Hepatitis A virus (HAV) is a common infection that is transmitted through the fecal-oral route, shed in the stool of infected individuals, and spread either by direct contact or by ingesting contaminated food or water. Each year, approximately 1.4 million acute cases are reported globally with a major risk factor for exposure being low household socioeconomic status. Recent trends show a decrease in anti-HAV antibodies in the general population, with concomitant increases in the numbers of HAV outbreaks.
KEYWORDS: hepatitis A virus, salivary antibodies, multiplex immunoassay, immunoprevalence, immunoconversion, coinfections, public health
ABSTRACT
Hepatitis A virus (HAV) is a common infection that is transmitted through the fecal-oral route, shed in the stool of infected individuals, and spread either by direct contact or by ingesting contaminated food or water. Each year, approximately 1.4 million acute cases are reported globally with a major risk factor for exposure being low household socioeconomic status. Recent trends show a decrease in anti-HAV antibodies in the general population, with concomitant increases in the numbers of HAV outbreaks. In line with a recreational water study, this effort aims to assess the prevalence of salivary IgG antibodies against HAV and subsequent incident infections (or immunoconversions) in visitors to a tropical beach impacted by a publicly owned treatment works (POTW). We applied a multiplex immunoassay to serially collected saliva samples gathered from study participants who recreated at Boquerón Beach, Puerto Rico. Analysis of assay results revealed an immunoprevalence rate of 16.17% for HAV with 1.43% of the cohort immunoconverting to HAV. Among those who immunoconverted, 10% reported chronic gastrointestinal symptoms and none experienced diarrhea. Tests on water samples indicated good water quality with low levels of fecal indicator bacteria; however, the collection and analysis of saliva samples afforded the ability to detect HAV infections in beachgoers. This rapid assay serves as a cost-effective tool for examining exposure to environmental pathogens and can provide critical information to policy makers, water quality experts, and risk assessment professionals seeking to improve and protect recreational water and public health.
INTRODUCTION
Hepatitis A virus (HAV) is a nonenveloped, RNA virus of the family Picornaviridae, genus Hepatovirus, that is spread primarily by the fecal-oral route either by direct contact with infected persons or by the ingestion of contaminated food or water (1). Although HAV infections are usually asymptomatic and subclinical in children (70% of children under age 6 often do not develop symptoms) (2, 3), 70% of adolescents and adults develop symptoms to the virus which is linked to liver failure and can cause death particularly in older adults (4). The incubation period for HAV is estimated at 14 to 49 days (5) with jaundice occurring in about 10% of infected children and 75% of infected adults (6). At about 28 days into the incubation period, patients usually exhibit nonspecific signs and symptoms (e.g., fever, malaise, anorexia, and jaundice), followed by gastrointestinal symptoms including nausea, abdominal discomfort, and diarrhea and genitourinary symptoms such as dark urine (7).
Low socioeconomic status, poor hygiene conditions, and lack of access to safe water have all been found to be associated with the incidence rate of the disease (4). In Puerto Rico, the median household income is around $19,000 ($19K), per capita income in the past 12 months (in 2017 dollars) is $12.8K, and the percentage of persons living in poverty is 43.1% (8). In comparison, the median household income for the same period for the mainland United States is $57.7K, with a per capita income of $31.1K and an 11.8% poverty rate (8). These data show that although Puerto Rico is a U.S. territory, its socioeconomic status is much lower than that of the mainland. High-income regions such as the United States, Europe, Canada, and Australia have very low endemicity levels and a high proportion of susceptible adults while low-income regions like sub-Saharan Africa and parts of South Asia have high endemicity levels and almost no susceptible adolescents and adults (9). Middle-income regions in Asia, Latin America, Eastern Europe, and the Middle East have been shown to have a mix of intermediate to low endemicity levels, suggesting that they may have an increasing burden of disease (9).
Currently, HAV infections are identified and diagnosed using immunological and molecular approaches. Since there are other types of viral hepatitis, it is critical that HAV is differentiated from the other hepatitis viruses. This differentiation is necessary for the proper diagnosis of HAV infection. One approach to correctly diagnosing HAV infection is through serological assays measuring the humoral immune response. Several commercial assays that measure IgM and total anti-HAV antibodies are available (10, 11). These serological assays are essential for diagnosis because HAV infection is practically indistinguishable clinically from disease caused by other hepatitis viruses (12). HAV serological assays include IgM for acute HAV infections (1), radioimmunoassay (13, 14), immunochemical staining (14), enzyme-linked immunosorbent assays (ELISAs) (15), immunoblotting (16), and dot blot immunogold filtration (17). Molecular detection methods for HAV include restriction fragment length polymorphism (RFLP) (18), single-strand conformational polymorphism (19), Southern blotting (20), and reverse transcription-PCR (RT-PCR) (21), among others. These methods have been deployed primarily to detect HAV in clinical specimens and food and environmental samples.
