Abstract
Background:
Low serum parathyroid hormone (PTH) level and secondary hyperparathyroidism (SHPT) are very common in hemodialysis patients. However, the outcomes of patients with low PTH level or SHPT have not been carefully compared. Therefore, in the present study, we compared the outcomes of hemodialysis patients with low PTH level or SHPT.
Methods:
This was a multi-center, prospective, cohort study of 647 patients. The patients were recruited between 1 September 2016 and 1 January 2017 and followed until 31 December 2018. The participants were allocated to a low PTH group [serum intact PTH (iPTH) concentration < 60 pg/ml] and an SHPT group (iPTH ⩾ 600 pg/ml) according to their mean iPTH concentration across the entire observation period, and the outcomes were compared between these groups. The primary outcome was a composite outcome, which comprised all-cause mortality, non-fatal acute myocardial infarction, non-fatal acute stroke, and acute heart failure.
Results:
A total of 197 hemodialysis patients were allocated to the two groups: 87 with low PTH level and 110 with SHPT; 450 patients with time-averaged iPTH concentrations of 60–600 pg/ml were excluded. Kaplan–Meier analysis of the composite endpoint revealed a significant difference between participants with low PTH level and those with SHPT (p = 0.002). Cox multiple regression showed that participants with low PTH level had a higher incidence of the composite endpoint than those with SHPT (relative risk: 1.337, 95% confidence interval: 1.059–1.688).
Conclusion:
Hemodialysis patients with low PTH level had a higher incidence of mortality and non-fatal cardiovascular events than those with SHPT, irrespective of whether the participants were age-matched.
Keywords: hemodialysis, parathyroid hormone, secondary hyperparathyroidism
Introduction
Chronic kidney disease-mineral-bone disorder is a major complication experienced by hemodialysis patients. One of its major characteristics is the abnormal metabolism of parathyroid hormone (PTH), such that patients may demonstrate either low PTH level or secondary hyperparathyroidism (SHPT).1 Both of these endocrine abnormalities are associated with higher cardiovascular and all-cause mortality.2,3
The prevalence of intact PTH (iPTH) concentration within the target range is very low in hemodialysis patients worldwide: it has been shown to be 26.2% in Europe and America,4 and <30% in China.5 This reflects the fact that it is very difficult to control PTH level completely using the available therapies. Total parathyroidectomy (PTX) is normally used to treat severe SHPT, and the incidence of recurrence is low, but low PTH level often develops subsequently. Therefore, we reasoned that, if patients with low PTH level have better outcomes than those with SHPT, patients with SHPT could be treated using total PTX, without physicians being concerned by the possibility that low PTH level might develop.
It is still unknown whether low PTH level (PTH < 60 pg/ml) or SHPT (PTH ⩾600 pg/ml) have poor prognoses. The results of the Dialysis Outcomes and Practice Patterns Study (DOPPS) suggested that a serum PTH concentration of ⩾600 pg/ml is associated with higher all-cause mortality, but that PTH < 100 pg/ml is associated with significantly greater cardiovascular mortality only in time-dependent models.6 Some previous studies have shown that patients who have low PTH level have a poorer prognosis than those who do not (PTH 100–300 or ⩾300 pg/ml).7,8 In addition, the results of many studies have suggested that a PTH concentration of ⩾500–600 pg/ml is associated with higher all-cause mortality,6,9,10 but that low PTH level is not. Therefore, in the present study, we compared the outcomes of hemodialysis patients with low PTH level with those of patients with SHPT.
Methods
Study design
This was a multi-center, prospective, cohort study. The patients were recruited at four centers: Beijing Friendship Hospital, Capital Medical University; Fuxing Hospital, Capital Medical University; the People’s Hospital of the Daxing District of Beijing; and the Hospital of the Shunyi District of Beijing.
Data were collected regarding the demographic characteristics, comorbidities, laboratory parameters, and the medical history of the participants. Follow-up data were obtained at approximately 2-month intervals. The incidences of all-cause mortality, non-fatal acute myocardial infarction, non-fatal acute stroke, and acute heart failure were recorded.
This study was approved by the Bioethics Committee of Beijing Friendship Hospital, Capital Medical University (Code: 2016-P2-044-01). Written informed consent was obtained from all the participants.
Grouping
The patients were allocated to two groups according to their mean serum iPTH concentration across the entire study period. Patients with time-averaged iPTH concentrations below the 25th percentile (<60 pg/ml)11 were allocated to the low PTH group, patients with concentrations above the 75th percentile (⩾600 pg/ml)12,13 were allocated to the SHPT group, and patients with iPTH concentrations of 60–600 pg/ml were excluded. The incidence of a composite outcome was then compared between the two groups.
Study population
We enrolled patients who were undergoing regular hemodialysis in the four hemodialysis centers between 1 September 2016 and 1 January 2017, then followed them until 31 December 2018, their death, their transfer to another center, or until they changed to peritoneal dialysis or underwent kidney transplantation.
The inclusion criteria were:
(1) Age: 18–80 years.
(2) Regular hemodialysis for ⩾3 months.
(3) Hemodialysis three times per week, with each session lasting 4 h. The flow rate of the dialysate was 500 ml/h, and the blood flow was 250–300 ml/min.
The exclusion criteria were:
(1) History of acute heart failure, acute myocardial infarction, or acute stroke in the preceding 3 months.
(2) Malignant tumor.
(3) Breastfeeding or pregnancy.
Outcome and follow up
The composite outcome comprised all-cause mortality, non-fatal acute myocardial infarction, non-fatal acute stroke, and acute heart failure.
Baseline data were collected by physicians in dialysis rooms, using a standardized questionnaire. The information collected comprised general demographic data (sex, age, and duration of dialysis), the primary kidney disease, and previous medical history, including hypertension, diabetes, cardio-or cerebrovascular disease, and oncological disease.
The following were measured every 2 months (±15 days) thereafter, until the end of the study: serum albumin (ALB), calcium (Ca), phosphorus (P), and iPTH concentrations, and hemoglobin (HGB) concentration. Serum calcium concentration was corrected for serum albumin concentration using the following formula: corrected calcium (mmol/l) = total calcium (mmol/l) − [0.025 × serum albumin (g/dl)] + 1 (Payne formula). All the blood samples were collected before a dialysis session. Follow up was terminated if a participant underwent kidney transplantation, changed to peritoneal dialysis, was transferred to another dialysis center, or at end of the study.
Statistical analysis
Continuous, normally distributed data are reported as means and standard deviations and non-normally distributed data as medians and interquartile ranges. Categorical data are reported as frequencies. Independent t-tests were used to compare sets of normally distributed data (age; duration of dialysis; ALB, Ca, P, and iPTH concentration; and HGB concentration) and the Wilcoxon rank sum test was used for skewed data (duration of follow up).
Survival analysis was performed using Cox regression and survival curves were plotted using the Kaplan–Meier method. The effects of iPTH concentration on the incidence of all-cause death or a non-fatal cardio- or cerebrovascular event were analyzed. Potential risk factors were corrected for in this analysis [Ca, P, ALB, and HGB concentrations; primary disease (diabetes or other); age; and duration of dialysis]. Data were analyzed using SPSS 21.0 software (IBM, Inc., Armonk, NY, USA), and p < 0.05 was considered to represent statistical significance.
Results
Patient characteristics
Characteristics of the enrolled patients
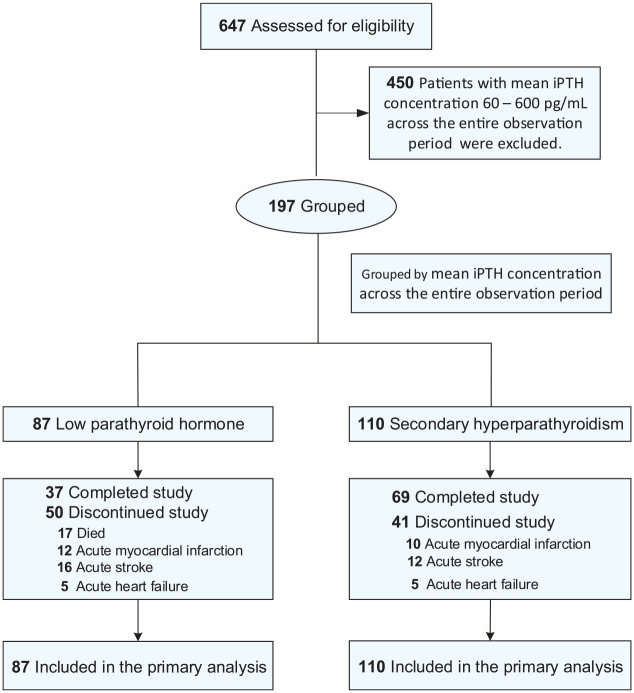
A total of 647 patients were initially enrolled in the study. During the study period, 10 patients underwent renal transplantation and 30 were transferred to other dialysis centers. After 24 months of follow up, a total of 197 hemodialysis patents had time-averaged iPTH concentrations of <60 pg/ml or ⩾600 pg/ml and met the inclusion criteria (87 with low PTH level and 110 with SHPT); 450 patients with time-averaged iPTH concentrations of 60–600 pg/ml were excluded. The disposition of the participants is shown in Figure 1 and their baseline characteristics are shown in Table 1.
Figure 1.
Flow diagram of the participants in the study.
iPTH, intact parathyroid hormone.
Table 1.
Comparison of the clinical characteristics of the two groups of participants.
| Variable | low PTH group (n = 87) | SHPT group (n = 110) | p value |
|---|---|---|---|
| Age(year) | 63.19 ± 10.63 | 55.05 ± 14.09 | <0.001 |
| Sex | 0.301 | ||
| Male [n (%)] | 45 (51.7%) | 65 (59.1%) | |
| Female [n (%)] | 42 (48.3%) | 45 (40.9%) | |
| Renal disease | 0.176 | ||
| Diabetic nephropathy [n (%)] | 30 (34.5%) | 27 (24.5%) | |
| Glomerulonephritis [n (%)] | 33 (37.9%) | 56 (20.9%) | |
| Polycystic kidney [n (%)] | 3 (3.4%) | 3 (2.7%) | |
| Hypertensive nephropathy [n (%)] | 13 (14.9%) | 20 (18.2%) | |
| Interstitial nephritis [n (%)] | 8 (9.2%) | 4 (3.6%) | |
| Comorbid conditions | |||
| Hypertension [n (%)] | 83 (95.4%) | 105 (95.5%) | 0.986 |
| Dyslipidaemia [n (%)] | 40 (45.9%) | 53 (48.2%) | 0.758 |
| Dialysis vintage (years) | 7.14 ± 6.83 | 7.24 ± 4.57 | 0.904 |
| Ca concentration in dialysate | 0.552 | ||
| 1.5 mmol/l [n (%)] | 55 (63.2%) | 74 (67.3%) | |
| 1.75 mmol/l [n (%)] | 32 (36.8%) | 36 (32.7%) | |
| Serum iPTH (pg/ml) | 30.88 ± 17.27 | 1236.64 ± 618.02 | |
| Serum Ca (mmol/l) | 2.29 ± 0.20 | 2.30 ± 0.27 | 0.798 |
| Serum P grouping | 0.006 | ||
| ⩾1.78 mmol/l | 28 (32.2%) | 57 (51.8%) | |
| < 1.78 mmol/l | 59 (67.8%) | 53 (48.2%) | |
| HGB (g/l) | 108.3 ± 15.8 | 111.3 ± 11.4 | 0.123 |
| ALB (g/l) | 38.38 ± 4.56 | 39.85 ± 2.76 | 0.006 |
| Survival time (month) | 15.74 ± 7.95 | 19.18 ± 6.86 | 0.001 |
| Follow-up time (month) | 17 | 24 | |
Mean ± standard deviation is shown for normally distributed data and median for non-normally distributed data.
ALB, serum albumin; Ca, calcium; HGB, hemoglobin; iPTH, intact parathyroid hormone; P, phosphorus; PTH, parathyroid hormone; SPTH, secondary hyperparathyroidism.
Treatment characteristics of the two groups
During the follow-up period, 89% of the patients in the SHPT group (n = 98) were treated with oral calcitriol, 16.8% (n = 20) were treated with oral calcimimetics, 76.3% (n = 84) were treated with a calcium-based phosphate-binder, and 30% (n = 33) were treated with a calcium-free phosphate-binder. In the Low PTH group, 71.3% of the patients (n = 62) were treated with a calcium-based phosphate-binder and 12.6% (n = 11) were treated with a calcium-free phosphate-binder. In the 43.7% (n = 38) of patients who were treated with oral calcitriol, the treatment was stopped when these patients had a PTH level of <60 pg/ml.
