Abstract
Objective
Mechanical ventilation (MV) has a complex interplay with the pathophysiology of aneurysmal subarachnoid hemorrhage (aSAH). We aim to provide a review of the physiology of MV in patients with aSAH, give recommendations based on a systematic review of the literature, and highlight areas that still need investigation.
Data sources
PubMed was queried for publications with the Medical Subject Headings (MeSH) terms “mechanical ventilation” and “aneurysmal subarachnoid hemorrhage” published between January 1, 1990, and March 1, 2020. Bibliographies of returned articles were reviewed for additional publications of interest.
Study selection
Study inclusion criteria included English language manuscripts with the study population being aSAH patients and the exposure being MV. Eligible studies included randomized controlled trials, observational trials, retrospective trials, case-control studies, case reports, or physiologic studies. Topics and articles excluded included review articles, pediatric populations, non-aneurysmal etiologies of subarachnoid hemorrhage, mycotic and traumatic subarachnoid hemorrhage, and articles regarding tracheostomies.
Data extraction
Articles were reviewed by one team member, and interpretation was verified by a second team member.
Data synthesis
Thirty-one articles met the inclusion criteria for this review.
Conclusions
We make recommendations on oxygenation, hypercapnia, PEEP, APRV, ARDS, and intracranial pressure monitoring.
Keywords: Mechanical ventilation, Aneurysm, Subarachnoid hemorrhage, APRV, Pressure control, Volume control
Background
Aneurysmal subarachnoid hemorrhage (aSAH) occurs in 10–15 patients per 100,000 annually and represents 10% of all strokes in the USA [1, 2]. Among ischemic, hemorrhagic, and aneurysmal strokes, aSAH is associated with the highest risk of requiring mechanical ventilation (MV) (RR, 3.9; 95% CI 3.8–4.0), with 38.5–65% of all aSAH patients requiring MV [3–6]. Pathologic processes such as neurogenic pulmonary edema (NPE), occurring in up to 20% of aSAH patients, illustrate the interconnectedness of the central nervous and pulmonary systems [7, 8]. Modulating oxygenation and ventilation can be particularly challenging due to a large percentage of aSAH patients suffering from one or more pulmonary complications, including pneumonia (22%) and pulmonary edema (23%) [9]. Additionally, 18–50% of patients with aSAH experience acute respiratory distress syndrome (ARDS) [10–12].
For a pathology that so frequently requires MV, intensivists and neurosurgeons alike are faced with questions regarding optimal management without an abundance of guiding evidence. Unlike other forms of stroke, the clinician must be increasingly cognizant of brain oxygen delivery and perfusion when considering the potential for delayed cerebral ischemia (DCI) and increased intracranial pressure from hydrocephalus (Fig. 1). Herein, we performed a systematic review of all aneurysmal subarachnoid studies with concomitant mechanical ventilation to review factors of ventilation that may be involved with brain-lung physiology. We outline the unique pulmonary pathophysiology of such patients, common conditions encountered, and how each element of mechanical ventilation can impact the complex disease processes of patients with aSAH. In addition, we make recommendations based on existing data and highlight gaps in knowledge for future research.
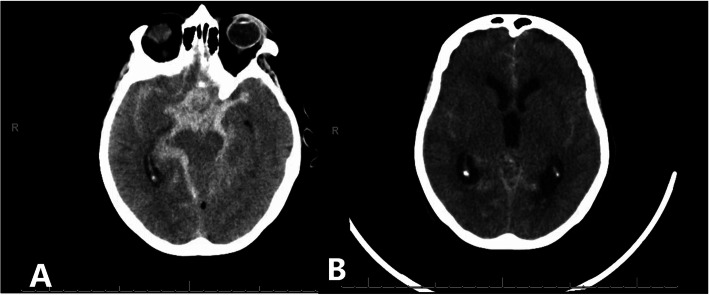
Fig. 1.
Head CT showing the unique challenges of an aneurysmal subarachnoid hemorrhage patient. a There is a significant amount of basilar cistern subarachnoid hemorrhage placing the patient at high risk for delayed cerebral ischemia. b There is prominent hydrocephalus needing CSF diversion to lower intracranial pressure
Methods
A PubMed database search was formulated based on the PICO (Participant, Intervention, Comparison, and Outcome) framework. The following Medical Subject Headings (MeSH) terms were used to define the participant and the intervention, respectively: “aneurysmal subarachnoid hemorrhage” and “mechanical ventilation.” Search parameters were limited to English language studies published between January 1, 1990, and March 1, 2020. The search was completed on March 1, 2018, and updated on March 8, 2020. Study inclusion criteria included English language manuscripts with the study population being aneurysmal subarachnoid hemorrhage and the exposure or intervention being mechanical ventilation. Eligible studies could include randomized controlled trials, observational trials, retrospective trials, case-control studies, case reports, or physiologic studies. We specifically assessed mechanical ventilation variables, brain physiologic measurements, and outcomes. We excluded topic review articles and articles concerning pediatric populations, non-aneurysmal etiologies of subarachnoid hemorrhage, mycotic and traumatic subarachnoid hemorrhage, and tracheostomies. The citations of the articles returned from the PubMed database search were examined, and any articles that appeared to pertain to our topic were reviewed and included, if appropriate. Bias was assessed individually given the type of study reported. Results found were not similar enough for combination or further statistical analysis.
