Abstract
Coronavirus disease (COVID-19) has aggressively spread across the United States with numerous fatalities. Risk factors for mortality are poorly described. This was a multicentered cohort study identifying patient characteristics and diagnostic markers present on initial evaluation associated with mortality in hospitalized COVID-19 patients. Epidemiological, demographic, clinical, and laboratory characteristics of survivors and non-survivors were obtained from electronic medical records and a multivariable survival regression analysis was conducted to identify risk factors of in-hospital death. Of 1629 consecutive hospitalized adult patients with confirmed COVID-19 from March 1st thru March 31, 2020, 1461 patients were included in final analysis. 327 patients died during hospitalization and 1134 survived to discharge. Median age was 62 years (IQR 50.0, 74.0) with 56% of hospitalized patients under the age of 65. 47% were female and 63% identified as African American. Most patients (55%) had either no or one comorbidity. In multivariable analysis, older age, admission respiratory status including elevated respiratory rate and oxygen saturation ≤ 88%, and initial laboratory derangements of creatinine > 1.33 mg/dL, alanine aminotransferase > 40 U/L, procalcitonin > 0.5 ng/mL, and lactic acid ≥ 2 mmol/L increased risk of in-hospital death. This study is one of the largest analyses in an epicenter for the COVID-19 pandemic. Older age, low oxygen saturation and elevated respiratory rate on admission, and initial lab derangements including renal and hepatic dysfunction and elevated procalcitonin and lactic acid are risk factors for in-hospital death. These factors can help clinicians prognosticate and should be considered in management strategies.
Keywords: COVID-19, Mortality, Predictors, Scoring system
Introduction
In December 2019, the novel coronavirus (severe acute respiratory syndrome coronavirus 2, SARS-CoV-2) was first detected in Wuhan, China. Since then, coronavirus disease 2019 (COVID-19) has spread rapidly to nearly every continent around the globe [1–3]. After the first case of this illness was discovered in the United States in January 2020 in Seattle, WA, it spread to every state in under 2 months [4, 5]. The United States quickly became the world leader in number of cases and deaths. As of April 30, 2020, over 3.1 million cases and 227,000 fatalities were identified worldwide, of which over 60,000 fatalities were in the United States [6].
COVID-19 patients can present with a range of symptoms, varying from asymptomatic to severe cases with acute respiratory failure requiring hospitalization [7–9]. Presently, patients with more dire illness are apparently older and have complicating comorbidities such as diabetes, hypertension, and obesity [10, 11]. There is currently no cure for COVID-19. Hospitalization results in supportive treatment with oxygen support and a myriad of medications to prevent multiple organ dysfunction and death [12]. Despite the increasing number of cases, there is limited data regarding the clinical characteristics of patients admitted to the hospital, outside of China, specifically in a diverse American population. Furthermore, while it is evident that this disease is highly contagious and virulent with local community and person-to-person transmission [13, 14], there is limited evidence that describes the predictors, particularly early in patients’ hospital course, of poor outcomes and mortality for hospitalized patients. An improved understanding is required to guide decisions regarding admissions, hospital capacity and therapeutic and operational resources.
As COVID-19 has spread, several regions in the United States have been disproportionately impacted. It is our objective to determine early risk factors for in-hospital death for COVID-19 patients in the largest health system in southeastern Michigan.
Methods
Study design and participants
This was an analysis of a multicentered cohort study assessing early predictors of mortality in hospitalized patients with COVID-19 comparing survivors to non-survivors. The study was conducted at Beaumont Health, an eight-hospital acute care regional health system caring for 2.2 million people across the communities within the Metro Detroit catchment area. The hospitals range from a large tertiary care academic center to intermediate-sized and smaller community hospitals. As the epidemic evolved, the health system converted one of the smaller hospitals to a complete COVID-19 center, including converting the emergency department into additional intensive care beds. As the surge in COVID-19 volume developed across the region, hospital systems collaborated to optimize the transfer process and accommodate capacity constraints. While many patients were transferred within Beaumont Health, many patients were also transferred to other hospitals.
The study was approved by the Institutional Review Board at the home institution. Written informed consent requirement was waived due to the rapid emergence of COVID-19. Data were analyzed and interpreted by the authors.
Data source
Data were obtained from the integrated electronic health record (EHR; Epic Systems, Verona, WI). Patients over 18 years of age who were admitted with COVID-19 from March 1st through March 31st 2020 were included. Data were collected until April 23, 2020. All patients had a laboratory confirmed case of COVID-19 as defined by a positive result on a reverse-transcriptase-polymerase-chain-reaction (RT-PCR) test of nasopharyngeal swab. Exclusions consisted of patients who left the hospital against medical advice, transfers to external hospitals, or if hospital course was ongoing beyond April 23, 2020. Transfers within Beaumont Health were included as investigators had full access to medical records. Epidemiological, demographic, radiological, therapeutic, clinical, and outcomes data were extracted. The integrity of the data was verified by two attending emergency medicine physicians.
Hospital admission was based on the clinical judgment of the treating emergency medicine provider. Laboratory and radiological testing was conducted at the discretion of the treating physicians. After initial COVID laboratory testing, patients were not routinely serially tested to evaluate for clearance of acute infection due to paucity of testing supplies. Discharge disposition post-hospitalization was based on patients’ clinical condition. Patients were either discharged to home, skilled nursing facility or rehabilitation, hospice, or expired in the hospital.
Admission data included demographics such as age, race, and gender. Clinical data included symptoms prompting presentation to the ED, time of onset (days) of symptoms prior to ED visit, comorbidities, body mass index (BMI), tobacco history, vital signs including lowest oxygen level on room air within first 24 h of admission and maximum temperature, laboratory findings, and admission floor type. Radiological data included chest x-ray and chest computed tomography (CT) impressions. Common laboratory analyses included complete blood count (CBC) with absolute lymphocyte count, metabolic chemistry panel (BMP/CMP), lactate dehydrogenase, lactic acid, procalcitonin, troponin, ferritin, and D-dimer. Laboratory and radiological data depicted the first test result occurring within the first 24 h of presentation to the ED.
Hospital treatment data included use of investigational or adjunctive therapies, renal replacement therapy, extracorporeal membrane oxygenation, oxygen and ventilation therapy, and intensive care unit (ICU) admission.
Study definitions
Fever was defined as an axillary temperature of ≥ 38.0 °C. Acute respiratory failure was defined as a partial pressure of oxygen (PaO2) < 60 mm Hg on blood gas assessment. Pulse oximetry was used as a surrogate for PaO2, with ≤ 88% on room air meeting the criteria. Acute kidney injury was defined per KDIGO clinical practice guidelines [15]. Acute cardiac injury was defined as an elevated cardiac biomarker (troponin I) > 99% of the upper reference limit [16]. Septic shock was defined as a patient with diagnosed sepsis requiring vasopressor therapy to maintain mean arterial blood pressure > 65 mmHg. Patients with a quick Sequential Organ Failure Assessment score of two or more were considered to have sepsis [17]. Extubation failure was defined as need for reintubation within 72 h [18].
