QuantiFERON-TB Gold Plus (QFT-Plus) is the most widely used interferon gamma release assay (IGRA) for the diagnosis of latent tuberculosis infection (LTBI). The aim of this study was to compare QFT-Plus results by enzyme-linked immunosorbent assay (ELISA) on the SkyLab system with those obtained with chemiluminescence immunoassay (CLIA) on the Liaison XL analyzer. Agreement between the two assays was evaluated on 419 QFT-Plus blood samples and was found to be substantial (75.4%); higher agreement was found for positive (95.
KEYWORDS: interferon gamma release assay, QuantiFERON-TB Gold Plus, agreement, chemiluminescent immunoassay, cutoff, enzyme-linked immunoassay, latent tuberculosis infection
ABSTRACT
QuantiFERON-TB Gold Plus (QFT-Plus) is the most widely used interferon gamma release assay (IGRA) for the diagnosis of latent tuberculosis infection (LTBI). The aim of this study was to compare QFT-Plus results by enzyme-linked immunosorbent assay (ELISA) on the SkyLab system with those obtained with chemiluminescence immunoassay (CLIA) on the Liaison XL analyzer. Agreement between the two assays was evaluated on 419 QFT-Plus blood samples and was found to be substantial (75.4%); higher agreement was found for positive (95.4%) and negative (80.4%) results, while most discordances were due to ELISA-indeterminate/CLIA-determinate results. According to Italian Clinical Microbiologist Association recommendations, in samples (n = 79) with a borderline result in ELISA (0.20 to 0.70 IU/ml), CLIA median values statistically increased (from 0.29 to 0.59 IU/ml for TB1 and from 0.32 to 0.60 IU/ml for TB2) but remained in the borderline range. Linear regression analysis indicated a substantial correlation between ELISA and CLIA for antigen tubes TB1 (Pearson’s r = 0.8666) and TB2 (Pearson’s r = 0.8728), but CLIA produced higher values than ELISA. Receiver operating characteristic (ROC) analysis showed that the optimal cutoff value in CLIA was 0.45 IU/ml for TB1 and 0.46 IU/ml for TB2. In conclusion, automated QFT-Plus with CLIA is comparable to QFT-Plus performed by ELISA. Within the linearity range of the test, CLIA detects higher quantitative values than ELISA, resulting in a higher number of determinate results and the conversion of samples that were close to the cutoff into positive borderline results. A higher cutoff for QFT-CLIA needs to be defined based on clinical diagnostic criteria.
INTRODUCTION
Latent tuberculosis infection (LTBI) is defined as a state of persistent immune response to Mycobacterium tuberculosis complex without clinically manifested evidence of active tuberculosis (TB) disease. About 1.7 billion people worldwide are estimated to have LTBI, and 5 to 10% of them are at risk of developing active TB during their lifetime (1–5).
The following two tests are available for the identification of LTBI: the tuberculin skin test (TST) and interferon gamma (IFN-γ) release assays (IGRAs). These are indirect markers of M. tuberculosis complex exposure and indicate a cellular immune response to M. tuberculosis complex. One IGRA, QuantiFERON-TB Gold Plus (QFT-Plus; Qiagen), measures IFN-γ released by T cells following stimulation by M. tuberculosis complex-specific antigens (6). QFT-Plus contains two M. tuberculosis complex-specific antigen tubes, TB1 and TB2. TB1 contains ESAT-6- and CFP-10-derived long peptides designed to elicit cell-mediated immune responses from CD4+ T-helper lymphocytes; TB2 contains the same long peptides as TB1 in addition to shorter peptides able to stimulate CD8 T cells (6–8). As CD8+ T-cell response seems to play a role in the early phase of M. tuberculosis complex infection and in reactivation from LTBI, the QFT-Plus test might be useful in identifying recent and remote LTBI, facilitating the decision to start LTBI treatment (9, 10). IFN-γ detection with QFT-Plus assay is almost exclusively performed with enzyme-linked immunosorbent assay (ELISA), which has some disadvantages in clinical laboratories, such as having labor-intense and time-consuming steps and requiring standard serial dilutions for each microplate.