All articles returned in the search were reviewed. Manuscripts’ abstracts were reviewed by one team member (a neurosurgery resident), and interpretation was verified by a second team member (a critical care physician). Those passing abstract review then underwent full review by two team members in the same manner.
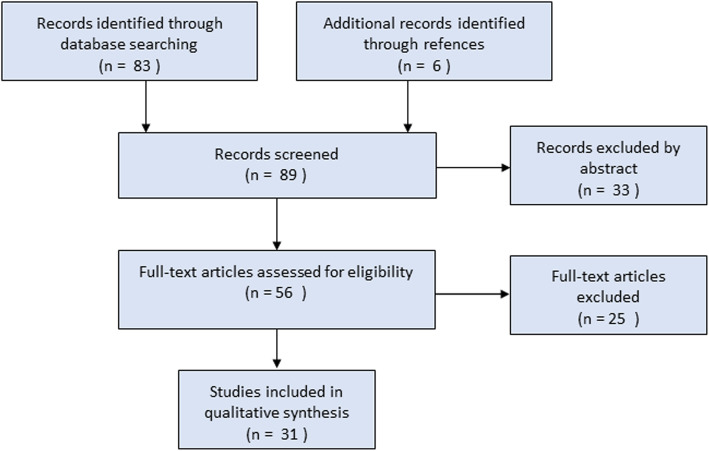
Our search yielded 83 manuscripts. Of these, 58 articles were ultimately excluded. An additional six manuscripts pertaining to MV in aSAH were identified upon examination of the references of the reviewed articles. Figure 2 is a flow diagram adapted from PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) [13]. The total number of articles relevant to this review was 31.
Fig. 2.
PRISMA flow sheet for systematic review
Oxygen delivery and carbon dioxide clearance
Oxygenation
Blood oxygen (O2) and, to a greater degree, carbon dioxide (CO2) are important variables to consider in the management of aSAH patients. Maintaining O2 delivery is critically important to avoid brain ischemia. MV must strike a balance between oxygenating blood and maintaining cardiac output.
While there is an accepted goal to avoid hypoxemia in any critically ill patient, the effect of hyperoxemia in aSAH is less well understood. A retrospective, multi-institutional database study of aSAH patients mechanically ventilated for a minimum of 24 h found no relationship between PaO2 level in the first 24 h of care, including moderate hyperoxemia (≥ 150 mmHg), and patient outcome [14].
Carbon dioxide
CO2 is an important and powerful vasomodulator, potentially having a dramatic effect on the delivery of blood to the brain in patients with intact cerebral autoregulation [15]. Studies, both in humans and animal models, have found functioning cerebrovascular reactivity to CO2 following aSAH [14, 16]. A retrospective, single-institution review of 102 aSAH patients found hypocapnia (defined as a partial pressure of CO2 (PaCO2) < 35) to be independently associated with unfavorable outcome (defined as Glasgow outcome scale (GOS) < 4) and DCI, but not mortality [17]. Conversely, PaCO2 levels above 37.5 mmHg in the first 24 h of care have been associated with a decreased risk of unfavorable outcome (defined as GOS of 1–3), suggesting that permissive mild hypercapnia may be beneficial in aSAH patients [18]. Westermaier et al. conducted a phase 1 clinical trial of controlled transient hypercapnia in aSAH. The authors examined six high-grade (Hunt and Hess (HH) 3–5 and Fisher grade (FG) 3) aSAH patients with multimodality monitoring, including intracerebral thermodilution probes, external ventricular drains (EVD), and near-infrared spectroscopy [19]. They found that PaCO2 levels of 30, 40, 50, and 60 mmHg resulted in baseline cerebral blood flow (CBF) changes of 79%, 98%, 124%, and 143%, respectively [19]. The cerebral tissue oxygenation for PaCO2 levels of 30, 40, 50, and 60 mmHg changed from baseline by 93%, 98%, 104%, and 111%, respectively [19]. Intracranial pressure (ICP) was not clinically affected by changes in PaCO2, but the average amount of cerebrospinal fluid (CSF) drained increased with increasing PaCO2 [19]. They experienced no rebound perfusion deficit upon return to baseline ventilator settings [19]. While the ICPs were not elevated in these patients, it was likely only due to the elevated CSF drainage and thus this may be an unsafe maneuver in patients without an EVD. Despite some investigation into permissive hypercapnia as a therapy, there is still significant work to be done in regard to safety and efficacy given that some believe that brain-injured patients should avoid hypercapnia due to the risk of increased ICP from the rise in CBF [20].
Recommendations
In summary, hypoxemia should be avoided in aSAH patients as with any critically ill patient. While hyperoxemia does not have strong clinical evidence of causing further brain injury, advanced intracranial monitoring to measure brain tissue oxygen pressure (PbtO2) can be used when titrating the fraction of inspired oxygen. Permissive hypercapnia is likely well tolerated in these patients, but it may safest to do so with intracranial pressure monitoring (ICPM) in place. Hypocapnia should generally be avoided unless there is an acute rise in ICP since it may incite ischemia.
