Abstract
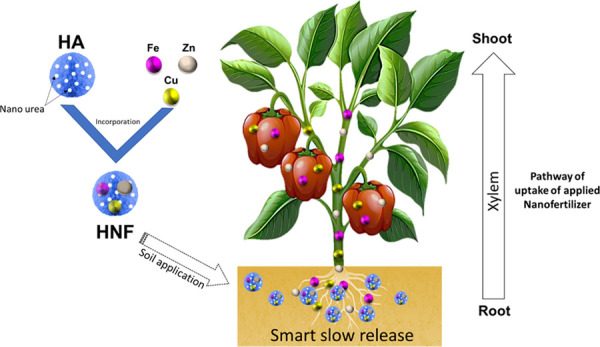
In this work, we have proposed a new formulation of a hybrid nanofertilizer (HNF) for slow and sustainable release of nutrients into soil and water. Urea-modified hydroxyapatite was synthesized, which is a rich source of nitrogen, calcium, and phosphate. Nanoparticles such as copper, iron, and zinc were incorporated into urea-modified hydroxyapatite to increase the efficiency of the proposed fertilizer. Different techniques including powder X-ray powder diffraction, Fourier-transform infrared spectroscopy, and scanning electron microscopy were used to get insight into the properties, morphology, and structure of the as-prepared fertilizer. The developed HNF was used in a field experiment on the ladies’ finger (Abelmoschus esculentus) plant. The slow release of HNF was observed during leaching studies and confirmed the availability of Ca2+, PO43–, NO2–, NO3–, Cu2+, Fe2+, and Zn2+. Furthermore, the presence of Cu2+, Fe2+, and Zn2+ nutrients in ladies’ finger was confirmed by the inductively coupled plasma-optical emission spectrometry (ICP-OES) experiment. A considerable increase in the physicochemical properties such as swelling ratio and water absorption and retention capacities of the proposed fertilizer was observed, which makes the fertilizer more attractive and beneficial compared with the commercial fertilizer. The composition of the proposed HNF was functionally valuable for slow and sustainable release of plant nutrients. The dose of prepared HNF applied was 50 mg/week, whereas the commercial fertilizer was applied at a dose of 5 g/week to A. esculentus. The obtained results showed a significant increase of Cu2+, Fe2+, and Zn2+ nutrient uptake in A. esculentus as a result of slow release from HNF.
1. Introduction
A fertilizer is a key source of soil nutrients, which enhances the plant growth and increases the productivity. Farmers have been using commercial fertilizers extensively for the last 50 years, which contain a balanced distribution of the three main essential nutrients needed for optimum plant growth: nitrogen, phosphorous, and potassium.1 The most commonly used commercial fertilizers are single superphosphate (SSP), triple superphosphate (TSP), urea, nitrogen–phosphorous–potassium (NPK), monoammonium phosphate (MAP), and diammonium phosphate (DAP), which supply the basic plant nutrients such as nitrogen, potassium, and phosphorus.2 The application of these fertilizers results in huge economic losses due to 40–70% of leaching-related problems, which cause dramatic losses in the soil nutrients and decrease the fertility of the soil.3 Furthermore, the use of large-scale commercial fertilizers for a long period is not an appropriate process to enhance the crop productivity because it causes remarkable damage to the soil microbial flora, soil structure, plants, and even to the ecosystem.4 Excess use of fertilizers causes environmental pollution as their residual and unused amounts become pollutants for air, water, and soil. On the other hand, the application of fertilizers that show slow and sustainable release of nutrients is thought to improve nutrient utilization as reported in the literature.5 Therefore, formulation of a new fertilizer is needed to release nutrients slowly and sustainably so that the soil and plants can take up nutrients easily.
Nanotechnology is a promising strategy with enormous potential to solve these agriculture-related problems like decline in land quality, low crop productivity, nutrient deficiency, leaching losses, etc.6 It has been reported that the nanostructure of nanofertilizers provides a high surface area-to-volume ratio that enables plants to take up nutrients slowly and sustainably as needed.7−10 Besides, nanofertilizers have many benefits such as improving soil fertility, reducing nutrient loss, increasing crop yield, lowering environmental pollution, and giving a feasible environment for microorganisms.11 Many researchers have formulated slow-release fertilizers by incorporating hydroxyapatite (HA) and urea to increase delivery of nutrients to the plants.12−14 In this background, we report here a hybrid nanofertilizer (HNF) composition with some extension, which is made by incorporating nanourea-modified hydroxyapatite nanoparticles into copper, iron, and zinc nanoparticles. A hydroxyapatite (Ca10(PO4)6(OH)2) nanoparticle is a continuous source of calcium and phosphate micronutrients, and it is used in surface modification for the formation of nanohybrids. According to the literature, hydroxyapatite (HA) has many biomedical and agricultural applications because of its excellent bioactivity and biocompatibility.15−18 Urea is a water-soluble fertilizer that is extensively used in agriculture as a source of nitrogen nutrients. In place of urea alone, the combination of hydroxyapatite nanoparticles with urea can perform dual roles, that is, the slow release of phosphorus and nitrogen fertilizers. The literature revealed that urea-modified hydroxyapatite nanoparticles with the capability of slow and sustainable release increase the nitrogen agronomic efficiency of plants and reduce the rate of decomposition of urea in the soil.19 Besides, there are different types of micronutrients such as zinc, iron, and copper that are essential for plant growth and each has its application in the agricultural sector. In the agricultural and horticultural sectors, the application of nanoparticles has a comprehensive role in healthy plant growth. Among them, zinc is an essential micronutrient that produces growth hormones and chloroplast.20 Iron nanoparticles have a potential role in plants as a fertilizer, as it can enhance photosynthesis efficiency and nutrient absorption.21−23 Copper nanoparticles play an important role as an antibacterial and antimicrobial agent in the formation of chlorophyll, enhancing porosity and taking part in some enzyme processes.24
The present work aims to produce HNF for the slow and sustainable release of micronutrients that can be made available to soil and fruits. All experiments are performed on ladies’ finger plants for a comparative study of prepared fertilizers with commercial fertilizers. It is shown that this proposed fertilizer will eliminate the leaching problem of commercial fertilizers as well as reduce the nutritional deficiencies of the plant and provide nutrient-rich fruits, which will help in reducing the nutritional deficiencies of human beings.
