LETTER
Clinical and public health laboratories throughout the world have rapidly expanded diagnostic testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (1–3). Supply chain shortages have necessitated redundant methods and workflows for SARS-CoV-2 testing in many laboratories. However, the interchangeability of instrumentation, especially for independent RNA extraction and nucleic acid amplification, remains largely unreported. This study compared the performance of three direct RNA extraction platforms and two thermocyclers as pivotal components within a single commercially available SARS-CoV-2 testing workflow.
Samples were deidentified nasopharyngeal swabs in universal transport medium (UTM; Quidel) collected originally as part of routine patient care in April 2020 at Barnes-Jewish Hospital in St. Louis, MO. Ten specimens were selected with original cycle threshold (CT) values ranging from 10 to 30 using the Lyra real-time PCR (RT-PCR) kit (Quidel) under Emergency Use Authorization by the FDA (4). The assay procedure subtracts the first 10 cycles from the raw CT values (i.e., when comparing with conventional methods, +10 CT correction factor should be applied to presented data). Specimens were diluted 1:10 in UTM and then processed following the manufacturers’ instructions on three extraction systems: KingFisher Flex purification system (Thermo Fisher), EZ1 Advanced XL (Qiagen), and NUCLISENS easyMAG (bioMérieux) (5–7). Eluates were amplified with reagents from the Lyra RT-PCR kit (8), which targets the gene encoding nonstructural polyprotein (pp1ab) of SARS-CoV-2. Eluates were run on the Rotor-Gene Q (RGQ) RT-PCR cycler (Qiagen) using software version 2.3.5 and the Applied Biosystems 7500 Fast RT-PCR system (Thermo Fisher) using software version 1.4. CT values were determined during clinical validation of the assays. External positive and negative controls from the Lyra kit were tested concurrently. Statistical analyses were performed in GraphPad Prism v8.0.
Comparisons of the extraction methods are shown in Fig. 1A to C, along with the theoretical trendline of an ideal 1:1 ratio. The KingFisher system resulted in lower CT values than the EZ1 on the RGQ and 7500 Fast (Fig. 1A, nearly all data points above the theoretical trendline) and differed from the easyMAG on the RGQ only (Fig. 1B, triangles above theoretical trendline). Although these three differences were statistically significant (P < 0.05, two-way analysis of variance [ANOVA]), they are unlikely to be clinically meaningful except potentially near the limit of detection, which is stated to be 8.00E-01 genomic RNA copies/μl (5). Importantly, all three extraction methods use the same sample input volume, but the KingFisher method elutes in half the volume of the other systems, and thus the RNA eluate is twice as concentrated, assuming 100% efficiency. This elution difference can account for the −1 CT trend in Fig. 1A and B. There was no significant difference between the EZ1 and easyMAG extraction methods using either thermocycler (Fig. 1C). There was also no significant difference by thermocycler when all three extraction methods were compared (Fig. 1D). The limits of detection for the 7500 Fast and RGQ systems correlate to CT values of 25.36 (standard deviation [SD] = 1.21) and 26.01 (SD = 0.40), respectively (data not shown). Although the limit of detection for each extraction system is not reported here, data are available for these systems for other viruses and microbes (9–12).
FIG 1.
Comparison of extraction methods using 10 clinical nasopharyngeal swab specimens. A single sample that was not detected is shown at 30 CT (the internal processing control [PRC] from the Lyra kit was detected in this sample). Statistical analyses performed in GraphPad Prism v8.0. Note that the Lyra assay removes the first 10 CT cycles (add +10 CT values to compare with conventional methods).
PCR efficiency and precision on the 7500 Fast system were assessed on a single positive patient specimen tested neat and at 1:10 and 1:100 dilutions in triplicate (Fig. 2). For all three extraction methods, PCR efficiency was determined by assessing the slopes of the observed CT values across dilutions. These slopes were statistically identical to the predicted slope based on dilution calculations and were statistically identical to each other. For precision, overall variation of the triplicates was low but increased per dilution. Two-way ANOVA comparisons revealed that the CT values of the KingFisher system were significantly lower than those of the EZ1 and easyMAG systems (both P < 0.05), likely for the same reason described above.
FIG 2.
