To the editor,
After SARS-CoV-2 first occurred in China in December of 2019, it set out to become a global pandemic. Critically ill patients constitute about 2–9% of all infected patients and progress from pneumonia and hypoxemia to multi-organ dysfunction, for which acute treatment options are scarce [1]. Currently, there is no clinical evidence supporting the efficacy and safety of a drug against any coronavirus in humans, including SARS-CoV-2. Here, we describe the empirical salvage treatment of critically ill COVID-19 patients in two German tertiary care University Hospitals with FX06 (F4 Pharma, Vienna, Austria), a naturally occurring peptide derived from the neo-N-terminus of fibrin (Bβ15-42). FX06 is known for its immunomodulatory properties [2] and was already investigated in clinical trials demonstrating convincing efficacy while being tolerated well with a favorable safety profile [3].
This observational case series includes six patients during their treatment in the intensive care unit. The respective institutions’ ethics committees approved the post hoc analysis of patient records for scientific purposes. The diagnosis of ARDS was based on the criteria put forth by the Berlin Definition.
Six mechanically ventilated patients suffering from moderate to severe ARDS upon ICU admission were treated with i.v. FX06 (400–600 mg per day; 3–7 days). Five out of these six patients additionally needed ECMO treatment during the course of their illness. Detailed clinical information is given in Table 1.
Table 1.
Demographics and clinical characteristics at admission and treatment of patients
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | ||
|---|---|---|---|---|---|---|---|
| Age (Y) | 52 | 78 | 63 | 51 | 71 | 55 | |
| Sex | Male | Male | Male | Female | Male | Male | |
| BMI | 31 | 35 | 26 | 54 | 28 | 37 | |
| Comorbidities | Obesity | Obesity, coronary artery disease, arterial hypertension | Bronchial asthma | Obesity, arterial hypertension, rheumatoid arthritis | Type 2 diabetes mellitus | Obesity, arterial hypertension | |
| Invasive ventilation | Yes | Yes | Yes | Yes | Yes | Yes | |
| Severity of ARDS at admission | Moderate | Moderate | Moderate | Moderate | Severe | Moderate | |
| Anti-infective therapy | Imipeneme | Imipeneme | Imipeneme, voriconazol | Piperacillin/tazobactam, ciprofloxa-cin, meropenem, vancomycin, anidulafun-gin | Merope-neme, co-trimoxazol | Ampicillin/sulbactam, cephazolin, caspofungin | |
| Days on ICU prior to FX06 treatment | 0 | 3 | 4 | 10 | 15 | 2 | |
| SAPS II Score | 57 | 75 | 43 | 68 | 63 | 59 | |
| PaO2/FiO2 ratio at admission | 186 | 141 | 131 | 154 | 85 | 122 | |
