Abstract
Background
Ambient air pollution can contribute to the development and exacerbation of COPD. However, the influence of air pollution on objective COPD phenotypes, especially from imaging, is not well studied. We investigated the influence of long-term exposure to air pollution on lung function and quantitative imaging measurements in a Korean cohort of participants with and without COPD diagnosis.
Methods
Study participants (N = 457 including 296 COPD cases) were obtained from the COPD in Dusty Areas (CODA) cohort. Annual average concentrations of particulate matter less than or equal to 10 μm in diameter (PM10) and nitrogen dioxide (NO2) were estimated at the participants’ residential addresses using a spatial air pollution prediction model. All the participants underwent volumetric computerized tomography (CT) and spirometry measurements and completed survey questionnaires. We examined the associations of PM10 and NO2 with FVC, FEV1, emphysema index, and wall area percent, using linear regression models adjusting for age, gender, education, smoking, height, weight, and COPD medication.
Results
The age of study participants averaged 71.7 years. An interquartile range difference in annual PM10 exposure of 4.4 μg/m3 was associated with 0.13 L lower FVC (95% confidence interval (CI), − 0.22- -0.05, p = 0.003). Emphysema index (mean = 6.36) was higher by 1.13 (95% CI, 0.25–2.02, p = 0.012) and wall area percent (mean = 68.8) was higher by 1.04 (95% CI, 0.27–1.80, p = 0.008). Associations with imaging phenotypes were not observed with NO2.
Conclusions
Long-term exposure to PM10 correlated with both lung function and COPD-relevant imaging phenotypes in a Korean cohort.
Keywords: Air pollution, COPD, CT, Lung function, Traffic
Introduction
Air pollution is an important risk factor for the mortality and morbidity of cardiorespiratory diseases globally [1]. Global estimates of premature deaths and disability-adjusted life-years from COPD by air pollution are 0.86 and 16.8 million in 2015 [2]. Increased short-term exposure to ambient air pollution for a few days is associated with respiratory mortality and exacerbation of respiratory diseases leading to hospital admission [3–5]. Long-term exposure to ambient air pollution for years has been associated with reduced lung function and also can contribute to the development and exacerbation of COPD [6–9]. These studies focused on concentrations of traffic-related air pollutants such as particulate matter less than or equal to 10 or 2.5 μm in diameter (PM10 or PM2.5) and nitrogen dioxide (NO2). In recent years, more refined methods have been developed to adequately estimate individual-level air pollution concentrations at residential addresses [10].
Recent advances in computed tomography (CT) measurement lead to understanding of the clinical implications of emphysema severity and airway wall thickening. Emphysema is an important structural feature of COPD and is associated with adverse outcomes with or without COPD [11, 12]. Airway wall thickening measured by CT was associated with cigarette smoking and disease severity [13]. However, only few studies have examined the effects of air pollution on these imaging phenotypes so far [14–16]. Previous studies were performed in Western countries. Genetic factors and nature of the PM may differ across regions. Studies based on a well-designed cohort including COPD patients, diverse environmental exposure data, and imaging measures can clarify the effects of air pollution on imaging phenotypes as well as lung function [17].
The COPD in Dusty Areas (CODA) cohort in South Korea was constructed focusing on the people living near cement plants in Gangwon and Chungbuk provinces, South Korea [18–20] and employed a recently-developed air pollution prediction model for improved exposure assessment at the individual level [21]. We investigated the association between traffic-related air pollution and both lung function and quantitative imaging phenotypes including emphysema severity and airway measurements. Some of these results have been previously presented as an abstract [22].
Methods
Study population
A total of 504 subjects who resided in areas near cement plants were recruited in the CODA cohort between 2012 and 2017 in South Korea. We recruited participants from affected administrative districts that were selected by the National Institute of Environmental Research of Korea based on the distances and wind direction to cement plants. We mailed an invitation and then subsequently called each subject whose address was located within our pre-defined area of study. Subjects include those having or not having airflow limitations based on spirometry. The protocols of data collection in the CODA cohort were previously described in detail [23–25]. In brief, we obtained data on demographic characteristics, medical history, and environmental exposures from participant questionnaires.
Spirometry and imaging procedures
Lung function was measured before and after administrating 400 μg of salbutamol using EasyOne (NDD, Zurich, Switzerland) and pulmonary function measures were selected according to ATS/ERS criteria [26]. We focused on FEV1 and FVC as the two lung function outcomes in this study. COPD status was defined as a post-bronchodilator FEV1/FVC less than 0.7 at baseline. CT measurements were obtained using a dual-source CT scanner (Somatom Definition, Siemens Healthcare, Forchheim, Germany) at full inspiration and expiration in the supine position. Emphysema index was calculated as the percentage of lung area below − 950 HU threshold, while wall area percent was defined as (100 x wall area/total bronchial area) to assess airway thickness and was measured near the origin of the right apical and left apicoposterior segmental bronchi using in-house software and the two measurements were averaged [25, 27, 28]. Functional small airway disease was calculated as a percentage of lung area between ≥ −950HU at inspiration and < −856HU at expiration after image co-registration of inspiratory and expiratory CT using an Aview® system (Coreline Soft Inc., Seoul, South Korea). Written informed consent was given by each participant. This study received ethical approval from the Kangwon National University Hospital IRB (KNUH 2012–06-007, clinical trial registration number KCT-0000552).
Air pollution exposure assessment
Annual average concentrations of PM10 and NO2 at participants’ home addresses were estimated from a previously-developed air pollution prediction model. The details of this model have been described previously [21]. Based on the air quality monitoring data for 2010 in South Korea, this model estimated annual average concentrations at any location in South Korea using a universal kriging framework that consists of summary predictors of about 300 geographic variables and spatial correlation of air pollution concentrations. The cross-validated R2 values indicating the prediction ability of the model were 0.45 and 0.82 for PM10 and NO2, respectively. This model performance was comparable to those of national-scale prediction models in North America and Europe [29–31].
Statistical analyses
To investigate the association of PM10 and NO2 with FEV1, FVC, emphysema, and wall area percent, we performed linear regression analysis adjusting for individual characteristics. Separate models were applied to each pair of two pollutants and four outcomes. We used two models to examine the sensitivity of our results to the progressively-added confounding variables. In model 1, we adjusted for age, gender, education, smoking, height, weight, occupation, and medication for COPD to our primary model. Smoking was identified as smoking status and smoking amount in pack-years. We analyzed job in 3 groups: cement worker (regular and higher dust exposure); farmer (less frequent and lower dust exposures), all other jobs (no dust exposure). Model 2 additionally included the calendar year of pulmonary function testing, and asthma history and COPD status were added in model 3. We presented the effect estimate for an interquartile increase (IQR) in each pollutant concentration to allow the comparison given the different scales of the two pollutants. We also performed subgroup analyses stratified by gender, the status of COPD, smoking, and overweight/obesity, and underwent statistical tests of interaction using product terms with PM10 or NO2. Smoking status was categorized to never vs. ever (combining former and current) smokers. Overweight/obesity was defined as a BMI ≥ 23 kg/m2, according to the World Health Organization Asia–Pacific criteria [32]. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). The p value < 0.05 was defined as indicating statistical significance.