2. Materials and Methods
2.1. Chemicals
All of the chemicals used in the study were at their highest integrity. Calcium hydroxide (Ca(OH)2) was purchased from Xilong Scientific Co., Ltd., (China). Orthophosphoric acid (H3PO4) and sodium hydroxide (NaOH) were purchased from Sigma-Aldrich (China). Trisodium citrate (Na3C6H5O7) was obtained from Hangzhou Dayangchem Co., Ltd. (China). Urea molecules (CO(NH2)2) and other chemicals including zinc chloride (ZnCl2), copper chloride (CuCl2), and ferrous chloride (FeCl2) were purchased from Zouping Zhijin New Material Technology Co., Ltd. (China) and used without any further purification. During the experimental period, ultrapure water (Evoqua Type-I, Germany; resistivity <18.2 MΩ) was used for all of the preparations and measurements.
2.2. Synthesis of Hydroxyapatite Nanoparticles
Hydroxyapatite nanoparticles were synthesized using an aqueous solution of calcium hydroxide and orthophosphoric acid. A suspension of Ca(OH)2 (19.29 g, 250 mL of distilled water) was prepared and allowed to mix for 25 min. By following the drop-cast method (10 mL/min), 250 mL of 0.6 M orthophosphoric acid solution was added from a buret to a Ca(OH)2 suspension and allowed to stir under mechanical agitation (1200 rpm) for 1 h.25 The chemical reaction that takes place is as follows
The resulting milky solution was refrigerated for 24 h to obtain the precipitated hydroxyapatite nanoparticles.
2.3. Synthesis of Nanourea
Nanourea was prepared by mixing urea molecules and trisodium citrate under optimum conditions. First, 0.30 g of urea molecules was mixed with 0.86 g of trisodium citrate in a beaker. The mixture was then heated gradually up to 90 °C for 1 h using a hot plate. Trisodium citrate worked as a physiologically active substance or nitrification inhibitor for nanourea production. After completion of the heating process, the color of the solution became ash, indicating the presence of nanourea.14 Further, the morphology of prepared nanourea was analyzed by scanning electron microscopy (SEM).
2.4. Preparation of Nanourea-Modified Hydroxyapatite Nanoparticles
The treated suspension of hydroxyapatite nanoparticles (100 mL) was mixed with 0.05 g of synthesized nanourea. The dispersion was prepared under ultrasound sonication (30 kHz for 1 h). Moreover, the mixture was allowed to settle, and the excess liquid was drained off. The resulting mixture was further centrifuged and washed three times with distilled water. Finally, nanourea-modified hydroxyapatite nanoparticles were dried at 100 °C for 2 h and finely powdered with a hand mortar. The powder was characterized using SEM, X-ray diffraction (XRD), and Fourier-transform infrared spectroscopy (FTIR).
2.5. Synthesis of Cu, Fe, and Zn Nanoparticles
NaOH (1.0 M) was dissolved in distilled water, and the solution was stirred at 90 °C. A 0.5 M CuCl2 solution was added dropwise to the NaOH solution for 26 min. This time, the mixture was stirred for 2 h at 90 °C and kept overnight to form a precipitate. The suspension was then filtered and washed with deionized water several times. After washing, the suspension was dried at 70 °C to obtain Cu nanoparticles.26,27 Similarly, Fe and Zn nanoparticles were synthesized by the chemical precipitation method using FeCl2 and ZnCl2, respectively.28
2.6. Preparation of HNF
For the preparation of HNF, 3 g of nanourea-modified hydroxyapatite nanoparticles was taken in a beaker and Cu, Fe, and Zn nanoparticles (5 g each) were gently mixed together. The resulting HNF was stored in an airtight container for further use.
2.7. Treatment of HNF on Abelmoschus esculentus Plants
For the experiment, soil and various types of plants were collected from a local tree nursery. A. esculentus was treated to assure the acceptability of the prepared HNF. Differential behavior was analyzed by applying 50 mg of synthesized HNF, and 5 g of commercial fertilizer was separately applied to A. esculentus plants.