Statistical analysis
Descriptive analyses were used to summarize epidemiological characteristics and clinical findings stratified by in-hospital survival status. Continuous and categorical variables were expressed as medians (interquartile ranges; IQR) and frequencies (percentages), respectively. Kruskal–Wallis test (continuous variables) and Chi-squared or Fisher’s exact test (categorical variables) were used to compare differences between non-survivors and survivors. To explore the association between risk factors and in-hospital mortality, discrete-time survival regression was employed for univariable and multivariable analyses. Missing data were imputed by the procedure of multiple imputation using PROC MI in SAS. To avoid collinearity and overfitting on multivariable model, the L1-penalized least absolute shrinkage and selection operator (LASSO) regression selection was employed to the analog with a logistic regression that fits the discrete-time survival, using a statistical package “glmnet” in R. Variables selected via LASSO regression and clinical representative variables in terms of the basis of relevant studies were applied to multivariable analysis. The effects of risk factors were combined from 20 imputed datasets, accounting for the additional variability introduced by the multiple imputation, through PROC MIANALYZE in SAS. The corresponding c-statistics and leave-one-out validation were further used to evaluate the performance of modeling in multivariable regression. To facilitate the assessment of risk on mortality from the multivariable model, a prediction risk score using categorical variables was built. The assigned score for each risk factor was determined according to estimates of regression coefficients multiplied by 10. Patients were classified into risk groups based on their risk score and the mortality rates of each risk group was calculated. The differences in survival among risk groups over study period were also assessed using Kaplan–Meier method with the log-rank test. A bootstrap cross-validation with 1000 bootstrap samples per imputed dataset was further employed to validate the performance of scoring predictivity by assessing the similarity of mortality rates on risk groups. All tests of statistical significance were two-sided with p value < 0.05 indicating a significant difference. Analyses were conducted using R-3.6.2 (R Foundation for Statistical Computing) and SAS v-9.4 (SAS Institute, Inc., Cary, NC).
Results
Epidemiological characteristics
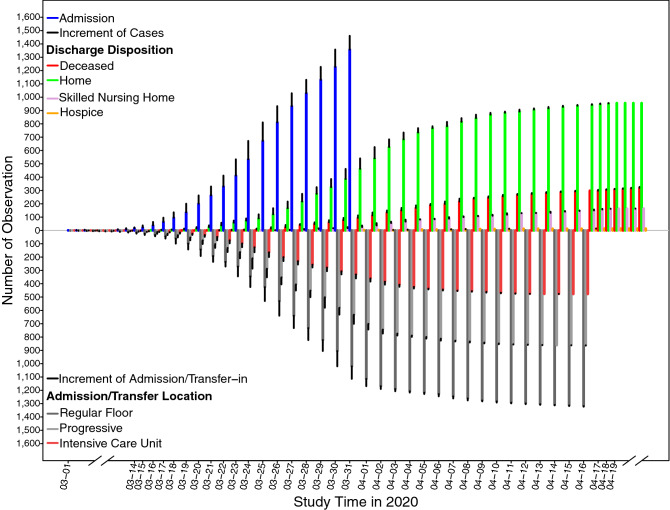
1629 patients were hospitalized at Beaumont Health with laboratory confirmed COVID-19 from March 1st thru March 31, 2020. 1461 patients were included in final analysis after exclusions (Fig. 1 shows enrollment scheme). 327 patients died during hospitalization and 1134 survived to discharge. Figure 2 illustrates admissions flow by unit type and discharge dispositions during the study period. The median age was 62 years (IQR 50.0–74.0) and 47.3% were female (Table 1). Almost two-thirds (63.0%) identified as Black or African American compared to 29.5% as White or Caucasian. Most patients (55.1%) had either no comorbidities (34.4%) or one comorbidity (20.7%). The most common pre-existing medical condition was hypertension (51.4%). The median BMI was 31.3 (IQR 27.1–37.1) with 41.8% of patients having a BMI of < 30. See Table 1 for full demographic and epidemiological data.
Fig. 1.
Flow diagram of study patients. Figure shows study inclusions and exclusions leading to the final cohort, and patient disposition
Fig. 2.
Daily patient influx by unit type and discharge disposition over the course of the study period. Figure shows probing daily change on hospital admission, locations of admission and transfer-in, and hospital discharge. In the upper panel, blue vertical lines stacked with black lines indicate the cumulative number of admitted patients prior to a specific date (blue lines) and the increment of patients on a specific date (black lines) from March 1st to March 31st, 2020. Other colorful vertical lines (red; green; plum; orange) stacked with black lines indicate the cumulative number on death, home discharge, skilled nursing home (SNF) discharge, and hospice discharge, respectively, prior to a specific date (red lines; green lines; plum lines; orange lines) and the increment of new occurrence (black lines) on a specific date until April 23rd, 2020. Similarly, in the bottom panel, colorful vertical lines (dark gray; gray; brown) stacked with black lines indicate the cumulative observations of admission/transfer-in to regular floor, progressive care, and intensive care unit (ICU), respectively, prior to a specific date (dark gray lines; gray lines; brown lines) and number of occurrence (black lines) on a specific date until April 23rd, 2020. Figure shows a daily influx of patients by unit type (Regular floor, Progressive floor, ICU) including admissions from the ED and transfers within the hospital and hospital system. Daily efflux of patients is also captured with discharge disposition including death, discharge to home, hospice, and skilled nursing facility/ rehabilitation units
Table 1.
Demographic, clinical, laboratory, and radiographic findings of the study patients
| Variablesa,b | All | Survival status | |||||
|---|---|---|---|---|---|---|---|
| Non-survivor | Survivor | p value | |||||
| 1461 | 327 | 1134 | |||||
| Demographics | |||||||
| Age, years | 62.0 (50.0–74.0) | 73.0 (63.0–81.0) | 59.0 (48.0–70.0) | < 0.001 | |||