Most serological tests involve the use of expensive, invasively acquired serum samples requiring the collection of blood using needles which are considered to be painful and undesirable by many, particularly children. As such, survey recruits are less likely to participate in studies that use invasive collection techniques. Conversely, saliva is an inexpensive, noninvasive, simple, and painlessly collected biofluid shown to be a suitable alternative to serum for measuring antibody responses to infectious organisms (22–26). It has emerging applications in research and clinical settings, and in fact, several studies have shown the efficacy of salivary antibodies as biomarkers of hepatitis A virus infections (27–29). Our team developed a bead-based, multiplex salivary antibody immunoassay to measure the prevalence of antibodies to multiple waterborne pathogens associated with drinking and recreational water contamination simultaneously (30, 31). Application of the assay has allowed us to measure immunoprevalence (32), immunoconversions (incident infections), coinfections (33), and asymptomatic infections (34) from exposure to various waterborne pathogens in visitors to Boquerón Beach, Puerto Rico. Immunoprevalence (the prevalence of circulating antibodies against specific pathogens) is an important aspect of these studies because it affords the ability to capture the baseline level of exposure at the beginning of a longitudinal study. An immunoconversion is defined as the development of detectable antibodies (typically within a few days of exposure) that can be tracked over time to examine the body’s immunological response during infection. Boquerón Beach is one of the water bodies studied as part of the U.S. Environmental Protection Agency’s (USEPA’s) National Epidemiologic and Environmental Assessment of Recreational (NEEAR) Water Studies (35) and was selected because of potential fecal contamination from a nearby discharging Publicly Owned Treatment Works (POTWs) (35). The NEEAR Water study involved water sampling and testing, epidemiological surveys, and the collection of saliva samples. Quantitative PCR (qPCR) and culture-based analyses indicated beach water quality was relatively good with low fecal indicator counts for enterococci and Bacteroidales (31). As part of the Boquerón Beach study, a total of 468 water samples were collected over 26 days and tested for Enterococcus CFU by USEPA method 1600. Results of the water quality studies showed that densities of fecal indicator bacteria were low, and no single day exceeded the USEPA geometric mean criterion of 35 CFU/100 ml for Enterococcus. The highest daily geometric mean was 27 CFU/100 ml (35). Complete results of the water quality study have been reported previously (35). While no specific analyses were performed to detect HAV in the water, researchers were interested in determining whether there was evidence of exposure to the virus in beachgoers as demonstrated by anti-HAV antibodies in the saliva of study participants. In this effort, three saliva samples were collected from consenting study participants with an initial sample (S1) collected at the beach and two follow-up samples self-collected by participants at home 10 to 14 (S2) and 30 to 40 (S3) days later. We employed our salivary antibody multiplex immunoassay to assess rates of immunoprevalence and immunoconversions (incident infections) to HAV in samples collected from beachgoers. Further, we examined linkages between possible exposure risk factors and immunoconversion rates.
MATERIALS AND METHODS
Reagents.
Polystyrene microspheres (5.6-μm bead) sets were obtained from Luminex Corp. (Austin, TX, USA) at a concentration of 12.5 × 106 beads/ml each. Biotinylated goat anti-human IgG (λ) secondary detection antibody was obtained from KPL (Gaithersburg, MD, USA). HAV grade II concentrate antigen was purchased from Meridian BioScience (Memphis, TN, USA) and coupled to one specific bead set in accordance with the optimized multiplex immunoassay. The assay was validated using characterized sera (10 positive and 10 negative) purchased from SeraCare (Milford, MA, USA) (31).
Antigen coupling and confirmation using animal-derived antibodies.
Beads were activated and coupled, as previously described, and serial dilutions of primary capture antibodies were used to confirm that the beads were coupled properly, thus ensuring that the dynamic range of the assay could be defined (30, 31). Briefly, coupled bead stocks were diluted in phosphate-buffered saline, pH 7.4, with 1% bovine serum albumin (PBS-BSA) to a final concentration of 100 beads/μl. Beads (5 × 103) from each bead set were added to individual wells of a prewet 96-well filter plate. An equal-volume 2-fold serial dilutions of anti-species IgG primary antibody (from 12.5 μg/ml to 0.1 μg/ml) was added to the beads, mixed gently, covered, and allowed to incubate in the dark, at room temperature for 30 min at 500 rpm on a VWR microplate shaker (Radnor, PA, USA).
After incubation, supernatant was vacuumed out, wells were washed twice with 100 μl of PBS (pH 7.4) containing 0.05% Tween 20 (PBS-T) (Sigma, St. Louis, MO, USA) and vacuumed again to remove excess buffer. Beads were resuspended in PBS-BSA buffer and incubated with 0.8 μg of biotinylated anti-species IgG secondary detection antibody. The filter plates were covered and allowed to incubate in the dark at room temperature for 30 min on a plate shaker. After a 30-minute incubation in the dark on a plate shaker to protect the beads from bleaching, the wells were washed twice as described above. Then, the samples were incubated for 30 min with 1.2 μg of streptavidin-R-phycoerythrin, vacuumed, washed twice, and resuspended in 100 μl of PBS-BSA. The plates were then analyzed on a Luminex 100 analyzer (Luminex Corporation, Austin, TX, USA).
Saliva collection, processing, and analysis.
During the summer of 2009, informed consent was obtained from subjects in accordance with Institutional Review Board approval (IRB no. 08-1844, University of North Carolina, Chapel Hill, NC, USA) and saliva samples were collected from 2,091 study participants at Boquerón Beach, Puerto Rico (Fig. 1). During the initial sample collection at the beach, study participants were guided on how to perform the sample collection and instructed to rub the Oracol saliva collection device (Malvern Medical Developments, Worcester, United Kingdom) against the gingival crevices of the oral mucosa (between the gums and teeth) to absorb saliva. Individuals who reported dental or any other illnesses were excluded from the study. Infants under 1 year old were also excluded at the time of the initial collection because of the potential for contamination by maternal antibodies and high rates of nonwaterborne infections. Within 2 days postcollection, participants shipped the second and third samples overnight on ice to USEPA in Cincinnati, OH, for storage at 4°C until ready for processing. Within 1 week of receipt, Oracol saliva collection devices were thawed to room temperature, centrifuged twice (first at 491 × g, 10°C, for 5 min to recover the saliva off the collection sponge and then at 1,363 × g, 10°C, for an additional 5 min to pellet debris from the saliva), and transferred to 1.5-ml microcentrifuge tubes. The samples were then centrifuged at 1,500 × g for 3 min, and the supernatant was transferred to a fresh 1.5-ml microcentrifuge tube and stored at −80°C.
FIG 1.
(A) Map of United States showing Puerto Rico. (B) Map of Puerto Rico showing Boquerón Beach (white arrow). Images courtesy of Google Maps: map data ©2020 Google, INEGI for the U.S. mainland (https://goo.gl/maps/wxUE7TQ7EW1DHXHU9), and Data LDEO-Columbia, NSF, NOAA Data SIO, NOAA, U.S. Navy, NGA, GEBCO Landsat/Copernicus for the map of Puerto Rico (t.ly/Wgcl). Last accessed 30 July 2020.