| Daily dose of FX06 | 500 mg | 600 mg | 400 mg | 400 mg | 400 mg | 400 mg | |
| Duration of FX06 treatment (days) | 7 | 7 | 4 | 3 | 4 | 4 | |
| vv-ECMO therapy | Yes | No | Yes | Yes | Yes | Yes | |
| Outcome | Rehabilitation care (after 35 days) | Death | Rehabilitation care (after 70 days) | Death | Rehabilitation care (after 48 days) | Rehabilitation care (after 44 days) | |
| Laboratory results at admission | Reference range | ||||||
| White blood cell count (cells per 106/L) | 14.02 | 15.56 | 6.26 | 7.9 | 14.2 | 11.2 | 3.92–9.81 |
| Lymphocyte (cells per 106/L) | 1.12 | 1.24 | 0.71 | 0.92 | 1.44 | 1.32 | 1.05–3.24 |
| Platelets | 320 | 147 | 171 | 161 | 272 | 255 | 146–328 |
| LDH U/L | 378 | 1277 | 417 | 611 | 516 | 609 | < 248 |
| Creatinine mg/dL | 0.72 | 2.34 | 0.43 | 0.50 | 0.82 | 0.88 | 0.7–1.2 |
| C-reactive protein (mg/dL) | 20.13 | 18.08 | 8.00 | 15.64 | 18.09 | 24.85 | < 0.5 |
| Ferritin ng/mL | 883 | 5505 | 3708 | 1114 | 4079 | 3503 (day 3) | 18–360 |
| Procalcitonin ng/mL | 0.15 | 0.30 | 0.78 | 0.09 | 1.32 | 2.44 | < 0.5 |
| Lactate mg/dL | 9.0 | 14 | 9.0 | 8.1 | 12.6 | 13.5 | 4.5–14.5 |
| IL-6 pg/mL | 92.3 | 25.4 | 250 | 2647.0 | 440.9 | 360.1 | < 7 |
| D-dimer ng/mL | 629 | 130,100 | 1056 | 450 | 2850 | 3750 | < 500 |
| aPTT (s) | 28 | 30 | 29 | 48.6 | 44.0 | 37.8 | 25–37 |
| vWF AG (%) | 283 | 446 | 311 | n/a | > 150 | > 150 | 60–150 |
Demographics and clinical characteristics at admission and treatment of patients
Y years, BMI body mass index, ARDS acute respiratory distress syndrome, SAPS simplified acute physiology score, LDH lactate dehydrogenase, U units, aPTT activated partial Thromboplastin time, VWF AG von Willebrand factor antigen, SAPS II Simplified Acute Physiology Score, PaO2 partial pressure arterial oxygen, FiO2 fraction of inspired oxygen, vv veno-venous, ECMO extracorporeal membrane oxygenation
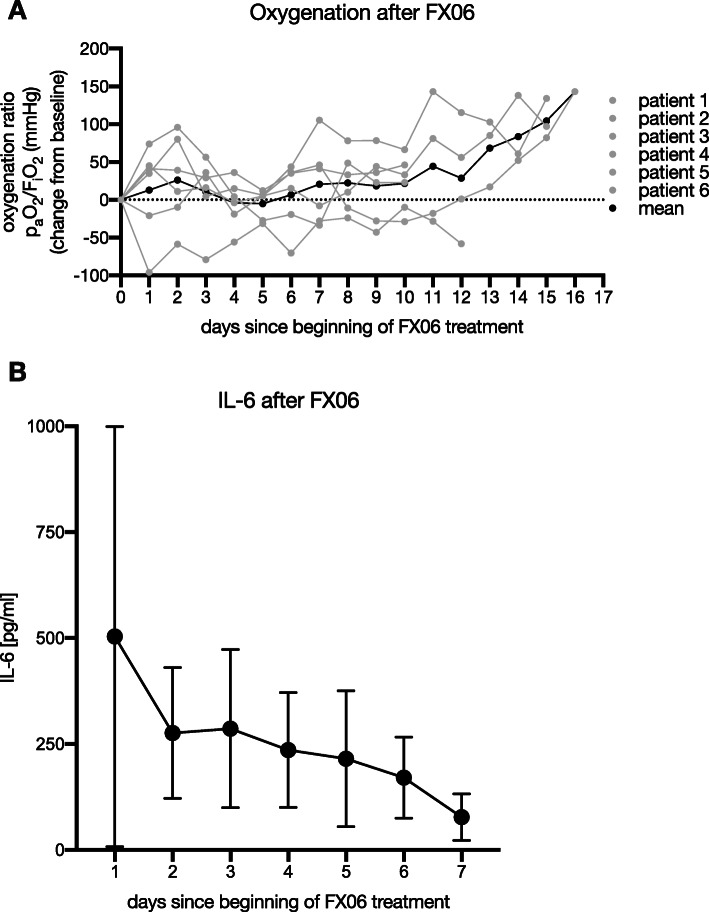
Mean oxygenation ratio improved over the first 3 days after the beginning of FX06 application, returned to baseline and increased steadily afterwards from day seven on (Fig. 1a). IL-6 serum concentrations as a marker of inflammation activity were instantly declining from day one (Fig. 1b). Norepinephrine dosages decreased initially after the initiation of FX06 therapy before returning to near-baseline values after some days (data not shown). Renal replacement therapy was necessary in four patients. Overall, four out of six patients survived. Both deceased patients (pats. 2 and 4 in Table 1) died from multi-organ failure due to septic shock most likely from secondary bacterial (co)infection. Hence, we saw no indication that the application of FX06 was in any way related to a patient’s death.
Fig. 1.