2.8. Characterization of HNF
FTIR spectroscopy was utilized to identify the presence a functional group in the HNF. To investigate the structural behavior and the formation of the HNF, an XRD study was performed. SEM images were taken to observe the surface morphology and calculate the size of different nanoparticles. The physical properties including pH and total dissolved solids (TDS) were measured using a conductivity meter (digital multiparameter tester kit, AI316-PC60).
2.9. Swelling Ratio and Equilibrium Water Content (EWC)
The swelling ratio (SR) is expressed as the raised weight of the sample due to water soaking. In this study, 0.30 g of HNF was dipped in a beaker and treated with 60 mL of distilled water. Then, the sample was swelled for 24 h at ambient temperature and pressure. Finally, the treated wet sample was filtered and the weight of the existing yields was measured.29
The SR and equilibrium water content (EWC) of the fertilizer can be determined by
| 1 |
| 2 |
where ws is the wet weight of the HNF and wd is the dry weight of the HNF.
2.10. Water Absorption Capacity (WAC) Analysis
Water absorption capacity (WAC) is the percentage of water that a plant can absorb the maximum amount of moisture for a certain period. To calculate the water absorption capacity, 0.3 g of the sample (w1) and two weighed beakers (w2) were kept in a desiccator in a wet environment for 5 days. After 5 days, the sample (w3) with the beakers was reweighed and kept in a desiccator, and WAC was then calculated. The water absorption capacity of the HNF was determined using the following equation30
| 3 |
where w1 is the weight of HNF, w2 is the weight of the beakers, and w3 is the weight of moist beakers with HNF after 5 days.
2.11. Water Retention Capacity Analysis
The water retention capacity (WRC) measures how much water a soil can hold for a while. First, the weight of a cup was measured through which water can seep. For the blank soil test, 50 g of soil and 0.5 g of HNF was mixed with distilled water in the cup. WRC was calculated using eq 4.(31) The procedure continued for the next several days.
| 4 |
where w1 is the weight of HNF and soil after soaking with water and w2 is the weight of HNF and soil after soaking with water after a 24 h interval.
2.12. Slow-Release Studies
Slow-release studies were performed for HNF to determine the leaching pattern of nutrients. For slow-release studies of tap water, 50 mg of the prepared HNF was mixed with 250 mL of normal tap water. Then, 100 mL water sample was collected from the mixture at the intervals of 1, 7, and 14 days, respectively. For soil tests, the collected soil was sieved and mixed with 50 mg of the prepared HNF. Further, 200 mL of tap water was added to the soil mixed with HNF. Water was added in such a way that the soil should be saturated with water. Then, the released water from the soil was collected to be analyzed. The nutrient release patterns of the sample were analyzed after 1, 7, and 14 days, respectively. Then, the measured values were compared with nutrient release patterns of the untreated soil.
2.13. A. esculentus Digestion
The real field experiments were carried out in a farming field using the control (no treatment) and the commercial fertilizer containing soluble micronutrients for comparison. Experiments were carried out at the Jashore district, Bangladesh, during the months of August to November, 2019. The temperature was about 30 °C in the day time, and the field trials were carried out following the randomized complete block design with an area of 15 m2. All of the trials were carried out in triplicate. The cultivated A. esculentus was separated, washed with deionized water, and dried under sunlight for a certain period. Then, the sample was dried in a horizontal drying oven at 60 °C for 14 h and weighed. After that, 1.5 g of the dried sample was added to 10 mL of nitric acid and heated at 95 °C. After heating, when the sample became 2 mL, it was cooled and 2 mL of hydrogen peroxide was added to the sample. The sample was diluted to a 50 mL solution, filtered, and stored in a refrigerator at 4 °C for further analysis. Isotope quantification such as that of copper, zinc, iron, and other nutrients in A. esculentus was carried out by inductively coupled plasma-optical emission spectrometry (ICP-OES) measurements.
3. Results and Discussion
3.1. Characterization of HNF
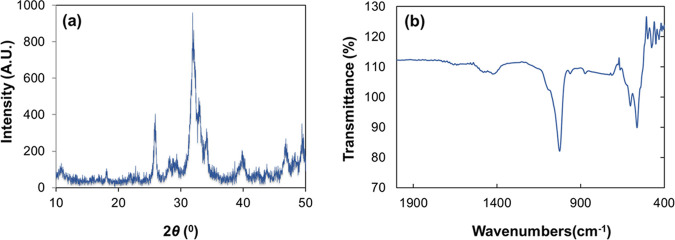
The powder X-ray diffraction (XRD) pattern of the nanourea-modified HA nanoparticles is shown in Figure 1a. XRD was performed to determine the crystalline nature of the nanourea-modified HA nanoparticles. From the XRD graph, the peaks observed at 2θ values of 25.9, 32.04, 32.98, and 34° are corresponding to the reflection plane structure of the crystalline HA nanoparticles.32 The XRD pattern of pure urea showed lower-intensity peaks at 23, 25, 32, 36, and 37°. Due to the strong interactions of urea with the HA nanoparticles, the intensity of the peak at the 2θ value of 22.1° corresponds to the significant breakdown of the crystalline structure of urea.33 The findings suggest that prominent bonding modes are metal–ligand interactions between N atoms in urea and Ca atoms in HA nanoparticles.
Figure 1.
XRD pattern (a) and FTIR spectra (b) of the nanourea-modified HA nanoparticles.