For analysis, a 1:4 dilution of the saliva samples in phosphate-buffered saline containing PBS-1% BSA was added to prewet and vacuumed 96-well filter plates (Millipore, Billerica, MA, USA). Beads (5 × 103) from each bead set and an equal volume of diluted saliva were loaded onto each well, resulting in a final dilution of 1:8 in a total volume of 100 μl per well. The loaded filter plates were processed as previously described, and reporter fluorescence was measured using a Luminex 100 analyzer and expressed as median fluorescence intensity (MFI) of at least 100 beads per bead set (30, 31). MFI readings are produced for every sample and serve as a proxy for antibodies present against the targeted pathogens. Each 96-well plate takes an average of 45 min to run the 29 targets/analytes we tested in each well on the Luminex 100 analyzer.
Assay controls, cross-reactivity, and SNR.
Assay controls have been described in detail elsewhere (31), but briefly stated, a unique, uncoupled bead set was added to the assay to evaluate nonspecific binding and sample-to-sample variability. These control beads were treated identically to antigen-conjugated beads and blocked with BSA but were not coupled to any antigen during the coupling step. Samples with reactivity to uncoupled control beads at ≥500 MFI were discarded to control for nonspecific binding and/or possible contamination of the saliva by serum from gum disease or other sources. Tests for cross-reactivity were performed in monoplex and duplex. Assay sensitivity was validated with characterized human plasma samples as previously described (30, 31), and a signal-to-noise ratio (SNR) was calculated by dividing the MFI of the specific antigen signals by the MFI of the uncoupled control beads for each sample (31, 36).
Defining immunoprevalence and immunoconversions.
Cutoff criteria were established in reference 32 [cutoff = 10mean (h) + 3 SD (h), where h = log10 (MFI of control beads)] to distinguish immunopositive and immunonegative samples and employed to measure immunopositivity and immunoprevalence (baseline immunopositivity) in the population. Immunoconversions are defined using the more stringent three-sample criterion presented by Simmons et al. (33), which extends the traditional 4-fold increase from S1 to S2 definition to ensure that the S2 sample is immunopositive (MFI ≥ cutoff point) and accounts for the fact that IgG levels are expected to remain relatively high and not drop to zero during the 30- to 40-day period after initial exposure; accordingly, the immunoconversion criteria are S2 ≥ 4 × S1, S2 ≥ cutoff, and S3 ≥ 3 × S1. Immunoconversions were computed only for study participants who provided all three samples.
Statistical analyses.
All data analyses were performed using Microsoft Excel 2016, JMP 14, and Matlab release 2018b. To examine possible risk factors of exposure, we used Fisher’s exact test to provide odds ratios and two-sided P values related to the association between HAV immunoconversions and general epidemiological survey data compiled during the NEEAR Water study on participant gender, age, consumption of undercooked meat or raw fish, contact with unknown animals, head immersion swimming, diarrhea at 10 to 14 days, contact with ill people, and chronic issues, including gastrointestinal (GI) disease, allergies, and asthma.
Approval was obtained from the University of North Carolina, Chapel Hill, NC, USA (IRB no. 08-1844), for the collection of saliva samples from beachgoers at Boquerón Beach, Puerto Rico, as part of the USEPA NEEAR Water study. Study subjects provided informed consent and were instructed on the use of the Oracol saliva collection device. Infants younger than 1 year were not included. Informed consent was obtained from parents of minors.
RESULTS
Beach selection and study population.
Figure 1A shows a map of the United States including Puerto Rico. Boquerón Beach, Puerto Rico, is in the beach town of Cabo Rojo in the southwest of the island (Fig. 1B) and is commonly attended by families on the island (70% of the visitors were locals who reported six or more visits per year). As discussed in the introduction, socioeconomically, Puerto Rico’s status falls within the low range with high endemicity levels of HAV infection. Study participants provided 5,533 serially collected saliva samples; however, 95 samples were removed from further analysis after quality assurance/quality control procedures discussed previously (34). The remaining 5,438 samples were broken down as follows: S1, 2,078; S2, 1,694; and S3, 1,666.
Bead coupling and confirmation.
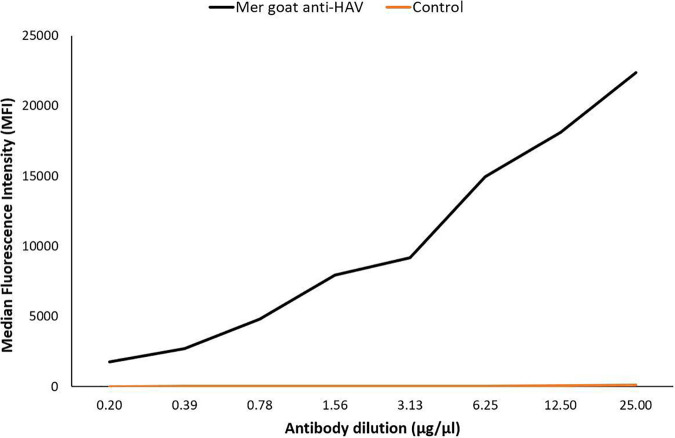
To confirm that the HAV antigen was sufficiently coupled to the carboxylated beads, anti-HAV polyclonal antibodies were exposed to the antigen-coupled beads as well as uncoupled control beads (Fig. 2).
FIG 2.
Coupling confirmation of duplex HAV antigen and uncoupled control beads using goat-anti-HAV polyclonal antibodies.
Prevalence of HAV exposure and incident infections in study participants.