Results
Characteristics of the CODA cohort participants
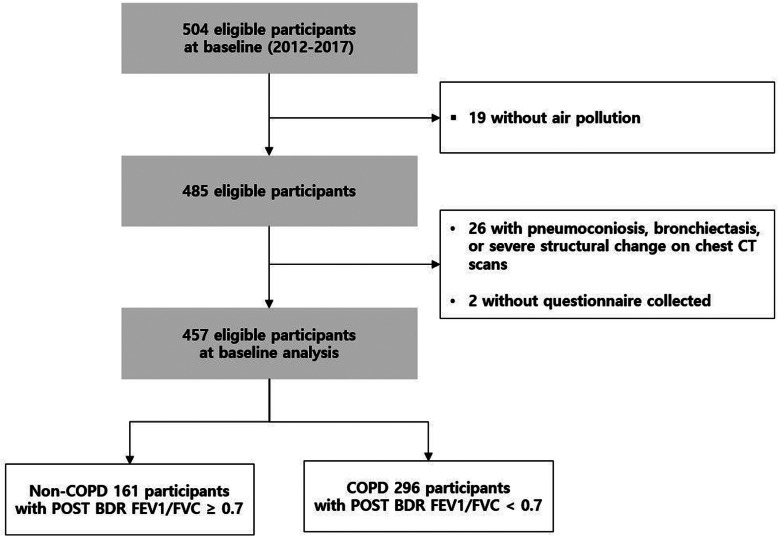
There were 457 participants included in our study. (Fig. 1) The mean age was 71.7 years and the mean BMI was 23.5 kg/m2. There were 165 never (36%), 194 former (43%), and 98 current smokers (21%). Among the participants, 170 subjects (38%) had an occupational history of a cement factory worker and 149 subjects had a history of a farmer. The average post-bronchodilator FEV1 and FVC were 1.96 and 3.02 L, respectively (Table 1). The average emphysema index was 6.36 and the mean wall area percent was 68.8%. Among all, 296 subjects (65%) were COPD patients and 161 subjects were non-COPD.
Fig. 1.
Flowchart for study participation in the COPD in Dusty Areas (CODA) cohort study
Table 1.
Participant characteristics at baseline in the Korean CODA cohort (n = 457)
| All (n = 457) | Non-COPD (n = 161) |
COPD (n = 296) |
|
|---|---|---|---|
| Gender | |||
| Male | 335 (73.3) | 97 (60.2) | 238 (80.4) |
| Female | 122 (26.7) | 64 (39.8) | 58 (19.6) |
| Age | 71.7 ± 7.3 | 70.8 ± 7.7 | 72.2 ± 7.1 |
| 44 ~ 59 yr | 29 (6.3) | 15 (9.3) | 14 (4.7) |
| 60 ~ 69 yr | 113 (24.7) | 42 (26.1) | 71 (24.0) |
| 70 ~ 79 yr | 260 (56.9) | 91 (56.5) | 169 (57.1) |
| 80 ~ 96 yr | 55 (12.0) | 13 (8.1) | 42 (14.2) |
| Education | |||
| < Elementary school | 143 (32.0) | 43 (27.7) | 100 (34.2) |
| Elementary school | 169 (37.8) | 67 (43.2) | 102 (34.9) |
| Middle school | 65 (14.5) | 23 (14.8) | 42 (14.4) |
| ≥ High school | 70 (15.7) | 22 (14.2) | 48 (16.4) |
| Income (x104won) | |||
| ≤ 49 | 280 (63.9) | 95 (62.5) | 185 (64.7) |
| 50–99 | 70 (16.0) | 22 (14.5) | 48 (16.8) |
| ≥ 100 | 88 (20.1) | 35 (23.0) | 53 (18.5) |
| Job | |||
| Cement factory | 170 (37.2) | 55 (34.2) | 115 (38.9) |
| farmer | 149 (32.6) | 62 (37.9) | 87 (29.3) |
| Others | 138 (30.2) | 44 (27.3) | 94 (31.8) |
| Smoking | |||
| Never-smoker | 165 (36.1) | 87 (54.0) | 78 (26.4) |
| Former-smoker | 194 (42.5) | 52 (32.3) | 142 (48.0) |
| Current-smoker | 98 (21.4) | 22 (13.7) | 76 (25.7) |
| Pack-years | 17.6 ± 23.4 | 12.0 ± 18.5 | 20.6 ± 25.2 |
| Height (cm) | 159.4 ± 9.3 | 157.8 ± 10.3 | 160.3 ± 8.6 |
| Weight (kg) | 59.7 ± 10.4 | 60.0 ± 10.6 | 59.6 ± 10.3 |
| BMI (kg/m2) | 23.5 ± 3.2 | 24.0 ± 3.3 | 23.2 ± 3.2 |
| < 23.0 | 207 (45.3) | 64 (39.8) | 143 (48.3) |
| 23.0 ~ 24.9 | 106 (23.2) | 40 (24.8) | 66 (22.3) |
| ≥ 25.0 | 144 (31.5) | 57 (35.4) | 87 (29.4) |
| History of COPD medications | |||
| No | 362 (79.2) | 149 (92.5) | 213 (72.0) |
| Yes | 95 (20.8) | 12 (7.5) | 83 (28.0) |
| Asthma, history of disease | |||
| No | 376 (83.9) | 136 (87.7) | 240 (81.9) |
| Yes | 72 (16.1) | 19 (12.3) | 53 (18.1) |
| Pulmonary outcome at baseline visit, Post BDR | |||
| FVC, L | 3.02 ± 0.81 | 2.88 ± 0.80 | 3.10 ± 0.81 |
| FVC, % predicted | 97.8 ± 19.1 | 96.9 ± 18.9 | 98.3 ± 19.3 |
| FEV1, L | 1.96 ± 0.60 | 2.19 ± 0.61 | 1.84 ± 0.56 |
| FEV1, % predicted | 87.3 ± 22.5 | 100.7 ± 21.1 | 80.0 ± 19.7 |
| FEV1/FVC | 0.65 ± 0.11 | 0.76 ± 0.05 | 0.59 ± 0.08 |
| Emphysema index, n = 414 | 6.36 ± 6.66 | 3.35 ± 3.60 | 7.64 ± 7.23 |
| Wall area %, n = 414 | 68.8 ± 5.2 | 67.5 ± 5.4 | 69.3 ± 5.0 |
Data are mean ± SD for continuous variables and n(%) for categorical variables
Exposure to air pollution
The summary statistics of the individual-level air pollution concentrations are shown in Table 2. Annual average concentrations of PM10 and NO2 predicted at 457 CODA cohort participants’ homes in 2010 were 43.1 ± 2.9 μg/m3 was 13.6 ± 2.1 ppb, respectively. These were lower than the South Korean national air quality standards for annual average concentrations of PM10 and NO2 (50 μg/m3 and 30 ppb, respectively). The correlation coefficient between the two pollutants was 0.44.
