Abstract
In this work, two types of residues (industrial fruit byproducts and agricultural wastes) were studies as promising adsorbents for cadmium uptake. Adsorption experiments using the evaluated biomasses (corn crops CC, palm bagasse PB, orange peels OP, and lemon peels LP) were conducted in batch mode by varying initial solution pH (2, 4, and 6) as well as the particle size (0.355, 0.5, and 1 mm). The optimum operating conditions were defined for further adsorption tests. The biomasses were chemically modified with alumina nanoparticles to evaluate the enhancement in adsorption capacities and how the nature of biomass contributes to successful incorporation of nanotechnology-based materials. The point of zero charges was ranged between 4 and 5 for all biomasses. Simultaneously, the Böehm titration method confirmed the presence of lactonic and carboxylic acid groups on the surfaces of the biomasses. Optimum operating conditions for batch cadmium adsorption experiments were observed at pH 6. Moreover, no significant changes were detected as a function of biomass size. For corn cob and lemon peels, removal percentages at 86 and 88% were reached using particle size = 0.5 mm. For palm bagasse and orange peels, the optimum parameters were 0.355 and 1 mm, respectively. Al2O3 nanoparticles with a crystal size of 58 ± 12 nm were obtained by applying the sol–gel methodology. A higher cadmium removal percentage was detected after using the biomasses modified with the Al2O3 nanoparticles, determining for the agricultural wastes an adsorption capacity of 91% (CC-Al2O3) and 92% (PB-Al2O3). In comparison, the industrial fruit byproducts exhibited a removal percentage of 93% (LP-Al2O3) and 96% (OP-Al2O3). The modification of industrial fruit byproducts (lemon peels and orange peels) showed increases in adsorption efficiencies around 12–6% after incorporating alumina nanoparticles, suggesting that this type of biomass is more suitable for adsorption property enhancement using nanomaterials.
1. Introduction
The presence of cadmium ions in aquatic ecosystems is associated with human activities such as mining, cement preparation, battery production, and electroplating.1 To drink contaminated water with cadmium ions (concentrations above 0.003 mg/L) may cause hypertension, cancer, damage of the peripheral nervous system, and dysfunction of kidneys and lung.2 The adverse consequences of poisoning have motivated the search for treatment alternatives that significantly reduce the content of cadmium in industrial wastewater before its emissions into the environment.3 Among the technologies used for this application, the biosorption of heavy metal ions is gaining scientific attention because it reaches high efficiencies depending on the type of the adsorbent.4,5
Despite the diversity of materials tested in batch adsorption experiments with promising results, there is still lack of cost-effective adsorbents that can remove most heavy metal ions from an aqueous solution but reduce the costs compared to conventional materials. To fulfill this need, recent work attempts to develop materials with high adsorption capacities such as a nanocomposite that is derived from incorporating nanoparticles into polymeric and biopolymeric matrices. As Awual et al.6 stated, these nanoscale materials offer several advantages such as pore geometry, shape, and particle morphology of the support carriers. For example, the adsorbent prepared by Saha et al.7 using alumina nanoparticles, chitosan, and polyacrylamide. They synthesized this material using reverse microemulsion, immobilization, and grafting via in situ dispersion. Its adsorption capacity was found to be 6.56 mg/g, which reveals the increase of adsorption properties for alumina nanoparticles after modification.
The high cost of nanoscale materials is balanced by economic support carriers such as biomasses from agricultural or industrial wastes. Previous contributions have shown the presence of functional groups in these biomasses that are responsible for the efficient removal of heavy metal ions.8 Their wide availability in agricultural regions is another strong motivation to be used as a biopolymeric matrix in nanocomposites.9 The type of biomasses may also affect the effectiveness of nanomaterial incorporation and the adsorption properties. They have been classified into woody (e.g., forestry residues), agricultural residues (e.g., husks, stalks, and stover), industrial byproducts (fruit skins and seeds), nonwood (grass), waste products, and marine.10 For industrial byproducts, lemon and orange peels (OP) are extensively studied due to the large volume of waste generation of fruit industries worldwide.11 Bhatnagar et al.12 used lemon peels (LP) for the adsorption of cobalt ions, reporting an adsorption capacity of 22.0 mg/g, which was higher compared to the natural zeolites and modified silica gel. Lasheen et al.13 modified the orange peels for the treatment of Cd(II), Cu(II), and Pb(II) ions. They contrasted the adsorption performance of modified and unmodified biomasses, achieving adsorption capacities of 11.2 and 6.94 mg/g, respectively.
Regarding the agricultural residues, contributions address the application of these type of biomasses for wastewater treatment. Kurniawan et al.14 analyzed the physicochemical properties of cassava peels for nickel uptake, showing an adsorption capacity of around 57 mg/g at pH = 4.5. The sugarcane was also selected as a biosorbent after chemical modifications with NaOH.15 Campos et al.16 prepared activated carbon from corn cob (CC) to evaluate its effectiveness during competitive adsorption of copper and nickel ions. They reached capacities at 0.39 and 0.28 mmol/g for Cu2+ and Ni2+, respectively. Due to the need for improving efficiencies of agrowastes and industrial byproducts, recent studies have been focused on incorporating nanomaterials for adsorbent synthesis; however, limited contributions are found in the literature about the comparison of the type of biomasses and its effects on the nanocomposite synthesis and performance during heavy metal removal.