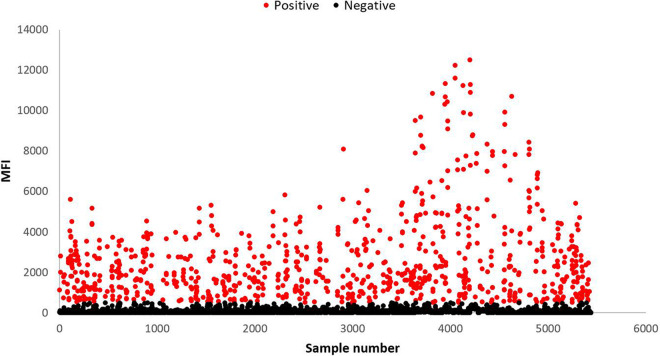
Figure 3 provides a scatterplot of the MFI response for all the saliva samples collected with the positive samples showing in red. To determine the baseline immune status of the beachgoers, HAV immunoprevalence was computed from saliva samples collected from participants at the beach (S1). Results indicate that beachgoers had a 16.17% (336/2,078) immunoprevalence rate. Nearly 70% of the participants gave all three samples (1,399/2,078), and analysis of samples from this cohort was used to determine immunoconversions (incident infections). Immunopositivity rates for this group remained relatively consistent with anti-HAV antibodies detected in approximately 16% of samples from S1 and subsequent samples (S1, 16.15% [226]; S2 and S3, 15.44% [216]) (Fig. 4).
FIG 3.
Scatterplot of anti-HAV responses measured in median fluorescence intensity (MFI) units for all saliva samples analyzed (n = 5,438). Positive samples (MFI ≥ cutoff) are shown in red (n = 849).
FIG 4.
Immunopositivity heatmap for study participants who returned all three samples (n = 1,399). Red lines denote immunopositive samples (MFI ≥ cutoff).
Analysis of MFI results indicated that 20 people (1.43%) immunoconverted to HAV. Epidemiological surveys were completed by most of the participants (n = 1,298) and, accordingly, used to assess possible linkages between immunoconversion rates and both demographic and exposure risk factors (Table 1). Most of the participants were female and did not consume undercooked meat or raw fish, nor did they have unknown animal contact. Furthermore, they did not swim in the previous 2 weeks, nor did they report diarrhea or contact with ill people. While most immersed their head when swimming, relatively few of the participants reported suffering from allergies, asthma, or chronic GI illness.
TABLE 1.
Evaluation of associations between HAV immunoconversions and potential risk factorsa
| Characteristic or risk factor (n) | IC (%n) | % IC |
|---|---|---|
| All (1,298) | 20 (1.54) | 100.0 |
| Gender | ||
| Male (548) | 11 (2.01) | 55.0 |
| Female (750) | 9 (1.2) | 45.0 |
| P value | 0.2611 | |
| Age (yr) | ||
| 0–4 (48) | 0 (0) | 0.0 |
| 5–11 (148) | 2 (1.35) | 10.0 |
| 12–19 (209) | 2 (0.96) | 10.0 |
| 20–34 (319) | 4 (1.25) | 20.0 |
| 35 and over (569) | 12 (2.11) | 60.0 |
| P value | 0.8121 | |
| Children under 7 yr | ||
| No (1,140) | 19 (1.67) | 95.0 |
| Yes (158) | 1 (0.63) | 5.0 |
| P value | 0.498 | |
| Undercooked meat consumption | ||
| No (1,263) | 20 (1.58) | 100.0 |
| Yes (34) | 0 (0) | 0.0 |
| P value | 1 | |
| Raw fish consumption | ||
| No (1,248) | 20 (1.6) | 100.0 |
| Yes (49) | 0 (0) | 0.0 |
| P value | 1 | |
| Unknown animal contact | ||
| No (1,214) | 18 (1.48) | 90.0 |
| Yes (48) | 1 (2.08) | 5.0 |
| P value | 0.5239 | |
| Swimming in previous 2 wk | ||
| No (884) | 14 (1.58) | 70.0 |
| Yes (414) | 6 (1.45) | 30.0 |
| P value | 1 | |
| Head immersion swimming | ||
| No (394) | 3 (0.76) | 15.0 |
| Yes (903) | 17 (1.88) | 85.0 |
| P value | 0.1495 | |
| Diarrhea at 10–12 days | ||
| No (1,248) | 19 (1.52) | 95.0 |
| Yes (20) | 0 (0) | 0.0 |
| P value | 1 | |
| Contact with ill people | ||
| No (1,221) | 20 (1.64) | 100.0 |
| Yes (75) | 0 (0) | 0.0 |
| P value | 0.6246 | |
| Allergies | ||
| No (1,127) | 17 (1.51) | 85.0 |
| Yes (171) | 3 (1.75) | 15.0 |
| P value | 0.7394 | |
| Asthma | ||
| No (1,171) | 18 (1.54) | 90.0 |
| Yes (127) | 2 (1.57) | 10.0 |
| P value | 1 | |
| Chronic GI illness | ||
| No (1,224) | 18 (1.47) | 90.0 |
| Yes (74) | 2 (2.7) | 10.0 |
| P value | 0.3173 | |
Fisher’s exact test was used to compute two-sided P values. In the table, IC (%n) is the percentage of people who immunoconverted and %IC is the percentage of immunoconversions. Note that 1,298 of the participants (n = 1,298) returned surveys but the numbers for each category may not add up to 1,298 (or 20 immunoconversions) due to nonresponse on individual questionnaires.
Individuals with HAV immunoconversions ranged in age from 6 to 88 years (mean = 39.7 years). Moreover, 60% (n = 12) of those who immunoconverted were over 35 years old. Although more females participated in the study, slightly more males experienced HAV infections, and nearly all the individuals with HAV immunoconversions immersed their head while swimming (85%). Figure 5 provides a visualization of the MFI responses from the baseline to final sample (S1 to S3) and associated chronic underlying conditions (CUCs) for those who immunoconverted. The black lines denote the 7 (35%) participants suffering from specific chronic conditions showing in the lower (Fig. 5B) panel (i.e., gastrointestinal [GI] issues, 2 [10%]; allergies, 3 [15%]; and asthma, 2 [10%]). Most (65%; 13/20) of the HAV immunoconversions were unaccompanied by the chronic conditions considered (denoted by the gray line/shading in Fig. 5B). Consequently, there was no statistically significant association (P values ≫0.05) between HAV immunoconversions and any of the demographic or exposure risk factors (Table 1).