Recently, new chemiluminescence immunoassays (CLIA) have been developed to detect IFN-γ in human plasma samples. However, to date, only a few studies have been published comparing QFT-Plus and the previous version QFT-TB Gold In-Tube by ELISA to CLIA (11–13). Among these, the study with the most relevant sample size (341 samples) reports a high degree of agreement (99.1%) between the two methods, using the AdvanSure I3 platform for CLIA (13).
A new fully automated CLIA detection system to measure IFN-γ in human plasma has recently been developed on the Liaison XL analyzer (DiaSorin, Italy). CLIA repeatability and reproducibility on this platform were studied by Brantestig et al., who found that the imprecision of the method is within an acceptable range and analysis of linearity showed acceptable recovery (12). Furthermore, a recent study by De Maertelaere et al. conducted on 92 samples showed that CLIA gave significantly higher values for TB1 and TB2 than ELISA (11).
The aim of this study was the head-to-head comparison of IFN-γ detection by ELISA on the SkyLab system and CLIA on the Liaison XL analyzer in a large number of plasma samples. Furthermore, we compared quantitative IFN-γ responses to M. tuberculosis complex antigens (TB1 and TB2) and mitogen detected by both methods.
MATERIALS AND METHODS
Samples.
In this study, 419 clinical specimens that had been submitted to the microbiology unit of S. Orsola-Malpighi University Hospital (Bologna, Italy) for QFT-Plus test by ELISA were also analyzed by CLIA.
Sample selection was based on the 3 categories of ELISA QFT-Plus results (positive, negative, indeterminate) according to the manufacturer’s cutoff and having a sufficient volume to perform CLIA. Furthermore, an additional category of samples defined borderline was included according to Italian Clinical Microbiologist Association recommendations. Sample size for each category was chosen to ensure a large enough number to perform the analysis on ELISA indeterminate and borderline results since they could be greatly influenced by different methods of measurement.
Samples were anonymized with an alphanumerical code according to the ELISA qualitative result.
Clinical data were not collected for this study. Informed consent was not required, as the data were analyzed anonymously. The study was conducted in accordance with the Declaration of Helsinki.
QuantiFERON-TB Gold Plus.
QFT-Plus samples (Qiagen, Germany) were analyzed by ELISA on the SkyLab automated system (Dasit, Italy) and by CLIA on the Liaison XL instrument (DiaSorin, Italy) according to the standard procedures recommended by the manufacturers (6, 14).
The clinical samples were handled according to the standard procedure for QFT-Plus assay, i.e., incubation at 37°C for 16 to 24 h within 16 h of sampling, followed by centrifugation at 2,700 × g at room temperature for 15 min and IFN-γ detection by ELISA. For this study, selected samples were promptly frozen at −20°C after ELISA to assure IFN-γ stability (15, 16). Before CLIA testing, frozen samples (range 1 to 101 days) were recentrifuged at 2,700 × g for 15 min to sediment the fibrin clots that can form during storage.
In accordance with the manufacturer’s interpretation, positive results were defined as background (nil)-corrected M. tuberculosis complex antigen (TB1 and/or TB2) values of ≥0.35 IU IFN-γ/ml; if the nil-corrected mitogen value was <0.50 IFN-γ IU/ml and/or if the nil value was >8.0 IFN-γ IU/ml, the test was considered indeterminate.
Furthermore, according to Italian Clinical Microbiologist Association recommendations, the category borderline was defined as nil-corrected M. tuberculosis complex antigens (TB1 and/or TB2) values within the range of 0.20 to 0.70 IFN-γ IU/ml (17).
Statistical analysis.
Cohen’s κ statistics were used to assess agreement between ELISA and CLIA QFT-Plus results as well as agreement between TB1 and TB2 results for each assay.