This work contributes to the current body of knowledge about selecting biomasses for adsorbent synthesis. Among the wide variety of biomasses, we can screen options based on the type of biomass and the synthesis of nanocomposites will follow reported improvements for such type. This work aimed at evaluating how two different types of biomass: agricultural residues (oil palm bagasse PB, and corn cob) and industrial byproducts (lemon and orange peels) may influence the properties of nanocomposites modified with alumina nanoparticles. The novelty of this work lies in the comparative analysis of biomass characteristics depending on its source to be supporters of nanoparticles during the synthesis of biosorbents. Most of the papers available in the literature are focused on testing the applicability of any material to uptake heavy metals, and the selection criteria for the biomasses follows an indeed revision of previous work. Due to the lack of comparative studies, the comparison of adsorption properties among published papers is not standardized with same operating conditions. In this sense, there would not be a fair selection depending on the adsorption yields since they are influenced by the operating conditions. With this work, researchers will be able to identify which types of biomass can achieve better results in modifications with nanoparticles and thus select the most promising one for a complete adsorption study.
2. Materials and Methods
2.1. Adsorbent Synthesis
The selected procedure for the preparation of adsorbents included the following steps: (i) biomass preparation, (ii) nanoparticles synthesis, and (iii) incorporation of alumina nanoparticles in biomass sample.
2.1.1. Biomass Preparation
The agricultural residues of African oil palm and corn crops were provided by a plant for crude oil production17 and a corn farm located in North-Colombia, respectively. The industrial fruit byproducts were collected from local markets with high production of lemon and orange fruits. These biomasses were washed thoroughly with water to remove impurities and subjected to sun drying. Then, they were dried in an oven at 60 °C and cut into small pieces. The particle size was adjusted to 0.355, 0.5, and 1 mm through grinding and sieve-meshing.
2.1.2. Synthesis of Nanoparticles
The Al2O3 nanoparticles were obtained according to the methodology of the sol–gel synthesis described by Herrera-Barros et al. The precursor of these nanoparticles was a 0.5 M aluminum nitrate [Al (NO3)3] solution, which was mixed with a 0.5 M citric acid solution under stirring at 60 °C. The appearance of a yellow color in the resulting mixture indicated the increasing temperature until gel formation. The gel was then sent to a muffle for 2 h and 200 °C to collect the powder with a crystal structure.18,19 Afterward, the Al2O3 nanoparticles were calcined at 1000 °C to obtain the stable α-Al2O3 phase.
2.1.3. Incorporation of Alumina Nanoparticles
The biomasses were modified with the alumina nanoparticles by mixing a suspension of 0.5 g of treated biomaterial in 20 mL of the organic solvent dimethylsulfoxide (DMSO). This mixture was stirred for 24 h at 120 rpm. Then, 3 mL of tetraethyl orthosilicate (TEOS) was added to the reaction media.20 The stirring was continued for 48 h at room temperature and 120 rpm. Afterward, 0.2 g of alumina nanoparticles were added and kept under stirring for 12 h. Finally, the resulting bioadsorbent modified with the Al2O3 nanoparticles was washed with ethanol, filtered under vacuum, and dried.21
2.2. Characterization Techniques
The lemon peels (LP), orange peels (OP), corn cob (CC), and palm bagasse (PB) were characterized using a point of zero charges (pHPZC) and Böehm titration to study the surface functionality of biomass. The modified materials with alumina nanoparticles were analyzed via Fourier-transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM) coupled with energy-dispersive X-ray spectroscopy (EDS), and X-ray diffraction (XRD).
The point of zero charge (pHPZC) relates the optimum pH conditions for further batch adsorption experiments, taking advantage of the negative and positive charge on the adsorbent surface.22 The procedure for determining the pHPZC is shown in Figure 1; distilled water was first pH-adjusted, and then the evaluated biomass was added. After 48 h of stirring, the final pH was measured and used to build a plot of initial vs final pH values.
Figure 1.
Experimental procedure for measuring the point of zero charges of the evaluated biomasses.
Böehm titration is used to identify the functional oxygen groups present on the surface of the evaluated materials, which can be involved in the adsorption of heavy metals. For this procedure, sodium hydroxide was used as a strong base to neutralize all phenolic groups, sodium carbonate was employed to neutralize carboxylic and lactonic groups, while sodium bicarbonate was used to neutralize carboxylic acids.23 Experimental steps for the Böehm titration follow previous studies.24
The X-ray diffraction technique was employed to determine the crystal size of the Al2O3 nanoparticles using the Scherrer equation (eq 1) and an X-Pert ProAnalytical diffractometer
| 1 |
where θ is the Bragg diffraction angle and corresponds to the angle of the peak at the maximum point, β is the width of the peak at the half of the maximum intensity, λ is the wavelength of the radiation emitted by the equipment (1.54 Å for radiation of Cu Kα), and D represents the diameter of the particle.
An IR Affinity-Shimadzu, model A213749 SN was used to determine the chemical groups present in the biomasses functionalized with the Al2O3 nanoparticles by cross-linking with the siloxane groups from the TEOS molecules. Measurements were recorded at the wavelength ranging from 4000 to 600 cm–1.
The modified biomasses were analyzed by SEM and XRD techniques to determine the morphology and elemental composition of the biosorbents after alumina nanoparticle incorporation. For this purpose, an EOL JSM-6490LV microscope was used coupled to an EDS recorded at quantification limits from 1000 μm to 50 nm and magnification limits from X30 to X100.000, following the ASTM E1508-12 and ASTM E766-14 methods.25
2.3. Adsorption Experiments
Adsorption experiments were carried out in a batch system as a function of pH (2, 4, and 6) and biomass particle size (0.355, 0.5, and 1.0 mm). The selection of levels of the treatments in the experimental design was based on the results achieved by authors in previous studies for similar agricultural and fruit peel biomasses adsorbing nickel ions.19,26 First, the unmodified biomasses were analyzed to determine the optimum operating conditions for the biosorbent particle size and pH. Then, the adsorption capacity of the biomasses modified with the Al2O3 nanoparticles was evaluated. All experiments were performed by duplicate. In a typical procedure, an aqueous solution of cadmium(II) ions was prepared at 100 ppm using cadmium sulfate (CdSO4). Afterward, pH was adjusted to 2, 4, and 6, employing 2 M solutions of hydrochloric acid and sodium hydroxide. Then, three aliquots of 100 mL were collected from each solution and mixed with 0.5 g of the unmodified biomasses with three different particle sizes (0.355, 0.5, and 1 mm). In total, 36 samples were collected from adsorption experiments, taking into account four biomasses (palm bagasse PB, corn cob CC, orange peels OP, and lemon peels LP). The resulting mixtures of synthetic wastewater and the biomasses were kept under continuous stirring for 2 h at room temperature. Once the adsorption process was completed, 10 mL of the supernatant was collected and analyzed. Cadmium adsorption was detected using an ICE 3000 Atomic spectrometer. The removal yield and adsorption capacity were quantified using eqs 2 and 3, respectively
| 2 |
| 3 |
where C0 (mg/L) and Ce (mg/L) are the initial and remaining concentrations of heavy metal ions in an aqueous solution, respectively, V is the solution volume, and m is the amount of adsorbent.