FIG 5.
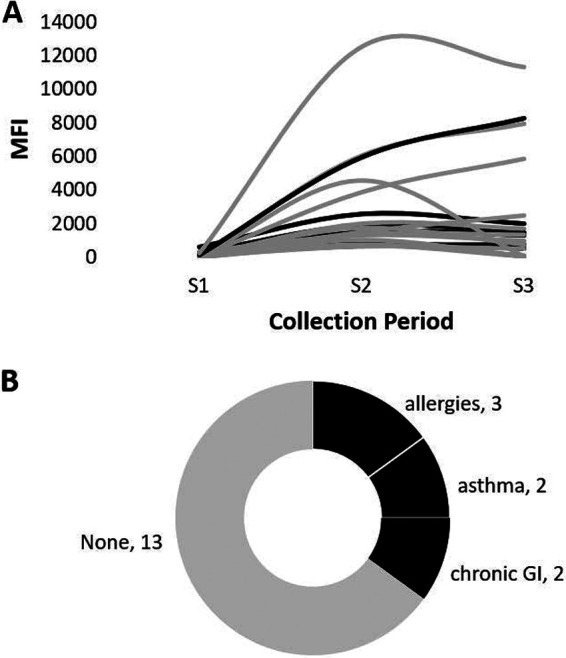
Summary of HAV immunoconversions and reported chronic underlying conditions (CUCs). (A) MFI response curves of the 20 individuals who immunoconverted. (B) Tree map of CUCs reported for individuals with HAV immunoconversions. The line color/shading is used to denote individuals with (black) and without (gray) CUCs.
DISCUSSION
The importance of population-based studies as a valuable tool for surveillance cannot be understated. These studies are essential in monitoring immunoprevalence rates over time to evaluate changes in epidemiological trends and provide important information regarding exposure susceptibility and potential future outbreaks, thereby facilitating the efforts of policy makers, public health practitioners, and environmental managers to adapt and/or adopt preventive measures (27). As such, rapid, noninvasive methods are needed to monitor changes in the population to determine the sources of exposures to these diseases. The bead-based salivary antibody immunoassay presented and applied in this study serves as a rapid screening test of HAV antibody prevalence and subsequent incident infections in a population. Moreover, the use of saliva greatly expands the applicability and future utility of the method. Saliva collection is less expensive and is neither invasive nor painful; hence, it is very well tolerated by children, a key group in epidemiologic studies. Relatively small sample volumes are needed, and trained personnel are not required to obtain samples (27).
In our study, we found a 16.17% immunoprevalence rate of anti-HAV antibodies in the beachgoers, which is about half of the overall immunoprevalence rate of 31.2% among U.S.-born persons ≥2 years of age between 2007 and 2012 (37). Researchers have shown that among U.S.-born persons ≥20 years old, there was a 24.2% decrease in the overall age-adjusted prevalence of anti-HAV antibodies during the same period, down from 29.5% between 1999 and 2006 (37). Only 1.43% of the participants who provided all three samples were found to have HAV immunoconversions. Of the 20 participants who immunoconverted, only 7 (35%) reported having underlying chronic conditions; none experienced diarrhea, and there was no statistically significant association between any of the demographic or exposure risk factors tested.
The low immunoconversion rate suggests that there is some level of immune protection in the population. Residents of the Cabo Rojo and Boquerón Beach area were the primary visitors to the beach (most participants reported multiple visits to the beach each year). In this study, we did not determine whether tourists at the beach were more likely to have become exposed or themselves displayed evidence of previous HAV infections in the initial S1 sample. This would have provided a valuable comparison in rates of immunoprevalence and incident infections between tourists and residents, as well as the efficacy of the HAV vaccine and the effectiveness of global vaccination programs. Although information regarding hepatitis A vaccination series completion rates is limited, low HAV vaccine series completion rates were observed among cohorts of commercial/Medicare (32%) and Medicaid (21%) enrollees in the United States (38). Additionally, adherence with and completion of recommended hepatitis vaccination schedules among adults in the United States have been described as suboptimal, leaving a substantial proportion of adults at risk (39). The same is true for adults in the United Kingdom, where adherence rates topped out at 23% (40). We may have observed higher rates of symptomatic infections in tourists than in residents who, through repeated exposures, would have been immunoprotected and therefore less likely to be symptomatic. We observed this phenomenon with norovirus GI.1 and GII.4 infections in the study population, where evidence of relatively high levels of antinorovirus antibodies was observed in the population without the expected symptoms of gastrointestinal illness (34).
Still, the estimated decrease in anti-HAV antibodies in those ≥20 years of age presents a public health challenge because it suggests that a substantial number of persons in the population remain susceptible to HAV infection at ages when the risk of morbidity and mortality from HAV infections is highest (41). Outbreaks occur because people have not been vaccinated or exposed or their immunity has declined over time. Accordingly, the observed decrease in anti-HAV antibodies in the population presents an ideal environment for outbreaks to occur.
Limitations of this study include nonspecific binding of antibodies in human saliva to the HAV antigen coupled to the beads, potential for cross-reactivity in the multiplex assay, and the difficulty in correlating water quality with antibody responses, symptomology, and incident infections. These limitations were addressed using a number of approaches (e.g., testing in monoplex and duplex and validating antigens using characterized samples) and were discussed in greater detail previously (30, 31).