Table 2.
Summary statistics and Pearson correlation coefficient of individual-level PM10 and NO2 concentrations estimated at participant homes in the Korean CODA cohort (n = 457)
| Mean ± SD | IQR | Percentiles | Correlation coefficient(r) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | PM10 | No2 | |||
| PM10 (ug/m3) | 43.1 ± 2.9 | 4.4 | 38.4 | 41.0 | 43.1 | 45.4 | 47.4 | - | 0.44*** |
| NO2 (ppb) | 13.6 ± 2.1 | 3.0 | 10.2 | 12.3 | 13.5 | 15.3 | 17.2 | - | |
***: p < 0.0001
Association between air pollution and lung function
Higher PM10 was significantly associated with lower FVC in all models; in our primary analysis adjusting for individual characteristics, a 4.4 μg/m3 IQR increase in PM10 concentration was associated with 0.13 L lower FVC (95% confidence interval (CI) = − 0.22 - -0.05, p = 0.003) (Table 3). The effect estimate for FEV1 was also negative but statistically non-significant in our primary model (regression coefficient = − 0.04, 95% CI = − 0.11 - 0.03, p = 0.29). Higher NO2 was significantly associated with lower FVC (regression coefficient = − 0.09, 95% CI = − 0.17 - -0.01, p = 0.035), while FEV1 was not associated with NO2 (Table 3).
Table 3.
Effect estimates and 95% confidence intervals of FVC, FEV1, emphysema index, and mean wall area % for interquartile range increases in PM10 (4.4 μg/m3) and NO2 (3.0 ppb) in the CODA cohort
| All (n = 457) | ||||
|---|---|---|---|---|
| PM10 | NO2 | |||
| β (95% CI) | P | β (95% CI) | P | |
| FVC, L | ||||
| Model 1a | −0.13 (− 0.22, − 0.05) | 0.003 | − 0.09 (− 0.17, − 0.01) | 0.035 |
| Model 2b | − 0.13 (− 0.22, − 0.03) | 0.011 | − 0.10 (− 0.18, − 0.02) | 0.017 |
| Model 3c | − 0.12 (− 0.22, − 0.02) | 0.015 | − 0.09 (− 0.17, − 0.01) | 0.029 |
| FEV1, L | ||||
| Model 1a | − 0.04 (− 0.11, 0.03) | 0.294 | 0.00 (− 0.06, 0.07) | 0.881 |
| Model 2b | − 0.02 (− 0.09, 0.06) | 0.647 | 0.00 (− 0.07, 0.06) | 0.950 |
| Model 3c | −0.07 (− 0.14, 0.01) | 0.078 | − 0.01 (− 0.07, 0.05) | 0.741 |
| Emphysema index | ||||
| Model 1a | 1.13 (0.25, 2.02) | 0.012 | 0.35 (−0.48, 1.19) | 0.406 |
| Model 2b | 1.08 (−0.08, 2.23) | 0.068 | 0.35 (−0.49, 1.18) | 0.412 |
| Model 3c | 1.13 (0.01, 2.25) | 0.048 | 0.26 (−0.54, 1.07) | 0.519 |
| Mean wall area % | ||||
| Model 1a | 1.04 (0.27, 1.80) | 0.008 | 0.37 (−0.35, 1.10) | 0.311 |
| Model 2b | 0.58 (−0.42, 1.58) | 0.253 | 0.37 (−0.35, 1.09) | 0.317 |
| Model 3c | 0.51 (−0.46, 1.49) | 0.302 | 0.32 (−0.38, 1.02) | 0.373 |
aModel 1 was adjusted for age, gender, education, height, weight, smoking, pack-years, medication use, and job
bModel 2 was adjusted for age, gender, education, height, weight, smoking, pack-years, medication use, job and calendar year at PFT test
cModel 3 was adjusted for age, gender, education, height, weight, smoking, pack-years, medication use, job, calendar year at PFT test, asthma and COPD
There were no significant interactions with the COPD status for the associations between either pollutant and lung function (Table 4). For PM10, there was a significant interactions with smoking status for FVC with association only in ever smokers, (P interaction = 0.011, Table 5) and with sex with associations existing only in the larger group of men (n = 335) (P interaction = 0.021, Table 6). We found no interaction with overweight/obesity.
Table 4.
Effect estimates and 95% confidence intervals of FVC, FEV1, emphysema index, and mean wall area % for interquartile range increases in PM10 (4.4 μg/m3) and NO2 (3.0 ppb) according to COPD status in the CODA cohort
| PM10 | P for interaction | NO2 | P for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Non-COPD (n = 161) | COPD (n = 296) | Non-COPD (n = 161) | COPD (n = 296) | |||||||
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |||
| FVC, L | ||||||||||
| Model 1a | −0.12 (−0.26, 0.03) | 0.117 | −0.13 (− 0.24, − 0.02) | 0.018 | 0.900 | −0.12 (− 0.25, 0.01) | 0.071 | −0.06 (− 0.15, 0.04) | 0.261 | 0.436 |
| Model 2b | −0.09 (− 0.25, 0.06) | 0.226 | − 0.11 (− 0.22, 0.00) | 0.060 | 0.875 | −0.15 (− 0.28, −.02) | 0.024 | −0.06 (− 0.16, 0.04) | 0.231 | 0.256 |
| Model 3c | −0.11 (− 0.26, 0.04) | 0.161 | − 0.13 (− 0.24, − 0.01) | 0.032 | 0.865 | − 0.15 (− 0.28, −.02) | 0.023 | −0.05 (− 0.15, 0.04) | 0.268 | 0.229 |
| FEV1, L | ||||||||||
| Model 1a | − 0.09 (− 0.20, 0.02) | 0.112 | − 0.04 (− 0.12, 0.04) | 0.359 | 0.451 | −0.03 (− 0.13, 0.06) | 0.515 | 0.00 (− 0.07, 0.08) | 0.912 | 0.550 |