Acute respiratory distress syndrome in aSAH
PEEP and intracranial pressure
ARDS is not uncommon in aSAH. A retrospective, single-institution cohort study of 620 aSAH patients found that 27% had a PaO2:FiO2 (fraction of inspired oxygen) ratio of ≤ 300 with 18% having a ratio ≤ 200 [11]. The diagnosis of lung injury occurred a median of 3 days from admission [11]. They found severity of illness, clinical grade of hemorrhage, red blood cell transfusions, and severe sepsis to be independently associated with developing ARDS [11]. Higher tidal volumes were not found to be associated with the subsequent development of ARDS [11]. ARDS was independently associated with mortality and longer hospital lengths of stay [11].
Another retrospective, single-institution observational study found that the development of lung injury was correlated with HH score (p < 0.001), with 30.4% of HH4 and 35.5% of HH5 patients experiencing severe lung injury (defined by the authors as a PaO2 to FiO2 ratio of ≤ 200) [6]. An additional retrospective, observational study of 62 patients with aSAH requiring MV found 50% of their cohort developed ARDS. Forty-five percent of the patients were diagnosed with ARDS on the first day of MV—suggesting hypoxia, not solely the need for airway protection, may contribute to the requirement for MV [12].
The hypothesized pathophysiology leading to the development of lung injury in aSAH patients is a “double-hit” model, with the first hit being an adrenergic surge and systemic inflammation incited by acute neurologic injury and the second from non-neurological stressors, such as infections, transfusions, and MV [21–23]. The best evidence for MV strategies to improve survival in ARDS involves lung-protective ventilation parameters described in an ARDS Network trial (ARDSNet) which includes tidal volumes of 6–8 mL/kg of predicted body weight to achieve a plateau pressure ≤ 30 cmH2O [24]. However, it is important to recognize that patients with elevated ICPs were excluded from the ARDSNet trial, which likely excluded many aSAH patients. Such an exclusion may limit the generalizability of this ventilation strategy to patients with aSAH.
When examining the utilization rates of the ARDSNet lung-protective ventilator strategies in aSAH patients, a retrospective, single-institution cohort study found that 58% of patients were maintained within ARDSNet parameters, including tidal volumes of ≤ 8 mL/kg, yet there were no ventilator settings that predicted the development of ARDS [12]. The presence of ARDS risk factors, defined as sepsis, shock, pneumonia, gastric aspiration, and transfusion, were the only findings associated with the development of ARDS. As opposed to other studies, the clinical severity of aSAH did not correlate with the development of ARDS [12]. The development of ARDS was associated with increased duration of intensive care unit (ICU) stay, but not mortality [12].
A prospective, single-center study of 499 patients with acute brain injury, including SAH, found that lower tidal volumes and higher positive end-expiratory pressure (PEEP) resulted in decreased duration of MV from 14.9 to 12.6 days and increased 90-day ICU days [25]. In a multicenter study of all acute brain injury patients including SAH, a protocol of low tidal volume (≤ 7 mL/kg), moderate PEEP (6–8 cmH2O), and early extubation was associated with a decrease in mortality and number of invasive ventilation-free days [26].
Alveolar collapse is a key pathophysiologic characteristic of ARDS and results in hypoxemia from intrapulmonary shunt [27]. By using PEEP to open collapsed lung units, alveolar recruitment is one strategy to maintain functional residual capacity and thereby improve oxygenation in ARDS [28, 29]. However, lung recruitment remains controversial, and high PEEP ventilation was associated with higher mortality compared to low PEEP ventilation in a randomized control trial [30]. The applicability of these trials to patients with aSAH is limited because they excluded patients with elevated ICP or acute brain injury.
To address this limitation, several small retrospective clinical studies have attempted to examine the relationship between lung-protective ventilation and ICP. One retrospective, single-institution review reported outcomes for 12 aSAH patients receiving lung-protective ventilation settings with resultant hypercapnia (defined as PaCO2 50–60 mmHg) and found that these patients had no increase in their ICP compared to patients with a PaCO2 of 40 mmHg [31]. The authors hypothesized that while pial arteries vasodilate in response to hypercapnia, there is some evidence that the major cerebral arteries and intracortical arteries constrict instead, possibly accounting for the unchanged ICP they observed [31, 32]. A randomized study of lung recruitment methods in aSAH patients with ARDS by Nemer et al. compared different alveolar recruitment maneuvers [33]. One arm was subjected to 35 cmH20 of continuous positive airway pressure for 40 s, termed continuous positive airway pressure recruitment maneuver (CRM), while the other underwent a pressure control recruitment maneuver (PCRM) of a PEEP of 15 cmH20 with pressure control above PEEP of 35 cmH20 for 2 min [33]. Compared to baseline, they found CRM to be associated with a higher ICP (20.50 ± 4.75 vs 13.13 ± 3.56 mmHg) and a lower cerebral perfusion pressure (CPP) (62.38 ± 9.81 vs 79.60 ± 6.8 mmHg) with no significant improvement in PaO2:FiO2 ratio (110.9 ± 24.7 to 112.6 ± 26.7). PCRM on the other hand had no significant effect on ICP but increased CPP (84.25 ± 5.48 to 79 ± 6.80 mmHg) [33]. PCRM was also associated with a clinically significant improvement in PaO2:FiO2 ratio (108.5 to 203.6) [33].