Although not specifically stated, a core goal of the overall effort is to link HAV incident infections to water quality. Because HAV infections are often asymptomatic in some populations, there is great difficulty in directly linking symptoms or water quality to HAV infection unless those symptoms had progressed to jaundice or HAV viral particles were isolated directly from the stool of the participants. An additional limitation is that symptomology information was collected only at S2 (10 to 14 days after beach visit) and can be highly subjective. As such, it would be difficult to link symptomology to hepatitis A infection because of the long incubation period and the fact that symptoms are not generally expressed until approximately day 28. These results dictate that symptomology data also be collected during the submission of both S2 and S3 samples to capture symptoms from pathogens with longer incubation periods. Previous testing of the same saliva samples detected evidence of exposure and immunoconversions against Helicobacter pylori, Campylobacter jejuni, Toxoplasma gondii, and noroviruses GI.1 and GII.4 (pathogens that produce similar GI symptoms), and the use of the immunoassay afforded the ability to examine exposure patterns even when symptoms or possible risk factors are absent. Further, linking water quality to HAV infections is difficult because investigators did not isolate HAV directly from the water samples. As a part of the NEEAR Water study, water quality was assessed at Boquerón Beach during the study period using Enterococcus CFU (CFU per 100 ml), Enterococcus CCE (quantitative PCR [qPCR] calibrator cell equivalents per 100 ml), and culture-based methods but was not analyzed specifically for HAV. Results indicated that the water quality was relatively good with low fecal indicator counts for enterococci and Bacteroidales (35). Wade et al. noted that any attempt to draw conclusions regarding the water quality data at Boquerón Beach would be questionable because of interference in the qPCR assay from an unknown source (35). Further, according to Wade et al., fecal indicator bacteria are used to monitor recreational waters because it is usually impractical to test these waters directly for the many and diverse pathogenic microorganisms associated with human-derived sewage (42). Accordingly, linking water quality to infection rates would require that other tests be performed to directly examine the presence of targeted organisms in water samples. In a recent study, researchers developed a reverse transcription plus nested or seminested PCR assay followed by sequencing and phylogenetic analysis to detect and genotype noroviruses and rotaviruses simultaneously in a wastewater treatment and reclamation system (43). Such an approach could be quite beneficial in linking water quality more directly with exposure health effects.
In summary, results from this effort demonstrate the utility and benefits of a rapid population-based, salivary antibody screening method in monitoring epidemiologic changes in the population. To better understand the potential cost and time savings afforded by the multiplex immunoassay, we compared it to an ELISA. While both methods can be used to analyze different types of proteins, the core difference lies in the fact that, unlike an ELISA which can assess only one analyte at a time, a multiplex immunoassay is a high-throughput method that possesses the ability to examine between 100 and 500 analytes, simultaneously. ThermoFisher estimates that the cost of analyzing one analyte is essentially the same for the two methods; however, the savings per target increases as the number of analytes increases (44). For example, while analyzing 29 analytes would cost nearly $9,000 U.S. and take about 120 h (5 days) using ELISA kits, multiplexing the analytes would cost roughly $3,700 U.S. and could be achieved in 45 min (44). The use of a multiplex immunoassay can facilitate the timely dissemination of information useful for public health officials and policy makers and could lead to measures such as more robust vaccination schedules and more stringent water and food quality advisories to reduce future exposures and corresponding incident infections. According to the U.S. Centers for Disease Control and Prevention (CDC), HAV surveillance can assist in (i) detecting and providing data to control outbreaks, (ii) identifying contacts of case-patients who require postexposure prophylaxis, (iii) characterizing changes in the epidemiology of infected populations and risk factors, and (iv) guiding vaccination policies and other prevention efforts (41). Hence, this bead-based salivary antibody assay can potentially be used as a rapid, inexpensive, noninvasive screening tool for HAV and other waterborne infections to help public health officials, policy makers, risk assessors, first responders, and the public in mitigating the health and financial burden posed by exposure to existing and emerging pathogens. Moreover, the reduced cost of multiplexing may be economically beneficial to developing and underdeveloped countries by providing a screening tool whereby antibody responses to multiple pathogens can be studied simultaneously, rapidly, and noninvasively.
ACKNOWLEDGMENTS
C.L.C. and M.K.D.R. were supported through an appointment to the Research Participation Program at the U.S. Environmental Protection Agency administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the U.S. Environmental Protection Agency.
Mention of trade names or commercial products does not constitute endorsement or recommendation by the United States Environmental Protection Agency for use.
The authors report no conflict of interest.
S.A.J.A., K.J.S., T.N.E., and T.J.W. designed the study. T.J.W. and E.A.S. provided the saliva samples. S.A.J.A., K.J.S., S.M.G., C.L.C., and M.K.D.R. conducted the laboratory experiments and processed the raw assay data. T.N.E. performed the data analysis. S.A.J.A. and T.N.E. wrote the original manuscript, and S.A.J.A., T.N.E., K.J.S., C.L.C., S.M.G., M.K.D.R., K.H.O., E.A.S., A.D., and T.J.W. reviewed, provided comments, and approved the final manuscript.
The United States Environmental Protection Agency through its Office of Research and Development funded and managed the research described here. It has been subjected to Agency’s administrative review and approved for publication.
REFERENCES
- 1.Cuthbert JA. 2001. Hepatitis A: old and new. Clin Microbiol Rev 14:38–58. doi: 10.1128/CMR.14.1.38-58.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armstrong GL, Bell BP. 2002. Hepatitis A virus infections in the United States: model-based estimates and implications for childhood immunization. Pediatrics 109:839–845. doi: 10.1542/peds.109.5.839. [DOI] [PubMed] [Google Scholar]
- 3.Tejada-Strop A, Zafrullah M, Kamili S, Stramer SL, Purdy MA. 2018. Distribution of hepatitis A antibodies in US blood donors. Transfusion 58:2761–2765. doi: 10.1111/trf.14916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koroglu M, Jacobsen KH, Demiray T, Ozbek A, Erkorkmaz U, Altindis M. 2017. Socioeconomic indicators are strong predictors of hepatitis A seroprevalence rates in the Middle East and North Africa. J Infect Public Health 10:513–517. doi: 10.1016/j.jiph.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 5.Hadler SC, Erben JJ, Matthews D, Starko K, Francis DP, Maynard JE. 1983. Effect of immunoglobulin on hepatitis A in day-care centers. JAMA 249:48–53. doi: 10.1001/jama.1983.03330250028023. [DOI] [PubMed] [Google Scholar]
- 6.Bull AR, Kimmance KJ, Parry JV, Perry KR. 1989. Investigation of an outbreak of hepatitis A simplified by salivary antibody testing. Epidemiol Infect 103:371–376. doi: 10.1017/s0950268800030715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nainan OV, Xia G, Vaughan G, Margolis HS. 2006. Diagnosis of hepatitis A virus infection: a molecular approach. Clin Microbiol Rev 19:63–79. doi: 10.1128/CMR.19.1.63-79.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Census Bureau. 2018. QuickFacts Puerto Rico. US Census Bureau, Washington, DC. https://www.census.gov/quickfacts/PR.