| Model 2b | −0.09 (− 0.20, 0.03) | 0.128 | − 0.04 (− 0.12, 0.05) | 0.387 | 0.452 | −0.04 (− 0.14, 0.06) | 0.419 | 0.00 (− 0.07, 0.08) | 0.930 | 0.474 |
| Model 3c | −0.10 (− 0.21, 0.02) | 0.091 | − 0.05 (− 0.13, 0.04) | 0.270 | 0.456 | −0.04 (− 0.14, 0.06) | 0.413 | 0.01 (− 0.07, 0.08) | 0.861 | 0.437 |
| Emphysema index | ||||||||||
| Model 1a | 0.65 (−0.89, 2.19) | 0.405 | 1.55 (0.52, 2.57) | 0.003 | 0.337 | −0.09 (−1.60, 1.38) | 0.908 | 0.47 (− 0.48, 1.42) | 0.332 | 0.523 |
| Model 2b | 0.24 (−1.50, 1.99) | 0.789 | 1.21 (− 0.02, 2.44) | 0.053 | 0.298 | −0.23 (−1.70, 1.23) | 0.756 | 0.51 (−0.43, 1.46) | 0.288 | 0.392 |
| Model 3c | 0.41 (−1.30, 2.15) | 0.641 | 1.39 (0.17, 2.62) | 0.026 | 0.291 | −0.22 (− 1.70, 1.23) | 0.764 | 0.46 (− 0.48, 1.40) | 0.337 | 0.429 |
| Mean wall area % | ||||||||||
| Model 1a | 2.33 (1.00, 3.66) | 0.001 | 0.64 (−0.25, 1.53) | 0.159 | 0.037 | 0.33 (−0.95, 1.61) | 0.614 | 0.34 (− 0.49, 1.17) | 0.417 | 0.985 |
| Model 2b | 1.61 (0.10, 3.12) | 0.037 | 0.05 (−1.00, 1.11) | 0.922 | 0.055 | 0.16 (− 1.10, 1.42) | 0.809 | 0.39 (−0.43, 1.21) | 0.346 | 0.751 |
| Model 3c | 1.65 (0.13, 3.16) | 0.033 | 0.09 (−0.97, 1.16) | 0.862 | 0.055 | 0.16 (−1.10, 1.42) | 0.807 | 0.38 (− 0.44, 1.20) | 0.360 | 0.764 |
aModel 1 was adjusted for age, gender, education, height, weight, smoking, pack-years, medication use, and job
bModel 2 was adjusted for age, gender, education, height, weight, smoking, pack-years, medication use, job and calendar year at PFT test
cModel 3 was adjusted for age, gender, education, height, weight, smoking, pack-years, medication use, job, calendar year at PFT test, and asthma
Table 5.
Effect estimates and 95% confidence intervals of FVC, FEV1, emphysema index, and mean wall area % for interquartile range increases in PM10 (4.4 μg/m3) and NO2 (3.0 ppb) according to smoking status in the CODA cohort
| PM10 | P for interaction | NO2 | P for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Never smoker (n = 165) |
Ever (former/current) smoker (n = 292) | Never smoker (n = 165) |
Ever (former/current) smoker (n = 292) | |||||||
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |||
| FVC, L | ||||||||||
| Model 1a | 0.02 (−0.13, 0.16) | 0.818 | − 0.21 (− 0.32, − 0.10) | 0.000 | 0.011 | −0.05 (− 0.18, 0.07) | 0.410 | −0.11 (− 0.21, − 0.01) | 0.038 | 0.510 |
| Model 2b | 0.02 (− 0.12, 0.17) | 0.760 | − 0.20 (− 0.32, − 0.09) | 0.001 | 0.012 | −0.07 (− 0.20, 0.05) | 0.264 | − 0.11 (− 0.21, − 0.01) | 0.028 | 0.626 |
| Model 3c | 0.03 (− 0.12, 0.18) | 0.683 | − 0.20 (− 0.31, − 0.08) | 0.001 | 0.010 | − 0.07 (− 0.20, 0.06) | 0.279 | − 0.10 (− 0.20, 0.00) | 0.049 | 0.708 |
| FEV1, L | ||||||||||
| Model 1a | 0.04 (− 0.07, 0.16) | 0.432 | − 0.09 (− 0.17, 0.00) | 0.046 | 0.064 | 0.00 (− 0.10, 0.10) | 0.965 | 0.01 (− 0.07, 0.09) | 0.825 | 0.861 |
| Model 2b | 0.06 (− 0.05, 0.18) | 0.302 | −0.07 (− 0.16, 0.02) | 0.151 | 0.071 | − 0.01 (− 0.12, 0.09) | 0.770 | 0.01 (− 0.07, 0.08) | 0.898 | 0.751 |
| Model 3c | 0.00 (−0.11, 0.11) | 0.967 | −0.10 (− 0.19, − 0.02) | 0.020 | 0.133 | −0.04 (− 0.14, 0.05) | 0.408 | 0.01 (− 0.07, 0.08) | 0.843 | 0.429 |
| Emphysema index | ||||||||||
| Model 1a | 1.16 (−0.37, 2.68) | 0.136 | 0.89 (− 0.18, 1.96) | 0.103 | 0.773 | 0.20 (−1.20, 1.62) | 0.781 | 0.42 (−0.61, 1.45) | 0.421 | 0.800 |
| Model 2b | 1.08 (−0.57, 2.73) | 0.200 | 0.79 (−0.56, 2.14) | 0.252 | 0.755 | 0.19 (−1.20, 1.60) | 0.794 | 0.42 (− 0.61, 1.45) | 0.421 | 0.790 |
| Model 3c | 1.45 (−0.16, 3.06) | 0.077 | 0.67 (−0.64, 1.98) | 0.318 | 0.388 | 0.28 (−1.10, 1.65) | 0.686 | 0.24 (−0.75, 1.24) | 0.631 | 0.964 |
| Mean wall area % | ||||||||||
| Model 1a | 0.75 (−0.55, 2.06) | 0.257 | 1.20 (0.28, 2.11) | 0.011 | 0.577 | 1.16 (− 0.05, 2.37) | 0.061 | −0.02 (− 0.90, 0.85) | 0.956 | 0.114 |
| Model 2b | 0.39 (− 1.00, 1.80) | 0.587 | 0.73 (−0.42, 1.88) | 0.215 | 0.755 | 1.14 (−0.06, 2.34) | 0.063 | −0.03 (− 0.90, 0.84) | 0.952 | 0.116 |
| Model 3c | 0.52 (−0.86, 1.90) | 0.459 | 0.53 (−0.60, 1.65) | 0.360 | 0.996 | 1.14 (−0.03, 2.31) | 0.057 | −0.10 (− 0.95, 0.75) | 0.810 | 0.088 |
aModel 1 was adjusted for age, gender, education, height, weight, pack-years, medication use, and job
bModel 2 was adjusted for age, gender, education, height, weight, pack-years, medication use, job and calendar year at PFT test
cModel 3 was adjusted for age, gender, education, height, weight, pack-years, medication use, job, calendar year at PFT test, asthma and COPD
Table 6.