| 18–50− | 349 (23.9) | 21 (6.4) | 328 (28.9) | < 0.001 | |||
| 50–65− | 462 (31.6) | 75 (22.9) | 387 (34.1) | ||||
| 65–80− | 438 (30.0) | 141 (43.1) | 297 (26.2) | ||||
| ≥ 80 | 212 (14.5) | 90 (27.5) | 122 (10.8) | ||||
| Gender | |||||||
| Female | 691 (47.3) | 147 (45.0) | 544 (48.0) | 0.34 | |||
| Male | 770 (52.7) | 180 (55.0) | 590 (52.0) | ||||
| Race | |||||||
| White/Caucasian | 431 (29.5) | 124 (37.9) | 307 (27.1) | 0.001 | |||
| Black/African American | 921 (63.0) | 182 (55.7) | 739 (65.2) | ||||
| Asian | 23 (1.6) | 7 (2.1) | 16 (1.4) | ||||
| Other | 86 (5.9) | 14 (4.3) | 72 (6.3) | ||||
| Smoke | |||||||
| Yes | 59 (4.0) | 14 (4.3) | 45 (4.0) | < 0.001 | |||
| Ever | 338 (23.1) | 113 (34.6) | 225 (19.8) | ||||
| Never | 731 (50.0) | 122 (37.3) | 609 (53.7) | ||||
| Unknown | 333 (22.8) | 78 (23.8) | 255 (22.5) | ||||
| BMI, kg/m2 (n = 1450/325/1125) | 31.3 (27.1–37.1) | 30.3 (25.9–35.3) | 31.8 (27.3–37.5) | 0.002 | |||
| < 25 | 222 (15.3) | 64 (19.7) | 158 (14.0) | 0.01 | |||
| 25–30− | 384 (26.5) | 94 (28.9) | 290 (25.8) | ||||
| 30–35− | 369 (25.4) | 80 (24.6) | 289 (25.7) | ||||
| 35–40− | 233 (16.1) | 36 (11.1) | 197 (17.5) | ||||
| ≥ 40 | 242 (16.7) | 51 (15.7) | 191 (17.0) | ||||
| Comorbidity | |||||||
| Asthma | 154 (10.5) | 30 (9.2) | 124 (10.9) | 0.36 | |||
| Cancer | 154 (10.5) | 44 (13.5) | 110 (9.7) | 0.05 | |||
| Coronary artery disease | 163 (11.2) | 59 (18.0) | 104 (9.2) | < 0.001 | |||
| Chronic heart failure | 100 (6.8) | 37 (11.3) | 63 (5.6) | < 0.001 | |||
| Chronic kidney disease | 75 (5.1) | 33 (10.1) | 42 (3.7) | < 0.001 | |||
| Chronic obstructive pulmonary disease | 132 (9.0) | 48 (14.7) | 84 (7.4) | < 0.001 | |||
| Cerebrovascular accident | 88 (6.0) | 30 (9.2) | 58 (5.1) | 0.01 | |||
| Diabetes mellitus | 430 (29.4) | 127 (38.8) | 303 (26.7) | < 0.001 | |||
| End-stage renal disease | 36 (2.5) | 15 (4.6) | 21 (1.9) | 0.01 | |||
| Human immunodeficiency virus | 8 (0.6) | 1 (0.3) | 7 (0.6) | 0.69 | |||
| Hypertension | 751 (51.4) | 200 (61.2) | 551 (48.6) | < 0.001 | |||
| Obstructive Sleep Apnea | 119 (8.2) | 29 (8.9) | 90 (7.9) | 0.59 | |||
| Pulmonary Hypertension | 12 (0.8) | 6 (1.8) | 6 (0.5) | 0.03 | |||
| Venous thromboembolism | 81 (5.5) | 29 (8.9) | 52 (4.6) | 0.003 | |||
| Comorbidity sum | |||||||
| 0 | 502 (34.4) | 88 (26.9) | 414 (36.5) | < 0.001 | |||
| 1 | 302 (20.7) | 45 (13.8) | 257 (22.7) | ||||
| 2 | 308 (21.1) | 73 (22.3) | 235 (20.7) | ||||
| 3 | 168 (11.5) | 55 (16.8) | 113 (10.0) | ||||
| ≥ 4 | 181 (12.4) | 66 (20.1) | 115 (10.1) | ||||
| Principal symptoms (n = 1422/313/1109) | |||||||
| Cough | 684 (48.1) | 122 (39.0) | 562 (50.7) | < 0.001 | |||
| Shortness of Breath | 787 (55.3) | 188 (60.1) | 599 (54.0) | 0.06 | |||
| Fever | 608 (42.8) | 115 (36.7) | 493 (44.5) | 0.01 | |||
| Gastrointestinal symptoms | 183 (12.9) | 35 (11.2) | 148 (13.4) | 0.31 | |||
| Altered mental status | 91 (6.4) | 45 (14.4) | 46 (4.2) | < 0.001 | |||
| Chest pain | 66 (4.6) | 8 (2.6) | 58 (5.2) | 0.05 | |||
| Weakness | 169 (11.9) | 43 (13.7) | 126 (11.4) | 0.25 | |||
| Chills | 28 (2.0) | 5 (1.6) | 23 (2.1) | 0.59 | |||
| Body aches | 82 (5.8) | 11 (3.5) | 71 (6.4) | 0.05 | |||
| Syncope | 61 (4.3) | 6 (1.9) | 55 (5.0) | 0.02 | |||
| Neurological symptoms | 29 (2.0) | 5 (1.6) | 24 (2.2) | 0.53 | |||
| Other | 104 (7.3) | 26 (8.3) | 78 (7.0) | 0.44 | |||
| Vital signs | |||||||
| Systolic blood pressure, mmHg | 130.0 (118.0–142.0) | 131.0 (117.0–147.0) | 129.5 (118.0–141.0) | 0.11 | |||
| Diastolic blood pressure, mmHg | 73.0 (65.0–81.0) | 71.0 (63.0–79.0) | 73.0 (65.0–82.0) | 0.02 | |||
| Pulse, beats per minute | 90.0 (80.0–101.0) | 90.0 (79.0–103.0) | 91.0 (80.0–100.0) | 0.89 | |||
| Respiratory rate, breaths per minute | 22.0 (19.0–25.0) | 24.0 (21.0–28.0) | 21.0 (19.0–24.0) | < 0.001 | |||
| Temperature, °F | 100.2 (99.0–101.7) | 100.1 (98.8–101.5) | 100.2 (99.0–101.7) | 0.39 | |||
| Blood oxygen saturation, % | 92.0 (88.0–95.0) | 88.0 (77.0–93.0) | 92.0 (89.0–95.0) | < 0.001 | |||
| ≤ 88 | 433 (29.6) | 165 (50.5) | 268 (23.6) | < 0.001 | |||
| 88 + to 94− | 523 (35.8) | 95 (29.0) | 428 (37.7) | ||||
| ≥ 94 | 505 (34.6) | 67 (20.5) | 438 (38.6) | ||||
| Laboratory findings | |||||||
| White blood cell count, 109/L (n = 1459/326/1133) | 6.3 (4.8–8.3) | 6.8 (5.1–9.7) | 6.1 (4.7–8.0) | < 0.001 | |||
| < 4 | 182 (12.5) | 31 (9.5) | 151 (13.3) | < 0.001 | |||
| 4–10 | 1,067 (73.1) | 220 (67.5) | 847 (74.8) | ||||
| > 10 | 210 (14.4) | 75 (23.0) | 135 (11.9) | ||||
| Lymphocyte count, 109/L (n = 1437/326/1111) | 0.9 (0.7–1.3) | 0.9 (0.6–1.2) | 1.0 (0.7–1.3) | < 0.001 | |||
| < 0.8 | 465 (32.4) | 134 (41.1) | 331 (29.8) | < 0.001 | |||
| ≥ 0.8 | 972 (67.6) | 192 (58.9) | 780 (70.2) | ||||
| Hemoglobin, g/dL (n = 1459/326/1133) | 13.2 (11.8–14.4) | 12.7 (11.3–14.3) | 13.3 (11.9–14.5) | 0.001 | |||
| Platelet count, 109/L (n = 1458/326/1132) | 196.0 (158.0–248.0) | 182.5 (149.0–234.0) | 199.0 (160.0–253.5) | < 0.001 | |||
| < 100 | 54 (3.7) | 22 (6.8) | 32 (2.8) | 0.001 | |||
| ≥ 100 | 1,404 (96.3) | 304 (93.2) | 1100 (97.2) | ||||
| ALT, U/L (n = 1335/319/1016) | 28.0 (19.0–48.0) | 30.0 (18.0–55.0) | 28.0 (19.0–46.5) | 0.18 | |||
| ≤ 40 | 901 (67.5) | 200 (62.7) | 701 (69.0) | 0.04 | |||
| > 40 | 434 (32.5) | 119 (37.3) | 315 (31.0) | ||||
| AST, U/L (n = 1335/319/1016) | 43.0 (29.0–66.0) | 53.0 (33.0–85.0) | 40.0 (27.0–60.0) | < 0.001 | |||
| ≤ 40 | 624 (46.7) | 110 (34.5) | 514 (50.6) | < 0.001 | |||
| > 40 | 711 (53.3) | 209 (65.5) | 502 (49.4) | ||||