One prospective, single-institution observational study evaluated the longitudinal effect of PEEP on ICP in aSAH patients [34]. The authors found that, compared to a group with PEEP of 5 cmH2O, patients with a PEEP of 20 cmH2O had no significant effect on ICP on post-bleed days 1 and 3, but did experience significantly higher ICP on post-bleed day 7 (19.5 vs 11 mmHg). Post-bleed day 7 is an important milestone in the natural history of aSAH because maximal vasospasm can occur between days 6 and 8 [35]. Severe vasospasm may lead to reduced CBF and cause cerebral ischemia and edema. The elevated PEEP group also experienced a decrease in mean arterial pressure (MAP) from baseline and subsequently a decrease in cerebral blood flow, thought to be a result of ineffective cerebral autoregulation [34]. The authors postulated that cerebral edema, in conjunction with elevated intracranial venous pressure and diminished intracranial venous outflow due to elevated PEEP, led to increased ICP [34].
A retrospective, single-institution review of patients with severe neurologic injuries (GCS < 9, 37.5% with aSAH) who required MV and ICP monitoring found no significant association between PEEP and ICP or CPP, except in patients with severe lung injury (PaO2/FiO2 < 100) [36]. On multivariate analysis of severe lung injury patients, every 1-cmH2O increase in PEEP was associated with a 0.31-mmHg increase in ICP (p = 0.04) and a 0.85-mmHg decrease in CPP (p = 0.02) [36]. The study did not report any subgroup analysis of the various pathologies included in their cohort or investigate if the mode of MV used had an effect on their results. On the other hand, a prospective study of 21 comatose patients with normal lung compliance and abnormal lung compliance were subjected to increases in PEEP while measuring central venous pressure (CVP), CPP, ICP, cerebral compliance, and mean middle cerebral artery velocity [37]. In those with normal lung compliance, PEEP increases caused an increase in CVP but reduced MAP, CPP, and mean velocities while ICP and cerebral compliances stayed the same. In those with low compliance, there was no variation in any of the variables with increases in PEEP.
Recommendations
In summary, the literature suggests that an increase in PEEP decreases MAP and increases intracranial pressure. However, since ARDS can present early in these patients, higher PEEPs may be safe early in the course of a patient with aSAH without evidence of intracranial hypertension or mass effect from hematoma. It is reasonable to use ICPM with ability of CSF diversion as the patient approaches the peak of the DCI period, prior to increasing PEEP to treat lung pathology.
Prone positioning
Prone position ventilation improves gas exchange in patients with ARDS and other pathologic states with ventilation-perfusion mismatch such as NPE. One retrospective study of sixteen patients with aSAH, HH grade III or higher with ICPM described proning [38]. With proning, there was a significant increase in PaO2 (from 97.3 ± 20.7 to 126.6 ± 31.7 Torr) and PbtO2 (from 26.8 ± 10.9 to 31.6 ± 12.2 Torr) along with ICP (from 9.3 ± 5.2 to 14.8 ± 6.7 mmHg) while CPP decreased (from 73.0 ± 10.5 to 67.7 ± 10.7 mmHg) [38]. In a retrospective review of 29 patients with ICPM and acute brain injury, the mean baseline ICP in a supine position was 9.5 ± 5.9 mmHg which increased significantly during prone positioning to 15.4 ± 6.2 mmHg [39]. They found no significant difference between CPP in a supine position (82 ± 14.5 mmHg) or a prone position (80.1 ± 14.1 mmHg) [39]. Another prospective study of proning in 8 patients with TBI and SAH found similar results as the prior study with a statistically significant increase in PaO2 (from 12.6 ± 1.4 to 15.7 ± 3.2 kPa) and ICP (from 12 ± 6 to 15 ± 4 mmHg) however with improvement in CPP (from 66 ± 7 to 73 ± 8 mmHg) [40]. MAP improved in these patients (from 78 ± 8 to 88 ± 8 mmHg) [40]. The authors postulate better venous return in the prone position improved MAP to a greater extent than ICP, resulting in improved CPP [40]. Finally, another prospective trial in 11 patients with TBI and SAH found that proning had no significant effect on ICP, CPP, or MAP but significantly increased PaO2 (from 13.2 ± 2.1 to 19.1 ± 6.1 kPa) [41]. The authors comment on increasing sedation on a patient who had an immediate increase in ICP on proning which highlights that results in non-controlled studies may be confounded. It is important to note neither of these studies had patients with ARDS.
Recommendations
Based on these studies, proning can be expected to raise ICP significantly however dramatically improves oxygenation. Patients with aSAH with ICPM who have demonstrated stable ICPs, have no mass effect from intracranial hematoma or edema, and who are experiencing ARDS can be considered for proning.
Alternative modes of ventilation
Airway pressure release ventilation (APRV), a pressure-limited, time-cycled mode of MV that allows spontaneous breathing, is another treatment modality in the management of ARDS. APRV utilizes inverse ratio ventilation (IRV), whereby the inspiratory time is longer than the expiratory time. This increases alveolar recruitment and improves oxygenation [42].