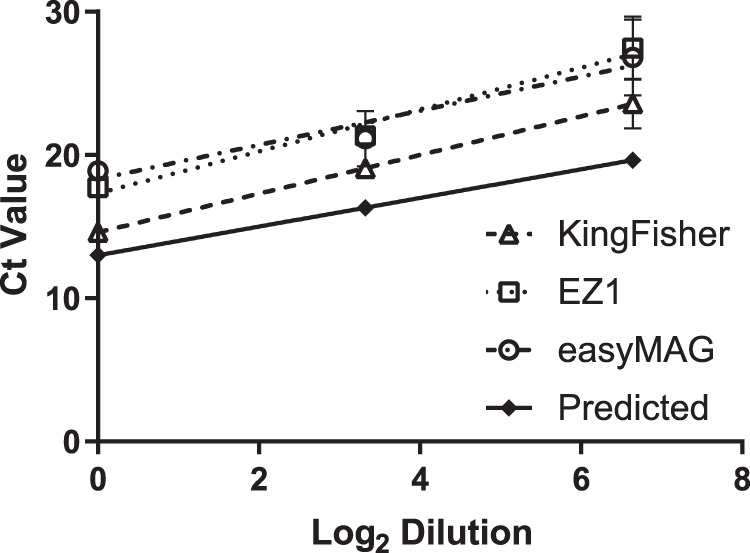
Precision and efficiency analyses. Technical replicates of a clinical sample: neat, 1:10, and 1:100. Samples were amplified on the 7500 Fast system. A single sample that was not detected is included at 30 CT (the internal processing control [PRC] from the Lyra kit was detected in this sample). Note that the Lyra assay removes the first 10 CT cycles (add +10 CT values to compare with conventional methods).
With the comparable analytical performance of the extraction and PCR systems tested in this study, other factors, such as cost, instrument or reagent availability, supply chain stability, throughput, and hands-on time, can be considered when selecting SARS-CoV-2 testing methods. The KingFisher system can extract up to 94 samples per run (excluding controls) but takes ∼90 min of technologist time to set up a full plate before the extraction run (∼30 min). The easyMAG and EZ1 have reduced setup times (∼30 min) but can extract only 22 and 12 patient samples per run (excluding controls), respectively, with run times of ∼45 min. Another common supply that is currently available in limited quantity is transport media. This study only evaluated one type (UTM), and future work is needed to evaluate others (e.g., viral transport media, Amies, and saline). Taken together, our data support the interchangeability of these methods, enabling optimization of laboratory workflows and facilitating a nimble response to fluctuations in testing capacity and supply chains.
REFERENCES
- 1.Xiao AT, Tong YX, Gao C, Zhu L, Zhang YJ, Zhang S. 2020. Dynamic profile of RT-PCR findings from 301 COVID-19 patients in Wuhan, China: a descriptive study. J Clin Virol 127:104346. doi: 10.1016/j.jcv.2020.104346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roser M, Ritchie H, Ortiz-Ospina E, Hasell J. 2020. Coronavirus pandemic (COVID-19). Our World in Data. https://ourworldindata.org/coronavirus. Accessed 2 June 2020.
- 3.Pabbaraju K, Wong AA, Douesnard M, Ma R, Gill K, Dieu P, Fonseca K, Zelyas N, Tipples GA. 2020. A public health laboratory response to the COVID-19 pandemic. J Clin Microbiol doi: 10.1128/JCM.01110-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mitchell SL, St. George K, Rhoads DD, Butler-Wu SM, Dharmarha V, McNult P, Miller MB. 2020. Understanding, verifying and implementing Emergency Use Authorization molecular diagnostics for the detection of SARS-CoV-2 RNA. J Clin Microbiol doi: 10.1128/JCM.00796-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher T. 2016. KingFisher Flex user manual. https://assets.thermofisher.com/TFS-Assets/BID/manuals/KingFisher%20Flex%20User%20Manual_English.pdf. Accessed 2 June 2020.
- 6.Qiagen. November 2017. EZ1 Advanced XL user manual. https://www.qiagen.com/us/resources/resourcedetail?id=c9ecd500-147b-4a8e-ae71-3dc86cd3d17a&lang=en. Accessed 5 June 2020.
- 7.Loens K, Bergs K, Ursi D, Goossens H, Ieven M. 2007. Evaluation of NucliSens easyMAG for automated nucleic acid extraction from various clinical specimens. J Clin Microbiol 45:421–425. doi: 10.1128/JCM.00894-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Quidel Corporation. 2020. Lyra SARS-CoV-2 Assay 9 instructions for use. https://www.fda.gov/media/136820/download. Accessed 5 June 2020.
- 9.Yang G, Erdman DE, Kodani M, Kools J, Bowen MD, Fields BS. 2011. Comparison of commercial systems for extraction of nucleic acids from DNA/RNA respiratory pathogens. J Virol Methods 171:195–199. doi: 10.1016/j.jviromet.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dundas N, Leos NK, Mitui M, Revell P, Rogers BB. 2008. Comparison of automated nucleic acid extraction methods with manual extraction. J Mol Diagn 10:311–316. doi: 10.2353/jmoldx.2008.070149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hindiyeh M, Mor O, Pando R, Mannasse B, Kabat A, Assraf-Zarfati H, Mendelson E, Sofer D, Mandelboim M. 2019. Comparison of the new fully automated extraction platform eMAG to the MagNA PURE 96 and the well-established easyMAG for detection of common human respiratory viruses. PLoS One 14:e0211079. doi: 10.1371/journal.pone.0211079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perry MD, White PL, Barnes RA. 2014. Comparison of four automated nucleic acid extraction platforms for the recovery of DNA from Aspergillus fumigatus. J Med Microbiol 63:1160–1166. doi: 10.1099/jmm.0.076315-0. [DOI] [PubMed] [Google Scholar]