Comparisons of basic characteristics of participants
The mean iPTH concentrations of the low PTH and SHPT groups were 30.88 ± 17.27 pg/ml and 1236.64 ± 618.02 pg/ml, respectively. The low PTH group had lower ALB and P concentrations than the SHPT group, but there were no significant differences in sex distribution, prevalence of diabetes, duration of dialysis, or serum Ca or HGB concentration between the groups (Table 1).
Outcomes
During the follow-up period, outcome events occurred in 50 patients (57.5%) in the low PTH group and 41 patients (37.3%) in the SHPT group.
There was a statistically significant difference in the composite outcome of mortality, or a cardio- or cerebrovascular event between participants in the low PTH group and those with SHPT (p = 0.002; Figure 2). However, Kaplan–Meier curve analysis is non-parametric, and thus does not rule out the presence of confounding factors. Therefore, a Cox regression model was used to analyze the risk factors for all-cause mortality. The results show that participants in the low PTH group had a greater risk of the composite outcome [hazard ratio (HR) 1.337; 95% confidence interval (CI) 1.059–1.688, p = 0.014] than participants in the SHPT group.
Figure 2.
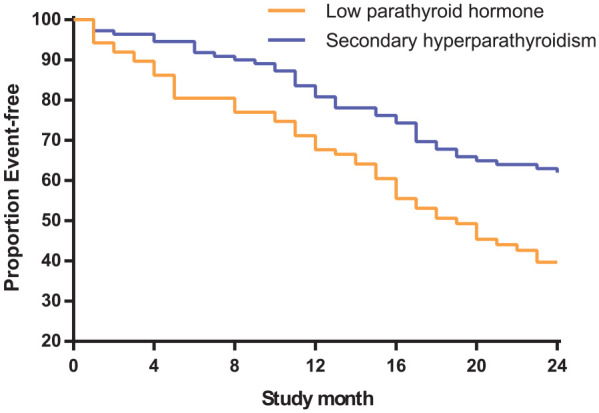
Comparison of the composite outcome in the two groups.
Kaplan–Meier curves are shown that compare the time to the composite outcome of mortality, or a cardio- or cerebrovascular event in the low PTH and SHPT groups.
Identification of prognostic factors in the two groups
Only four of the analyzed parameters were associated with all-cause mortality after adjustment for potential confounding factors (age; sex; ALB, Ca, and P concentration; HGB concentration; duration of dialysis; and the primary disease) (Table 2). Serum phosphorus concentration ⩾1.78 mmol/l [relative risk (RR): 0.318, 95% CI: 0.143–0.709], the presence of diabetes (RR: 0.756, 95% CI: 0.605–0.945), low serum iPTH concentration (RR: 1.337, 95% CI: 1.059–1.688), and hypoalbuminemia (RR: 0.882, 95% CI: 0.827–0.942) were found to be independent risk factors for mortality, or a cardio- or cerebrovascular event in patients undergoing hemodialysis.
Table 2.
Cox regression analysis to identify prognostic factors in the two groups.
| Variable | HR | 95% CI | p value |
|---|---|---|---|
| Low PTH group (vs. SHPT) | 1.337 | 1.059~1.688 | 0.014 |
| Low serum P (<1.78 mmol/l) | 0.318 | 0.143~0.709 | 0.005 |
| ALB | 0.882 | 0.827~0.942 | <0.001 |
| Age | 1.014 | 0.995~1.032 | 0.143 |
| Dialysis vintage | 1.019 | 0.985~1.054 | 0.278 |
| Gender | 0.835 | 0.670~1.040 | 0.107 |
| Serum Ca | 0.556 | 0.228~1.358 | 0.198 |
| HGB | 0.999 | 0.982~1.016 | 0.912 |
| Non-diabetes | 0.756 | 0.605~0.945 | 0.014 |
ALB, serum albumin; Ca, calcium; CI, confidence interval; HGB, hemoglobin; HR, hazard ratio; P, phosphorus; PTH, parathyroid hormone; SPTH, secondary hyperparathyroidism.