| Creatinine, mg/dL (n = 1458/326/1132) | 1.18 (0.92–1.66) | 1.57 (1.11–2.37) | 1.12 (0.89–1.47) | < 0.001 | |||
| ≤ 1.33 | 901 (61.8) | 131 (40.2) | 770 (68.0) | < 0.001 | |||
| > 1.33 | 557 (38.2) | 195 (59.8) | 362 (32.0) | ||||
| Lactate dehydrogenase, U/L (n = 884/241/643) | 421.5 (312.5–584.5) | 511.0 (353.0–717.0) | 396.0 (304.0–531.0) | < 0.001 | |||
| ≤ 245 | 90 (10.2) | 18 (7.5) | 72 (11.2) | 0.10 | |||
| > 245 | 794 (89.8) | 223 (92.5) | 571 (88.8) | ||||
| Troponin, ng/mL (n = 1,115/297/818) | 0.02 (0.01–0.05) | 0.04 (0.02–0.12) | 0.01 (0.01–0.03) | < 0.001 | |||
| ≤ 0.3 | 1,052 (93.3) | 260 (87.5) | 792 (96.8) | < 0.001 | |||
| > 0.3 | 63 (5.7) | 37 (12.5) | 26 (3.2) | ||||
| D-dimer, ng/mL FEU (n = 803/254/549) | 1,064.0 (637.0–2,181.0) | 1441.5 (903.0–5041.0) | 885.0 (568.0–1,691.0) | < 0.001 | |||
| ≤ 500 | 125 (15.6) | 18 (7.1) | 107 (19.5) | < 0.001 | |||
| 500–1000 | 263 (32.7) | 61 (24.0) | 202 (36.8) | ||||
| > 1000 | 415 (51.7) | 175 (68.9) | 240 (43.7) | ||||
| Procalcitonin, ng/mL (n = 974/251/723) | 0.17 (0.08–0.48) | 0.47 (0.18–1.40) | 0.12 (0.07–0.31) | < 0.001 | |||
| < 0.1 | 324 (33.3) | 31 (12.3) | 293 (40.5) | < 0.001 | |||
| 0.1–0.25 | 272 (27.9) | 55 (21.9) | 217 (30.0) | ||||
| 0.25–0.5 | 143 (14.7) | 44 (17.5) | 99 (13.7) | ||||
| > 0.5 | 235 (24.1) | 121 (48.2) | 114 (15.8) | ||||
| C reactive protein, mg/L (n = 1,002/269/733) | 106.4 (59.1–177.1) | 155.9 (89.5–218.8) | 94.8 (51.7–153.0) | < 0.001 | |||
| < 50 | 201 (20.1) | 23 (8.5) | 178 (24.3) | < 0.001 | |||
| 50–100 | 258 (25.7) | 51 (19.0) | 207 (28.2) | ||||
| > 100 | 543 (54.2) | 195 (72.5) | 348 (47.5) | ||||
| Lactic acid, mmol/L (n = 806/262/544) | 1.4 (1.0–1.9) | 1.7 (1.2–2.4) | 1.3 (1.0–1.7) | < 0.001 | |||
| < 2 | 612 (75.9) | 160 (61.1) | 452 (83.1) | < 0.001 | |||
| ≥ 2 | 194 (24.1) | 102 (38.9) | 92 (16.9) | ||||
| CT/Chest x-ray (n = 1,442/326/1,116) | |||||||
| Patchy ground-glass opacities | 1,048 (72.7) | 236 (72.4) | 812 (72.8) | 0.90 | |||
| Pleural effusion | 17 (1.2) | 3 (0.9) | 14 (1.3) | 0.78 | |||
| Pulmonary embolism | 118 (8.2) | 50 (15.3) | 68 (6.1) | < 0.001 | |||
BMI body mass index, ALT alanine aminotransferase, AST aspartate aminotransferase, CT computerized tomography
aFor continuous variables, medians (Interquartile Ranges) were presented. For categorical variables, frequencies (percentages) were presented
bFor any missing on variables, the corresponding number of observations was presented as n = the number of observations for all/the number of observations for non-survivors/the number of observations for survivors within parentheses
Clinical features
Shortness of breath was the most common presenting complaint present in 55.3% of patients followed closely by cough and fever. The median admission oxygen saturation and respiratory rate were 92.0% (IQR 88.0–95.0) and 21.0 (IQR 19.0–24.0) for survivors and 88.0% (77.0–93.0) and 24.0 (21.0–28.0) for non-survivors, respectively (p < 0.001). Renal impairment was more common in non-survivors (Cr = 1.57 mg/dL; IQR 1.11–2.37) compared to survivors (Cr = 1.12 mg/dL; IQR 0.89–1.47; p < 0.001). Procalcitonin level was higher in non-survivors (0.47 ng/mL; IQR 0.18–1.40) compared to survivors (0.12 ng/mL; IQR 0.07–0.31); p < 0.001). Lactic acid was elevated in non-survivors (1.7 mmol/L; IQR 1.2–2.4) compared to survivors (1.2 mmol/L; IQR 1.0–1.7; p < 0.001). Patchy ground glass opacities on imaging were commonly seen in patients (72.7%) (Table 1).
Respiratory failure was the most common patient outcome complicating 52.5% of admissions and present in 93.9% of non-survivors. High-flow oxygen therapy via nasal cannula was used in 28.7% of patients, more commonly employed in non-survivors (63.0%) compared to survivors (18.8%). Invasive mechanical ventilation was employed in 308 patients (21.1%). It was used in 67.0% of non-survivors compared to 7.9% of survivors (p < 0.001). Overall, 71.1% of patients did not survive to hospital discharge when invasive mechanical ventilation was utilized. Acute kidney injury was noted in 382 patients and developed in 71.3% of non-survivors compared to 13.1% of survivors (p < 0.001). Of these patients, 20.9% required hemodialysis. Acute cardiac injury was a less frequent complication occurring in 9.1% of cases but was a more common complication in non-survivors (26.0%). Septic shock requiring vasopressors occurred in 16.2% of patients, occurring in 59.9% of non-survivors compared to 3.6% of survivors (p < 0.001). Of the 237 patients classified as septic shock, only 30 patients (12.65%) had positive blood cultures. 25 patients had blood cultures that identified a bacterial source and six patients had blood cultures that grew Candida albicans, treated with an antifungal. 7 patients had more than one bacteria isolated, and one patient had bacteria and Candida isolated. 207 patients had blood cultures without any growth. The most common isolates were gram positive bacteria, such as several different species of Staphylococcus, including methicillin resistant Staphylococcus aureus, Enterococcus faecalis and Streptococcus viridans. There were less gram negative isolates but included Haemophilus influenzae, Pseudomonas aeruginosa, Klebsiella pneumoniae and Escherichia coli.
The median length of illness (symptom onset) for survivors was 12.0 days compared to 15.0 days for non-survivors. The median hospital stay was 7 days. Intensive care admission was required in 25.6% of patients with a median ICU length of stay of 7.0 days (Table 2).
Table 2.