FTIR absorption spectrum of the synthesized urea-modified HA nanoparticles is shown in Figure 1b. The P–O stretching of PO43– ions in hydroxyapatite was represented as a sharp intensity peak with the wavenumber value of 1050 cm–1. After the modification process, the N–H bending motion of urea shifted from 1593 to 1614 cm–1. This shift indicates the presence of free N–H bonds in the urea-modified HA nanoparticles. The carbonyl stretching vibration is observed at 1657 cm–1 in urea-modified HA nanoparticles. This is attributed to affecting the C=O group assigned to the N–H hydrogen bonding of HA. It is worth mentioning that the N–C–N stretching vibration peak of urea shifted to a lower intensity of 472 cm–1 in the urea-modified HA nanoparticles.
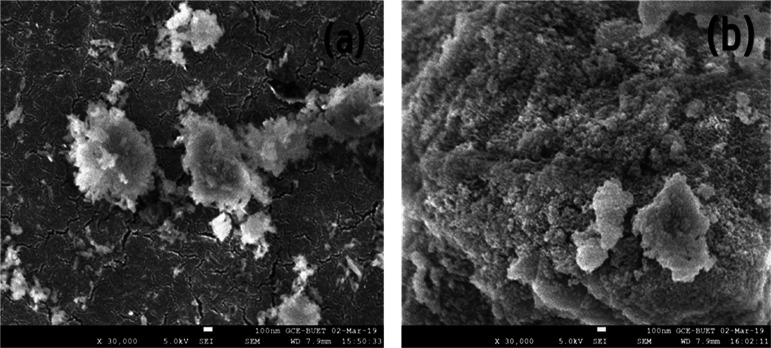
SEM analysis was conducted to get insight into the morphologies of the synthesized nanourea and nanourea-modified HA nanoparticles, which are presented in Figure 2a,b. According to the analysis, nanourea particles were of different sizes and possessed a fiberlike structure (Figure 2a). The average size of nanourea particles was calculated to be 39.76 nm. However, the average size of the urea-modified HA nanoparticles was calculated to be 38.21 nm (Figure 2b).
Figure 2.
SEM micrographs of synthesized (a) nanourea and (b) nanourea-modified HA nanoparticles.
3.2. Physical Parameter Test
3.2.1. Swelling Ratio, Water Absorption Capacity, and Equilibrium Water Content
After analyzing the sample, the weight of the HNF was observed to be increased. The SR and EWC of the HNF were calculated to be 2.784 and 74%, respectively, by following eqs 1 and 2 with respect to the used quantity of the prepared sample. These values show that the water content and the released nutrients remain constant and that the nutrients were delivered slowly and efficiently. The water absorption capacity of the HNF was calculated to be 80%, which is an excellent capacity. This results in increasing the long-term water holding capacity of the plant and crop nutrients, which is a beneficial characteristic of the proposed fertilizer. HNF has high porosity due to which it can hold water and nutrients for a longer time.34
3.2.2. Water Retention Capacity
A comparative study on WRC of the HNF-mixed soil and soil without HNF is graphically illustrated in Figure 3. WRCs of the soil without HNF were calculated to be 82, 69, and 51.3% on 5th, 10th, and 15th days, respectively. However, in the case of HNF, the water retention capacities of the soil for the same reference days were calculated to be 97, 75.6, and 57.8%, respectively. This study shows that HNF has higher water accessibility and availability to the soil, which makes this fertilizer an effective alternative for enhancing plant health.
Figure 3.
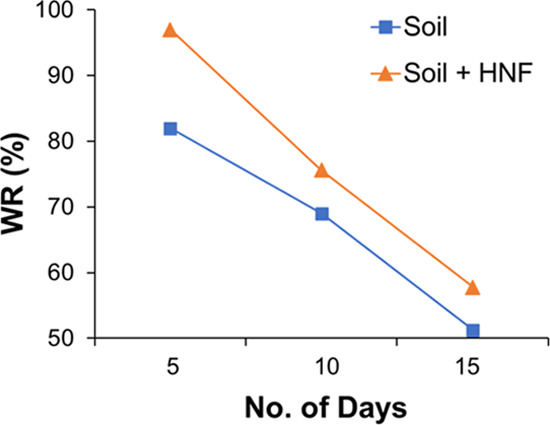
WRC of soil without (blue line) and with (orange line) HNF.
3.2.3. pH, Conductivity, and Total Dissolved Solids (TDSs)
The pH level of the soil indicates its acidic level, which is necessary to be considered because different plants require different pH levels for proper growth. If the pH level of the composite is not maintained, it can negatively affect the plant’s growth. It was noted that the pH level decreased after incorporating the nanoparticles into the urea-modified HA nanoparticles. However, when water was treated with the prepared HNF, the conductivity significantly increased. The total dissolved solids (TDSs) in the tap water without and with the addition of HNF were measured to be 400 and 800 mg/L, respectively, and TDSs in the soil without and with the addition of the HNF were 800 and 1200 mg/L, respectively. The conductivity measurement was also carried out for water and soil with and without HNF addition. The results are summarized in Table 1.
Table 1. Physicochemical Properties of Soil and Water with and without HNF.
| soil with HNF |
water with HNF |
|||||||
|---|---|---|---|---|---|---|---|---|
| parameter | blank soil | tap water | 1 day | 7 days | 14 days | 1 day | 7 days | 14 days |
| pH | 8.0 | 7.8 | 7.9 | 7.8 | 7.7 | 7.6 | 7.6 | 7.5 |
| conductivity (μS/cm) | 905 | 732 | 1050 | 1127 | 1187 | 960 | 1015 | 1090 |
| TDS (mg/L) | 800 | 400 | 1200 | 1400 | 1600 | 800 | 1000 | 1200 |
3.3. Nutrient Release Studies
The release study is related to analyses of nutrient release patterns of the synthesized HNF. The studies were performed on both normal tap water and soil water. After incorporating the fertilizer, the nutrient release data were collected daily and compared with tap water and fertilizer-free soil water. For selecting a suitable fertilizer for plants, we found this analysis to be an efficient approach. The results obtained by this investigation show the exact release of specific nutrients present in the HNF. Further, the concentration of the release pattern was investigated by measuring the release percentage in the following period.
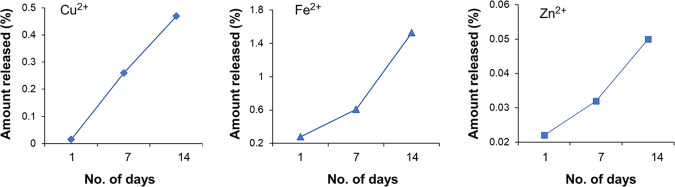
Release studies were performed for 14 days in pre-analyzed soil, which show that the release of Cu2+ nutrients increases with a sequential increase in time interval as can be seen in Figure 4. Initially, the release percentage of both Fe2+ and Zn2+ nutrients increases with time slightly, but after 7 days, it increases more rapidly.
Figure 4.
HNF release (percentage) pattern for nutrients (Cu2+, Fe2+, and Zn2+) in the soil for 14 day studies.
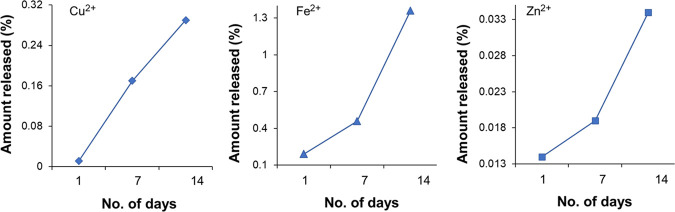
In the case of tap water, the 14 day release studies show the same result as in soil. The release of Cu2+ nutrients increases gradually with time, but for Fe2+ and Zn2+ nutrients, the percentage of release rapidly increases after 7 days as shown in Figure 5.
Figure 5.
HNF release (percentage) pattern for nutrients (Cu2+, Fe2+, and Zn2+) in water for 14 days.
The obtained result shows that the increase of nutrients such as calcium, phosphate, nitrite, and nitrate for the initial day is quite normal due to the porous structure of nanourea-modified hydroxyapatite nanoparticles and nutrient holding capacity. The 7 day release pattern shows the continuity of increasing nutrients with time as can be seen in Figure 6. This gradually increases the nutrients, which are greater than those in normal tap water and fertilizer-free soil water. The 14 day analysis showed the same trend of a continual supply of nutrients to plants as observed in the previous studies. These studies ensure the long-term accessibility of nutrients to the plants, which enhances the healthy growth of plants that is lacking in the traditional fertilizer. The HNF provides abundant nutrients that help plants for early flowering and fruiting. Moreover, the use of HNF is more beneficial than the use of the traditional fertilizer.
Figure 6.
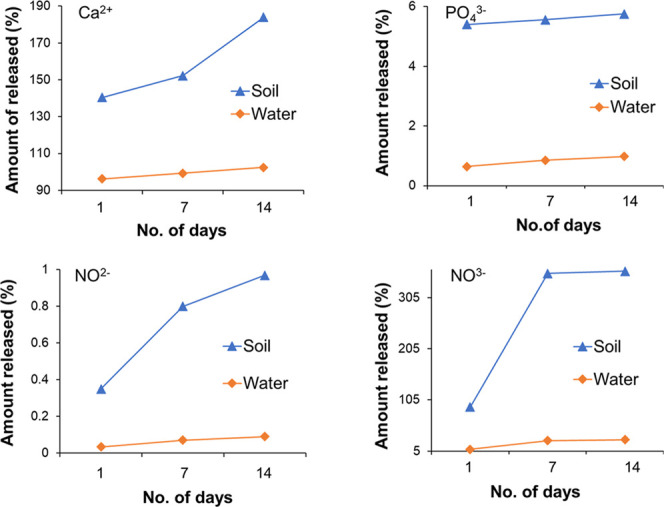
Nanourea-modified hydroxyapatite nanoparticles’ release (percentage) pattern for nutrients (Ca2+, PO43–, NO2–, NO3–) in the soil and water for 14 days.
3.4. Availability of Nutrients in Fruit
The nutritional food quality in the grain is improved by micronutrients, which are needed in small amounts. Micronutrients mainly include copper, zinc, iron, cobalt, nickel, chlorine, boron, and manganese. HNF possesses three key micronutrients, that is, copper, iron, and zinc, in a single compound, whereas the other fertilizers provide individual micronutrients. This single fertilizer provides a slow release of major micronutrients, which is more beneficial to the plants. Here, slow-release applications of these micronutrients were performed on A. esculentus. A great increase in total uptake of copper, iron, zinc, and other nutrients was observed in the fruit by following the application of HNF, as presented in Table 2.
Table 2. Nutrient Uptake by Plant A. esculentus in ppm.
|
A. esculentus |
||
|---|---|---|
| parameters | commercial fertilizer | HNF |
| As | –0.2789 | –0.3688 |
| Cu | 0.0203 | 0.3269 |
| Fe | 1.1639 | 3.3319 |
| Pb | –0.0224 | 0.2331 |
| Cd | 0.0908 | 0.1671 |
| Zn | 0.0091 | 1.3335 |
| Na | 26.2776 | 15.5195 |
| Mg | 48.1892 | 44.5961 |
| K | 9.1269 | 14.8050 |
| Ca | 14.6292 | 14.9706 |
| Ni | 0.0340 | 0.1215 |
| Se | –0.0922 | –0.1148 |
The increased nutrient uptake efficiency of A. esculentus at a dose of 50 mg/week of HNF is statistically significant. The proposed nanofertilizer increases the uptake ratio of nutrients in both soil and water (Figures 4 and 5) for which a small dosage of HNF was effective for the plant. It also saves fertilizer resources and reduces the expenditure on fertilizers. Nutrients appeared to have stabilized at this dosage. Increases in Pb, Cd, K, Ca, Ni, and Se nutrient uptake were observed at that dosage of the slow-release fertilizer.
The HNF fertilizer has a particle size less than the pore size of leaves as well as roots of the A. esculentus plant, which can rise percolation into the plant from the soil and enhance the uptake efficiency of nutrients in the fruit. Plant fertilized with HNF presented the highest Cu, Fe, and Zn contents in fruits, but a comparatively small amount of these contents was found in the fruit fertilized with the commercial fertilizer, as graphically presented in Figure 7. The percentages of Cu, Fe, and Zn nutrients in the commercial fertilizer are 0.0203, 1.1639, and 0.0091, respectively, whereas in HNF, these values are significantly increased to 0.3269, 3.3319, and 1.33, respectively. Moreover, HNF helps to produce nutrient-rich fruits that compensate for the deficiency of these nutrients in the human body.
Figure 7.
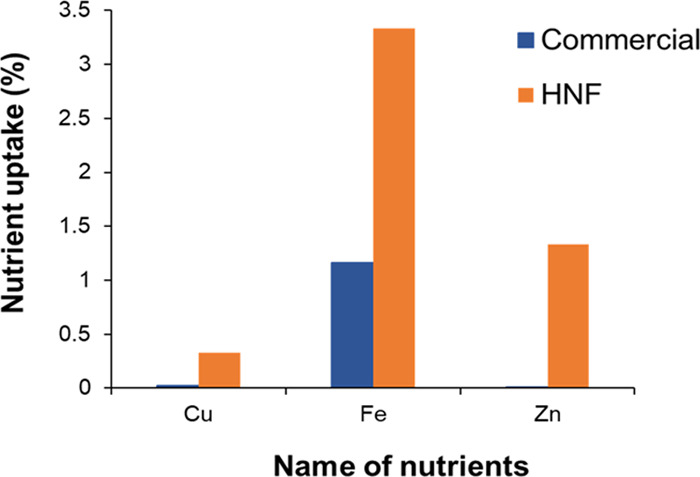
Total uptake of Cu, Fe, and Zn nutrients by A. esculentus.
4. Conclusions
We have successfully synthesized HNF that is functionally valuable for the slow and sustained release of urea and nutrients into the soil. Our result suggests that HNF has potential for a slow release of Ca2+, PO43–, NO2–, NO3–, Cu2+, Fe2+, and Zn2+ nutrients. This nanofertilizer was applied on A. esculentus and showed maximum nutrient use efficiency and higher yields. It was noticed that HNF increases Cu2+, Fe2+, and Zn2+ nutrient uptake efficiency more than the commercial fertilizer within a few days. The slow-release study of HNF was conducted for up to 14 days. This work concluded that HNF has great advantages as a fertilizer including the slow and sustainable nutrient release, low dosing (50 mg/week), low cost, nutrient-rich fruits, and negligible land contamination.
Acknowledgments
This project was done with financial support from the Ministry of Education, Bangladesh (Project ID: PS-2018774).
The authors declare no competing financial interest.