Comparison of age-matched participants in low PTH and SHPT groups
Further comparisons were performed between the 18 participants in the low PTH (mean serum iPTH concentration 15.21 pg/ml) group who underwent total PTX and the 18 participants in the SHPT group who were matched on the basis of age and duration of dialysis ± 1 year (serum iPTH concentration 1131.22 pg/ml). The duration of previous SHPT in 18 participants who underwent total PTX was 17.5 ± 6.32 months, with a mean PTH level of 1328.89 ± 480.57 pg/ml. The highest dose of vitamin D receptor activator that had been administered was 10.12 ± 3.84 μg. These comparisons showed that the mean duration of dialysis was longer in the low PTH group than in the SHPT group (11.67 ± 5.18 versus 10.17 ± 3.85 years; p < 0.05), but there were no other statistically significant differences (Table 3).
Table 3.
Comparisons of the clinical characteristics of participants with low PTH level who had undergone total PTX and paired participants with SHPT.
| PTX-low PTH group (n = 18) | Paired-SHPT group (n = 18) | p value | |
|---|---|---|---|
| Age (year) | 58.17 ± 7.73 | 57.89 ± 7.84 | 0.311 |
| Sex | 0.305 | ||
| Male | 13 (72.2) | 9 (50) | |
| Female | 5 (27.8) | 9 (50) | |
| Diabetes [n (%)] | 16 (88.9) | 15 (83.3) | 1.000 |
| Dialysis vintage (year) | 11.67 ± 5.18 | 10.17 ± 3.85 | 0.016 |
| Serum iPTH (pg/ml) | 15.21 | 1131.22 | |
| Serum Ca (mmol/l) | 2.21 ± 0.20 | 2.34 ± 0.21 | 0.072 |
| Serum P Grouping | 1.000 | ||
| ⩾1.78 mmol/l | 8 (44.4) | 9 (50) | |
| < 1.78 mmol/l | 10 (55.6) | 9 (50) | |
| HGB (g/l) | 108.3 ± 15.8 | 111.3 ± 11.4 | 0.123 |
| ALB (g/l) | 41.29 ± 3.76 | 39.14 ± 2.19 | 0.062 |
| Composite endpoint events [n (%)] | 9 (50) | 5 (27.8) | 0.305 |
| Survival time (month) | 17.83 ± 8.08 | 19.56 ± 7.44 | 0.534 |
ALB, serum albumin; Ca, calcium; HGB, hemoglobin; iPTH, intact parathyroid hormone; P, phosphorus; PTH, parathyroid hormone; PTX, parathyroidectomy; SPTH, secondary hyperparathyroidism.
Kaplan–Meier analysis showed that there was no statistically significant difference in the composite outcome of mortality or a cardio- or cerebrovascular event in patients with low PTH level who had undergone total parathyroidectomy and those with SHPT (p = 0.2) (Figure 3).
Figure 3.
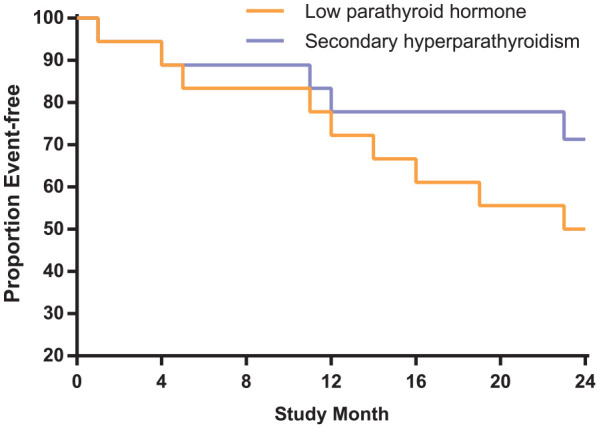
Comparison of the composite outcome in participants with low PTH level who had undergone total parathyroidectomy and age-matched participants with low PTH level who had not.
PTH, parathyroid hormone.
Kaplan–Meier curves are shown for the time to the composite outcome of mortality, or a cardio- or cerebrovascular event (p = 0.2).