The Mann-Whitney test was used to compare medians of nil-corrected IFN-γ responses to TB1 and TB2 and mitogen. Since the ELISA QFT-Plus test cannot accurately determine IFN-γ values >10 IU/ml, a value of 10 IU/ml was attributed to plateau values in all of the analyses by convention as already adopted in the literature (18).
Samples with TB1 and TB2 IFN-γ levels within the analytical range of each assay (<10 IU/ml), excluding indeterminate results, were used to assess the correlation between ELISA and CLIA. Correlation was expressed by Pearson’s correlation coefficient (r). For this group, the optimal cutoff values of CLIA for TB1 and TB2 were determined from receiver operating characteristic (ROC) curve analysis assuming the positive result of the ELISA method as true LTBI or TB.
Statistical analysis was performed using GraphPad Prism version 8.0.1 (USA). Statistical significance was set at P < 0.05.
RESULTS
Agreement between ELISA and CLIA QFT-Plus results.
A total of 419 QFT-TB Plus samples analyzed by ELISA were included in this study with the following results: 153 (36.5%) positive, 168 (40.1%) negative, 97 (23.2%) indeterminate due to low nil-corrected mitogen value (<0.50 IFN-γ IU/ml), and 1 (0.2%) indeterminate due to high nil value (>8.0 IFN-γ IU/ml) according to the manufacturer’s cutoff. The same QFT-Plus samples were then processed by CLIA and produced the following results: 182 (43.4%) positive, 197 (47.0%) negative, 34 (8.1%) indeterminate due to low nil-corrected mitogen value, and 6 (1.5%) indeterminate due to high nil value.
The comparison of the results obtained by both assays is reported in Table 1. Concordant results were obtained for 316 out of 419 samples (agreement, 75.4%; κ = 0.61; 95% confidence interval [CI], 0.55 to 0.67). The agreement between TB1 and TB2 results was 95.7% for ELISA (κ = 0.91; 95% CI, 0.86 to 0.95) and 95.0% for CLIA (κ = 0.90; 95% CI, 0.85 to 0.94).
TABLE 1.
Results and agreement of QFT-Plus assay performed by ELISA and CLIA according to the manufacturer’s cutoff
| QFT-Plus CLIA | QFT-Plus ELISA |
|||
|---|---|---|---|---|
| No. positive | No. negative | No. indeterminate | Total no. | |
| No. positive | 146 | 33 | 3 | 182 |
| No. Negative | 2 | 135 | 60 | 197 |
| No. Indeterminate | 5 | 0 | 35 | 40 |
| Total no. | 153 | 168 | 98 | 419 |
| Agreement (%) | 95.4 | 80.4 | 35.7 | 75.4 |
Of the 103 (24.6%) samples with discordant results, 63 (61.2%) were due to indeterminate ELISA results, which were determinate with CLIA (60 negative and 3 positive). Median mitogen IFN-γ value in these samples was 0.34 IU/ml in ELISA and 0.94 IU/ml in CLIA. In contrast, median mitogen IFN-γ value in 34 samples which remained indeterminate in CLIA was 0.16 IU/ml in ELISA and 0.27 IU/ml in CLIA, excluding 1 indeterminate case due to high nil value. These differences were statistically significant (P < 0.0001).
The discordant cases with a determinate ELISA result were as follows: 2 ELISA-positive/CLIA-negative (both with only 1 M. tuberculosis complex antigen tube positive in ELISA), 5 ELISA-positive/CLIA-indeterminate (all due to high nil values in ELISA with a median value of 6.78 IU/ml), and 33 ELISA-negative/CLIA-positive (median values of 0.21 and 0.24 IU/ml with ELISA statistically lower than median values of 0.49 and 0.51 IU/ml with CLIA for TB1 and TB2, respectively; P < 0.0001).
