak H, Baran AI, Karsen H, Evirgen O, Akdeniz H. 2010. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int J Infect Dis 14:e469. doi: 10.1016/j.ijid.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 10.Reguera JM, Alarcon A, Miralles F, Pachon J, Juarez C, Colmenero JD. 2003. Brucella endocarditis: clinical, diagnostic, and therapeutic approach. Eur J Clin Microbiol Infect Dis 22:647–650. doi: 10.1007/s10096-003-1026-z. [DOI] [PubMed] [Google Scholar]
- 11.Guven T, Ugurlu K, Ergonul O, Celikbas AK, Gok SE, Comoglu S, Baykam N, Dokuzoguz B. 2013. Neurobrucellosis: clinical and diagnostic features. Clin Infect Dis 56:1407–1412. doi: 10.1093/cid/cit072. [DOI] [PubMed] [Google Scholar]
- 12.Kayaaslan B, Bastug A, Aydin E, Akinci E, But A, Aslaner H, Yetkin MA, Bodur H. 2016. A long-term survey of brucellosis: is there any marker to predict the complicated cases? Infect Dis (Lond) 48:215–221. doi: 10.3109/23744235.2015.1107187. [DOI] [PubMed] [Google Scholar]
- 13.Purwar S, Metgud SC, Gokale SK. 2012. Exceptionally high titres in atypical presentation of occult epididymo-orchitis due to brucellosis. J Med Microbiol 61:443–445. doi: 10.1099/jmm.0.034892-0. [DOI] [PubMed] [Google Scholar]
- 14.Baldane S, Sivgin S, Alkan TS, Kurnaz F, Pala C, Keklik M, Karaman A, Kaynar L. 2012. An atypical presentation of brucellosis in a patient with isolated thrombocytopenia complicated with upper gastrointestinal tract bleeding. Case Rep Med 2012:473784. doi: 10.1155/2012/473784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Solera J. 2010. Update on brucellosis: therapeutic challenges. Int J Antimicrob Agents 36(Suppl 1):S18–S20. doi: 10.1016/j.ijantimicag.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 16.Sen P, Demirdal T, Nemli SA. 2019. Predictive value of inflammation markers in brucellosis. Arch Iran Med 22:640–645. [PubMed] [Google Scholar]
- 17.Lee Y, Lim J, Choi SW, Han S, Park B, Youm JY. 2019. Changes of biomarkers before and after antibiotic treatment in spinal infection. Korean J Neurotrauma 15:143–149. doi: 10.13004/kjnt.2019.15.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu N, Wang W, Chen F, Li W, Wang G. 2020. ELISA is superior to bacterial culture and agglutination test in the diagnosis of brucellosis in an endemic area in China. BMC Infect Dis 20:11. doi: 10.1186/s12879-019-4729-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gönen S, Dizbay M, Söylemezoğlu O. 2017. Investigation of human leukocyte antigen in osteoarticular brucellosis. Turk J Med Sci 47:1505–1508. doi: 10.3906/sag-1612-105. [DOI] [PubMed] [Google Scholar]
- 20.Zheng R, Xie S, Lu X, Sun L, Zhou Y, Zhang Y, Wang K. 2018. A systematic review and meta-analysis of epidemiology and clinical manifestations of human brucellosis in China. Biomed Res Int 2018:5712920. doi: 10.1155/2018/5712920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adetunji SA, Ramirez G, Foster MJ, Arenas-Gamboa AM. 2019. A systematic review and meta-analysis of the prevalence of osteoarticular brucellosis. PLoS Negl Trop Dis 13:e0007112. doi: 10.1371/journal.pntd.0007112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bosilkovski M, Kamiloski V, Miskova S, Balalovski D, Kotevska V, Petrovski M. 2018. Testicular infection in brucellosis: report of 34 cases. J Microbiol Immunol Infect 51:82–87. doi: 10.1016/j.jmii.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 23.Rossi M, Tascini C, Carannante N, Di Caprio G, Sofia S, Iacobello C. 2018. Neurobrucellosis: diagnostic and clinical management of an atypical case. New Microbiol 41:165–167. [PubMed] [Google Scholar]
- 24.Koruk ST, Erdem H, Koruk I, Erbay A, Tezer-Tekce Y, Erbay AR, Dayan S, Deveci O, Inan A, Engin DO, Guner R, Dikici N, Doyuk-Kartal E, Kurtaran B, Pehlivanoglu F, Sipahi OR, Yalci A, Yemisen M, Alp-Cavus S, Gencer S, Guzel G, Oncul O, Parlak M, Kazak E, Tulek N, Ulcay A, Savasci U. 2012. Management of Brucella endocarditis: results of the Gulhane study. Int J Antimicrob Agents 40:145–150. doi: 10.1016/j.ijantimicag.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Erdem H, Inan A, Elaldi N, Tekin R, Gulsun S, Ataman-Hatipoglu C, Beeching N, Deveci O, Yalci A, Bolukcu S, Dagli O, Brucellosis Study Group. 2014. Respiratory system involvement in brucellosis: the results of the Kardelen study. Chest 145:87–94. doi: 10.1378/chest.13-0240. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y, Gao H, Pappas G, Chen Q, Li M, Xu J, Lai S, Liao Q, Yang W, Yi Z, Rouzi Z, Yu H. 2018. Clinical features of 2041 human brucellosis cases in China. PLoS One 13:e0205500. doi: 10.1371/journal.pone.0205500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Balin SO, Tartar AS, Akbulut A. 2018. The predictive role of haematological parameters in the diagnosis of osteoarticular brucellosis. Afr Health Sci 18:988–994. doi: 10.4314/ahs.v18i4.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu J, Zhao X. 2017. Clinical features and serum profile of inflammatory biomarkers in patients with brucellosis. J Infect Dev Ctries 11:840–846. doi: 10.3855/jidc.8872. [DOI] [PubMed] [Google Scholar]
- 29.Hashemi SH, Esna-Ashari F, Nemat Gorgani F, Tayebinia H, Mamani M. 2018. Increased serum levels of hepcidin and C-reactive protein in patients with brucellosis. Trans R Soc Trop Med Hyg 112:509–512. doi: 10.1093/trstmh/try092. [DOI] [PubMed] [Google Scholar]
- 30.da Cunha S. 1995. Adenosine deaminase in cerebrospinal fluid during Brucella meningitis. J Infect 31:82–83. doi: 10.1016/S0163-4453(95)91829-9. [DOI] [PubMed] [Google Scholar]
- 31.Sisirak M, Hukic M. 2015. Osteoarticular complications of brucellosis: the diagnostic value and importance of detection matrix metalloproteinases. Acta Med Acad 44:1–9. doi: 10.5644/ama2006-124.121. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.