Treatments, complications, and clinical outcomes
| Variablesa,b | All | Survival status | |||||
|---|---|---|---|---|---|---|---|
| Non-survivor | Survivor | p value | |||||
| 1461 | 327 | 1134 | |||||
| Treatments | |||||||
| Hydroxychloroquine | 1073 (73.4) | 290 (88.7) | 783 (69.1) | < 0.001 | |||
| Corticosteroids | 617 (42.2) | 211 (64.5) | 406 (35.8) | < 0.001 | |||
| NSAIDs | 103 (7.1) | 12 (3.7) | 91 (8.0) | 0.01 | |||
| Vitamin C | 407 (27.9) | 116 (35.5) | 291 (25.7) | < 0.001 | |||
| Zinc | 372 (25.4) | 111 (33.9) | 261 (23.0) | < 0.001 | |||
| ACE inhibitors | 209 (14.3) | 28 (8.6) | 181 (16.0) | 0.001 | |||
| ARBs or Sartanics | 104 (7.1) | 23 (7.0) | 81 (7.1) | 0.95 | |||
| Renal replacement therapy | 82 (5.6) | 50 (15.3) | 32 (2.8) | < 0.001 | |||
| Non-invasive ventilation | 106 (7.3) | 53 (16.2) | 53 (4.7) | < 0.001 | |||
| High flow oxygen therapy | 419 (28.7) | 206 (63.0) | 213 (18.8) | < 0.001 | |||
| Inv. Mechanical ventilation | 308 (21.1) | 219 (67.0) | 89 (7.9) | < 0.001 | |||
| Extubation (n = 308/219/89) | |||||||
| No extubation trial | 175 (56.8) | 146 (66.7) | 29 (32.6) | < 0.001 | |||
| Unsuccessful wean | 63 (20.5) | 62 (28.3) | 1 (1.1) | ||||
| Success | 70 (22.7) | 11 (5.0) | 59 (66.3) | ||||
| Outcomes | |||||||
| Respiratory failure | 767 (52.5) | 307 (93.9) | 460 (40.6) | < 0.001 | |||
| Septic shock | 237 (16.2) | 196 (59.9) | 41 (3.6) | < 0.001 | |||
| Acute cardiac injury | 133 (9.1) | 85 (26.0) | 48 (4.2) | < 0.001 | |||
| Acute kidney injury | 382 (26.2) | 233 (71.3) | 149 (13.1) | < 0.001 | |||
| ICU admission | 374 (25.6) | 211 (64.5) | 163 (14.4) | < 0.001 | |||
| ICU length of stay, days | 7.0 (4.0–12.0) | 7.0 (4.0–11.0) | 8.0 (4.0–14.0) | 0.32 | |||
| Hospital length of stay, days | 7.0 (4.0–11.0) | 10.0 (6.0–15.0) | 6.0 (3.0–10.0) | < 0.001 | |||
| Time from illness onset to ICU admission, days | 8.0 (5.0–10.0) | 8.0 (5.0–11.0) | 8.0 (4.0–10.0) | 0.31 | |||
| Time from illness onset to hydroxychloroquine therapy, days | 7.0 (5.0–9.0) | 6.0 (4.0–9.0) | 8.0 (5.0–10.0) | < 0.001 | |||
| Time from illness onset to corticosteroids therapy, days | 9.0 (6.0–13.0) | 8.0 (5.0–12.0) | 9.0 (6.0–13.0) | 0.11 | |||
| Time from illness onset to high flow oxygen therapy, days | 8.0 (6.0–11.0) | 8.0 (5.0–10.0) | 9.0 (6.0–12.0) | < 0.001 | |||
| Time from illness onset to mechanical ventilation, days | 8.0 (6.0–12.0) | 8.0 (6.0–11.0) | 9.0 (7.0–13.0) | 0.11 | |||
| Time from illness onset to death or discharge, days | 13.0 (9.0–18.0) | 15.0 (10.0–21.0) | 12.0 (8.0–17.0) | < 0.001 | |||
NSAIDs nonsteroidal anti-inflammatory drugs, ACE angiotensin-converting enzyme, ARB angiotensin II receptor blocker, ICU intensive unit care
aFor continuous variables, medians (Interquartile Ranges) were presented. For categorical variables, frequencies (percentages) were presented
bFor results of extubation, only 308 patients who received mechanical ventilation, including 219 non-survivors and 89 survivors, were assessed
The most commonly employed investigational treatment was hydroxychloroquine, used in 73.4% of patients with the average time from illness onset to therapy start of 7.0 days (IQR 5.0–9.0). Systemic glucocorticoids were utilized in 42.2% of patients with median time from illness onset to therapy start of 9.0 days (IQR 6.0–13.0).
In univariable analysis, epidemiological and clinical characteristics listed on Table 1 were utilized to illustrate the unadjusted effects on the hazard of in-hospital patient death. In multivariable survival model 1, older age (HR 1.05, 95% CI 1.04 to 1.06), admission elevated respiratory rate (HR 1.06, 95% CI 1.04–1.09), pulse oximetry oxygen saturation less than or equal to 88% (HR 1.82, 95% CI 1.33–2.50) and initial laboratories: creatinine > 1.33 mg/dL (HR 1.41, 95% CI 1.08–1.85), ALT > 40 U/L (HR 1.36, 95% CI 1.05–1.77), procalcitonin > 0.5 ng/mL (HR 2.14, 95% CI 1.37–3.36), and lactic acid ≥ 2.0 mmol/L (HR 1.57, 95% CI 1.17 to 2.12) were predictors of in-hospital death. Similar results were found when using age groups as a risk factor in multivariable survival model 2 (Table 3).
Table 3.
Risk factors associated with study patients deceased in-hospital
| Univariable model | Multivariable model 1a | Multivariable model 2a | ||||
|---|---|---|---|---|---|---|
| Variables | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value |
| Demographics | ||||||
| Age, years | 1.06 (1.04–1.06) | < 0.001 | 1.05 (1.04–1.06) | < 0.001 | ||
| Age, years | ||||||
| 18–50- | 1 [Reference] | 1 [Reference] | ||||
| 50–65- | 1.93 (1.18–3.14) | 0.01 | 1.89 (1.14–3.15) | 0.01 | ||
| 65–80- | 3.40 (2.14–5.41) | < 0.001 | 3.29 (1.99–5.44) | < 0.001 | ||
| ≥ 80 | 6.62 (4.09–10.74) | < 0.001 | 7.13 (4.14–12.27) | < 0.001 | ||
| Gender | ||||||
| Male | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Female | 1.11 (0.89–1.39) | 0.36 | 1.11 (0.86–1.45) | 0.42 | 1.14 (0.88–1.48) | 0.31 |
| Race | ||||||
| Black/African American | 0.67 (0.53–0.85) | 0.001 | 0.72 (0.55–0.94) | 0.01 | 0.69 (0.53–0.89) | 0.006 |
| White/Caucasian | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Other | 0.57 (0.36–0.91) | 0.02 | 0.57 (0.35–0.93) | 0.03 | 0.54 (0.33–0.89) | 0.01 |
| BMI, kg/m2 | 0.98 (0.96–0.99) | 0.002 | 1.01 (0.99–1.02) | 0.30 | 1.00 (0.99–1.02) | 0.68 |
| Comorbidity | ||||||
| Asthma | 0.97 (0.66–1.43) | 0.89 | ||||
| Cancer | 1.05 (0.76–1.46) | 0.77 | ||||
| Coronary artery disease | 1.69 (1.26–2.27) | < 0.001 | 1.23 (0.89–1.70) | 0.21 | 1.32 (0.95–1.83) | 0.10 |
| Chronic heart failure | 1.49 (1.05–2.13) | 0.02 | ||||
| Chronic kidney disease | 1.62 (1.10–2.36) | 0.01 | ||||
| Chronic obstructive Pulmonary disease | 1.77 (1.29–2.43) | < 0.001 | ||||
| Cerebrovascular accident | 1.38 (0.94–2.04) | 0.10 | ||||
| Diabetes mellitus | 1.33 (1.06–1.67) | 0.01 | 1.08 (0.83–1.41) | 0.56 | 1.08 (0.83–1.41) | 0.55 |
| End-stage renal disease | 1.23 (0.71–2.14) | 0.46 | ||||
| Human immunodeficiency virus | 0.55 (0.08–4.00) | 0.55 | ||||
| Hypertension | 1.20 (0.96–1.51) | 0.11 | 0.84 (0.64–1.10) | 0.20 | 0.89 (0.68–1.17) | 0.41 |
| Obstructive sleep apnea | 0.99 (0.67–1.46) | 0.95 | ||||
| Pulmonary hypertension | 1.12 (0.49–2.56) | 0.79 | ||||
| Venous thromboembolism | 1.18 (0.80–1.75) | 0.40 | ||||
| Vital sign | ||||||
| Systolic blood pressure, mmHg | 1.00 (1.00–1.01) | 0.34 | ||||
| Diastolic blood pressure, mmHg | 0.99 (0.98–1.00) | 0.21 | ||||
| Pulse, beats per minute | 1.00 (0.99–1.01) | 0.82 | ||||
| Respiratory rate, breaths per minute | 1.06 (1.03–1.08) | < 0.001 | 1.06 (1.04–1.09) | < 0.001 | 1.06 (1.04–1.09) | < 0.001 |
| Temperature, °F | 0.95 (0.89–1.02) | 0.14 | ||||
| Blood oxygen saturation, % | ||||||
| ≤ 88 | 2.01 (1.50–2.69) | < 0.001 | 1.82 (1.33–2.50) | < 0.001 | 1.81 (1.32–2.49) | < 0.001 |
| 88 + to 94− | 1.25 (0.90–1.72) | 0.18 | 1.27 (0.90–1.78) | 0.17 | 1.28 (0.92–1.80) | 0.14 |
| ≥ 94 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| Principal symptoms | ||||||
| Cough | 0.65 (0.51–0.82) | < 0.001 | ||||
| Shortness of Breath | 1.27 (1.01–1.61) | 0.04 | ||||
| Fever | 0.80 (0.63–1.01) | 0.07 | ||||
| Gastrointestinal symptoms | 0.87 (0.61–1.26) | 0.47 | ||||
| Altered mental status | 2.09 (1.50–2.92) | < 0.001 | ||||
| Chest pain | 0.83 (0.41–1.68) | 0.60 | ||||
| Weakness | 1.22 (0.88–1.70) | 0.24 | ||||
| Chills | 0.65 (0.27–1.59) | 0.35 | ||||
| Body aches | 0.61 (0.33–1.12) | 0.11 | ||||
| Syncope | 0.66 (0.29–1.50) | 0.32 | ||||
| Neurological symptoms | 0.78 (0.32–1.89) | 0.58 | ||||
| Other | 1.07 (0.70–1.63) | 0.75 | ||||
| Laboratory findings | ||||||
| White blood cell count × 109/L | ||||||
| < 4 | 0.86 (0.58–1.27) | 0.45 | 1.05 (0.70–1.58) | 0.80 | 1.01 (0.68–1.52) | 0.94 |
| 4–10 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| > 10 | 1.35 (1.03–1.77) | 0.03 | 0.72 (0.53–0.97) | 0.03 | 0.74 (0.54–1.01) | 0.06 |
| Lymphocyte count × 109/L | ||||||
| < 0.8 | 1.30 (1.04–1.63) | 0.02 | ||||
| ≥ 0.8 | 1 [Reference] | |||||
| Hemoglobin, g/dL | 0.94 (0.90–0.99) | 0.02 | 0.94 (0.89–1.00) | 0.05 | 0.94 (0.89–0.99) | 0.04 |
| Platelet count × 109/L | ||||||
| < 100 | 1.76 (1.13–2.75) | 0.01 | ||||
| ≥ 100 | 1 [Reference] | |||||
| ALT, U/L | ||||||
| ≤ 40 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| > 40 | 1.27 (1.01–1.60) | 0.04 | 1.36 (1.05–1.77) | 0.02 | 1.41 (1.09–1.83) | 0.01 |
| AST, U/L | ||||||
| ≤ 40 | 1 [Reference] | |||||
| > 40 | 1.28 (1.01–1.62) | 0.04 | ||||
| Creatinine, mg/dL | ||||||
| ≤ 1.33 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| > 1.33 | 1.79 (1.43–2.25) | < 0.001 | 1.41 (1.08–1.85) | 0.01 | 1.38 (1.05–1.82) | 0.02 |
| Lactate dehydrogenase, U/L | ||||||
| ≤ 245 | 1 [Reference] | |||||
| > 245 | 0.93 (0.60–1.45) | 0.75 | ||||
| Troponin, ng/mL | ||||||
| ≤ 0.3 | 1 [Reference] | |||||
| > 0.3 | 1.88 (0.76–4.67) | 0.16 | ||||
| D-dimer, ng/mL FEU | ||||||
| ≤ 500 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| 500–1000 | 1.24 (0.73–2.08) | 0.43 | 1.00 (0.59–1.68) | 0.99 | 1.06 (0.63–1.79) | 0.81 |
| > 1000 | 1.97 (1.20–3.22) | 0.007 | 1.08 (0.64–1.81) | 0.78 | 1.14 (0.68–1.93) | 0.61 |
| Procalcitonin, ng/mL | ||||||
| < 0.1 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| 0.1–0.25 | 1.45 (0.94–2.21) | 0.09 | 1.23 (0.78–1.95) | 0.36 | 1.23 (0.78–1.94) | 0.37 |
| 0.25–0.5 | 1.88 (1.21–2.92) | 0.005 | 1.38 (0.84–2.25) | 0.20 | 1.37 (0.84–2.23) | 0.20 |
| > 0.5 | 3.17 (2.18–4.60) | < 0.001 | 2.14 (1.37–3.36) | 0.001 | 2.11 (1.34–3.31) | 0.001 |
| C reactive protein, mg/L | ||||||
| < 50 | 1 [Reference] | |||||
| 50–100 | 1.28 (0.81–2.02) | 0.30 | ||||
| > 100 | 1.60 (1.06–2.41) | 0.03 | ||||
| Lactic acid, mmol/L | ||||||
| < 2 | 1 [Reference] | 1 [Reference] | 1 [Reference] | |||
| ≥ 2 | 2.15 (1.65–2.80) | < 0.001 | 1.57 (1.17–2.12) | 0.003 | 1.59 (1.19–2.14) | 0.002 |
| CT/Chest x-ray | ||||||
| Pulmonary embolism | 1.76 (1.29–2.40) | < 0.001 | ||||
ALT alanine aminotransferase, AST aspartate aminotransferase, HR hazard ratio, CI confidence interval
aThe model assessment indicated the predictive ability (c statistics) on in-hospital survival status was as high as 0.78 and the leave-one-out cross-validated results showed that there was no overfitting in the multivariable model
To facilitate the assessment of risk on mortality, a multivariable survival model using categorical variables for predicting mortality is shown in Table 4 with a scoring system depicted in Table 5. According to our model, a score of ≤ 12, 13–26, and ≥ 27 carries a mortality risk of 3.91%, 17.98%, and 51.50%, respectively, with the significant differences among risk-scoring groups (p < 0.001, Fig. 3).
Table 4.
Multivariable survival model using categorical variables for predicting mortality
| Variablesb | Log-HR (β) | SE | p value | Assigned pointsa |
|---|---|---|---|---|
| Age | ||||
| 50 to 65 years | 0.58 | 0.26 | 0.02 | 6 |
| 65 to 80 years | 1.15 | 0.25 | < 0.001 | 12 |
| ≥ 80 years | 1.96 | 0.27 | < 0.001 | 20 |
| Female | 0.14 | 0.13 | 0.27 | 1 |
| Race | ||||
| Black/African American | − 0.36 | 0.13 | 0.01 | − 4 |
| Other | − 0.52 | 0.25 | 0.04 | − 5 |
| BMI ≥ 30 kg/m2 | 0.14 | 0.13 | 0.26 | 1 |
| Comorbidity | ||||
| Coronary artery disease | 0.27 | 0.17 | 0.10 | 3 |
| Diabetes Mellitus | 0.09 | 0.13 | 0.49 | 1 |
| Hypertension | − 0.12 | 0.14 | 0.38 | − 1 |
| Respiratory rate ≥ 24 breaths per minute | 0.50 | 0.12 | < 0.001 | 5 |
| Blood oxygen saturation | ||||
| ≤ 88% | 0.62 | 0.16 | < 0.001 | 6 |
| 88 + to 94− % | 0.29 | 0.17 | 0.09 | 3 |
| White blood cell count | ||||
| < 4 × 109/L | 0.07 | 0.21 | 0.74 | 1 |
| > 10 × 109/L | − 0.24 | 0.16 | 0.13 | − 2 |
| Hemoglobin ≤ 11 g/dL | 0.34 | 0.15 | 0.03 | 3 |
| ALT > 40 U/L | 0.36 | 0.13 | 0.01 | 4 |
| Creatinine > 1.33 mg/dL | 0.30 | 0.14 | 0.03 | 3 |
| D-dimer | ||||
| 500–1000 ng/mL FEU | 0.07 | 0.26 | 0.78 | 1 |
| > 1000 ng/mL FEU | 0.10 | 0.27 | 0.71 | 1 |
| Procalcitonin | ||||
| 0.1–0.25 ng/mL | 0.22 | 0.23 | 0.33 | 2 |
| 0.25–0.5 ng/mL | 0.35 | 0.25 | 0.16 | 4 |
| > 0.5 ng/mL | 0.79 | 0.23 | < 0.001 | 8 |
| Lactic acid ≥ 2 mmol/L | 0.46 | 0.15 | 0.002 | 5 |
ALT alanine aminotransferase, HR hazard ratio, SE standard error
aEstimates of regression coefficient (β) were pooled from survival models fitted on 20 imputed datasets. Points associated with the presence of a given level of a risk factor (variable) were assigned by multiplying β by 10 and rounding to the nearest integer. The reference level was assigned zero point. The theoretical ranges of total score were − 8 and 62
bUsing bootstrap cross-validation with 1000 bootstrap samples per imputed dataset, the estimate of the concordance index (c-statistics) was 0.78 (95% CI 0.75–0.81)
Table 5.