References
- Glass A. D. M. Nitrogen Use Efficiency of Crop Plants: Physiological Constraints upon Nitrogen Absorption. Crit. Rev. Plant Sci. 2003, 22, 453–470. 10.1080/07352680390243512. [DOI] [Google Scholar]
- Lubkowski K. Environmental impact of fertilizer use and slow release of mineral nutrients as a response to this challenge. Pol. J. Chem. Technol. 2016, 18, 72–79. 10.1515/pjct-2016-0012. [DOI] [Google Scholar]
- Ditta A.; Arshad M. Applications and perspectives of using nanomaterials for sustainable plant nutrition. Nanotechnol. Rev. 2016, 5, 209–229. 10.1515/ntrev-2015-0060. [DOI] [Google Scholar]
- Solanki P.; Bhargava A.; Chhipa H.; Jain N.; Panwar J.. Nano-fErtilizers and Their Smart Delivery System. In Nanotechnologies in Food and Agriculture; Springer International Publishing, 2015; pp 81–101. [Google Scholar]
- Duan L.-L.; Zhang M.; Liu G.; Shang Z.-C.; Yang Y. Nutrient release characteristics and use efficiency of slow? and controlled release fertilizers. Chin. J. Appl. Ecol. 2009, 20, 1118–1124. [PubMed] [Google Scholar]
- He X.; Deng H.; Hwang H.-m. The current application of nanotechnology in food and agriculture. J. Food Drug Anal. 2019, 27, 1–21. 10.1016/j.jfda.2018.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein M. Z.; Mohamad Jaafar A.; Yahaya A. H.; Zainal Z. Inorganic-based phytohormone delivery vector of 2-chloroethylphosphonate nanohybrid: a new stimulating compound with controlled release property to increase latex production. J. Exp. Nanosci. 2010, 5, 310–318. 10.1080/17458080903531013. [DOI] [Google Scholar]
- Sastry R. K.; Rashmi H. B.; Rao N. H.; Ilyas S. M. Integrating nanotechnology into agri-food systems research in India: A conceptual framework. Technol. Forecast. Soc. Change 2010, 77, 639–648. 10.1016/j.techfore.2009.11.008. [DOI] [Google Scholar]
- Hossain K.-Z.; Monreal C. M.; Sayari A. Adsorption of urease on PE-MCM-41 and its catalytic effect on hydrolysis of urea. Colloids Surf., B 2008, 62, 42–50. 10.1016/j.colsurfb.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Monreal C. M.; Derosa M.; Mallubhotla S. C.; Bindraban P. S.; Dimkpa C. Nanotechnologies for increasing the crop use efficiency of fertilizer-micronutrients. Biol. Fertil. Soils 2016, 52, 423–437. 10.1007/s00374-015-1073-5. [DOI] [Google Scholar]
- Liu M.; Liang R.; Zhan F.; Liu Z.; Niu A. Synthesis of a slow-release and superabsorbent nitrogen fertilizer and its properties. Polym. Adv. Technol. 2006, 17, 430–438. 10.1002/pat.720. [DOI] [Google Scholar]
- Xiong L.; Wang P.; Hunter M. N.; Kopittke P. M. Bioavailability and movement of hydroxyapatite nanoparticles (HA-NPs) applied as a phosphorus fertiliser in soils. Environ. Sci.: Nano 2018, 5, 2888–2898. 10.1039/C8EN00751A. [DOI] [Google Scholar]
- Rop K.; Karuku G. N.; Mbui D.; Michira I.; Njomo N. Formulation of slow release NPK fertilizer (cellulose-graft-poly(acrylamide)/nano-hydroxyapatite/soluble fertilizer) composite and evaluating its N mineralization potential. Ann. Agric. Sci. 2018, 63, 163–172. 10.1016/j.aoas.2018.11.001. [DOI] [Google Scholar]
- Subbaiya M. R.; Priyanka M.; Selvam M. M. Formulation of Green Nano-Fertilizer to Enhance the Plant Growth through Slow and Sustained Release of Nitrogen. J. Pharm. Res. 2012, 5, 5178–5183. [Google Scholar]
- Cao H.; Zhang L.; Zheng H.; Wang Z. Hydroxyapatite nanocrystals for biomedical applications. J. Phys. Chem. C. 2010, 114, 18352–18357. 10.1021/jp106078b. [DOI] [Google Scholar]
- Teng S.-H.; Lee E.-J.; Wang P.; Jun S.-H.; Han C.-M.; Kim H.-E. Functionally gradient chitosan/hydroxyapatite composite scaffolds for controlled drug release. J. Biomed. Mater. Res., Part B 2008, 90B, 275–282. 10.1002/jbm.b.31283. [DOI] [PubMed] [Google Scholar]
- Mateus A. Y. P.; Barrias C. C.; Ribeiro C.; Ferraz M. P.; Monteiro F. J. Comparative study of nanohydroxyapatite microspheres for medical applications. J. Biomed. Mater. Res., Part A. 2008, 86A, 483–493. 10.1002/jbm.a.31634. [DOI] [PubMed] [Google Scholar]
- Ferraz M. P.; Mateus A. Y.; Sousa J. C.; Monteiro F. J. Nanohydroxyapatite microspheres as delivery system for antibiotics: Release kinetics, antimicrobial activity, and interaction with osteoblasts. J. Biomed. Mater. Res., Part A 2007, 81A, 994–1004. 10.1002/jbm.a.31151. [DOI] [PubMed] [Google Scholar]