Effect estimates and 95% confidence intervals of FVC and FEV1, emphysema index, and mean wall area % for interquartile range increases in PM10 (4.4 μg/m3) and NO2 (3.0 ppb) by gender in the CODA cohort
| PM10 | P for interaction | NO2 | P for interaction | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male (n = 335) | Female (n = 122) | Male (n = 335) | Female (n = 122) | |||||||
| β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | β (95% CI) | P | |||
| FVC, L | ||||||||||
| Model 1a | −0.20 (−0.30, − 0.10) | 0.000 | 0.02 (− 0.14, 0.19) | 0.762 | 0.021 | −0.11 (− 0.20, − 0.02) | 0.023 | −0.03 (− 0.18, 0.12) | 0.704 | 0.365 |
| Model 2b | −0.19 (− 0.30, − 0.08) | 0.001 | 0.03 (− 0.13, 0.19) | 0.727 | 0.022 | −0.12 (− 0.21, − 0.02) | 0.015 | −0.05 (− 0.20, 0.10) | 0.527 | 0.436 |
| Model 3c | −0.18 (− 0.29, − 0.07) | 0.001 | 0.03 (− 0.13, 0.20) | 0.697 | 0.024 | −0.11 (− 0.20, − 0.02) | 0.022 | −0.04 (− 0.19, 0.11) | 0.608 | 0.430 |
| FEV1, L | ||||||||||
| Model 1a | −0.07 (− 0.15, 0.01) | 0.080 | 0.05 (− 0.08, 0.18) | 0.423 | 0.103 | −0.01 (− 0.08, 0.07) | 0.856 | 0.03 (− 0.08, 0.15) | 0.556 | 0.546 |
| Model 2b | −0.05 (− 0.14, 0.03) | 0.236 | 0.06 (− 0.06, 0.19) | 0.325 | 0.122 | −0.01 (− 0.08, 0.06) | 0.756 | 0.02 (− 0.09, 0.14) | 0.695 | 0.613 |
| Model 3c | −0.09 (− 0.17, − 0.01) | 0.034 | 0.00 (− 0.13, 0.12) | 0.945 | 0.231 | −0.01 (− 0.08, 0.06) | 0.833 | −0.02 (− 0.13, 0.09) | 0.764 | 0.885 |
| Emphysema index | ||||||||||
| Model 1a | 1.15 (0.14, 2.17) | 0.026 | 1.08 (−0.64, 2.80) | 0.219 | 0.942 | 0.42 (−0.54, 1.37) | 0.391 | 0.15 (−1.50, 1.84) | 0.861 | 0.786 |
| Model 2b | 1.09 (−0.21, 2.40) | 0.100 | 1.04 (−0.77, 2.85) | 0.261 | 0.957 | 0.43 (−0.52, 1.38) | 0.374 | 0.08 (−1.60, 1.77) | 0.922 | 0.723 |
| Model 3c | 0.95 (−0.31, 2.21) | 0.139 | 1.54 (−0.22, 3.31) | 0.086 | 0.549 | 0.26 (−0.67, 1.18) | 0.586 | 0.29 (−1.30, 1.93) | 0.724 | 0.968 |
| Mean wall area % | ||||||||||
| Model 1a | 1.30 (0.42, 2.18) | 0.004 | 0.25 (−1.20, 1.74) | 0.744 | 0.227 | 0.19 (−0.64, 1.01) | 0.657 | 0.98 (−0.49, 2.45) | 0.190 | 0.352 |
| Model 2b | 0.86 (−0.27, 1.98) | 0.135 | −0.06 (−1.60, 1.51) | 0.945 | 0.300 | 0.21 (−0.62, 1.03) | 0.624 | 0.89 (−0.57, 2.34) | 0.231 | 0.418 |
| Model 3c | 0.67 (−0.43, 1.77) | 0.234 | 0.15 (−1.40, 1.69) | 0.846 | 0.550 | 0.11 (−0.69, 0.91) | 0.784 | 0.98 (−0.44, 2.40) | 0.177 | 0.295 |
aModel 1 was adjusted for age, education, height, weight, smoking, pack-years, medication use, and job
bModel 2 was adjusted for age, education, height, weight, smoking, pack-years, medication use, job and calendar year at PFT test
cModel 3 was adjusted for age, education, height, weight, smoking, pack-years, medication use, job, calendar year at PFT test, asthma and COPD
Association between air pollution and CT features
For CT features, both the emphysema index and wall area percent were significantly associated with PM10. For an IQR increase in PM10, the emphysema index increased by 1.13 (95% CI = 0.25–2.02, p = 0.012) and the wall area percent increased by 1.04 (95% CI = 0.27–1.80, p = 0.008, Table 3) in our primary model. However, there was no association between NO2 and the CT phenotypes. We repeated the analysis by including the calendar year of the pulmonary function measurement and history of asthma or COPD as a covariate and the associations for PM10 remained significant with the emphysema index, but not with the wall area% (Table 3). We also performed analysis on functional small airway disease and did not find any significant association (regression coefficient = 0.26, 95% CI = − 2.10 - 2.62, p = 0.83).
Stratified analysis by COPD status showed a stronger association of PM10 with the wall area percent among individuals without COPD (P interaction = 0.037, Table 4) There was no significant interaction with smoking status or gender (Tables 5 and 6).
Discussion
In this study, we found that PM10 was associated with lung function, emphysema index, and wall area percent in the Korean CODA cohort. Higher long-term PM10 exposure was related to lower FVC and this association appeared to be limited to men or ever-smokers. We also found significantly different associations between PM10 and wall area percent by COPD status. There was significant association between NO2 and FVC. However, there was no association between NO2 and imaging phenotypes.
While most previous studies of long-term air pollution and lung function in older adults were based on general populations, the current study used a cohort including healthy subjects as well as a substantial proportion of COPD subjects and found that the association with FVC was also significant in the COPD subgroup. Increased ambient air pollution including PM10 and NO2 was associated with decreased lung function in healthy adults from the Study on Air Pollution And Lung Disease In Adults in Switzerland [33]. In middle-aged men and women from the Atherosclerosis Risk in Communities study in the United States, increased traffic-related air pollution was associated with decreased FEV1 and FVC [34]. In middle- to old-aged participants from the Framingham Heart study in the Northeastern United States, long-term exposure to traffic emission and PM2.5 was associated with decreased FEV1 as well as FEV1 decline [35]. In Japanese women, living in areas with a high level of air pollution was associated with large FEV1 decline [36]. In the National Emphysema Treatment Trial study, one of a few studies focusing on COPD patients, an increase in PM2.5 was associated with a rapid decline of FEV1 [37]. Our study suggests that the influence of PM air pollution could be larger for COPD patients than for the general population.
In the current study, a significant association of PM10 was observed with FVC, while no association was found with FEV1. Some studies reported the consistent patterns of stronger associations with FVC than FEV1, while others found the reverse pattern. A recent paper in UK reported higher effect estimates on FVC than FEV1 for PM10, but higher estimates on FEV1 for PM2.5 [9]. Whether PM is associated differently with lung volume or airflow limitation according to the size of the particles should be further investigated.
NO2 is an important marker of traffic-related air pollution and was associated with various endpoints including COPD in previous studies, although we did not find associations with imaging phenotypes. Our cohort of fewer than 500 participants might have not provided sufficient statistical power for detecting an association, although our results showed an association of PM10 with both lung function and CT measurements. Another possible explanation could be different features of pollution sources related to traffic between the two pollutants. With respect to traffic, PM results from re-suspended road dust generated by moving vehicles, tire and brake wear, and tailpipe exhaust, whereas NO2 is mainly emitted in vehicle exhaust. The low correlation coefficient between the two pollutant concentrations (0.44) also supports this explanation. The model performance for NO2 was better than for PM10, which can be explained by the large impact of local pollution sources on NO2 as opposed to PM10 affected by regional sources. The local sources are better characterized by geographic variables which are major input data of our prediction model. R2 values for PM10 are under 0.50 in other national models.
The effects of air pollution and lung function may vary by various factors such as gender, genetics, smoking status, diet, medication, and obesity. Modification by these factors is inconsistent according to the literature. In a previous general population study in Taiwan, the association between air pollution and lung function was stronger in females, the obese, and nonsmokers [38]. However, in the current study, we saw some evidence that men were more susceptible as found in previous studies, possibly because men are likely to spend more time outdoors [9, 39, 40]. However, our study had more men than women to begin with, and more male subjects smoked with a history of COPD, which may have affected our findings. Our results showed a significant association between PM10 and lung function in ever-smokers, but not in never smokers. This is consistent with the findings of the Framingham Heart study showing that former smokers are more susceptible to air pollution [35]. We did not find a significant interaction with overweight in the association with PM10, although there are reports that obesity is a risk factor for air pollution susceptibility. The modifying effects differ according to the population.
Recent studies have revealed that imaging features are associated with adverse clinical outcomes in COPD [11]. To our knowledge, this is the first study to investigate the association between air pollution and CT features in COPD subjects. There were at least three studies based on the general population. The Multi-Ethnic Study of Atherosclerosis (MESA) including 6515 participants showed only weak evidence of the association between PM and NOx and percent emphysema from cardiac CT scans [15]. The MESA study also showed significant associations between long-term exposure to air pollutants and emphysema progression [16]. Among 2545 nonsmoking Framingham CT sub-study participants, there was no evidence of the association between ambient air pollution and radiographic measures of emphysema or airway disease, whereas the odds of emphysema in former smokers increased for living near major roads [14]. In the current study, PM10 exposure was associated with increased emphysema index and wall area percent in participants with or without COPD. The depth of inspiration affects the results of the CT-derived airway measurements. An increase in the depth of inspiration results in a larger airway lumen and smaller airway thickness [41]. The influence of the inspiration level in the upper bronchus is significantly lower than that in the lower bronchus [42]. Therefore, airways were measured in the right apical and left apicoposterior segmental bronchi in our study to standardize the assessment of airway wall thickness, a measure of a chronic bronchitis phenotype. The association with wall thickness differed according to COPD status. PM10 exposure was associated with wall area percent especially in the non-COPD group. Occupational dust/fume exposure was associated with air trapping, and airway wall thickness in men [43] and our previous study of biomass exposure showed an association with wall area percent in smokers [44]. Our current results suggest that ambient air pollution can also influence airway thickening as well as worsen emphysema.
Our study has some limitations to address. First, we used modeled annul-average concentrations of air pollution at subjects’ home addresses at baseline as individual-level long-term exposure to air pollution, without incorporating early exposures in the life course. Household exposure and exposure varying by time-activities were not accounted for either. Future analyses considering highly-resolved exposure estimates with longitudinal address information and time activity data may address the impact of these limitations. We also used annual-average concentrations in the year of 2010 and applied to our cohort data started in 2012. We assumed that the spatial distribution of air pollution concentrations is consistent throughout the study period. Since this is a cohort study which relies on the spatial contrast of air pollution across participants, a change of concentrations over 5 years may not matter as much compared to the change in spatial ranking of high and low pollution areas. Our previous study showed high correlation (Pearson correlation coefficient = 0.94) between 4-year averages for 2009–2012 and annual averages in 2010 across about 300 air quality regulatory monitoring sites [45]. Annual average concentration of PM10 and NO2 were below the South Korean national air quality standard (50 μg/m3 and 30 ppb, respectively). However, these are still higher than the average concentrations and the air quality standards in the US and Europe where many studies reported the associations with respiratory outcomes. Secondly, as some previous epidemiological studies reported, PM2.5 may be strongly associated with COPD compared to PM10 or NO2. It is not feasible to include PM2.5 to this study because national-scale PM2.5 regulatory monitoring data are available since 2015. The sample size is relatively small. However, our strength using standardized spirometry and quantitative CT measurement using a single CT scanner could have allowed us to detect the association. This cohort recruited participants near cement plants, generalizability to areas without such point source may be reduced.
Conclusions
In conclusion, both lung function and imaging phenotypes (emphysema and airway wall thickening) were associated with PM10 exposure in this population of older adults. We found evidence of differences in associations by sex, smoking and COPD status.
Acknowledgements
We thank CT technologists at the Department of Radiology, Kangwon National University Hospital.
Abbreviations
- BMI
Body mass index
- CODA cohort
Chronic obstructive pulmonary disease in dust areas cohort
- COPD
Chronic obstructive pulmonary disease
- CT
Computed tomography
- FEV1
Forced expiratory volume in 1 s
- FVC
Forced vital capacity
- PM
Particulate matter
- HU
Hounsfield unit
Authors’ contributions
WJK, SYK contributed to the study design. Data collection was performed by SHH, SHB, YJH. Data analysis was carried out by SOK. Data interpretation was performed by WJK, SYK, JK, MKL, and SJL. All authors were involved in the preparation of the manuscript. The authors read and approved the final manuscript.
Funding
This study was supported by the Korean Ministry of Environment and National Research Foundation (2017R1A2B4003790, 2018R1A2B6004608)(WJK, SYK), and the National Cancer Center of Korea (NCC-1810220-01) (SYK), and Intramural Research Program of the NIH, National Institute of Environmental Health Sciences (MKL and SJL, ZO1 ES043012).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the Institutional Review Board of the Kangwon National University Hospital, and all participants provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Woo Jin Kim and Sun-Young Kim contributed equally to this work.
Contributor Information
Sung Ok Kwon, Email: kamelon@hanmail.net.
Seok Ho Hong, Email: seokhohong1972@gmail.com.
Young-Ji Han, Email: youngji@kangwon.ac.kr.
So Hyeon Bak, Email: arsgnm17@gmail.com.
Junghyun Kim, Email: jhkimd29@gmail.com.
Mi Kyeong Lee, Email: mikyeong.lee@nih.gov.
Stephanie J. London, Email: london2@niehs.nih.gov
Woo Jin Kim, Email: pulmo2@kangwon.ac.kr.