- 9.Jacobsen GD, Jacobsen KH. 2011. Health awareness campaigns and diagnosis rates: evidence from National Breast Cancer Awareness Month. J Health Econ 30:55–61. doi: 10.1016/j.jhealeco.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Dignani MC, Miceli MH, Rosa CM, Gatica J, Martínez-Rolón J, Pizzolato M. 2003. Loss of hepatitis A virus (HAV) antibodies after peripheral stem cell transplantation (PSCT). Bone Marrow Transplant 31:809–812. doi: 10.1038/sj.bmt.1704028. [DOI] [PubMed] [Google Scholar]
- 11.Jindal M, Rana SS, Gupta RK, Das K, Kar P. 2002. Serological study of hepatitis A virus infection amongst the students of a medical college in Delhi & evaluation of the need of vaccination. Indian J Med Res 115:1–4. [PubMed] [Google Scholar]
- 12.Stapleton JT. 1995. Host immune response to hepatitis A virus. J Infect Dis 171(Suppl 1):S9–S14. doi: 10.1093/infdis/171.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- 13.Purcell RH, Wong DC, Moritsugu Y, Dienstag JL, Routenberg JA, Boggs JD. 1976. A microtiter solid-phase radioimmunoassay for hepatitis A antigen and antibody. J Immunol 116:349–356. [PubMed] [Google Scholar]
- 14.Huang SN, Lorenz D, Gerety RJ. 1979. Electron and immunoelectron microscopic study on liver tissues of marmosets infected with hepatitis A virus. Lab Invest 41:63–71. [PubMed] [Google Scholar]
- 15.Delem AD. 1992. Comparison of modified HAVAB and ELISA for determination of vaccine-induced anti-HAV response. Biologicals 20:289–291. doi: 10.1016/s1045-1056(05)80049-1. [DOI] [PubMed] [Google Scholar]
- 16.Wang CH, Tschen SY, Heinricy U, Weber M, Flehmig B. 1996. Immune response to hepatitis A virus capsid proteins after infection. J Clin Microbiol 34:707–713. doi: 10.1128/JCM.34.3.707-713.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shao ZJ, Xu DZ, Yan YP, Li JH, Zhang JX, Zhang ZY, Pan BR. 2003. Detection of anti-HAV antibody with dot immunogold filtration assay. World J Gastroenterol 9:1508–1511. doi: 10.3748/wjg.v9.i7.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goswami BB, Burkhardt W III, Cebula TA. 1997. Identification of genetic variants of hepatitis A virus. J Virol Methods 65:95–103. doi: 10.1016/S0166-0934(97)02179-4. [DOI] [PubMed] [Google Scholar]
- 19.Fujiwara K, Yokosuka O, Ehata T, Imazeki F, Saisho H. 2000. PCR-SSCP analysis of 5′-nontranslated region of hepatitis A viral RNA: comparison with clinicopathological features of hepatitis A. Dig Dis Sci 45:2422–2427. doi: 10.1023/a:1005607512633. [DOI] [PubMed] [Google Scholar]
- 20.Buti M, Jardi R, Bosch A, Rodriguez F, Sanchez G, Pinto R, Costa X, Sanchez-Avila JF, Cotrina M, Esteban R, Guardia J. 2001. Assessment of the PCR-Southern blot technique for the analysis of viremia in patients with acute hepatitis A. Gastroenterol Hepatol 24:1–4. (In Spanish.) doi: 10.1016/S0210-5705(01)70124-0. [DOI] [PubMed] [Google Scholar]
- 21.Cromeans TL, Nainan OV, Margolis HS. 1997. Detection of hepatitis A virus RNA in oyster meat. Appl Environ Microbiol 63:2460–2463. doi: 10.1128/AEM.63.6.2460-2463.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKie A, Vyse A, Maple C. 2002. Novel methods for the detection of microbial antibodies in oral fluid. Lancet Infect Dis 2:18–24. doi: 10.1016/s1473-3099(01)00169-4. [DOI] [PubMed] [Google Scholar]
- 23.Moorthy M, Daniel HD, Kurian G, Abraham P. 2008. An evaluation of saliva as an alternative to plasma for the detection of hepatitis C virus antibodies. Indian J Med Microbiol 26:327–332. doi: 10.4103/0255-0857.42116. [DOI] [PubMed] [Google Scholar]
- 24.Mortimer PP, Parry JV. 1988. The use of saliva for viral diagnosis and screening. Epidemiol Infect 101:197–200. doi: 10.1017/s0950268800054108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nokes DJ, Enquselassie F, Nigatu W, Vyse AJ, Cohen BJ, Brown DWG, Cutts FT. 2001. Has oral fluid the potential to replace serum for the evaluation of population immunity levels? A study of measles, rubella and hepatitis B in rural Ethiopia. Bull World Health Organ 79:588–595. [PMC free article] [PubMed] [Google Scholar]
- 26.O’Connell T, Thornton L, O’Flanagan D, Staines A, Connell J, Dooley S, McCormack G. 2001. Oral fluid collection by post for viral antibody testing. Int J Epidemiol 30:298–301. doi: 10.1093/ije/30.2.298. [DOI] [PubMed] [Google Scholar]
- 27.Quoilin S, Hutse V, Vandenberghe H, Claeys F, Verhaegen E, De Cock L, Van Loock F, Top G, Van Damme P, Vranckx R, Van Oyen H. 2007. A population-based prevalence study of hepatitis A, B and C virus using oral fluid in Flanders, Belgium. Eur J Epidemiol 22:195–202. doi: 10.1007/s10654-007-9105-6. [DOI] [PubMed] [Google Scholar]
- 28.Stuart JM, Majeed FA, Cartwright KA, Room R, Parry JV, Perry KR, Begg NT. 1992. Salivary antibody testing in a school outbreak of hepatitis A. Epidemiol Infect 109:161–166. [PMC free article] [PubMed] [Google Scholar]