Results interpreted according to Italian Clinical Microbiologist Association recommendations by introducing the category “borderline” (TB1 and/or TB2 values within 0.20 to 0.70 IFN-γ IU/ml) are reported in Table 2. Concordant results were obtained for 295 of the 419 samples (70.4%; κ = 0.59; 95% CI, 0.54 to 0.65). In samples with a borderline result in ELISA (n = 79), CLIA median values statistically increased from 0.29 to 0.59 IU/ml for TB1 and from 0.32 to 0.60 IU/ml for TB2 (P < 0.0001).
TABLE 2.
Results and agreement of QFT-Plus performed by ELISA and CLIA according to Italian Clinical Microbiologist Association recommendations
| QFT-Plus CLIA | QFT-Plus ELISA |
||||
|---|---|---|---|---|---|
| No. positive | No. borderline | No. negative | No. Indeterminate | Total no. | |
| No. positive | 104 | 41 | 0 | 0 | 145 |
| No. Borderline | 2 | 34 | 8 | 4 | 48 |
| No. negative | 1 | 4 | 122 | 59 | 186 |
| No. indeterminate | 5 | 0 | 0 | 35 | 40 |
| Total no. | 112 | 79 | 130 | 98 | 419 |
| Agreement (%) | 92.8 | 43.0 | 93.8 | 35.7 | 70.4 |
Correlation between ELISA and CLIA TB1 and TB2 IFN-γ levels.
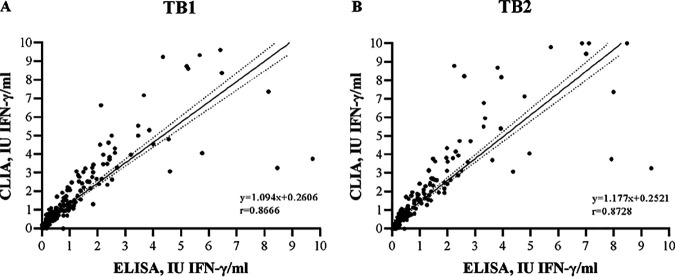
For M. tuberculosis complex antigen tube results within the linearity range of 0 to 10 IU/ml and excluding indeterminate results (n = 301), linear regression analysis showed that there was substantial correlation between the two tests, both for TB1 (Pearson’s r = 0.8666) and TB2 (Pearson’s r = 0.8728) (Fig. 1A and B). Furthermore, the regression slopes (1.094 for TB1, 1.177 for TB2) and the intercepts (+0.2606 for TB1, +0.2521 for TB2) indicated that CLIA produces significantly higher values both for TB1 and TB2 than ELISA (P < 0.0001). In fact, in this group, median IFN-γ values of M. tuberculosis complex antigen tubes were statistically higher in CLIA than in ELISA both for TB1 (0.42 versus 0.21 IU/ml; P = 0.0039) and TB2 (0.40 versus 0.22 IU/ml; P = 0.0047).
FIG 1.
Regression analysis of TB1 (A) and TB2 (B) IFN-γ levels between ELISA and CLIA QFT-Plus. Regression line (solid) and 95% confidence intervals (dotted) for the nil-subtracted antigen tubes within the range of 0 to 10 IU/ml are plotted. r, Pearson’s correlation coefficient.
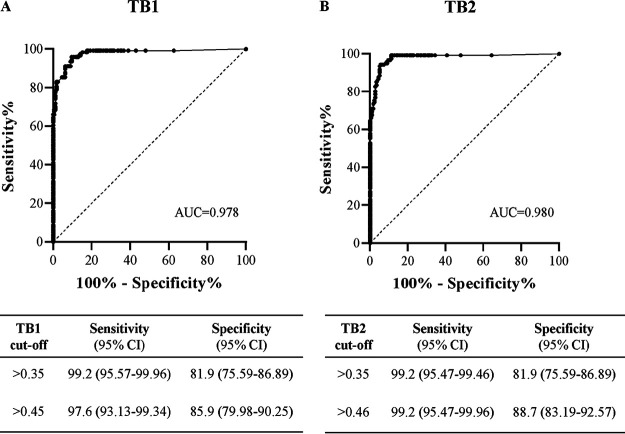
Area under the curve (AUC) results for TB1 and TB2 are reported in Fig. 2A and B, respectively. For TB1, AUC was 0.978 (95% CI, 0.962 to 0.994; P < 0.0001), and the cutoff value with the maximal sum of sensitivity and specificity was >0.45 IFN-γ IU/ml (sensitivity, 97.6%; specificity, 85.9%). Using the manufacturer’s suggested cutoff value of 0.35 IFN-γ IU/ml, the results showed a comparable sensitivity of 99.2% but a lower specificity of 81.9%.