3. Results and Discussion
3.1. Biomass Characterization
The point of zero charges ranged between 4.0 and 4.80 for the biomasses. As this property was below 7.0 (corresponding to a neutral surface), the agricultural and industrial byproduct wastes showed an acidic surface. The lowest pHPZC (4.06) was reached by the orange peels, indicating that they contain more acidic groups than basic groups, and consequently, the surface is more acidic. The lemon peels and corn cob reported similar pHPZC (4.75 and 4.79, respectively), while the palm bagasse reached the second lowest value (4.29). This property plays an important role in selecting optimum pH conditions, for all of these biomasses, it is recommended to work at pH values greater than pHPZC to increase adsorption yields. This is explained by the availability of more functional groups like carboxyl and hydroxyl and the biomasses are thus suitable for adsorption of cationic ions.27,28 No trends were observed for the fruit peels and agricultural waste types, and consequently, no generalized assertations can be made about the tendency of citrus fruit wastes toward high or low pH environments. These results were also compared with previous contributions using the same biomasses. For lemon peels, Singh et al.27 reported a pHPZC at 4.45, while Pathak et al.28 attained 4.10 for orange peels. Panumati et al.29 also measured the point of zero charge for palm bagasse with a value at 4.2. According to the above discussion, the pHPZC of palm bagasse and orange peels was quite similar to the values found in the literature; however, the corn cob showed a deviation from the work performed by Berber-Villamar et al.30 with a property value at 6.83. This can be attributed to the varieties of corn and the conditions in which they are grown.
Figure 2 shows the amount of phenols, lactones, and carboxylic acid groups found in the evaluated biomasses via titration. The lactone groups showed the highest peaks for agricultural wastes (corn cob and palm bagasse) with 18 890 μmole, suggesting a similar trend for different sources ascribed to this types of biomass. The carboxylic acids appeared in a range between 2362 and 3306 μmole, where corn cob reported a greater value than palm bagasse. As stated by Shafeeyan et al.,31 this presence of functional groups of carboxylic acid and lactones is a source of surface acidity, which is consistent with the results of point of zero charges. The absence of phenolic groups in all of the biomasses could refer to the lack of lactone hydrolysis, which produces some of the phenols normally available in lignocellulosic materials.32 Uner et al.33 prepared activated carbons from watermelon residues and similar consistency between point of zero charges and Böehm titration showed a predominance of acidic groups.33 At neutral or basic environments for adsorption, it is expected that the fruit peels and agricultural wastes are propellant to uptake metallic ions.34
Figure 2.
Böehm titration results.
The adsorption process can be increased after using materials with a high surface area. Nanomaterials are characterized by having a high surface area due to their small size. Thus, the incorporation of metal oxide nanoparticles onto a biomass surface can enhance its adsorption effectiveness, increasing the surface area of the biosorbent, while providing additional active sites to attach metal cations, such as cadmium(II) present in aqueous media. In this work, the crystal size of alumina nanoparticles was determined using the X-ray diffraction pattern displayed in Figure 3 and the Scherrer equation (eq 1).
Figure 3.

X-ray diffraction of alumina nanoparticles calcined at 1000 °C.
The intensity of the peaks observed in Figure 3 is an indicative of the formation of alumina nanoparticles with the α-Al2O3 phase, displaying representative peaks at the angles of 25.56, 35.12, 43.31, 52.5, 57.45, 66.46, and 68.15°:10 A crystal size of 58 ± 12 nm was estimated for the synthesized Al2O3 nanoparticles after the thermal treatment at 1000 °C, which compares very well with the size reported in the literature for the synthesis of nanomaterials by the sol–gel methodology. An average surface area (SAave) of about 2.6 m2/g was estimated for the synthesized alumina nanoparticles, assuming a spherical shape and relating the total area of the nanoparticles with its mass (SAAve = 6/ρD), taking into account a density (ρ) of 3950 kg/m3 for alumina nanoparticles. This surface area compares very well with the value of 6.0 m2/g reported by Trung-Nguyen and co-workers for α-Al2O3 nanoparticles with a size of 40 nm.35
Al2O3 nanoparticles were grafted onto the biomass’s surfaces using TEOS, which led to the formation of siloxane Si–O–Si groups in the presence of DMSO, as the solvent media.13 FTIR spectroscopy was used to identify the functional groups present in the biomasses and the chemical modification with the metal oxide nanoparticles, which can promote the uptake of cadmium ions during the adsorption process. Figure 4 shows the FTIR spectra obtained for the biomasses after the incorporation of the Al2O3 nanoparticles. From these measurements, it was observed that all biomasses exhibited peaks at 1700, 1650, and 1040 cm–1, related to the stretching bands of the functional groups C=O, C=C, and C–OH, present in cellulosic materials. Moreover, all samples displayed peaks around 1550, 1100, and 940 cm–1, which can be attributed to the chemical groups Al–C=O, Si–O–Si, and Al–O–Al, confirming the grafting of the nanoparticles onto the biomass surfaces.36,37
Figure 4.