Sun-Young Kim, Email: sykim@ncc.re.kr.
References
- 1.Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, Brauer M, Burnett R, Cercy K, Charlson FJ, et al. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990-2013; a systematic analysis for the global burden of disease study 2015. Lancet. 2016;388:1659–1724. doi: 10.1016/S0140-6736(16)31679-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the global burden of diseases study 2015. Lancet. 2017;389:1907–1918. doi: 10.1016/S0140-6736(17)30505-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thurston GD, Kipen H, Annesi-Maesano I, Balmes J, Brook RD, Cromar K, De Matteis S, Forastiere F, Forsberg B, Frampton MW, et al. A joint ERS/ATS policy statement: what constitutes an adverse health effect of air pollution? An analytical framework. Eur Respir J. 2017;49:1600419. doi: 10.1183/13993003.00419-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jo YS, Lim MN, Han Y-J, Kim WJ. Epidemiological study of PM(2.5) and risk of COPD-related hospital visits in association with particle constituents in Chuncheon, Korea. Int J Chron Obstruct Pulmon Dis. 2018;13:299–307. doi: 10.2147/COPD.S149469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen R, Yin P, Meng X, Liu C, Wang L, Xu X, Ross JA, Tse LA, Zhao Z, Kan H, Zhou M. Fine particulate air pollution and daily mortality. A nationwide analysis in 272 Chinese cities. Am J Respir Crit Care Med. 2017;196:73–81. doi: 10.1164/rccm.201609-1862OC. [DOI] [PubMed] [Google Scholar]
- 6.Eisner MD, Anthonisen N, Coultas D, Kuenzli N, Perez-Padilla R, Postma D, Romieu I, Silverman EK, Balmes JR. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2010;182:693–718. doi: 10.1164/rccm.200811-1757ST. [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Zhou Y, Liu S, Chen X, Zou W, Zhao D, Li X, Pu J, Huang L, Chen J, et al. Association between exposure to ambient particulate matter and chronic obstructive pulmonary disease: results from a cross-sectional study in China. Thorax. 2017;72:788–795. doi: 10.1136/thoraxjnl-2016-208910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim WJ, Lee CY. Environmental exposures and chronic obstructive pulmonary disease. Mol Cell Toxicol. 2017;13:251–255. [Google Scholar]
- 9.Doiron D, de Hoogh K, Probst-Hensch N, Fortier I, Cai Y, De Matteis S, Hansell AL. Air pollution, lung function and COPD: results from the population-based UK biobank study. Eur Respir J. 2019;54:1802140. doi: 10.1183/13993003.02140-2018. [DOI] [PubMed] [Google Scholar]
- 10.Hoek G. Methods for assessing long-term exposures to outdoor air pollutants. Curr Environ Health Rep. 2017;4:450–462. doi: 10.1007/s40572-017-0169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bhatt SP, Washko GR, Hoffman EA, Jr, Newell JD, Jr, Bodduluri S, Diaz AA, Galban CJ, Silverman EK, Estépar RSJ, Lynch DA, Investigators ftC Imaging advances in chronic obstructive pulmonary disease: insights from COPDGene. Am J Respir Crit Care Med. 2019;199:286–301. doi: 10.1164/rccm.201807-1351SO. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oelsner EC, Hoffman EA, Folsom AR, Carr JJ, Enright PL, Kawut SM, Kronmal R, Lederer D, Lima JAC, Lovasi GS, et al. Association between emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort StudyEmphysema-like lung on CT and all-cause mortality. Ann Intern Med. 2014;161:863–873. doi: 10.7326/M13-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charbonnier J-P, Pompe E, Moore C, Humphries S, van Ginneken B, Make B, Regan E, Crapo JD, van Rikxoort EM, Lynch DA. Airway wall thickening on CT: relation to smoking status and severity of COPD. Respir Med. 2019;146:36–41. doi: 10.1016/j.rmed.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rice MB, Li W, Dorans KS, Wilker EH, Ljungman P, Gold DR, Schwartz J, Koutrakis P, Kloog I, Araki T, et al. Exposure to traffic emissions and fine particulate matter and computed tomography measures of the lung and airways. Epidemiology. 2018;29:333–341. doi: 10.1097/EDE.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adar SD, Kaufman JD, Diez-Roux AV, Hoffman EA, D’Souza J, Stukovsky KH, Rich SS, Rotter JI, Guo X, Raffel LJ, et al. Air pollution and percent emphysema identified by computed tomography in the multi-ethnic study of atherosclerosis. Environ Health Perspect. 2015;123:144–151. doi: 10.1289/ehp.1307951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang M, Aaron CP, Madrigano J, Hoffman EA, Angelini E, Yang J, Laine A, Vetterli TM, Kinney PL, Sampson PD, et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung FunctionLong-term exposure to ambient air pollution and change in emphysema and lung FunctionLong-term exposure to ambient air pollution and change in emphysema and lung function. JAMA. 2019;322:546–556. doi: 10.1001/jama.2019.10255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulin L, Hansel N. Particulate air pollution and impaired lung function [version 1; referees: 3 approved]. F1000Research. 2016;5.201. [DOI] [PMC free article] [PubMed]
- 18.Kim S, Lim M-N, Hong Y, Han S-S, Lee S-J, Kim WJ. A cluster analysis of chronic obstructive pulmonary disease in dusty areas cohort identified three subgroups. BMC Pulm Med. 2017;17:209. doi: 10.1186/s12890-017-0553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee H, Hong Y, Lim MN, Bak SH, Kim M-J, Kim K, Kim WJ, Park HY. Inflammatory biomarkers and radiologic measurements in never-smokers with COPD: a cross-sectional study from the CODA cohort. Chr Respir Dis. 2018;15:138–145. doi: 10.1177/1479972317736293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee MK, Xu C-J, Carnes MU, Nichols CE, Ward JM, Kwon SO, Kim S-Y, Kim WJ, London SJ. The Bc: Genome-wide DNA methylation and long-term ambient air pollution exposure in Korean adults. Clin Epigenetics. 2019;11:37. doi: 10.1186/s13148-019-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim S-Y, Song I. National-scale exposure prediction for long-term concentrations of particulate matter and nitrogen dioxide in South Korea. Environ Pollut. 2017;226:21–29. doi: 10.1016/j.envpol.2017.03.056. [DOI] [PubMed] [Google Scholar]
- 22.Kim W, Kwon S, Han Y, Hong S, Bak SH, Lee M, Kim SY. Long-term exposure to ambient air pollution and CT measurements in a Korean COPD cohort. Am J Respir Crit Care Med. 2019;199:A4913. [Google Scholar]