Identification of prognostic factors in patients with low PTH level
Further analysis of the risk factors for mortality in participants with low PTH level showed that serum phosphorus concentration ⩾1.78 mmol/l (RR: 0.114, 95% CI: 0.034–0.379) and hypoalbuminemia (RR: 0.901, 95% CI: 0.830, 0.978) were independent risk factors for mortality, or a cardio- or cerebrovascular event (Table 4).
Table 4.
Cox regression analysis of potential prognostic factors in patients with low PTH level.
| Variable | HR | 95% CI | p value |
|---|---|---|---|
| Low serum P grouping | 0.112 | 0.034–0.373 | <0.001 |
| ALB | 0.899 | 0.827–0.977 | 0.012 |
| Age | 1.006 | 0.979–1.034 | 0.646 |
| Dialysis vintage | 1.017 | 0.973–1.062 | 0.454 |
| Gender | 0.565 | 0.310–1.031 | 0.069 |
| Serum Ca | 0.195 | 0.031–1.240 | 0.083 |
| HGB | 0.992 | 0.968–1.018 | 0.549 |
| Non-diabetes | 0.598 | 0.307–1.167 | 0.132 |
ALB, serum albumin; Ca, calcium; CI, confidence interval; HGB, hemoglobin; HR, hazard ratio; P, phosphorus; PTH, parathyroid hormone.
Discussion
The findings of the present study suggest that patients with low PTH level have a poorer prognosis than those with SHPT, irrespective of age. Both very low and high serum iPTH concentration may be associated with higher mortality in dialysis patients. Because of their very low PTH concentration, patients with low PTH level demonstrate low bone turnover and formation, and a reduction in the buffering of circulating calcium and phosphorus, which predisposes toward arterial vascular calcification, and therefore mortality.14,15
In the DOPPS study, a PTH concentration >600 pg/ml was associated with significantly greater all-cause mortality, but patients with PTH concentrations of <100 pg/ml or 301–600 pg/ml did not have a higher mortality than those with a PTH concentration of 101–300 pg/ml.6 However, the outcomes of patients with PTH < 100 pg/ml and ⩾600 pg/ml were not compared. Rhee et al. claimed that persistently low iPTH concentration (<60 pg/ml) is an independent risk factor for both aortic arch calcification and mortality in hemodialysis patients,16 but only patients with iPTH <60 pg/ml and iPTH 150–300 pg/ml were compared in this study. Therefore, it remained to be established whether a very low iPTH concentration (<60 pg/ml) is associated with a higher mortality than an iPTH ⩾600 pg/ml. Jean et al. suggested that a very low PTH concentration (<50 pg/ml) is associated with a significantly higher risk of mortality than PTH ⩾50 pg/ml,7 which appears to be consistent with the results of the DOPPS study.6 Cozzolino et al. suggested that a low PTH concentration (<150 pg/ml) is associated with a significantly higher risk of mortality than PTH ⩾150 pg/ml.17 However, the differences in outcomes in patients with low PTH level or SHPT remained unclear.
The findings of the present study suggest that patients with low PTH level (iPTH < 60 pg/ml) have a poorer prognosis than those with SHPT (iPTH ⩾ 600 pg/ml). Because there was a significant difference in age between the two groups, we also compared age-matched participants with low PTH level or SHPT. However, the incidence of the composite outcome remained significantly higher in the Low PTH group than in the SHPT group (50% versus 27.8%), implying that this may be of clinical relevance. Therefore, because total PTX frequently causes low PTH level, it should be performed with caution.18
We also found that age, the presence of diabetes, and the serum P and ALB concentrations were risk factors for low PTH level in patients undergoing hemodialysis. This finding is consistent with those of previous studies.19–21 All these factors are risk factors for death and cardio- and cerebrovascular events,22–24 and therefore may have been confounding factors in the present study. Therefore, we corrected for these parameters in the multivariate Cox regression analysis. This analysis showed that age-matched participants with low PTH level or SHPT had similar prevalences of diabetes, and serum P and ALB concentrations. These results remained consistent, with low PTH level being associated with a poorer prognosis than SHPT.
There were some limitations to the present study. First, it was limited by the relatively small sample and short follow-up time, which would have affected the outcomes of the statistical analyses to some extent. Second, the age difference between the two groups may have confounded the results. Although we corrected for potential confounding factors in multivariate Cox regression analysis and analyzed age-matched low PTH and SHPT subgroups in an attempt to minimize confounding, further studies should be performed to confirm the present findings.