Mortality assessment using the predictor scoring system
| Scoring groupa,b | Mortality rate (95% confidence interval) | Hazard raito (95% confidence interval) | p value |
|---|---|---|---|
| ≤ 12 (Low Risk) | 3.91% (1.88–5.94%) | 1 [Reference] | |
| 13–26 | 17.98% (15.01–20.95%) | 2.82 (15.59–5.00) | < 0.001 |
| ≥ 27 (High Risk) | 51.50% (46.16%–56.83%) | 9.15 (5.19–16.14) | < 0.001 |
aThe observed ranges of total score were − 4 and 58 on 20 imputed datasets. The median score was 19 and the 25th and 75th percentiles (IQR) were 12 and 26, respectively. The 10th and 90th percentiles were 7 and 33, respectively. Mortality rates and the corresponding estimates of hazard ratios were pooled from 20 imputed datasets
bUsing bootstrap cross-validation with 1000 bootstrap samples per imputed dataset, estimates of mortality rates from low risk to high risk were 3.92% (95% CI 2.03–6.05%), 17.98% (95% CI 15.04–21.01%) and 51.48% (95% CI 46.15–56.80%), respectively
Fig. 3.
Kaplan–Meier survival curve for mortality for three risk groups. Figure shows overall survival of study patients associated with three risk groups (score: ≤ 12, 13 to 26, ≥ 27) during study period. The estimated survival cures were pooled from 20 imputed datasets
Discussion
In this study, we described the characteristics of 1461 patients requiring hospitalization for laboratory-confirmed COVID-19 in Metro Detroit.
We also delineated the average clinical course and identified risk factors for mortality that were present early in the hospitalization course. Overall, mortality exceeded 22% for hospitalized patients and is consistent with other published reports, ranging between 14 and 28% [14, 19–21]. Specifically older age, abnormal respiratory parameters on presentation, and initial laboratory results demonstrating renal insufficiency, liver injury, and elevated procalcitonin and lactic acid levels were risk factors for in-hospital death. These factors can help clinicians prognosticate so a scoring system was developed based on these variables among others to help predict inpatient mortality. It was found that a score of less than 12 was low risk of mortality, while a score ≥ 27 represented greater than 50% likelihood of death. This scoring system is based on the clinical features in our cohort and could help physicians estimate mortality and possibly aid in resource allocation, although has not been validated outside of this cohort.
In this analysis, we identified a strong association of demographic and epidemiologic characteristics with hospitalization and mortality. Age was a significant predictor of death as median age of fatalities was 73 compared to 59 years for survivors. This is consistent with other reports citing that the geriatric population is at the highest risk in this pandemic [14]. Interestingly, though, while mortality predominantly occurs in the elderly population, younger adults remain at high risk for hospitalization as nearly 56% of patients in our study were between 18 and 64 years. Gender vulnerability of males has also been a commonly reported descriptive within investigations, with male gender comprising 73% of deaths and 82% of ICU admissions in Chinese and Italian publications, respectively [20, 21]. It is postulated that women may be partially protected due to genetic, biological, and hormonal factors [22–24]. We, however, did not find a male predominance to the COVID-19 illness within our large sample size. While males made up slightly more hospital admissions (53%) than females, there was no mortality difference between genders. Race was another relevant variable. In our study, we found that African Americans were over twice as likely to be hospitalized due to COVID-19 compared to Caucasians. In a quality analysis of demographics at Beaumont Health, African Americans accounted for only 22% of hospitalizations in 2019, yet they accounted for 63% of COVID-19 hospitalizations. The African American community had a higher prevalence of hospitalizations but not mortality, leading us to speculate that other factors such as inability to socially distance due to multi-generational households, the need to work outside the home, and other similar characteristics, put this population at greater risk to be exposed to the illness.
Pre-existing conditions are described as significant predictors of COVID-19 disease outcomes in the literature [14, 19, 20, 25, 26]. Specifically, coronary artery disease and hypertension have been identified as risk factors for mortality. In our study, 34% of hospitalized patients had no comorbidities and 55% had one or no pre-existing medical conditions. This prevalence demonstrates the virulence of COVID-19 in a seemingly healthier population. Unlike other publications, our multivariable analysis did not provide supportive evidence for an association between specific comorbidities and mortality. Additionally, obesity has reportedly been associated with poor outcomes in COVID-19. In a large recent analysis based in New York, BMI greater than 30 was associated with critical illness and a threshold over 40 was implicated as a variable associated with hospitalization [14]. Our cohort had a median BMI of 31; however, while obesity is considered a pro-inflammatory state, in a multivariable analysis, this did not present a significant positive association with mortality.
There are several clinical variables that increase the risk of in-hospital death. Laboratory analysis revealed that elevated lactic acid and procalcitonin levels were risk factors for death. These tests have been described in other investigations as predictors of mortality in COVID-19 patients likely due to relationship of these markers to severe sepsis and secondary bacterial infections [27–29].
Our study also identified renal insufficiency on initial laboratories as a prognosticator for severe illness and mortality. This has also been documented in Wuhan, where the presence of kidney disease was associated with in-hospital mortality. It has been postulated this is multifactorial but could be due to the novel coronavirus using angiotensin converting enzyme 2 (ACE2) receptor for renal cell entry [30, 31]. It is also thought that virus-induced cytokines could cause indirect effects on renal cells via shock or hypoxia [30, 32].
Not surprisingly, there was a strong association between hypoxia with both hospitalization and death. The hospitalized patient was hypoxic at baseline, with median admission pulse oximetry of 92%. Additionally, admission pulse oximetry ≤ 88% was predictive of demise. The use of high flow oxygenation and mechanical ventilation were common interventions employed in 29% and 21% of patients, respectively. Patients receiving invasive mechanical ventilation had poor outcomes with a 71% case fatality rate in this cohort. In other smaller case series, reported mortality associated with invasive ventilation was also dismal with 97% mortality in one cohort of 32 patients and 100% in a 17-patient series [19, 20]. The utilization of invasive mechanical ventilation represents a complicated provider decision based on oxygen saturation, dyspnea, respiratory rate, chest x-ray, and other factors [33]. In our cohort this decision was also likely influenced by factors including guidelines supporting early intubation and cautioning the use of noninvasive mechanical ventilation strategies [34]. Noninvasive strategies such as bilevel positive airway pressure were used in less than 10% of cases due to the potential risk of viral aerosolization [35]. Other countries used non-invasive ventilation treatments more liberally and some research suggests a possible benefit [36]. In a Chinese study of 113 fatalities, only 6% of patients received invasive treatment compared to 37% receiving noninvasive ventilation [20]. It is unclear whether this strategy was employed purposefully or due to limited resources, and the impact of this choice on mortality is unclear. Given the high mortality rates associated with invasive mechanical ventilation, the overall strategy for oxygenation and ventilation deserves additional investigation with full consideration of all modes of oxygen delivery, both invasive and noninvasive ventilation, delayed intubation with permissive hypoxia, positioning maneuvers, among other therapies.
The study has several limitations. Patients are from a single geographic region, treated within a single health system and factors that are associated with hospitalization and oxygen requirements could differ in other regions, although the region is relatively diverse. While the health system services a range of urban to suburban populations, the rural community is relatively absent from this cohort. Second, ED provider discretion regarding need for admission created potential variability in admission characteristics of patients. Third, data from the EHR were occasionally incomplete. Finally, the case fatality rate likely underrepresents true mortality as transfers out of the health system often needed intensive care resources and ICU admission [19, 37]. Further, we quantified in-hospital mortality through the designated follow-up date of April 23rd. We recently queried clinical status for all patients and found that an additional 17 patients who were discharged home or to a skilled nursing facility had also expired.
This study represents one of the largest cohort analyses in a region considered an epicenter for the COVID-19 pandemic. Older age, low admission saturation ≤ 88%, elevated respiratory rate, and initial laboratory derangements including renal and hepatic injury, and elevated procalcitonin and lactic acid levels were the most significant risk factors for in-hospital death. These factors, in addition to our scoring system, can help clinicians prognosticate and should be considered in management strategies.
Compliance with ethical standards
Conflict of interest
This manuscript in part or in full has not been submitted or published anywhere. No authors have any relevant conflict of interest disclosures.
Statement of human and animal rights
The study was approved by the Institutional Review Board at the home institution.
Informed consent
Written informed consent requirement was waived due to the rapid emergence of COVID-19.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zhu N, Zhang D, Wang W, et al. China Novel Coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polosa R, Spinicci M, Prisco D. COVID-19: diagnosis, management and prognosis: a new topical collection of internal and emergency medicine. Intern Emerg Med. 2020;15:747–750. doi: 10.1007/s11739-020-02461-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (2020) Novel coronavirus—China. January 12, 2020 . Geneva: World Health Organization. https://www.who.int/csr/don/12-january-2020-novel-coronavirus-china/en/. Accessed 30 Apr 2020
- 4.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Holshue ML, DeBolt C, Lindquist S, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382:929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roser M, Ritchie H, Ortiz-Ospina E, Hasell J (2020) Coronavirus Disease (COVID-19)—statistics and research. Our World in Data 2020. https://ourworldindata.org/coronavirus. Accessed 30 Apr 2020
- 7.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan. China JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feng Y, Ling Y, Bai T, et al. COVID-19 with different severity: a multi-center study of clinical features. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202002-0445OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jin Y, Cai L, Chen Z, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version) Mil Med Res. 2020;7:4. doi: 10.1186/s40779-020-0233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan JF, Yuan S, Kok KH, et al. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395:514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrilli CM, Jones SA, Yang J, et al. Factors associated with hospitalization and critical illness among 4,103 patients with COVID-19 disease in New York City. BMJ. 2020 doi: 10.1101/2020.04.08.20057794. [DOI] [Google Scholar]
- 15.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Prac. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. J Am Coll Cardiol. 2012;60:1581–1598. doi: 10.1016/j.jacc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 17.Gul F, Arslantas MK, Cinel I, et al. Changing definitions of sepsis. Turk J Anaesthesiol Reanim. 2017;45(3):129–138. doi: 10.5152/TJAR.2017.93753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smina M, Salam A, Khamiees M, Gada P, Amoateng-Adjepong Y, Manthous CA. Cough peak flows and extubation outcomes. Chest. 2003;124(1):262–268. doi: 10.1378/chest.124.1.262. [DOI] [PubMed] [Google Scholar]
- 19.Zhou F, Ting Y, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T, Wu D, Chen H, et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: a retrospective study. BMJ. 2020;368:m1091. doi: 10.1136/bmj.m1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grasselli G, Zangrillo A, Zanella A, et al. COVID-19 Lombardy ICU Network. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region. Italy. JAMA. 2020;323(26):1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman M, Kleine-Weber H, Schroeder S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel SK, Velkoska E, Burrell LM. Emerging markers in cardiovascular disease: where does angiotension-converting enzyme 2 fit in? Clin Exp Pharmacol Physiol. 2013;40(8):551–559. doi: 10.1111/1440-1681.12069. [DOI] [PubMed] [Google Scholar]
- 24.Channappanavar R, Fett C, Mack M, Ten Eyck PP, Meyerholz DK, Perlman S. Sex-based differences in susceptibility to SARS-CoV infection. J Immunol. 2017;198(10):4046–4053. doi: 10.4049/jimmunol.1601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Team CC-R Preliminary estimates of the prevalence of selected underlying health conditions among patients with Coronavirus Disease 2019—United States, February 12-March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382–386. doi: 10.15585/mmwr.mm6913e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.COVID-19 Surveillance Group (2020) Characteristics of COVID-19 patients dying in Italy: report based on available data on March 20th, 2020. Rome, Italy: Instituto SuperioreDiSanita; 2020. https://www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_20_marzo_eng.pdf. Accessed 20 Mar 2020
- 27.Peng YD, Meng K, Guan HQ, et al. Clinical characteristics and outcomes of 112 cardiovascular patients infected by 2019-nCoV. Zhonghua Xin Xue Guan Bing Za Zhi. 2020;48:E004. doi: 10.3760/cma.j.cn112148-20200220-00105. [DOI] [PubMed] [Google Scholar]
- 28.Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: A systematic literature review and meta-analysis [published online ahead of print, 2020 Apr 23] J Infect. 2020 doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuetz P, Birkhahn R, Sherwin R, et al. Serial Procalcitonin Predicts Mortality in Severe Sepsis Patients: Results From the Multicenter Procalcitonin MOnitoring SEpsis (MOSES) Study. Crit Care Med. 2017;45(5):781–789. doi: 10.1097/CCM.0000000000002321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng Y, Luo R, Wang K, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Albini A, Di Guardo G, Noonan DM, et al. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern Emerg Med. 2020;15:759–766. doi: 10.1007/s11739-020-02364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Durvasula R, Wellington T, McNamara E, Watnick S. Covid-19 and kidney failure in the acute care setting: our experience from Seattle. AJKD. 2020 doi: 10.1053/j.ajkd.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tobin MJ. Basing respiratory management of coronavirus on physiological principles. Am J Respir Crit Care Med. 2020 doi: 10.1164/rccm.202004-1076ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.WHO (2020) Interim guidance: Clinical management of severe acute respiratory infection (SARI) when COVID-19 disease is suspected. Geneva: World Health Organization. https://www.who.int/publications-detail/clinical-management-of-severe-acute-respiratory-infection-when-novel-coronavirus-(ncov)-infection-is-suspected. Accessed 20 Mar 2020
- 35.Yu IT, Xie ZH, Tsoi KK, et al. Why did outbreaks of severe acute respiratory syndrome occur in some hospital wards but not in others? Clin Infect Dis. 2007;44(8):1017–1025. doi: 10.1086/512819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Privitera D, Angaroni L, Capsoni N, et al. Flowchart for non-invasive ventilation support in COVID-19 patients from a northern Italy Emergency Department. Intern Emerg Med. 2020;15:767–771. doi: 10.1007/s11739-020-02370-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatraju PK, Ghassemieh BJ, Nichols M, et al. Covid-19 in Critically Ill Patients in the Seattle Region- Case Series. N Engl J Med. 2020 doi: 10.1056/NEJMoa2004500. [DOI] [PMC free article] [PubMed] [Google Scholar]