FTIR spectra of biomasses after incorporation of Al2O3 nanoparticles (a) palm bagasse, (b) corn cob, (c) orange peels, and (d) lemon peels.
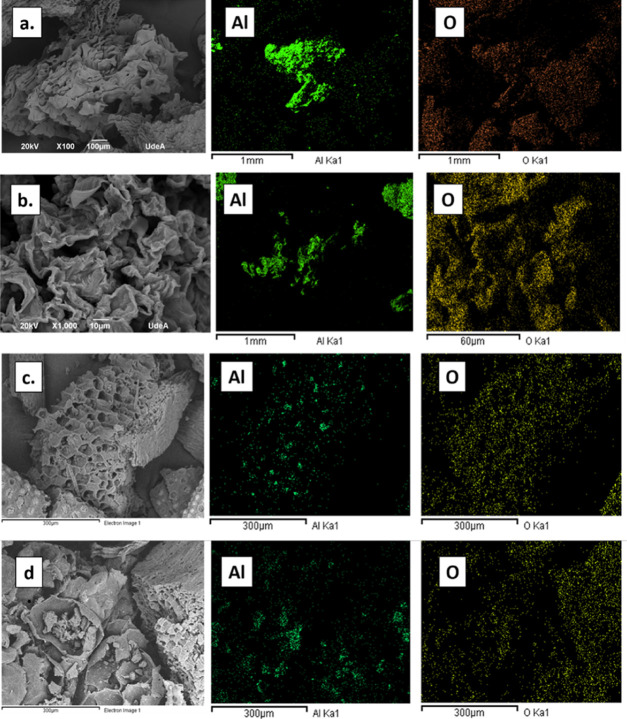
As shown in the micrographs present in Figure 5, all biomasses exhibit an amorphous and porous structure, characteristic of cellulosic materials. Alumina nanoparticles are dispersed on the biomasses surface, forming small agglomerates, as observed from the EDS mapping images, highlighting the presence of the aluminum and oxygen atoms, constituents of this metal oxide nanomaterial. Besides, Table 1 summarizes the weight percentage composition, evidencing for almost all samples the presence of the silicon atom, as previously observed from FTIR analysis. The irregular geometry of the lemon peels with alumina nanoparticles is also observed in the micrograph of unmodified biomass reported by Villen-Guzman et al.38 The modified orange peels showed a more amorphous appearance than for lemon peels, which may cause an increase in adsorption capacities for heavy metal uptake. A more porous structure was observed for agricultural wastes modified with the nanoparticles compared to the fruit peels.
Figure 5.
SEM micrographs and EDS mapping for biomasses chemically modified with Al2O3 nanoparticles: (a) lemon peels, (b) orange peels, (c) palm bagasse, and (d) corn cob.
Table 1. EDS Analysis for Biomasses Functionalized with Alumina Nanoparticles.
| weight
% |
||||
|---|---|---|---|---|
| elements | lemon peels-Al2O3 | orange peels-Al2O3 | palm bagasse-Al2O3 | corn cob-Al2O3 |
| C | 47.57 | 50.66 | 46.92 | 44.01 |
| O | 45.99 | 40.80 | 48.32 | 47.31 |
| Al | 4.67 | 7.52 | 2.27 | 7.0 |
| Si | 0.16 | 2.48 | 1.68 | |
3.2. Batch Adsorption Experiments
3.2.1. Selection of Optimum Operating Conditions
The batch adsorption experiments allowed us to obtain optimum operating conditions for the selected biomasses before modification with alumina nanoparticles. The adsorption process showed to be highly pH-dependent, according to Figure 6. The adsorption capacities reached the highest values when the pH of the solution was adjusted to 6. These results were expected as this pH environment is above the point of zero charges for all of the biomasses. The dependence between heavy metals uptake and the pH conditions is derived from the production of cationic and anionic forms of cadmium ions in the presence of water as described below39
Similar adsorption favorability toward pH around 6 was found in previous contributions. Schiewer et al.40 studied the equilibrium during heavy metal adsorption with pH-sensitive isotherms using different citrus peels. They observed higher adsorption capacities for pH = 5 than for pH = 2 attributed to the presence of hydronium ions, leading to a global positive charge on the biomass surface.41 Mahato et al.42 also reviewed several studies about the biosorption of metals, dyes, and emerging contaminants using citrus processing wastes. They found a strong tendency for pH ranged 5–6 for the fruit peels when binding metals like cobalt, cadmium, and nickel.42
Figure 6.
Influence of the solution pH on adsorption performance.
Considering Figure 7, the analysis provided a nondetermining trend to establish which particle size was the most favorable; the smallest size (0.355 mm) was chosen for further experiments with modified biomasses, based on the fact that this size could provide a larger surface area and, in theory, can be the most favorable for the metal ion uptake process. These results were compared with those available in the literature and are summarized in Table 2. The removal yield of corn is approximately 7% lower than the corn cob biomass employed in this work at similar pH environments. The grapefruit power reached similar values than orange peels at lower pH conditions and particle sizes. The fact that higher adsorption yields are observed for the agricultural and fruit peel biomasses compared to other biosorbents, even the commercial activated carbon suggests possible promising results when modifying these materials with alumina nanoparticles.
Figure 7.
Effect of the particle size on adsorption performance.
Table 2. Optimum Operating Conditions of Cadmium Adsorption Using Agricultural and Fruit Biomasses.
| biomass type | biomass | removal yield (%) | pH | particle size (mm) | initial concentration (ppm) | dosage (g/L) | temperature (°C) | reference |
|---|---|---|---|---|---|---|---|---|
| agricultural | corn power | 79.36 | 6.5 | 0.105 | 25 | 10 | 25 | (43) |
| activated carbon | 83.00 | 5.7 | 20 | 2.5 | (44) | |||
| fruit peel | grapefruit power | 85.94 | 4.5 | 0.25 | 100 | 5 | 25 | (45) |
| agricultural | corn cob | 86.00 | 6 | 0.50 | 100 | 5 | 25 | this work |
| agricultural | palm bagasse | 87.00 | 6 | 0.355 | 100 | 5 | 25 | this work |
| fruit peel | lemon peels | 88.00 | 6 | 0.50 | 100 | 5 | 25 | this work |
| fruit peel | orange peels | 85.00 | 6 | 1.00 | 100 | 5 | 25 | this work |
3.2.2. Comparative Analysis of Biomass Modifications with Alumina Nanoparticles
After selecting the optimum operating conditions, the batch adsorption experiments were conducted for the modified biomasses to quantify the enhancement provided by nanocompounds. Figure 8 shows the adsorption yields reached by the modified biomass with alumina nanoparticles, as well as the increase in efficiencies when incorporating the nanocompounds. The highest removal yield was obtained by the orange peel-Al2O3 adsorbent (OP-Al), followed by the lemon peel-Al2O3 adsorbent (LP-Al). The agricultural residues of palm bagasse and corn cob showed the lowest removal yields after biomass modification (PB-Al and CC-Al), which suggested that industrial fruit byproducts are more suitable in the enhancement of adsorption properties with nanoparticles. An increase in the removal yields of 12% was observed for OP-Al compared with the unmodified orange peels. The BP-Al and CC-Al showed the lowest increases (5.77 and 5.88%, respectively), confirming an insignificant contribution of alumina nanoparticles in the biomass performance.
Figure 8.
Adsorption performance of modified biomasses.
The adsorption capacity of these modified materials was calculated using eq 3 and compared with the values found in the literature for different adsorbents. As summarized in Table 3, the selected biomasses with alumina nanoparticles have higher capacities to uptake cationic ions compared to magnetic nanoparticles or funcionalized silica. As the modified orange and lemon peel biomasses showed more promising adsorption properties, it can be stated that fruit peels are more suitable for modification with nanoparticles instead of some lignocellulosic wastes to prepare an efficient adsorbent.
Table 3. Comparison of Adsorption Capacities of Modified Biomasses with Previous Studies.
| adsorbent | heavy metal | maximum adsorption capacity (mg/g) | reference |
|---|---|---|---|
| thiol-functionalized silica | Cd(II) | 13.87 | (46) |
| Fe3O4@SiO2 | Cu(II) | 12.71 | (47) |
| nanosized TiO2 | Cr(VI) | 12.60 | (48) |
| SiO2-corn cob | Cr(VI) | 90.01 | (49) |
| magnetite-corn cob silica | Cr(VI) | 11.10 | (50) |
| SiO-orange peels | Pb(II) | 200.0 | (51) |
| magnetite-orange peels | As(III) | 10.30 | (52) |
| Fe3O4 nanoparticles | Cu(II) | 14.50 | (53) |
| OP-Al2O3 nanoparticles | Cd(II) | 19.12 | this work |
| LP-Al2O3 nanoparticles | Cd(II) | 18.66 | this work |
| PB-Al2O3 nanoparticles | Cd(II) | 18.40 | this work |
| CC-Al2O3 nanoparticles | Cd(II) | 18.20 | this work |
A possible adsorption mechanism for biomasses modified with alumina nanoparticles refers to the site-binding theory, where electrolyte ion adsorption of cadmium ions on the nanomaterials takes place.54 For the Cd(II) ions, the adsorption mechanism onto the surface of the modified materials is illustrated in Figure 9. The surface of the alumina nanoparticles hydrolyzed in an aqueous environment, and for pH around 6.0–7.0, the deprotonation of Al–OH surface groups occurs forming Al–O–, which is more attractive for cation binding.55 The OH groups on the biomass surface also offer sites for bonds with cationic ions, as follows25
Figure 9.
Schematic representation of the possible adsorption mechanism of cadmium ions onto modified biomasses.
4. Conclusions
The present work attempted to analyze how the type of biomass either agricultural or fruit peel residues influences the adsorption properties of heavy metals when modifying biomass with alumina nanoparticles. These findings contribute to the scientific community as it provides a comparative study under the same conditions of pH, temperature, and dosage of any increase in adsorption yields when adding nanoparticles to biomasses from different sources. The characterization techniques of unmodified biomasses showed favorable pH environments for adsorption above 4.0–4.8, as this was the range observed for the point of zero charges. The acidic surface of all of the materials was confirmed by the Böehm titration technique, showing a high content of lactones and carboxylic acid groups. The SEM micrographs exhibited an amorphous and a porous structure of modified biomasses, which is attractive for high adsorption efficiencies. The effect of operating conditions such as pH and particle size for the raw materials allowed us to define the optimum conditions to carry out the experiments for modified biomasses (i.e., initial pH = 6). The removal yields increased by 12.47, 6.03, 5.77, and 5.88% for OP, LP, PB, and CC, respectively, when adding alumina nanoparticles to the biomass matrix. The maximum adsorption capacities were observed for orange peel and lemon peel biomass with alumina nanoparticles reaching values of approximately 18–19 mg/g, which suggested that industrial fruit peels are more suitable for this purpose compared to agricultural wastes.
Acknowledgments
The authors express their gratitude to the University of Cartagena for providing financial support to conclude this work.
The authors declare no competing financial interest.