Our search yielded one single case report describing the use of APRV in aSAH, resulting in improvement in oxygenation, alveolar ventilation, and cerebral blood flow with a negligible increase in ICP [43]. One study that examined IRV in a rabbit aSAH model compared to normal ratio ventilation did not find CPP to be significantly different, but did find significantly elevated mean airway pressures and slightly elevated ICP above baseline [44]. In another study, 22 Yorkshire swine undergoing controlled lung injury to mimic ARDS and intracranial pathology with ICP elevation to 30–40 with intracranial balloon were randomized to ARDSNet, APRV, or sham, and blood gases, quantitative histopathology, and cerebral microdialysis were assessed [45]. The investigators found no difference in FiO2, CVP, end-tidal CO2, MAP, CPP, and ICP between the groups, but statistically improved P/F ratio and higher mean airway pressures in the APRV group [45]. They also found no differences in arterial pH, PaCO2, PaO2, and SaO2 at the common carotid or venous pH, lactate, SvO2, or PvO2 at femoral and jugular sites with the only difference of APRV having lower PvCO2 at the jugular site [45]. Cerebral dialysis showed lower lactate in the APRV group but lactate pyruvate ratios insignificantly different [45]. The two main limitations of this study are the dropout bias due to death of six animals not included and analysis for only 6.5 h.
Recommendations
Consider APRV in this population if there is concern for vent asynchrony or ARDS but need to maintain ICP.
Delayed cerebral ischemia
Ventilation
DCI is one of the most dreaded complications of aSAH and is a significant contributor to long-term morbidity [46]. Angiographic vasospasm can be seen independently or in conjunction with DCI in patients with aSAH [35]. There is significant heterogeneity in the literature among definitions and endpoints in studies describing the phenomenon of post-aSAH ischemia, with some studies using vasospasm as a surrogate marker or interchangeably with DCI [46]. We found existing data describing DCI, vasospasm, and MV to be contradictory, possibly due to this heterogeneity. One retrospective, single-institution review found DCI to be independently associated with prolonged MV (HR 1.61; 95% CI 1.02–2.56) [47]. A relationship between pulmonary complications and DCI was echoed by a second retrospective, single-institution study, which determined pneumonia was independently associated with the development of DCI (adjusted OR, 2.0; 95% CI 1.1–3.7) [48]. An understanding of this relationship is not firmly established, however. A retrospective, single-institution cohort study found the development of ARDS did not appear to increase the risk of vasospasm [11]. One prospective, observational study examined the effect of cerebral vasospasm on CBF in the setting of elevated PEEP, finding that vasospasm did not appear to influence MAP or CBF at PEEP up to 20 cmH20 [34]. However, increasing PEEP resulted in codependent decreases in MAP and CBF in patients regardless of the presence of vasospasm. Additionally, MAP and CBF responded as expected to medical therapy in both populations [49]. This suggests that application of PEEP is no worse for patients with vasospasm than without, though this cannot necessarily be applied to patients with DCI, and further studies are needed to elucidate this.
Recommendations
In summary, one of the most challenging scenarios in managing high-grade aSAH patients is preventing the occurrence of DCI while there is concurrent severe ARDs. Higher PEEPs may decrease CBF in this group of patients; thus, simultaneous use of ionotropic agents such as milrinone may offset the decreased venous return from higher PEEPs. These patients are typically ideal candidates for advanced intracranial monitoring with PbtO2, potentially allowing for more nuanced ventilator titration.
Conclusions
Overall, there is a paucity of high-level data on the effects of MV on patients with aSAH, with the result that no definitive management statements can be made. However, we can summarize the data along with our experience with the following suggestions:
Hypercapnia may be effective in reducing DCI and improving outcomes, but these patients should have some form of ICPM if higher PaCO2 will be targeted.
ARD protocol ventilation should be followed in patients with aSAH allowing for higher PEEPs if early in the bleed course and after aneurysm has been secured. These patients should have ICPM, especially during the DCI period, and advance intracranial monitoring can help guide ventilator titration to strike a balance between oxygenation, ventilation, PEEP, and cerebral perfusion.
Spontaneous modes of ventilation such as APRV may be considered in patients with concomitant ARDs and in the DCI period. This may lower sedation requirements, which, in addition to ionotropic agents, may meet goals of cerebral perfusion. Hypercapnia during this period as discussed above may be beneficial.
Acknowledgements
Not applicable
Abbreviations
- APRV
Airway pressure release ventilation
- ARDS
Acute respiratory distress syndrome
- aSAH
Aneurysmal subarachnoid hemorrhage
- CBF
Cerebral blood flow
- CSF
Cerebrospinal fluid
- CO2
Carbon dioxide
- CPP
Cerebral perfusion pressure
- CRM
Continuous positive airway pressure recruitment maneuver
- CVP
Central venous pressure
- DCI
Delayed cerebral ischemia
- EVD
External ventricular drain
- FG
Fisher grade
- FiO2
Fraction of inspired oxygen
- HH
Hunt Hess
- ICP
Intracranial pressure
- ICPM
Intracranial pressure monitoring
- ICU
Intensive care unit
- IRV
Inverse ratio ventilation
- MAP
Mean arterial pressure
- MV
Mechanical ventilation
- NPE
Neurogenic pulmonary edema
- O2
Oxygen
- PaCO2
Partial pressure of CO2
- PbtO2
Brain partial oxygen pressure
- PCRM
Pressure control recruitment maneuver
- PEEP
Positive end-expiratory pressure
- VAP
Ventilator-acquired pneumonia
Authors’ contributions
JT: conception, design, analysis, and drafting
RR: conception, design, analysis, and drafting
CZ: conception and drafting
IRK: drafting
DAP: drafting
TB: drafting
DER: conception, design, and drafting
The authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
Not applicable
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
James E. Towner and Redi Rahmani contributed equally to this work.