- Gao X.; Li C.; Zhang M.; Wang R.; Chen B. Controlled release urea improved the nitrogen use efficiency, yield and quality of potato (Solanum tuberosum L.) on silt loamy soil. Field Crops. Res. 2015, 181, 60–68. 10.1016/j.fcr.2015.07.009. [DOI] [Google Scholar]
- Palmer C. M.; Guerinot M. L. Facing the challenges of Cu, Fe and Zn homeostasis in plants. Nat. Chem. Biol. 2009, 5, 333–340. 10.1038/nchembio.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui M.; Ma C.; Hao Y.; Guo J.; Rui Y.; Tang X.; Zhao Q.; Fan X.; Zhang Z.; Hou T.; Zhu S. Iron Oxide Nanoparticles as a Potential Iron Fertilizer for Peanut (Arachis hypogaea). Front. Plant Sci. 2016, 7, 815 10.3389/fpls.2016.00815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombuloglu H.; Slimani Y.; Tombuloglu G.; Almessiere M.; Baykal A. Uptake and translocation of magnetite (Fe3O4) nanoparticles and its impact on photosynthetic genes in barley (Hordeum vulgare L.). Chemosphere 2019, 226, 110–122. 10.1016/j.chemosphere.2019.03.075. [DOI] [PubMed] [Google Scholar]
- Rajabi H. R.; Khani O.; Shamsipur M.; Vatanpour V. High-performance pure and Fe3+-ion doped ZnS quantum dots as green nanophotocatalysts for the removal of malachite green under UV-light irradiation. J. Hazard. Mater. 2013, 250–251, 370–378. 10.1016/j.jhazmat.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Abbasifar A.; Shahrabadi F.; ValizadehKaji B. Effects of green synthesized zinc and copper nano-fertilizers on the morphological and biochemical attributes of basil plant. J. Plant Nutr. 2020, 43, 1104–1118. 10.1080/01904167.2020.1724305. [DOI] [Google Scholar]
- Kottegoda N.; Sandaruwan C.; Priyadarshana G.; Siriwardhana A.; Rathnayake U. A.; Berugoda Arachchige D. M.; Kumarasinghe A. R.; Dahanayake D.; Karunaratne V.; Amaratunga G. A. J. Urea-Hydroxyapatite Nanohybrids for Slow Release of Nitrogen. ACS Nano 2017, 11, 1214–1221. 10.1021/acsnano.6b07781. [DOI] [PubMed] [Google Scholar]
- Luna I. Z.; Hilary L. N.; Chowdhury A. M. S.; Gafur M. A.; Khan N.; Khan R. A. Preparation and Characterization of Copper Oxide Nanoparticles Synthesized via Chemical Precipitation Method. OALib 2015, 2, 1–8. 10.4236/oalib.1101409. [DOI] [Google Scholar]
- Yusoff A. H. M.; Salimi M. N.; Jamlos M. F.. Synthesis and Characterization of Biocompatible Fe3O4 Nanoparticles at Different pH, AIP Conference Proceedings; American Institute of Physics Inc., 2017; p 020010.
- Wu C.; Qiao X.; Chen J.; Wang H.; Tan F.; Li S. A novel chemical route to prepare ZnO nanoparticles. Mater. Lett. 2006, 60, 1828–1832. 10.1016/j.matlet.2005.12.046. [DOI] [Google Scholar]
- He X.-s.; Liao Z.-w.; Huang P.-z.; Duan J.-x.; Ge R.-s.; Li H.-b.; Geng Z.-c. Characteristics and Performance of Novel Water-Absorbent Slow Release Nitrogen Fertilizers. Agric. Sci. China 2007, 6, 338–346. 10.1016/S1671-2927(07)60054-6. [DOI] [Google Scholar]
- Hamid N. N. A.; Mohamad N.; Hing L. Y.; Dimin M. F.; Azam M. A.; Hassan M. H. C.; Ahmad M. K. S. M.; Shaaban A. The effect of chitosan content to physical and degradation properties of biodegradable urea fertilizer. J. Sci. Innovative Res. 2013, 2, 893–902. [Google Scholar]
- Mohamad N.; Nor Nadiah A. H.; Jeefferie A. R.; Mohd Fairuz D. Effect of chitosan gelatinization temperature on water absorption and water retention of chitosan-based urea fertilizer. Int. J. Automot. Mech. Eng. 2013, 8, 1357–1366. 10.15282/ijame.8.2013.23.0111. [DOI] [Google Scholar]
- Surmenev R. A.; Shkarina S.; Syromotina D. S.; Melnik E. V.; Shkarin R.; Selezneva I. I.; Ermakov A. M.; Ivlev S. I.; Cecilia A.; Weinhardt V.; Baumbach T.; Rijavec T.; Lapanje A.; Chaikina M. V.; Surmeneva M. A. Characterization of biomimetic silicate- and strontium-containing hydroxyapatite microparticles embedded in biodegradable electrospun polycaprolactone scaffolds for bone regeneration. Eur. Polym. J. 2019, 113, 67–77. 10.1016/j.eurpolymj.2019.01.042. [DOI] [Google Scholar]
- Giroto A. S.; Fidélis S. C.; Ribeiro C. Controlled release from hydroxyapatite nanoparticles incorporated into biodegradable, soluble host matrixes. RSC Adv 2015, 5, 104179–104186. 10.1039/C5RA17669G. [DOI] [Google Scholar]
- Wu L.; Liu M.; Liang R. Preparation and properties of a double-coated slow-release NPK compound fertilizer with superabsorbent and water-retention. Bioresour. Technol. 2008, 99, 547–554. 10.1016/j.biortech.2006.12.027. [DOI] [PubMed] [Google Scholar]