References
- Vilela P. B.; Matias C. A.; Dalalibera A.; Becegato V. A.; Paulino A. T. Polyacrylic acid-based and chitosan-based hydrogels for adsorption of cadmium: Equilibrium isotherm, kinetic and thermodynamic studies. J. Environ. Chem. Eng. 2019, 7, 103327 10.1016/j.jece.2019.103327. [DOI] [Google Scholar]
- Thabede P. M.; Shooto N. D.; Xaba T.; Naidoo E. B. Adsorption studies of toxic cadmium (II) and chromium (VI) ions from aqueous solution by activated black cumin (Nigella sativa) seeds. J. Environ. Chem. Eng. 2020, 8, 104045 10.1016/j.jece.2020.104045. [DOI] [Google Scholar]
- Bulgariu L.; Bulgariu D. Functionalized soy waste biomass - A novel environmental-friendly biosorbent for the removal of heavy metals from aqueous solution. J Cleaner Prod. 2018, 197, 875–885. 10.1016/j.jclepro.2018.06.261. [DOI] [Google Scholar]
- Amro A. N.; Abhary M. K.; Shaikh M. M.; Ali S. Removal of lead and cadmium ions from aqueous solution by adsorption on a low-cost Phragmites biomass. Processes 2019, 7, 406 10.3390/pr7070406. [DOI] [Google Scholar]
- Abbar A. H.; Salman R. H.; Abbas A. S. Cadmium removal using a spiral-wound woven wire meshes packed bed rotating cylinder electrode. Environ.Technol. Innovation 2019, 13, 233–243. 10.1016/j.eti.2018.12.005. [DOI] [Google Scholar]
- Awual M. R.; Khraisheh M.; Alharthi N. H.; Luqman M.; Islam A.; Rezaul M.; Rahman M. M.; Khaleque A. Efficient detection and adsorption of cadmium (II) ions using innovative nano-composite materials. Chem. Eng. J. 2018, 343, 118–127. 10.1016/j.cej.2018.02.116. [DOI] [Google Scholar]
- Saha S.; Sarkar P. Arsenic remediation from drinking water by synthesized nano-alumina dispersed in chitosan-grafted polyacrylamide. J. Hazard. Mater. 2012, 227–228, 68–78. 10.1016/j.jhazmat.2012.05.001. [DOI] [PubMed] [Google Scholar]
- Afroze S.; Sen T. K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water, Air, Soil Pollut. 2018, 229, 225 10.1007/s11270-018-3869-z. [DOI] [Google Scholar]
- Herrera-Barros A.; Tejada-Tovar C.; Villabona-Ortíz Á.; González-Delgado Á.; Reyes-Ramos A. Adsorption study of Ni (II) and Pb (II) onto low-cost agricultural biomasses chemically modified with TiO2 nanoparticles. Indian J. Sci. Technol. 2018, 11, 1–9. 10.17485/ijst/2018/v11i21/123248. [DOI] [Google Scholar]
- Brown R.; Wang K.. Fast Pyrolysis of Biomass: Advances in Science and Technology; Royal Society of Chemistry, 2017. [Google Scholar]
- Romero-Cano L. A.; García-Rosero H.; Gonzalez-Gutierrez L. V.; Baldenegro-Pérez L. A.; Carrasco-Marín F. Functionalized adsorbents prepared from fruit peels: Equilibrium, kinetic and thermodynamic studies for copper adsorption in aqueous solution. J. Cleaner Prod. 2017, 162, 195–204. 10.1016/j.jclepro.2017.06.032. [DOI] [Google Scholar]
- Bhatnagar A.; Minocha A.; Sillanpää M. Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem. Eng. J. 2010, 48, 181–186. 10.1016/j.bej.2009.10.005. [DOI] [Google Scholar]
- Lasheen M. R.; Ammar N.; Ibrahim H. Adsorption/desorption of Cd(II), Cu(II) and Pb(II) using chemically modified orange peel: Equilibrium and kinetic studies. Solid State Sci. 2012, 14, 202–210. 10.1016/j.solidstatesciences.2011.11.029. [DOI] [Google Scholar]
- Kurniawan A.; Natasia A.; Febrianto J.; Ju Y.; Sunarso J.; Indraswati N.; Ismadji S. Evaluation of cassava peel waste as lowcost biosorbent for Ni-sorption: Equilibrium, kinetics, thermodynamics and mechanism. Chem. Eng. J. 2011, 172, 158–166. 10.1016/j.cej.2011.05.083. [DOI] [Google Scholar]
- de Oliveira A. V. B.; Rizzato T. M.; Barros B. C. B.; Favaro S. L.; Caetano W.; Hioka N.; Batistela V. R. Physicochemical modifications of sugarcane and cassava agro-industrial wastes for applications as biosorbents. Bioresour. Technol. Rep. 2019, 7, 100294 10.1016/j.biteb.2019.100294. [DOI] [Google Scholar]
- Campos N. F.; Guedes G. A. J. C.; Oliveira L. P. S.; Gama B. M. V.; Sales D. C. S.; Rodríguez-díaz J. M.; Barbosa C. M. B. M.; Duarte M. M. M. B. Competitive adsorption between Cu2+ and Ni2+ on corn cob activated carbon and the difference of thermal effects on mono and bicomponent systems. J. Environ. Chem. Eng. 2020, 8, 104232 10.1016/j.jece.2020.104232. [DOI] [Google Scholar]
- Moreno-Sader K.; Alarcón-Suesca C.; González-Delgado Á.-D. Application of environmental and hazard assessment methodologies towards the sustainable production of crude palm oil in North-Colombia. Sustainable Chem. Pharm. 2020, 15, 100221 10.1016/j.scp.2020.100221. [DOI] [Google Scholar]
- Li J.; Pan Y.; Xiang C.; Ge Q.; Guo J. Low temperature synthesis of ultrafine a-Al2O3 powder by a simple aqueous sol–gel process. Ceram. Int. 2006, 32, 587–591. 10.1016/j.ceramint.2005.04.015. [DOI] [Google Scholar]
- Herrera-Barros A.; Tejada-Tovar C.; Villabona-Ortíz A.; González-Delgado A. D.; Alvarez-Calderon J. Adsorption of Nickel and Cadmium by Corn Cob Biomass Chemically Modified with Alumina Nanoparticles. Indian J. Sci. Technol. 2018, 11, 1–11. 10.17485/ijst/2018/v11i22/126125. [DOI] [Google Scholar]
- Sanchez-Valente J.; Bokhimi X.; Toledo J. Synthesis and catalytic properties of nanotructured aluminas obtained by the sol-gel method. Appl. Catal., A 2004, 264, 175–181. 10.1016/j.apcata.2003.12.041. [DOI] [Google Scholar]
- Herrera A. P.; Barrera C.; Rinaldi C. Synthesis and functionalization of magnetite nanoparticles with aminopropylsilane and carboxymethyldextran. J. Mater. Chem. 2008, 18, 3650–3654. 10.1039/b805256e. [DOI] [Google Scholar]
- Martin M.Caracterización y aplicación de biomasa residual a la eliminación de metales pesados; Universidad de Granada, 2008. [Google Scholar]
- Goertzen S. L.; The′riault K. D.; Oickle A. M.; Tarasuk A. C.; Andreas H. A. Standardization of the Boehm titration. Part I. CO2 expulsion and endpoint determination. Carbon 2010, 48, 1252–1261. 10.1016/j.carbon.2009.11.050. [DOI] [Google Scholar]
- Herrera-Barros A.; Tejada-Tovar C.; Villabona-Ortíz A.; Gonzalez-Delgado Á.; Mejia-Meza R. Assessment of the Effect of Al2O3 and TiO2 Nanoparticles on Orange Peel Biomass and its Application for Cd (II) and Ni (II) Uptake. Trans. ASABE 2019, 62, 139–147. 10.13031/trans.12966. [DOI] [Google Scholar]
- Herrera-Barros A.; Tejada-Tovar C.; Villabona-Ortíz A.; Gonzalez-Delgado A. D.; Benitez-Monroy J. Cd (II) and Ni (II) uptake by novel biosorbent prepared from oil palm residual biomass and Al2O3 nanoparticles. Sustainable Chem. Pharm. 2020, 15, 100216 10.1016/j.scp.2020.100216. [DOI] [Google Scholar]
- Tejada-Tovar C.; Gallo-Mercado J.; Moscote J.; Villabona A.; Acevedo-Correa D. Competitive adsorption of lead and nickel onto yam husk and palm bagasse in continuous system. Biotecnol. Sect. Agropecu. Agroind. 2018, 16, 52–61. 10.18684/BSAA(16)52-61. [DOI] [Google Scholar]
- Singh S. A.; Shukla S. R. Adsorptive removal of cobalt ions on raw and alkali-treated lemon peels. Int. J. Environ. Sci. Technol. 2016, 13, 165–178. 10.1007/s13762-015-0801-6. [DOI] [Google Scholar]
- Pathak P. D.; Mandavgane S. A.; Kulkarni B. D. Fruit peel waste: characterization and its potential uses. Curr. Sci. 2017, 113, 444–454. 10.18520/cs/v113/i03/444-454. [DOI] [Google Scholar]
- Panumati S.; Chudecha K.; Vankhaew P.; Choolert V.; Chuenchom L.; Innajitara W.; Sirichote O. Adsorption of phenol from diluted aqueous solutions by activated carbons obtained from bagasse, oil palm shell and pericarp of rubber fruit. Songklanakarin J. Sci. Technol. 2008, 30, 185–189. [Google Scholar]
- Berber-Villamar N. K.; Netzahuatl-Muñoz A.; Morales-Barrera L.; Chávez-Camarillo G.; Flores-Ortiz M.; Cristiani-Urbina E. Corncob as an effective, eco-friendly, and economic biosorbent for removing the azo dye Direct Yellow 27 from aqueous solutions. PLoS One. 2018, 13, e0196428 10.1371/journal.pone.0196428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafeeyan M. S.; Wan W.; Houshmand A.; Shamiri A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. 10.1016/j.jaap.2010.07.006. [DOI] [Google Scholar]
- Rutherford D.; Wershaw R.; Reeves J.. Development of Acid Functional Groups and Lactones During the Thermal Degradation of Wood and Wood Components, 2007.