FIG 2.
ROC curve of the CLIA QFT-Plus TB1 (A) and TB2 (B) to diagnose latent tuberculosis infection. Sensitivity and specificity according to manufacturer’s cutoff and to the cutoff defined by AUC analysis are reported. Infection was assessed based on the results of ELISA QFT-Plus.
For TB2, AUC was 0.980 (95% CI, 0.964 to 0.996; P < 0.0001), and the cutoff value with the maximal sum of sensitivity and specificity was >0.46 IFN-γ IU/ml (sensitivity, 99.2%; specificity, 88.7%). Using the manufacturer’s suggested cutoff value of 0.35 IFN-γ IU/ml, the results showed the same sensitivity of 99.2% but a lower specificity of 81.9%.
DISCUSSION
In this study, we compared the QFT-Plus routinely performed by ELISA in our laboratory with the automated CLIA performed on the Liaison XL instrument on a large number of selected samples (n = 419). In particular, we focused our analysis on ELISA indeterminate and borderline results since they could be greatly influenced by different methods of measurement.
We found substantial agreement (75.4%) between the assays interpreted according to the manufacturer’s cutoff. Most discordant results (61.2%; n = 63) were due to indeterminate ELISA results, which were determinate in CLIA. In literature, only a few studies have been published regarding this comparison on a smaller number of samples, reporting higher agreement between the two tests. De Maertelaere et al. described a population of 92 samples with 4.3% indeterminate ELISA results and found an overall agreement of 95% (11); Brantestig et al. analyzed 125 samples with 8% indeterminate ELISA results and found an agreement of 96.8% (12); Kim and colleagues reported an overall agreement of 99.12% on 341 samples (13). The near perfect agreement obtained by Kim et al. was probably due to the lack of indeterminate cases (0.3%) in their sample population. In contrast, in our population, indeterminate ELISA results due to low mitogen value (<0.50 IFN-γ IU/ml) accounted for 23.2%.
The high number of indeterminate results selected for this study allowed us to show that the ELISA median mitogen IFN-γ value in samples that converted to a determinate result in CLIA was 0.34 IU/ml, significantly higher than those that remained indeterminate in CLIA (0.16 IU/ml).
Further discordant results (32%; n = 33) were ELISA-negative/CLIA-positive, confirming that CLIA detects higher quantitative values than ELISA. However, in this group, ELISA median TB1 and TB2 values were close to the cutoff (0.21 and 0.24 IFN-γ IU/ml for TB1 and TB2, respectively) as were the corresponding CLIA values (0.49 and 0.51 IFN-γ IU/ml for TB1 and TB2, respectively).
The Italian Clinical Microbiologist Association recently suggested defining borderline results as TB1 and/or TB2 values within the range of 0.20 to 0.70 IFN-γ IU/ml and recommended retesting borderline samples. According to this recommendation, agreement between the two assays was moderate (70.4%), lower than the agreement observed when the manufacturer’s cutoff was used. Similarly, Brantestig et al. found a lower agreement (88%) when using the Swedish National recommendations that define a broad borderline range (0.20 to 0.99 IFN-γ IU/ml) than when applying the manufacturer’s cutoff (96.8%) (12). However, in our study, among the ELISA borderline samples (n = 79), the increased CLIA values remained in the borderline range (0.29 versus 0.59 IU/ml for TB1 and 0.32 versus 0.60 IU/ml for TB2), suggesting that this range may not need to be revised for CLIA.
Linear regression analysis indicated substantial correlation between ELISA and CLIA despite the two different methods of measurement; however, CLIA produced significantly higher values both for TB1 and TB2 than ELISA. This is in agreement with previous data on CLIA performance in a smaller study population (11). In our opinion, this difference is not due to preanalytical factors but rather to the intrinsic chemistry of the assay based on chemiluminescence technology with paramagnetic microparticle solid phase, allowing the detection of very low levels of IFN-γ (13, 19).
AUC analysis indicated that cutoff values of 0.45 IU/ml for TB1 and 0.46 IU/ml for TB2 returned the maximal sum of sensitivity and specificity, suggesting the need for a higher cutoff for QFT-Plus with CLIA compared to ELISA.
The limitation of our study is the lack of clinical data; further studies on a larger sample size with medical records available should be performed to more clearly define the CLIA threshold on the Liaison XL system.
In conclusion, QFT-Plus performed with CLIA showed substantial agreement with ELISA. The Liaison XL analyzer has several advantages, such as rapid turnaround time, high analytical measurement ranges, and good precision. Within the linearity range of the test, CLIA detects higher quantitative values than ELISA, resulting in a higher number of determinate results and the conversion of negative samples close to the cutoff into positive borderline results. A higher cutoff for QFT-CLIA needs to be defined based on clinical diagnostic criteria.
ACKNOWLEDGMENTS
We thank DiaSorin for providing reagents for the Liaison instrument; Paola Monari, Eleonora Gatti, and Giorgio Venturelli for technical support; and Jackie Leeder for English language editing.
REFERENCES
- 1.Cohen A, Mathiasen VD, Schon T, Wejse C. 2019. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur Respir J 54:1900655. doi: 10.1183/13993003.00655-2019. [DOI] [PubMed] [Google Scholar]
- 2.Houben RM, Dodd PJ. 2016. The global burden of latent tuberculosis infection: a re-estimation using mathematical modelling. PLoS Med 13:e1002152. doi: 10.1371/journal.pmed.1002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girardi E, Sabin CA, d'Arminio Monforte A, Hogg B, Phillips AN, Gill MJ, Dabis F, Reiss P, Kirk O, Bernasconi E, Grabar S, Justice A, Staszewski S, Fätkenheuer G, Sterne JA, Antiretroviral Therapy Cohort Collaboration. 2005. Incidence of tuberculosis among HIV-infected patients receiving highly active antiretroviral therapy in Europe and North America. Clin Infect Dis 41:1772–1782. doi: 10.1086/498315. [DOI] [PubMed] [Google Scholar]
- 4.Sotgiu G, Goletti D, Matteelli A. 2019. Global tuberculosis prevention: should we start from the beginning? Eur Respir J 54:1901394. doi: 10.1183/13993003.01394-2019. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. 2019. Global tuberculosis report 2019. World Health Organization, Geneva, Switzerland: https://www.who.int/tb/publications/global_report/en/. [Google Scholar]
- 6.Qiagen. 2014. QuantiFERON-TB Gold Plus ELISA package insert. Qiagen, Hilden, Germany: https://www.quantiferon.com/wp-content/uploads/2020/01/L1083163-R06-QF-TB-Gold-Plus-ELISA-IFU-CE.pdf. [Google Scholar]
- 7.Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, Barcellini L, Palmieri F, Cirillo DM, Ippolito G, Goletti D. 2016. Characterization of the CD4 and CD8 T-cell response in the QuantiFERON-TB Gold Plus kit. Int J Mycobacteriol 5:S25–S26. doi: 10.1016/j.ijmyco.2016.09.063. [DOI] [PubMed] [Google Scholar]
- 8.Sotgiu G, Saderi L, Petruccioli E, Aliberti S, Piana A, Petrone L, Goletti D. 2019. QuantiFERON TB Gold Plus for the diagnosis of tuberculosis: a systematic review and meta-analysis. J Infect 79:444–453. doi: 10.1016/j.jinf.2019.08.018. [DOI] [PubMed] [Google Scholar]
- 9.Barcellini L, Borroni E, Brown J, Brunetti E, Codecasa L, Cugnata F, Dal Monte P, Di Serio C, Goletti D, Lombardi G, Lipman M, Rancoita PM, Tadolini M, Cirillo DM. 2016. First independent evaluation of QuantiFERON-TB Plus performance. Eur Respir J 47:1587–1590. doi: 10.1183/13993003.02033-2015. [DOI] [PubMed] [Google Scholar]
- 10.Petruccioli E, Chiacchio T, Pepponi I, Vanini V, Urso R, Cuzzi G, Barcellini L, Cirillo DM, Palmieri F, Ippolito G, Goletti D. 2016. First characterization of the CD4 and CD8 T-cell responses to QuantiFERON-TB Plus. J Infect 73:588–597. doi: 10.1016/j.jinf.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 11.De Maertelaere E, Vandendriessche S, Verhasselt B, Coorevits L, André E, Padalko E, Boelens J. 2020. Evaluation of QuantiFERON-TB Gold Plus on Liaison XL in a low-tuberculosis-incidence setting. J Clin Microbiol 58:e00159-20. doi: 10.1128/JCM.00159-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brantestig S, Kinnunen A, Almeflo S, Restorp K, Ahlqvist J, Dyrdak R. 2020. Comparative evaluation of CLIA and EIA for Quantiferon-TB Gold Plus. APMIS 128:343–349. doi: 10.1111/apm.13025. [DOI] [PubMed] [Google Scholar]
- 13.Kim JJ, Park Y, Choi D, Kim HS. 2020. Performance evaluation of a new automated chemiluminescent immunoanalyzer-based interferon-gamma releasing assay AdvanSure I3 in comparison with the QuantiFERON-TB Gold In-Tube assay. Ann Lab Med 40:33–39. doi: 10.3343/alm.2020.40.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qiagen. 2019. DiaSorin LIAISON QuantiFERON-TB Gold Plus. Qiagen, Hilden, Germany: https://www.quantiferon.com/products/liaison-quantiferon-tb-gold-plus/. [Google Scholar]
- 15.Lee JE, Kim SY, Shin SY. 2015. Effect of repeated freezing and thawing on biomarker stability in plasma and serum samples. Osong Public Health Res Perspect 6:357–362. doi: 10.1016/j.phrp.2015.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohen L, Keegan A, Melanson SEF, Walt DR. 2019. Impact of clinical sample handling and processing on ultra-low level measurements of plasma cytokines. Clin Biochem 65:38–44. doi: 10.1016/j.clinbiochem.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 17.Tortoli E, Camaggi A, Cirillo D, Costa D, Fattorini L, Frizzera E, Marchetti D, Pecorari M, Piana F, Piersimoni C, Scarparo C, Gruppo di Lavoro Micobatteri, Associazione Microbiologi Clinici Italiani. 2019. Refertazione Quantiferon-Plus: considerazioni emerse durante la tavola rotonda “Nuovi approcci clinici, microbiologici e diagnostici alla TB latente”. XLVII Congresso AMCLI 2019. http://www.amcli.it/documenti/consensus/.
- 18.Lombardi G, Petrucci R, Corsini I, Bacchi Reggiani ML, Visciotti F, Bernardi F, Landini MP, Cazzato S, Dal Monte P. 2017. Quantitative analysis of interferon-γ release assay response in children with latent and active tuberculosis. J Clin Microbiol 56:e01360-17. doi: 10.1128/JCM.01360-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang QY, Chen H, Lin Z, Lin JM. 2012. Comparison of chemiluminescence enzyme immunoassay based on magnetic microparticles with traditional colorimetric ELISA for the detection of serum alpha-fetoprotein. J Pharm Anal 2:130–135. doi: 10.1016/j.jpha.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]