References
- 1.Shea AM, Reed SD, Curtis LH, Alexander MJ, Villani JJ, Schulman KA. Characteristics of nontraumatic subarachnoid hemorrhage in the United States in 2003. Neurosurgery. 2007;61(6):1131–1137. doi: 10.1227/01.neu.0000306090.30517.ae. [DOI] [PubMed] [Google Scholar]
- 2.Labovitz DL, Halim AX, Brent B, Boden-Albala B, Hauser WA, Sacco RL. Subarachnoid hemorrhage incidence among Whites, Blacks and Caribbean Hispanics: the Northern Manhattan study. Neuroepidemiology. 2006;26(3):147–150. doi: 10.1159/000091655. [DOI] [PubMed] [Google Scholar]
- 3.Lahiri S, Mayer SA, Fink ME, Lord AS, Rosengart A, Mangat HS, et al. Mechanical ventilation for acute stroke: a multi-state population-based study. Neurocrit Care. 2015;23(1):28–32. doi: 10.1007/s12028-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 4.Mayer SA, Copeland D, Bernardini GL, Boden-Albala B, Lennihan L, Kossoff S, et al. Cost and outcome of mechanical ventilation for life-threatening stroke. Stroke. 2000;31(10):2346–2353. doi: 10.1161/01.str.31.10.2346. [DOI] [PubMed] [Google Scholar]
- 5.Udy AA, Vladic C, Saxby ER, Cohen J, Delaney A, Flower O, et al. Subarachnoid hemorrhage patients admitted to intensive care in Australia and New Zealand: a multicenter cohort analysis of in-hospital mortality over 15 years. Crit Care Med. 2017;45(2):e138–ee45. doi: 10.1097/CCM.0000000000002059. [DOI] [PubMed] [Google Scholar]
- 6.Gruber A, Reinprecht A, Gorzer H, Fridrich P, Czech T, Illievich UM, et al. Pulmonary function and radiographic abnormalities related to neurological outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 1998;88(1):28–37. doi: 10.3171/jns.1998.88.1.0028. [DOI] [PubMed] [Google Scholar]
- 7.Busl KM, Bleck TP. Neurogenic pulmonary edema. Crit Care Med. 2015;43(8):1710–1715. doi: 10.1097/CCM.0000000000001101. [DOI] [PubMed] [Google Scholar]
- 8.Vespa PM, Bleck TP. Neurogenic pulmonary edema and other mechanisms of impaired oxygenation after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2004;1(2):157–170. doi: 10.1385/NCC:1:2:157. [DOI] [PubMed] [Google Scholar]
- 9.Solenski NJ, Haley EC, Jr, Kassell NF, Kongable G, Germanson T, Truskowski L, et al. Medical complications of aneurysmal subarachnoid hemorrhage: a report of the multicenter, cooperative aneurysm study. Participants of the Multicenter Cooperative Aneurysm Study. Crit Care Med. 1995;23(6):1007–1017. doi: 10.1097/00003246-199506000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Veeravagu A, Chen YR, Ludwig C, Rincon F, Maltenfort M, Jallo J, et al. Acute lung injury in patients with subarachnoid hemorrhage: a nationwide inpatient sample study. World Neurosurg. 2014;82(1–2):e235–e241. doi: 10.1016/j.wneu.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 11.Kahn JM, Caldwell EC, Deem S, Newell DW, Heckbert SR, Rubenfeld GD. Acute lung injury in patients with subarachnoid hemorrhage: incidence, risk factors, and outcome. Crit Care Med. 2006;34(1):196–202. doi: 10.1097/01.ccm.0000194540.44020.8e. [DOI] [PubMed] [Google Scholar]
- 12.Marhong JD, Ferguson ND, Singh JM. Ventilation practices in subarachnoid hemorrhage: a cohort study exploring the use of lung protective ventilation. Neurocrit Care. 2014;21(2):178–185. doi: 10.1007/s12028-014-0014-8. [DOI] [PubMed] [Google Scholar]
- 13.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romner B, Brandt L, Berntman L, Algotsson L, Ljunggren B, Messeter K. Simultaneous transcranial Doppler sonography and cerebral blood flow measurements of cerebrovascular CO2-reactivity in patients with aneurysmal subarachnoid haemorrhage. Br J Neurosurg. 1991;5(1):31–37. doi: 10.3109/02688699108998444. [DOI] [PubMed] [Google Scholar]
- 15.Ainslie PN, Duffin J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: mechanisms of regulation, measurement, and interpretation. Am J Physiol Regul Integr Comp Physiol. 2009;296(5):R1473–R1495. doi: 10.1152/ajpregu.91008.2008. [DOI] [PubMed] [Google Scholar]
- 16.Diringer MN, Kirsch JR, Hanley DF, Traystman RJ. Altered cerebrovascular CO2 reactivity following subarachnoid hemorrhage in cats. J Neurosurg. 1993;78(6):915–921. doi: 10.3171/jns.1993.78.6.0915. [DOI] [PubMed] [Google Scholar]
- 17.Solaiman O, Singh JM. Hypocapnia in aneurysmal subarachnoid hemorrhage: incidence and association with poor clinical outcomes. J Neurosurg Anesthesiol. 2013;25(3):254–261. doi: 10.1097/ANA.0b013e3182806465. [DOI] [PubMed] [Google Scholar]