In summary, we first tried to compare the survival of patients with low PTH level and SHPT, and the results of the study suggest that hemodialysis patients with low PTH level have a poorer prognosis than those with SHPT. The deleterious effects of low PTH levels are not only due to the reduction in turnover, because PTH also plays crucial roles in directing hematopoietic stem cell renewal in the bone marrow and stem cells to peripheral tissues. Low PTH levels can cause abnormal cell function, which leads to vascular calcification.25 This suggests that we should treat those patients with low PTH level more carefully. In addition, total parathyroidectomy should be used with caution for the treatment of hemodialysis patients with SHPT, and when using drugs to treat hemodialysis patients with SHPT, PTH level should be closely monitored. In the present study, we did not alter the treatment of the patients. The results show that 71.3% of patients in the low PTH group were taking a Ca-based phosphate-binder, which may explain the higher mortality in this group. This suggests that patients who have a low PTH concentration and use Ca-free binders may have a better prognosis. Further studies should be performed to confirm these findings in the future.
Acknowledgments
We thank Mark Cleasby, from Liwen Bianji, Edanz Group China (http://www.liwenbianji.cn/ac), for editing the English text of drafts of this manuscript.
Footnotes
Author contribution(s): Yue Yu: Data curation; Investigation; Methodology; Writing-original draft.
Zongli Diao: Formal analysis; Funding acquisition; Methodology; Software; Validation; Visualization; Writing-original draft; Writing-review & editing.
Ying Wang: Data curation; Formal analysis; Investigation; Writing-review & editing.
Peiyi Zhou: Data curation; Investigation; Writing-review & editing.
Rui Ding: Data curation; Investigation; Writing-review & editing.
Wenhu Liu: Conceptualization; Resources; Writing-review & editing.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Beijing Municipal Administration of Hospitals Incubating Program (grant number PX2017013) and Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding (grant number ZYLX201824).
ORCID iD: Wenhu Liu  https://orcid.org/0000-0002-6532-4394
https://orcid.org/0000-0002-6532-4394
Contributor Information
Yue Yu, Department of Nephrology, Beijing Friendship Hospital, Capital Medical University, Beijing, China; Department of Nephrology, Fu Xing Hospital, Capital Medical University, Beijing, China.
Zongli Diao, Department of Nephrology, Beijing Friendship Hospital, Capital Medical University, 95 Yong’An Road, Beijing 100050, China.
Ying Wang, Department of Nephrology, Fu Xing Hospital, Capital Medical University, Beijing, China.
Peiyi Zhou, Department of Nephrology, People’s Hospital of Beijing Daxing District, Beijing, China.
Rui Ding, Department of Nephrology, The Hospital of Shunyi District Beijing, Beijing, China.
Wenhu Liu, Department of Nephrology, Beijing Friendship Hospital, Capital Medical University, 95 Yong’An Road, Beijing 100050, China.
References
- 1. Jeloka T, Mali M, Jhamnani A, et al. Are we overconcerned about secondary hyperparathyroidism and underestimating the more common secondary hypoparathyroidism in our dialysis patients? J Assoc Physicians India 2017; 60: 102–105. [PubMed] [Google Scholar]
- 2. Sarnak MJ, Amann K, Bangalore S, et al. Chronic kidney disease and coronary artery disease: JACC state-of-the-art review. J Am Coll Cardiol 2019; 74: 1823–1838. [DOI] [PubMed] [Google Scholar]
- 3. Tentori F, Wang M, Bieber BA, et al. Recent changes in therapeutic approaches and association with outcomes among patients with secondary hyperparathyroidism on chronic hemodialysis: the DOPPS study. Clin J Am Soc Nephrol 2015; 10: 98–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tentori F. Mineral and bone disorder and outcomes in hemodialysis patients: results from the DOPPS. Semin Dial 2010; 23: 10–14. [DOI] [PubMed] [Google Scholar]
- 5. Kong X, Zhang L, Zhang L, et al. Mineral and bone disorder in Chinese dialysis patients: a multicenter study. BMC Nephrol 2012; 13: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tentori F, Blayney MJ, Albert JM, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2008; 52: 519–530. [DOI] [PubMed] [Google Scholar]