- 23.Bak SH, Kim S, Hong Y, Heo J, Lim M-N, Kim WJ. Quantitative computed tomography features and clinical manifestations associated with the extent of bronchiectasis in patients with moderate-to-severe COPD. Int J Chron Obstruct Pulmon Dis. 2018;13:1421–1431. doi: 10.2147/COPD.S157953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han Y, Heo Y, Hong Y, Kwon SO, Kim WJ. Correlation between physical activity and lung function in dusty areas: results from the chronic obstructive pulmonary disease in dusty areas (CODA) cohort. Tuberc Respir Dis. 2019;82:311–318. doi: 10.4046/trd.2019.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bak SH, Kwon SO, Han S-S, Kim WJ. Computed tomography-derived area and density of pectoralis muscle associated disease severity and longitudinal changes in chronic obstructive pulmonary disease: a case control study. Respir Res. 2019;20:226. doi: 10.1186/s12931-019-1191-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CPM, Gustafsson P, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 27.Heussel CP, Herth FJF, Kappes J, Hantusch R, Hartlieb S, Weinheimer O, Kauczor HU, Eberhardt R. Fully automatic quantitative assessment of emphysema in computed tomography: comparison with pulmonary function testing and normal values. Eur Radiol. 2009;19:2391–2402. doi: 10.1007/s00330-009-1437-z. [DOI] [PubMed] [Google Scholar]
- 28.Nakano Y, Muro S, Sakai H, Hirai T, Chin K, Tsukino M, Nishimura K, Itoh H, Paré PD, Hogg JC, Mishima M. Computed tomographic measurements of airway dimensions and emphysema in smokers. Am J Respir Crit Care Med. 2000;162:1102–1108. doi: 10.1164/ajrccm.162.3.9907120. [DOI] [PubMed] [Google Scholar]
- 29.Hart JE, Yanosky JD, Puett RC, Ryan L, Dockery DW, Smith TJ, Garshick E, Laden F. Spatial modeling of PM10 and NO2 in the continental United States, 1985-2013;2000. Environ Health Perspect. 2009;117:1690–1696. doi: 10.1289/ehp.0900840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vienneau D, de Hoogh K, Beelen R, Fischer P, Hoek G, Briggs D. Comparison of land-use regression models between Great Britain and the Netherlands. Atmos Environ. 2010;44:688–696. [Google Scholar]
- 31.Young MT, Sandler DP, DeRoo LA, Vedal S, Kaufman JD, London SJ. Ambient air pollution exposure and incident adult asthma in a Nationwide cohort of U.S. Women. Am J Respir Crit Care Med. 2014;190:914–921. doi: 10.1164/rccm.201403-0525OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Consultation WE. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 33.Ackermann-Liebrich U, Leuenberger P, Schwartz J, Schindler C, Monn C, Bolognini G, Bongard JP, Brändli O, Domenighetti G, Elsasser S, et al. Lung function and long term exposure to air pollutants in Switzerland. Study on air pollution and lung diseases in adults (SAPALDIA) team. Am J Respir Crit Care Med. 1997;155:122–129. doi: 10.1164/ajrccm.155.1.9001300. [DOI] [PubMed] [Google Scholar]
- 34.Kan H, Heiss G, Rose KM, Whitsel E, Lurmann F, London SJ. Traffic exposure and lung function in adults: the atherosclerosis risk in communities study. Thorax. 2007;62:873–879. doi: 10.1136/thx.2006.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rice MB, Ljungman PL, Wilker EH, Dorans KS, Gold DR, Schwartz J, Koutrakis P, Washko GR, O’Connor GT, Mittleman MA. Long-term exposure to traffic emissions and fine particulate matter and lung function decline in the Framingham heart study. Am J Respir Crit Care Med. 2015;191:656–664. doi: 10.1164/rccm.201410-1875OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sekine K, Shima M, Nitta Y, Adachi M. Long term effects of exposure to automobile exhaust on the pulmonary function of female adults in Tokyo, Japan. Occup Environ Med. 2004;61:350–357. doi: 10.1136/oem.2002.005934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kariisa M, Foraker R, Pennell M, Buckley T, Diaz P, Criner GJ, Wilkins JR. Short- and long-term effects of ambient ozone and fine particulate matter on the respiratory health of chronic obstructive pulmonary disease subjects. Arch Environ Occup Health. 2015;70:56–62. doi: 10.1080/19338244.2014.932753. [DOI] [PubMed] [Google Scholar]
- 38.Guo C, Zhang Z, Lau AKH, Lin CQ, Chuang YC, Chan J, Jiang WK, Tam T, Yeoh E-K, Chan T-C, et al. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planetary Health. 2018;2:e114–e125. doi: 10.1016/S2542-5196(18)30028-7. [DOI] [PubMed] [Google Scholar]
- 39.Tashkin DP, Detels R, Simmons M, Liu H, Coulson AH, Sayre J, Rokaw S. The UCLA population studies of chronic obstructive respiratory disease: XI. Impact of air pollution and smoking on annual change in forced expiratory volume in one second. Am J Respir Crit Care Med. 1994;149:1209–1217. doi: 10.1164/ajrccm.149.5.8173761. [DOI] [PubMed] [Google Scholar]
- 40.Rice MB, Ljungman PL, Wilker EH, Gold DR, Schwartz JD, Koutrakis P, Washko GR, O’Connor GT, Mittleman MA. Short-term exposure to air pollution and lung function in the Framingham heart study. Am J Respir Crit Care Med. 2013;188:1351–1357. doi: 10.1164/rccm.201308-1414OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petersen J, Wille MMW, Rakêt LL, Feragen A, Pedersen JH, Nielsen M, Dirksen A, de Bruijne M. Effect of inspiration on airway dimensions measured in maximal inspiration CT images of subjects without airflow limitation. Eur Radiol. 2014;24:2319–2325. doi: 10.1007/s00330-014-3261-3. [DOI] [PubMed] [Google Scholar]
- 42.Bakker ME, Stolk J, Reiber JHC, Stoel BC. Influence of inspiration level on bronchial lumen measurements with computed tomography. Respir Med. 2012;106:677–686. doi: 10.1016/j.rmed.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 43.Marchetti N, Garshick E, Kinney GL, McKenzie A, Stinson D, Lutz SM, Lynch DA, Criner GJ, Silverman EK, Crapo JD. Association between occupational exposure and lung function, respiratory symptoms, and high-resolution computed tomography imaging in COPDGene. Am J Respir Crit Care Med. 2014;190:756–762. doi: 10.1164/rccm.201403-0493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji W, Lim MN, Bak SH, Hong S-H, Han S-S, Lee S-J, Kim WJ, Hong Y. Differences in chronic obstructive pulmonary disease phenotypes between non-smokers and smokers. Clin Respir J. 2018;12:666–673. doi: 10.1111/crj.12577. [DOI] [PubMed] [Google Scholar]
- 45.Yi S, Eum Y, Kim H, Sy K. Exploration and application of regulatory PM10 measurement data for developing long-term prediction models in South Korea. J Korean Soc Atmos Environ. 2016;32:114–126. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.