Oxygenation and IL-6 serum concentrations after FX06 treatment. a The difference in oxygenation compared to baseline (before FX06 treatment). paO2, partial pressure arterial oxygen; FiO2, fraction of inspired oxygen. b The course of interleukin 6 during the treatment with FX06. Data are presented as mean ± standard deviation
In summary, we observed substantial improvement in lung function following FX06 administration, which may be attributed to its immunomodulatory properties [3] and its function to preserve the endothelial barrier [4]. Patients treated with FX06 displayed a remarkable increase of their oxygenation indices, which we consider to be indicative of the normalization of the pulmonary vascular walls through the aforementioned underlying mechanisms. This was also mirrored in the radiographic diagnostics in five out of all six patients, reflecting a normalization of the interface between the alveolar space and an enhanced tissue integrity. Various coagulation factors, including fibrin degradation products, modulate the inflammatory response by influencing leukocyte migration and cytokine production [5, 6]. The decrease in IL-6 after FX06 is therefore considered to be attributed to these immunomodulatory effects.
Based on our experience, the salvage use of FX06 in severe COVID-19-associated ARDS could be an effective therapy to improve pulmonary function and vascular leakage in the most severely ill patients. A prospective randomized, controlled study to better elucidate this hypothesis is on preparation.
Acknowledgements
We are extremely thankful for all our staff nurses and support personal enabling us to successfully treat the high number of patients with Sars-Cov-2-induced ARDS.
This work was performed at Frankfurt University Hospital, Department of Anesthesiology and Intensive Care Medicine, Frankfurt, Germany, and Wuerzburg University Hospital, Department of Anesthesiology, Wuerzburg, Germany.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- SARS-CoV-2
Severe Acute Respiratory Syndrome Coronavirus 2
- ICU
Intensive care unit
- IL-6
Interleukin 6
- ECMO
Extracorporeal membrane oxygenation
Authors’ contributions
KZ and PM designed the study. EA, BS, and PM analyzed and interpreted the patient data and wrote the manuscript. MS, TS, and HN aided in interpreting the results and worked on the manuscript. All authors read and approved the final manuscript.
Funding
This study was funded by institutional funds of both University hospitals. FX06 was provided by the manufacturer (F4 Pharma, Vienna, Austria). No further external funding, especially none through the pharmaceutical manufacturer, was provided. The Universities of Frankfurt and Wuerzburg are in part owners of the patent of FX06.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The study was approved by the local ethics committee (University Hospital Frankfurt, Frankfurt, Germany) (#20-643).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Elisabeth H. Adam and Benedikt Schmid share first authorship.
Kai Zacharowski and Patrick Meybohm share senior authorship.
References
- 1.Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henning R, Zacharowski K, Petzelbauer P. FX06 (fibrin-derived peptide Bbeta15-42)-a potential candidate for myocardial reperfusion therapy. Drugs Future. 2006;31(9):811–818. doi: 10.1358/dof.2006.031.09.1025670. [DOI] [Google Scholar]
- 3.Atar D, Petzelbauer P, Schwitter J, Huber K, Rensing B, Kasprzak JD, et al. Effect of intravenous FX06 as an adjunct to primary percutaneous coronary intervention for acute ST-segment elevation myocardial infarction results of the F.I.R.E. (Efficacy of FX06 in the Prevention of Myocardial Reperfusion Injury) trial. J Am Coll Cardiol. 2009;53(8):720–729. doi: 10.1016/j.jacc.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 4.Gröger M, Pasteiner W, Ignatyev G, Matt U, Knapp S, Atrasheuskaya A, et al. Peptide Bβ15-42 preserves endothelial barrier function in shock. PLoS One. 2009;4(4):e5391. https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0005391. [DOI] [PMC free article] [PubMed]
- 5.Pawlinski R, Mackman N. Cellular sources of tissue factor in endotoxemia and sepsis. Thromb Res. 2010;125:S70–SS3. doi: 10.1016/j.thromres.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jensen T, Kierulf P, Sandset PM, Klingenberg O, Joø GB, Godal HC, et al. Fibrinogen and fibrin induce synthesis of proinflammatory cytokines from isolated peripheral blood mononuclear cells. Thromb Haemost. 2007;97(05):822–829. doi: 10.1160/TH07-01-0039. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.