- Üner O.; Geçgel Ü.; Bayrak Y. Preparation and characterization of mesoporous activated carbons from waste watermelon rind by using the chemical activation method with zinc chloride. Arabian J. Chem. 2019, 12, 3621–3627. 10.1016/j.arabjc.2015.12.004. [DOI] [Google Scholar]
- Hossain M. A.; Ngo H. H.; Guo W. S.; Nguyen T. V. Palm oil fruit shells as biosorbent for copper removal from water and wastewater: Experiments and sorption models. Bioresour Technol. 2012, 113, 97–101. 10.1016/j.biortech.2011.11.111. [DOI] [PubMed] [Google Scholar]
- Nguyen N. T.; Dao T. H.; Truong T. T.; Nguyen T. M. T.; Pham T. D. Adsorption characteristic of ciprofloxacin antibiotic onto synthesized alpha alumina nanoparticles with surface modification by polyanion. J. Mol. Liq. 2020, 309, 113150 10.1016/j.molliq.2020.113150. [DOI] [Google Scholar]
- Fatimah I.; Prakoso N. I.; Sahroni I.; Musawwa M. M.; Sim Y.-L.; Kooli F.; Muraza O. Physicochemical characteristics and photocatalytic performance of TiO2/SiO2 catalyst synthesized using biogenic silica from bamboo leaves. Heliyon 2019, 5, e02766 10.1016/j.heliyon.2019.e02766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi S. D. A.; Wang C.; Shao Q.; Gao J.; Zhu S.; Yuan H.; Chen J. Polymer-free electrospun separator film comprising silica nanofibers and alumina nanoparticles for Li-ion full cell. J. Energy Chem. 2020, 42, 217–226. 10.1016/j.jechem.2019.06.018. [DOI] [Google Scholar]
- Villen-Guzman M.; Guitierrez-Pinilla D.; Gomez-Lahoz C.; Vereda-Alonso C.; Rodriguez-Maroto J.; Arhoun B. Optimization of Ni (II) biosorption from aqueous solution on modified lemon peel. Environ. Res. 2019, 179, 108849 10.1016/j.envres.2019.108849. [DOI] [PubMed] [Google Scholar]
- Cheraghi E.; Ameri E.; Moheb A. Adsorption of cadmium ions from aqueous solutions using sesame as a low-cost biosorbent: kinetics and equilibrium studies. Int. J. Environ. Sci. Technol. 2015, 12, 2579–2592. 10.1007/s13762-015-0812-3. [DOI] [Google Scholar]
- Schiewer S.; Patil S. B. Modeling the effect of pH on biosorption of heavy metals by citrus peels. J. Hazard. Mater. 2008, 157, 8–17. 10.1016/j.jhazmat.2007.12.076. [DOI] [PubMed] [Google Scholar]
- Krika F.; Azzouz N.; Ncibi M. C. Adsorptive removal of cadmium from aqueous solution by cork biomass: Equilibrium, dynamic and thermodynamic studies. Arabian J. Chem. 2016, 9, S1077–S1083. 10.1016/j.arabjc.2011.12.013. [DOI] [Google Scholar]
- Mahato N.; Sharma K.; Sinha M.; Raj E.; Koteswararao R.; Dhyani A.; Hwan M.; Cho S. Bio-sorbents, industrially important chemicals and novel materials from citrus processing waste as a sustainable and renewable bioresource: A review. J. Adv. Res. 2020, 23, 61–82. 10.1016/j.jare.2020.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal P.; Srivastava S. Characterization of novel Zea Mays based biomaterial designed for toxic metals biosorption. J. Hazard. Mater. 2009, 172, 1206–1211. 10.1016/j.jhazmat.2009.07.125. [DOI] [PubMed] [Google Scholar]
- Basso M.; Cukierman A.. Empleo de un biosorbente para el tratamiento de agua. Avances en Energías Renovables y Medio Ambiente 2003, 7. [Google Scholar]
- Rebollo J.Eliminación de Cadmio (II) de efluentes urbanos tratados mediante procesos de bioadsorcion: El efecto competitivo de otros metales pesados; Universidad Politecnica de Cartagena, 2012.
- Rostamian R.; Najafi M.; Abbas A. Synthesis and characterization of thiol-functionalized silica nano hollow sphere as a novel adsorbent for removal of poisonous heavy metal ions from water: Kinetics, isotherms and error analysis. Chem. Eng. J. 2011, 171, 1004–1011. 10.1016/j.cej.2011.04.051. [DOI] [Google Scholar]
- Zhang J.; Zhai S.; Li S.; Xiao Z.; Song Y.; An Q.; Tian G. Pb (II) removal of Fe3O4 @ SiO2 – NH2 core – shell nanomaterials prepared via a controllable sol – gel process. Chem. Eng. J. 2013, 215–216, 461–471. 10.1016/j.cej.2012.11.043. [DOI] [Google Scholar]
- Seisenbaeva G. S.; Geoffrey D.; Jean-Marie N.; Yurii G.; Vadim K. High surface area ordered mesoporous nano-titania by a rapid surfactant-free approach. J. Mater. Chem. 2012, 22, 20374–20380. 10.1039/c2jm33977c. [DOI] [Google Scholar]
- Dutta D. P.; Nath S. Low cost synthesis of SiO2/C nanocomposite from corn cobs and its adsorption of uranium (VI), chromium (VI) and cationic dyes from wastewater. J. Mol. Liq. 2018, 269, 140–151. 10.1016/j.molliq.2018.08.028. [DOI] [Google Scholar]
- Kumari D.; Goswami R.; Kumar M.; mazumder P.; Kataki R.; Shim J. Removal of Cr (VI) ions from the aqueous solution through nanoscale zero- valent iron (nZVI) Magnetite Corn Cob Silica (MCCS): A bio-waste based water purification perspective. Groundwater Sustainable Dev. 2018, 7, 470–476. 10.1016/j.gsd.2017.12.007. [DOI] [Google Scholar]
- Saini J.; Garg V. K.; Gupta R. K. Green synthesized SiO2 @ OPW nanocomposites for enhanced Lead (II) removal from water. Arabian J. Chem. 2020, 13, 2496–2507. 10.1016/j.arabjc.2018.06.003. [DOI] [Google Scholar]
- Shehzad K.; Xie C.; He J.; Cai X.; Xu W.; Liu J. Facile synthesis of novel calcined magnetic orange peel composites for efficient removal of arsenite through simultaneous oxidation and adsorption. J. Colloid Interface Sci. 2018, 511, 155–164. 10.1016/j.jcis.2017.09.110. [DOI] [PubMed] [Google Scholar]
- Giraldo L.; Erto A.; Moreno-Piraja J. C. Magnetite nanoparticles for removal of heavy metals from aqueous solutions: synthesis and characterization. Adsorption 2013, 19, 465–474. 10.1007/s10450-012-9468-1. [DOI] [Google Scholar]
- Visa M.; Duta A. TiO2/fly ash novel substrate for simultaneous removal of heavy metals and surfactants. Chem. Eng. J. 2013, 223, 860–868. 10.1016/j.cej.2013.03.062. [DOI] [Google Scholar]
- Ali S.; Abbas Y.; Zuhra Z.; Butler L. Synthesis of g-alumina (Al2O3) nanoparticles and their potential for use as an adsorbent in the removal of methylene blue dye from industrial wastewater. Nanoscale Adv. 2019, 1, 213–218. 10.1039/C8NA00014J. [DOI] [PMC free article] [PubMed] [Google Scholar]