- 7. Jean G, Lataillade D, Genet L, et al. Association between very low PTH levels and poor survival rates in haemodialysis patients: results from the French ARNOS cohort. Nephron Clin Pract 2011; 118: 211–216. [DOI] [PubMed] [Google Scholar]
- 8. Lee SA, Lee MJ, Ryu GW, et al. Low serum intact parathyroid hormone level is an independent risk factor for overall mortality and major adverse cardiac and cerebrovascular events in incident dialysis patients. Osteoporos Int 2016; 27: 2717–2726. [DOI] [PubMed] [Google Scholar]
- 9. Palmer SC, Hayen A, Macaskill P, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. J Am Med Assoc 2011; 305: 1119–1127. [DOI] [PubMed] [Google Scholar]
- 10. Naves-Diaz M, Passlick-Deetjen J, Guinsburg A, et al. Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant 2011; 26: 1938–1947. [DOI] [PubMed] [Google Scholar]
- 11. Fukagawa M, Yokoyama K, Koiwa F, et al. Clinical practice guideline for the management of chronic kidney disease-mineral and bone disorder. Ther Apher Dial 2013; 17: 247–288. [DOI] [PubMed] [Google Scholar]
- 12. Group KDIGO (CUW). KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 2017; 7: 1–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nephrology Branch of Chinese Medical Association. Guidelines for diagnosis and treatment of mineral and bone abnormalities in chronic kidney disease. Chin J Nephrol Dial Transpl 2019; 28: 52–57. [Google Scholar]
- 14. Hong YA, Kim JH, Kim YK, et al. Low parathyroid hormone level predicts infection-related mortality in incident dialysis patients: a prospective cohort study. Korean J Intern Med 2020; 35: 160–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vervloet M, Cozzolino M. Vascular calcification in chronic kidney disease: different bricks in the wall? Kidney Int 2017; 91: 808–817. [DOI] [PubMed] [Google Scholar]
- 16. Rhee H, Song SH, Kwak IS, et al. Persistently low intact parathyroid hormone levels predict a progression of aortic arch calcification in incident hemodialysis patients. Clin Exp Nephrol 2012; 16: 433–441. [DOI] [PubMed] [Google Scholar]
- 17. Cozzolino M, Brancaccio D, Cannella G, et al. VDRA therapy is associated with improved survival in dialysis patients with serum intact PTH ⩽150 pg/mL: results of the Italian FARO Survey. Nephrol Dial Transplant 2012; 27: 3588–3594. [DOI] [PubMed] [Google Scholar]
- 18. Ayuk J, Cooper MS, Gittoes NJ. New perspectives in the management of primary hyperparathyroidism. Ther Adv Endocrinol Metab 2010; 1: 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Drechsler C, Krane V, Grootendorst DC, et al. The association between parathyroid hormone and mortality in dialysis patients is modified by wasting. Nephrol Dial Transplant 2009; 24: 3151–3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Saran R, Li Y, Robinson B, et al. US renal data system 2015 annual data report: epidemiology of kidney disease in the United States. Am J Kidney Dis 2016; 67: 1–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hedgeman E, Lipworth L, Lowe K, et al. International burden of chronic kidney disease and secondary hyperparathyroidism: a systematic review of the literature and available data. Int J Nephrol 2015; 2015: 184–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collins AJ, Foley RN, Chavers B, et al. United States renal data system 2011 annual data report: atlas of chronic kidney disease & end-stage renal disease in the United States. Am J Kidney Dis 2012; 59: e1–420. [DOI] [PubMed] [Google Scholar]
- 23. Kalantar-Zadeh K, Shah A, Duong U, et al. Kidney bone disease and mortality in CKD: revisiting the role of vitamin D, alkaline phosphatase and minerals. Kidney Int Suppl 2010; 117: S10–S21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fouque D, Pelletier S, Mafra D, et al. Nutrition and chronic kidney disease. Kidney Int 2011; 80: 348–357. [DOI] [PubMed] [Google Scholar]
- 25. Cianciolo G, Capelli I, Cappuccilli M, et al. Calcifying circulating cells: an uncharted area in the setting of vascular calcification in CKD patients. Clin Kidney J 2016; 9: 280–286. [DOI] [PMC free article] [PubMed] [Google Scholar]