- 18.Lang M, Raj R, Skrifvars MB, Koivisto T, Lehto H, Kivisaari R, et al. Early moderate hyperoxemia does not predict outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2016;78(4):540–545. doi: 10.1227/NEU.0000000000001111. [DOI] [PubMed] [Google Scholar]
- 19.Westermaier T, Stetter C, Kunze E, Willner N, Holzmeier J, Kilgenstein C, et al. Controlled transient hypercapnia: a novel approach for the treatment of delayed cerebral ischemia after subarachnoid hemorrhage? J Neurosurg. 2014;121(5):1056–1062. doi: 10.3171/2014.7.JNS132611. [DOI] [PubMed] [Google Scholar]
- 20.Reinges MH. Pros and cons of permissive hypercapnia in patients with subarachnoid haemorrhage and ARDS. Acta Neurochir. 2010;152(12):2173–2174. doi: 10.1007/s00701-010-0760-0. [DOI] [PubMed] [Google Scholar]
- 21.Kapinos G, Chichra A. Lung-protective ventilation for SAH patients: are these measures truly protective? Neurocrit Care. 2014;21(2):175–177. doi: 10.1007/s12028-014-0058-9. [DOI] [PubMed] [Google Scholar]
- 22.Stevens RD, Lazaridis C, Chalela JA. The role of mechanical ventilation in acute brain injury. Neurol Clin. 2008;26(2):543–563. doi: 10.1016/j.ncl.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Mascia L. Acute lung injury in patients with severe brain injury: a double hit model. Neurocrit Care. 2009;11(3):417–426. doi: 10.1007/s12028-009-9242-8. [DOI] [PubMed] [Google Scholar]
- 24.Acute Respiratory Distress Syndrome N, Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, et al. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 25.Roquilly A, Cinotti R, Jaber S, et al. Implementation of an evidence-based extubation readiness bundle in 499 brain-injured patients. a before-after evaluation of a quality improvement project. Am J Respir Crit Care Med. 2013;188(8):958-966. 10.1164/rccm.201301-0116OC. [DOI] [PubMed]
- 26.Asehnoune K, Mrozek S, Perrigault PF, Seguin P, Dahyot-Fizelier C, Lasocki S, et al. A multi-faceted strategy to reduce ventilation-associated mortality in brain-injured patients. The BI-VILI project: a nationwide quality improvement project. Intensive Care Med. 2017;43(7):957–970. doi: 10.1007/s00134-017-4764-6. [DOI] [PubMed] [Google Scholar]
- 27.Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, et al. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354(17):1775–1786. doi: 10.1056/NEJMoa052052. [DOI] [PubMed] [Google Scholar]
- 28.Hodgson CL, Tuxen DV, Davies AR, Bailey MJ, Higgins AM, Holland AE, et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care. 2011;15(3):R133. doi: 10.1186/cc10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, et al. Open lung approach for the acute respiratory distress syndrome: a pilot, randomized controlled trial. Crit Care Med. 2016;44(1):32–42. doi: 10.1097/CCM.0000000000001383. [DOI] [PubMed] [Google Scholar]
- 30.Writing Group for the Alveolar Recruitment for Acute Respiratory Distress Syndrome Trial I. Cavalcanti AB, Suzumura EA, Laranjeira LN, Paisani DM, Damiani LP, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petridis AK, Doukas A, Kienke S, Maslehaty H, Mahvash M, Barth H, et al. The effect of lung-protective permissive hypercapnia in intracerebral pressure in patients with subarachnoid haemorrhage and ARDS. A retrospective study. Acta Neurochir. 2010;152(12):2143–2145. doi: 10.1007/s00701-010-0761-z. [DOI] [PubMed] [Google Scholar]
- 32.McHedlishvili GI, Ormotsadze LG, Nikolaishvili LS, Baramidze DG. Reaction of different parts of the cerebral vascular system in asphyxia. Exp Neurol. 1967;18(2):239–252. doi: 10.1016/0014-4886(67)90045-3. [DOI] [PubMed] [Google Scholar]
- 33.Nemer SN, Caldeira JB, Azeredo LM, Garcia JM, Silva RT, Prado D, et al. Alveolar recruitment maneuver in patients with subarachnoid hemorrhage and acute respiratory distress syndrome: a comparison of 2 approaches. J Crit Care. 2011;26(1):22–27. doi: 10.1016/j.jcrc.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 34.Muench E, Bauhuf C, Roth H, Horn P, Phillips M, Marquetant N, et al. Effects of positive end-expiratory pressure on regional cerebral blood flow, intracranial pressure, and brain tissue oxygenation. Crit Care Med. 2005;33(10):2367–2372. doi: 10.1097/01.ccm.0000181732.37319.df. [DOI] [PubMed] [Google Scholar]