- 29.Thieme T, Yoshihara P, Piacentini S, Beller M. 1992. Clinical evaluation of oral fluid samples for diagnosis of viral hepatitis. J Clin Microbiol 30:1076–1079. doi: 10.1128/JCM.30.5.1076-1079.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Augustine SA, Eason TN, Simmons KJ, Curioso CL, Griffin SM, Ramudit MK, Plunkett TR. 2016. Developing a salivary antibody multiplex immunoassay to measure human exposure to environmental pathogens. J Vis Exp (115):54415. doi: 10.3791/54415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Augustine SAJ, Simmons KJ, Eason TN, Griffin SM, Curioso CL, Wymer LJ, Shay Fout G, Grimm AC, Oshima KH, Dufour A. 2015. Statistical approaches to developing a multiplex immunoassay for determining human exposure to environmental pathogens. J Immunol Methods 425:1–9. doi: 10.1016/j.jim.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Augustine SAJ, Simmons KJ, Eason TN, Curioso CL, Griffin SM, Wade TJ, Dufour A, Fout GS, Grimm AC, Oshima KH, Sams EA, See MJ, Wymer LJ. 2017. Immunoprevalence to six waterborne pathogens in beachgoers at Boqueron Beach, Puerto Rico: application of a microsphere-based salivary antibody multiplex immunoassay. Front Public Health 5:84. doi: 10.3389/fpubh.2017.00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simmons KJ, Eason TN, Curioso CL, Griffin SM, Ramudit MKD, Oshima KH, Sams EA, Wade TJ, Grimm A, Dufour A, Augustine SAJ. 2019. Visitors to a tropical marine beach show evidence of immunoconversions to multiple waterborne pathogens. Front Public Health 7:231. doi: 10.3389/fpubh.2019.00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wade TJ, Augustine SAJ, Griffin SM, Sams EA, Oshima KH, Egorov AI, Simmons KJ, Eason TN, Dufour AP. 2018. Asymptomatic norovirus infection associated with swimming at a tropical beach: a prospective cohort study. PLoS One 13:e0195056. doi: 10.1371/journal.pone.0195056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wade TJ, Sams EA, Haugland R, Brenner KP, Li Q, Wymer W, Molina M, Oshima K, Dufour A. 2009. Report on 2009 national epidemiologic and environmental assessment of recreational water epidemiology studies. US Environmental Protection Agency, Washington, DC. [Google Scholar]
- 36.Cretich M, Daaboul GG, Sola L, Unlu MS, Chiari M. 2015. Digital detection of biomarkers assisted by nanoparticles: application to diagnostics. Trends Biotechnol 33:343–351. doi: 10.1016/j.tibtech.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Klevens RM, Denniston MM, Jiles-Chapman RB, Murphy TV. 2015. Decreasing immunity to hepatitis A virus infection among US adults: findings from the National Health and Nutrition Examination Survey (NHANES), 1999–2012. Vaccine 33:6192–6198. doi: 10.1016/j.vaccine.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 38.Ghaswalla PK, Patterson BJ, Cheng WY, Duchesneau E, Macheca M, Duh MS. 2018. Hepatitis A, B, and A/B vaccination series completion among US adults: a claims-based analysis. Hum Vaccin Immunother 14:2780–2785. doi: 10.1080/21645515.2018.1489189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trantham L, Kurosky SK, Zhang D, Johnson KD. 2018. Adherence with and completion of recommended hepatitis vaccination schedules among adults in the United States. Vaccine 36:5333–5339. doi: 10.1016/j.vaccine.2018.05.111. [DOI] [PubMed] [Google Scholar]
- 40.Johnson KD, Lu X, Zhang D. 2019. Adherence to hepatitis A and hepatitis B multi-dose vaccination schedules among adults in the United Kingdom: a retrospective cohort study. BMC Public Health 19:404. doi: 10.1186/s12889-019-6693-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hofmeister MG, Klevens M, Nelson N. 2018. Hepatitis A. In Roush SW, Baldy LM, Kirkconnell Hall MA (ed), Manual for the surveillance of vaccine-preventable diseases. National Center for Immunization and Respiratory Diseases, CDC, Atlanta, GA: https://www.cdc.gov/vaccines/pubs/surv-manual/. [Google Scholar]
- 42.Wade TJ, Sams E, Brenner KP, Haugland R, Chern E, Beach M, Wymer L, Rankin CC, Love D, Li Q, Noble R, Dufour AP. 2010. Rapidly measured indicators of recreational water quality and swimming-associated illness at marine beaches: a prospective cohort study. Environ Health 9:66. doi: 10.1186/1476-069X-9-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ji Z, Wang XC, Xu L, Zhang C, Rong C, Rachmadi AT, Amarasiri M, Okabe S, Funamizu N, Sano D. 2019. Fecal source tracking in a wastewater treatment and reclamation system using multiple waterborne gastroenteritis viruses. Pathogens 8:170. doi: 10.3390/pathogens8040170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thermo Fisher Scientific. 2016. The power of multiplexing. Thermo Fisher Scientific, Waltham, MA: https://www.thermofisher.com/content/dam/LifeTech/global/technical-reference-library/s2s/dbourdon/The%20power%20of%20multiplexing.pdf. [Google Scholar]