- 35.Weir B, Grace M, Hansen J, Rothberg C. Time course of vasospasm in man. J Neurosurg. 1978;48(2):173–178. doi: 10.3171/jns.1978.48.2.0173. [DOI] [PubMed] [Google Scholar]
- 36.Boone MD, Jinadasa SP, Mueller A, Shaefi S, Kasper EM, Hanafy KA, et al. The effect of positive end-expiratory pressure on intracranial pressure and cerebral hemodynamics. Neurocrit Care. 2017;26(2):174–181. doi: 10.1007/s12028-016-0328-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Caricato A, Conti G, Della Corte F, Mancino A, Santilli F, Sandroni C, et al. Effects of PEEP on the intracranial system of patients with head injury and subarachnoid hemorrhage: the role of respiratory system compliance. J Trauma. 2005;58(3):571–576. doi: 10.1097/01.ta.0000152806.19198.db. [DOI] [PubMed] [Google Scholar]
- 38.Reinprecht A, Greher M, Wolfsberger S, Dietrich W, Illievich UM, Gruber A. Prone position in subarachnoid hemorrhage patients with acute respiratory distress syndrome: effects on cerebral tissue oxygenation and intracranial pressure. Crit Care Med. 2003;31(6):1831–1838. doi: 10.1097/01.CCM.0000063453.93855.0A. [DOI] [PubMed] [Google Scholar]
- 39.Roth C, Ferbert A, Deinsberger W, Kleffmann J, Kastner S, Godau J, et al. Does prone positioning increase intracranial pressure? A retrospective analysis of patients with acute brain injury and acute respiratory failure. Neurocrit Care. 2014;21(2):186–191. doi: 10.1007/s12028-014-0004-x. [DOI] [PubMed] [Google Scholar]
- 40.Nekludov M, Bellander BM, Mure M. Oxygenation and cerebral perfusion pressure improved in the prone position. Acta Anaesthesiol Scand. 2006;50(8):932–936. doi: 10.1111/j.1399-6576.2006.01099.x. [DOI] [PubMed] [Google Scholar]
- 41.Thelandersson A, Cider A, Nellgard B. Prone position in mechanically ventilated patients with reduced intracranial compliance. Acta Anaesthesiol Scand. 2006;50(8):937–941. doi: 10.1111/j.1399-6576.2006.01037.x. [DOI] [PubMed] [Google Scholar]
- 42.Daoud EG, Farag HL, Chatburn RL. Airway pressure release ventilation: what do we know? Respir Care. 2012;57(2):282–292. doi: 10.4187/respcare.01238. [DOI] [PubMed] [Google Scholar]
- 43.Marik PE, Young A, Sibole S, Levitov A. The effect of APRV ventilation on ICP and cerebral hemodynamics. Neurocrit Care. 2012;17(2):219–223. doi: 10.1007/s12028-012-9739-4. [DOI] [PubMed] [Google Scholar]
- 44.Taplu A, Gokmen N, Erbayraktar S, Sade B, Erkan N, Karadibak K, et al. Effects of pressure- and volume-controlled inverse ratio ventilation on haemodynamic variables, intracranial pressure and cerebral perfusion pressure in rabbits: a model of subarachnoid haemorrhage under isoflurane anaesthesia. Eur J Anaesthesiol. 2003;20(9):690–696. doi: 10.1017/s0265021503001121. [DOI] [PubMed] [Google Scholar]
- 45.Davies SW, Leonard KL, Falls RK, Jr, Mageau RP, Efird JT, Hollowell JP, et al. Lung protective ventilation (ARDSNet) versus airway pressure release ventilation: ventilatory management in a combined model of acute lung and brain injury. J Trauma Acute Care Surg. 2015;78(2):240–249. doi: 10.1097/TA.0000000000000518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vergouwen MD. Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid H. Vasospasm versus delayed cerebral ischemia as an outcome event in clinical trials and observational studies. Neurocrit Care. 2011;15(2):308–311. doi: 10.1007/s12028-011-9586-8. [DOI] [PubMed] [Google Scholar]
- 47.Gessler F, Mutlak H, Lamb S, Hartwich M, Adelmann M, Platz J, et al. The impact of tracheostomy timing on clinical outcome and adverse events in poor-grade subarachnoid hemorrhage. Crit Care Med. 2015;43(11):2429–2438. doi: 10.1097/CCM.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 48.Frontera JA, Fernandez A, Schmidt JM, Claassen J, Wartenberg KE, Badjatia N, et al. Impact of nosocomial infectious complications after subarachnoid hemorrhage. Neurosurgery. 2008;62(1):80–87. doi: 10.1227/01.NEU.0000311064.18368.EA. [DOI] [PubMed] [Google Scholar]
- 49.Rondeau N, Cinotti R, Rozec B, Roquilly A, Floch H, Groleau N, et al. Dobutamine-induced high cardiac index did not prevent vasospasm in subarachnoid hemorrhage patients: a randomized controlled pilot study. Neurocrit Care. 2012;17(2):183–190. doi: 10.1007/s12028-012-9732-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable