Abstract
Multicomponent reaction (MCR) is often used to rapidly assemble complex compounds for drug screening. Povarov MCR has been used to prepare a new library containing tetrahydro-3H-pyrazolo[4,3-f]quinoline core and the library tested against MDA-MB-231 (a triple-negative breast cancer, TNBC, cell line). A few of the tetrahydro-3H-pyrazolo[4,3-f]quinoline-containing compounds, bearing 3-aminoindazolyl group, potently inhibited MDA-MB-231. The most active compound, HSD1787, was evaluated against NCI60 cell lines and this compound inhibited melanoma, renal, breast, ovarian, and leukemia cancer cell lines with GI50 values as low as 0.1 μM. The tetrahydro-3H-pyrazolo[4,3-f]quinoline core is therefore a new scaffold that could be developed into potent anticancer therapeutics against difficult-to-treat cancers.
Introduction
Multicomponent reactions (MCRs), such as Ugi,1 Gewald,2 Groebke–Blackburn–Bienaymé,3 Hantzsch,4 Biginelli,5 Passerini,6 etc., have been routinely used to make diverse libraries for biological screening and many compounds with anticancer, antibacterial, antiviral, etc., properties have been discovered from such libraries.3,7−10 In addition to facilitating compound library synthesis, MCRs have also been used to streamline drug synthesis, highlighted by the classic synthesis of the blockbuster drug nifedipine via a Hantzsch three-component reaction (3CR).11
Other pertinent examples are the syntheses of HIV protease inhibitors, crixivan and telaprevir, which can be synthesized on scale utilizing Ugi reactions.12 The Groebke–Blackburn–Bienaymé,4 a relatively newer MCR, was used for the synthesis of GLPG1690, an autotaxin inhibitor that is in clinical development for the treatment of idiopathic pulmonary fibrosis (IPF).13 The Povarov reaction is another robust multicomponent reaction, which produces tetrahydroquinoline core (a common scaffold found in many biologically active compounds or drugs, see Figure 1).14−24 For example, the FDA-approved drug talazoparib (Talzenna, Pfizer Inc.), a poly-(ADP-ribose) polymerase-1/2 (PARP-1/2) orally bioavailable inhibitor, contains a tetrahydroquinoline core (see Figure 1A). Talazoparib is used for the treatment of germline BRCA-mutated HER2-negative locally advanced and metastatic breast cancer.25 BMS-593214, another compound that contains the tetrahydroquinoline core (Figure 1A), is a factor V11a inhibitor and an anticoagulant compound. Others have used the Povarov reaction to make compounds with various biological properties. For example, Jiang and co-workers reported that the furano[3,2-c] tetrahydroquinoline 10a, with all cis stereochemistries across the tetrahydroquinoline stereogenic centers (Figure 1A), inhibited various cancer cell lines with IC50 values of 2.5–50 μM.26 Almansa and co-workers also reported novel hexahydro-2H-pyrano[3,2-c]quinolones, synthesized via Povarov, which are selective σ1 receptor ligands with potential application as analgesics.27
Figure 1.
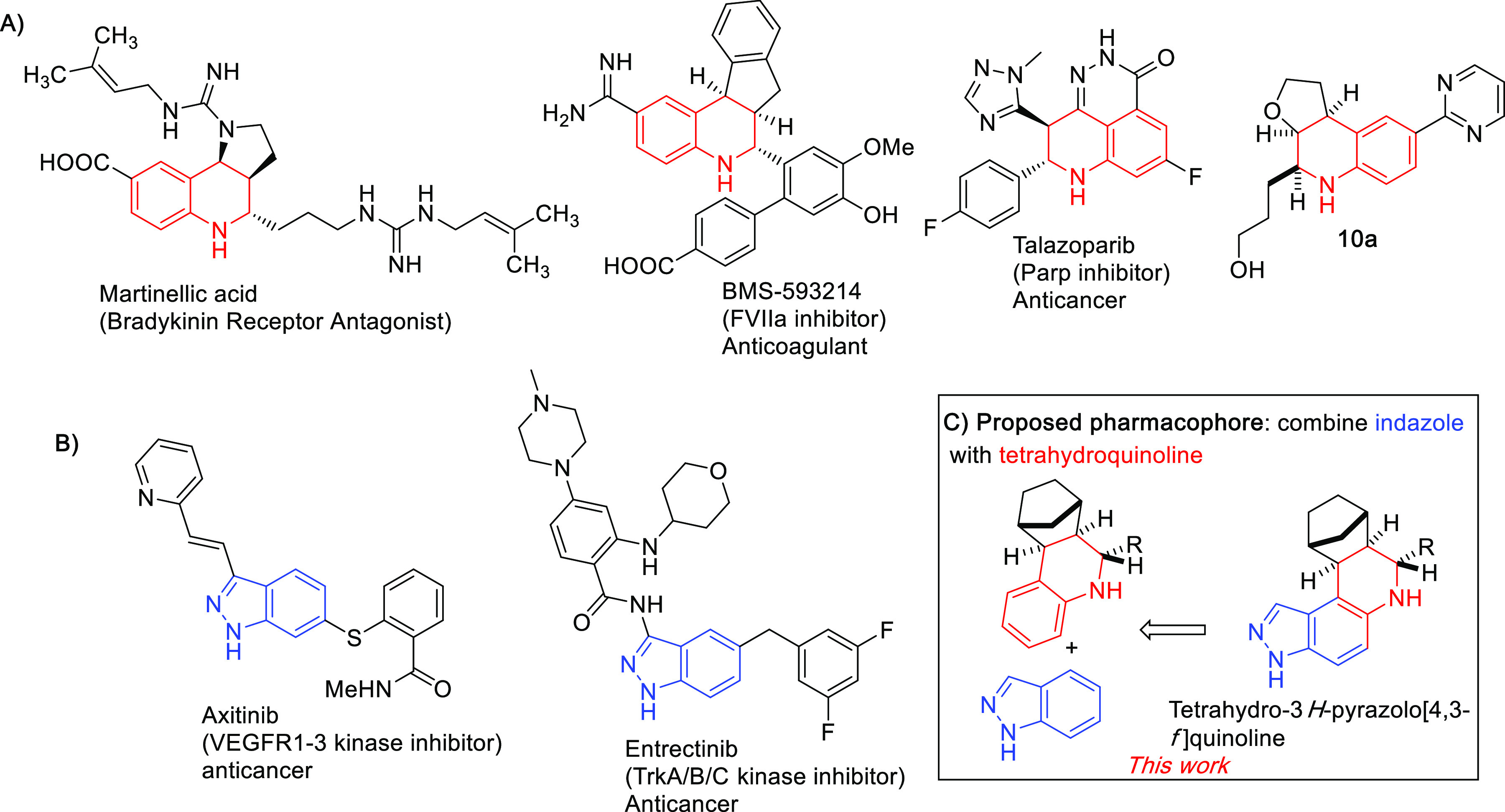
(A) Structure of biologically active tetrahydroquinoline core containing compounds, synthesized using Povarov reaction; (B) indazole-containing drugs; and (C) combining the privileged indazole with tetrahydroquinoline to afford tetrahydro-3H-pyrazolo[4,3-f]quinoline.
Results and Discussion
We decided to make a novel library for anticancer activity screening by combining the privileged indazole (see Figure 1B for FDA-approved drugs containing indazole core) with tetrahydroquinoline to afford tetrahydro-3H-pyrazolo[4,3-f]quinoline, which we rationalized could be readily synthesized via Povarov reaction. The first series of compounds were initially screened for growth inhibition of MDA-MB-231 (a triple-negative breast cancer, TNBC, cell line). We selected TNBC cell for initial screening because TNBC patients (who comprise ∼11% of all breast cancer patients) have worst prognosis.28 For metastatic TNBC, the median survival is only 13 months,29 and therefore we were motivated to find lead compounds for this indication.
Library Synthesis
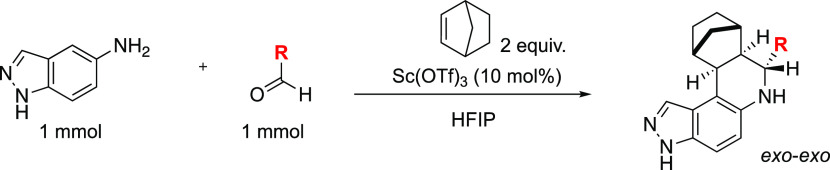
For the synthesis of the Povarov library, we followed previously reported protocols30,31 but with a slight modification. Instead of using the typical dichloromethane or acetonitrile solvent, we used hexafluroisopropanol (HFIP) as solvent and 10 mol % scandium triflate as Lewis catalyst. The switch to HFIP was needed because our starting amine, 5-aminoindazole and its intermediate, imine, had limited solubility in the traditional solvents dichloromethane and acetonitrile. Treating 5-aminoindazole and corresponding aldehydes and bicyclo[2.2.1]hept-2-ene (norbornene) as an activated alkene source afforded tetrahydroquinoline-containing compounds in 33–66% yields after 8–12 h of stirring at room temperature.
X-ray Crystallographic Studies
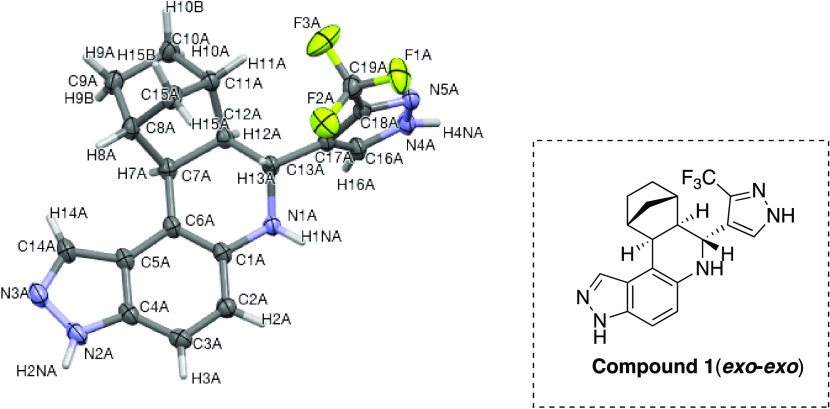
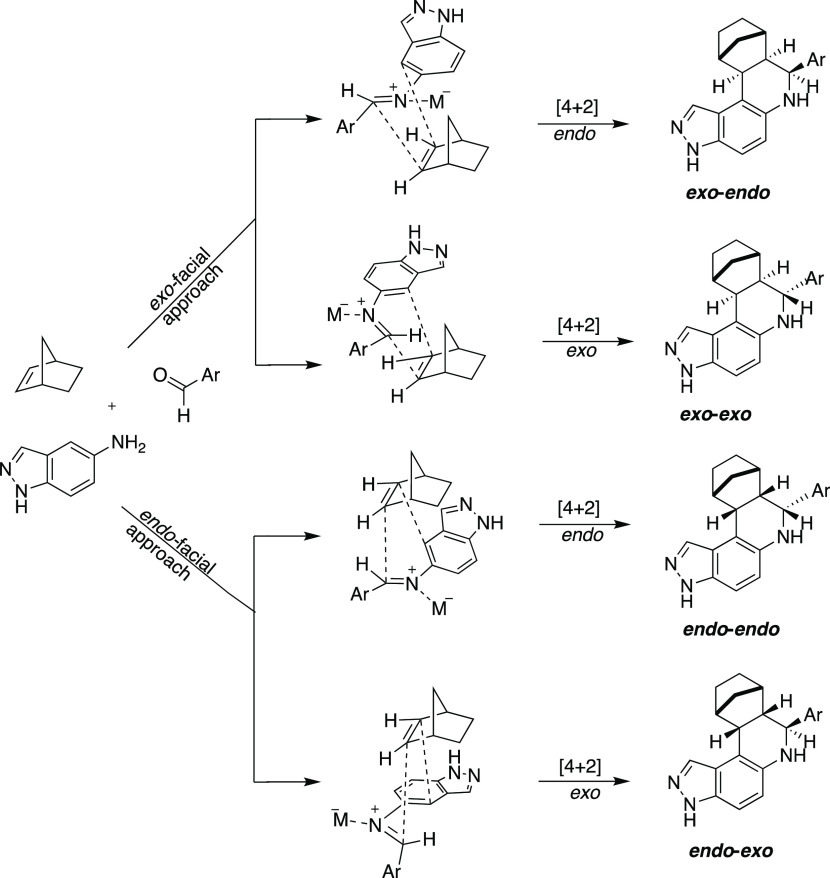
Povarov reaction with norbornene can lead to four possible diastereoisomers (see Figure 2) of compound 1. Excitingly, only one diastereomer (see Figure 2) was obtained in the Povarov reactions after column chromatography (up to 66% yield) and single-crystal X-ray diffraction analysis was used to confirm the stereochemical assignments of compounds. Crystals of compound 1 were obtained by slow diffusion of acetone in an ethanol solution. X-ray diffraction analysis of compound 1 indicated that the tetrahydro-pyrazolo-quinoline ring acquires a half-chair conformation and the H7A and H12A protons are trans to H13A proton in the major diastereomer, which has exo–exo relative sterochemistry (see Figure 2). The torsional angle for H-13A–C-13A–C12A–H12A, H-7A–C-7A–C12A–H12A and C-7A–C-12A–C13A–C17A are 166.7, −9.9, and 169.9° respectively. The bond angle C-12A–C-13A–C-17A was determined to be 112.8°. Out of four possible diasteroisomer products in the Povarov reaction of 5-aminoindazole with 1-norbornene, exo–exo diastereomer was observed as a major product probably because of favorable exo-facial approach of cyclization (see Scheme 1). This result is in agreement with literature precedent, which also utilized norbornene in Povarov reactions.30
Figure 2.
ORTEP diagram showing the molecular structure of compound 1. Disorder omitted for clarity (for details, see the Supporting Information).
Scheme 1. Three-Component Povarov Reaction of 5-Aminoindazole, Aldehyde, and Norbornene.
Biological Evaluation
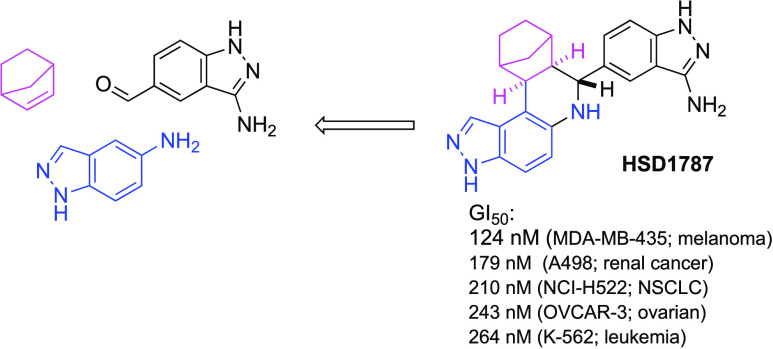
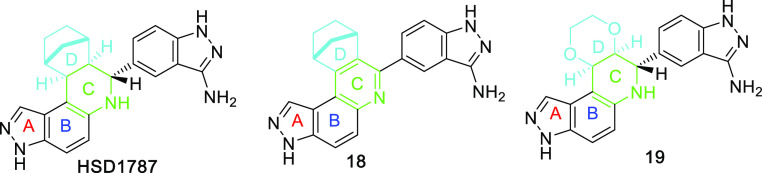
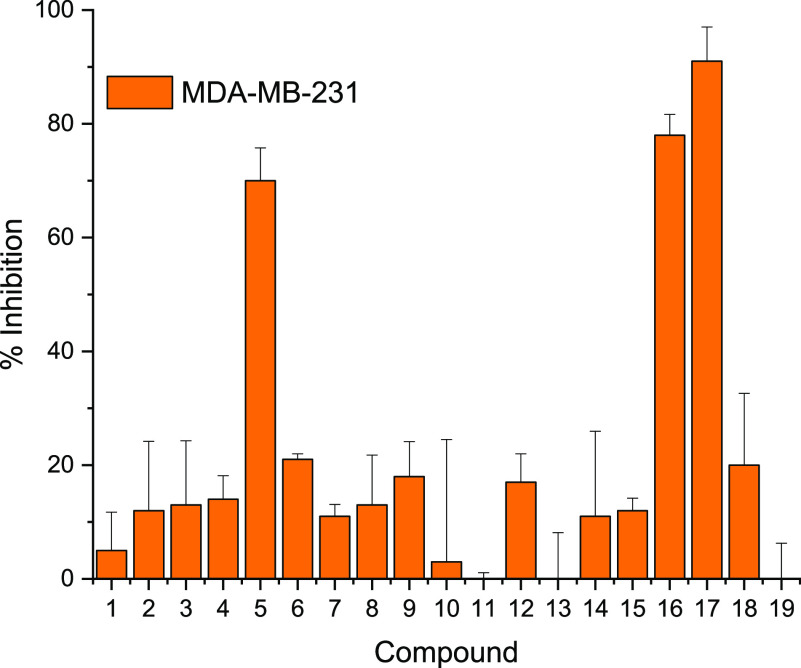
The series of 17 compounds were obtained by the reaction of 5-aminoindazole and norbornene with 17 different aldehydes (see Table 1). The inhibitions of MDA-MB-231 viability by the compounds at 1 μM concentration were evaluated by first treating the cancer cell line with compounds and incubating for 72 h and using CellTiter-Blue cell viability assay to evaluate growth inhibition. The anticancer properties of the compounds (see Table 1 and Figure 3) depended heavily on the nature of the starting aldehyde used for the synthesis (Figure 4). Compounds 16 and 17, bearing 5-indazolyl and 6-indazolyl groups, respectively, at the C13A position were the most potent inhibitors (percent growth inhibition at 1 μM of 78 and 91% for 16 and 17, respectively). The aminoindazole analog of compound 17, HSD1787 (see Figure 3), inhibited MDA-MB-231 at 95% at 1 μM concentration.
Table 1. Synthesis of First Series Analogs.
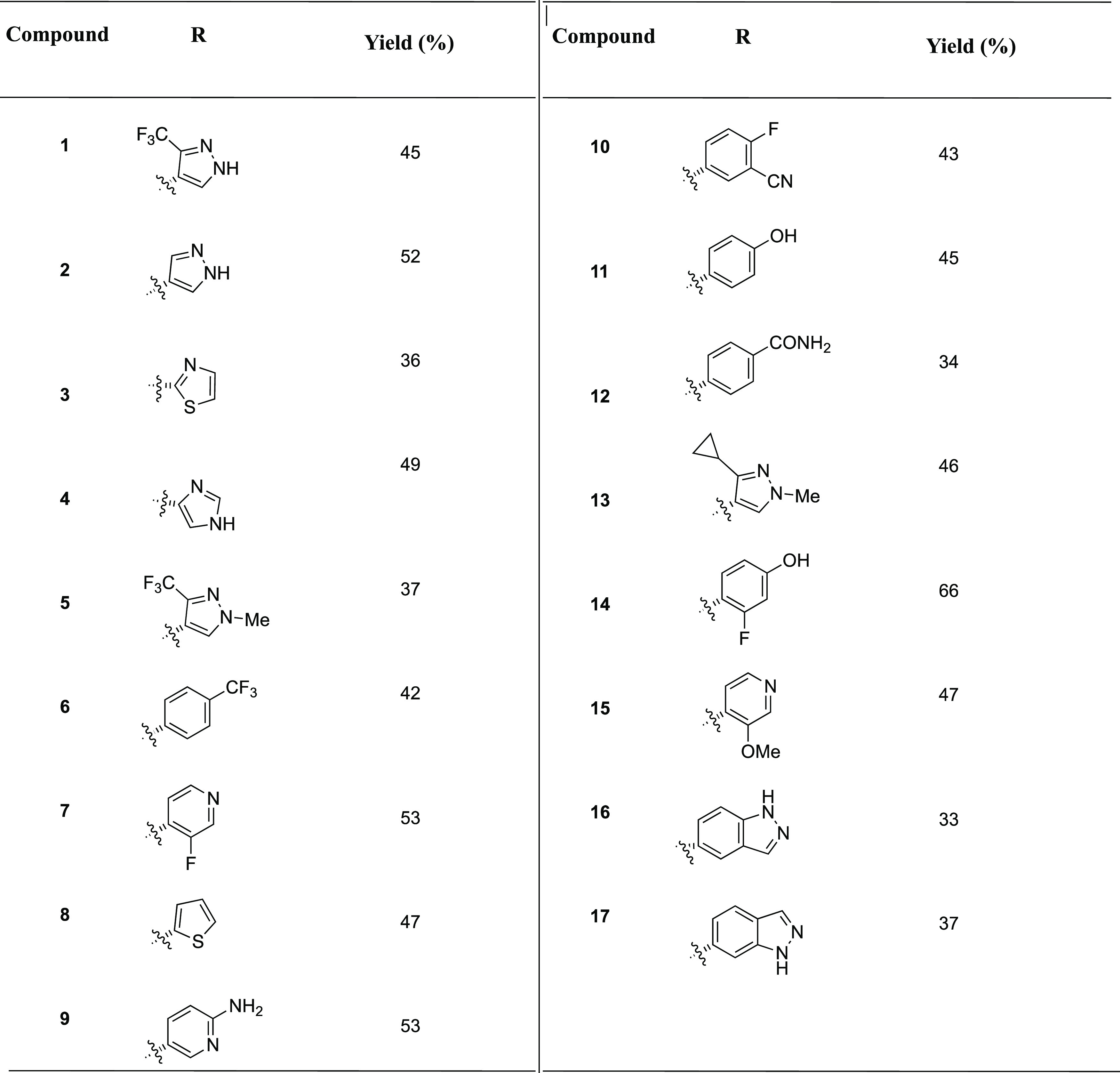
Figure 3.
Further analogs of HSD1787 analogs, which were synthesized and tested for growth inhibition of MDA-MB-231.
Figure 4.
Inhibition of MDA-MB-231 cell lines by compounds (concentration: 1 μM). Experiments were done in triplicate.
Drugs that contain high fraction sp3 carbons are generally considered as more druglike than analogs that contain a higher degree of polycyclic aromatic moieties.32−36 Therefore, we also designed a compound to investigate how the fraction sp3 of the compound (compare HSD1787 and compound 18, Figure 3) affected anticancer activities. Also, we designed compound 19, which did not contain the bicyclo[2.2.1]heptan-2-yl moiety in ring D but instead contained 2,3-dihydro-1,4-dioxine,37 to investigate if the bridged bicyclic, bicyclo[2.2.1]heptan-2-yl group found in HSD1787 and analogs is critical for anticancer activities. Analog 18, with less sp3 carbon than HSD1878 inhibited MDA-MB-231 at only 19%, highlighting that a higher fraction sp3 is important for anticancer activities for this series. Compound 19, which did not contain the bicyclo[2.2.1]heptan-2-yl moiety in ring D but instead contained 2,3-dihydro-1,4-dioxine showed no activity.
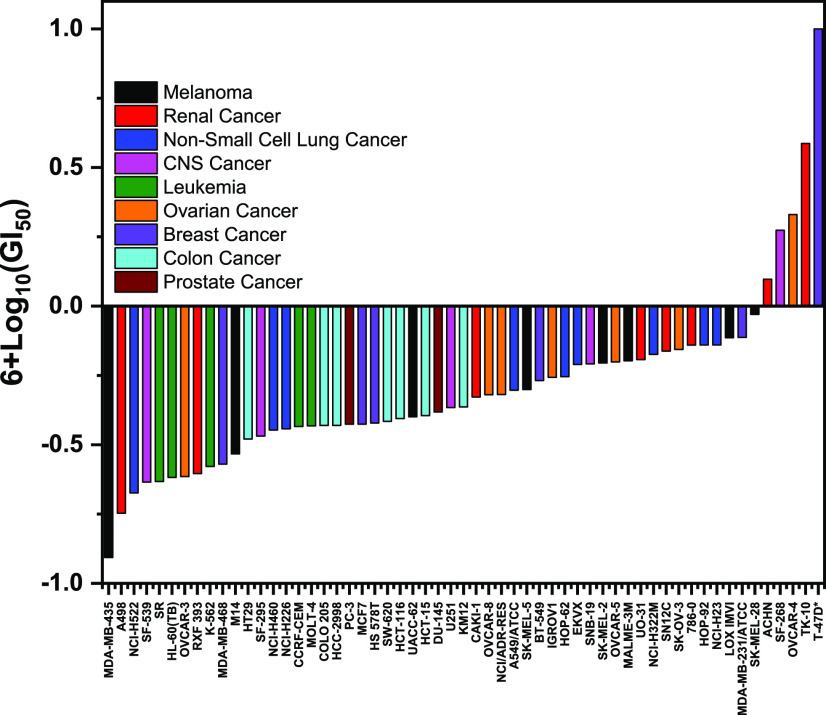
Compound HSD1787 was sent to the National Cancer Institute (NCI), Bethesda, MD (Drug Evaluation Branch), to evaluate the effects on the NCI-60 cell panel. According to the NCI protocol, HSD1787 was evaluated at five concentrations (10-fold dilutions) with the highest concentration being 100 μM and the incubation period was 48 h. A sulforhodamine B assay was used to assay the effects of the compound on the cancer cells. HSD1787 exhibited sub-micromolar GI50 (the concentration that causes 50% growth inhibition) against the majority of the cell lines in the NCI-60 panel (see Figure 5). The most sensitive cell lines (see Table 2) were MDA-MB-435 (melanoma), SF-539 (glioma), A498 (renal), MDA-MB-468 and MCF7 (breast), OVCAR-3 and OVCAR-8 (ovarian), and NCI-H522 (nonsmall cell lung cancer). On the other hand, UACC-257 (melanoma) and T-47D (breast) were resistant to HSD1787 with GI50 greater than 10 μM (Table 2). Thus, the inhibitions of the cancer cell lines by HSD1787 do not necessarily depend on the tissue type but probably on specific cancer drivers. This is in line with current appreciation that tumor mutational burden and not necessarily the anatomical origin of the tumor determines treatment strategies or outcomes. Compounds that do not grossly kill all cell types but are selective for dysregulated pathways tend to be better tolerated by patients.
Figure 5.
In vitro NCI60 cell lines vs GI50 representation for HSD1787. Asterisk represents poor dose response for T-47D so accurate GI50 could not be determined but at 10 μM, percent inhibition is less than 50%.
Table 2. GI50 by HSD1787 Against Select Cell Linesa.
| entry | cell line | cancer type | GI50(μM) |
|---|---|---|---|
| i | MDA-MB-435 | melanoma | 0.12 |
| ii | A498 | renal | 0.18 |
| iii | NCI-H522 | non-small cell lung | 0.21 |
| iv | SF-539 | CNS | 0.23 |
| v | SR | lymphoma | 0.23 |
| vi | Ovcar-3 | ovarian | 0.24 |
| vii | K-562 | leukemia | 0.26 |
| viii | MDA-MB-468 | breast | 0.27 |
| ix | SF-295 | CNS | 0.34 |
| x | MCF7 | breast | 0.38 |
| xi | Ovcar-8 | ovarian | 0.48 |
| xii | NCI/ADR-RES | ovarian | 0.48 |
| xiii | SK-MEL-28 | melanoma | 0.93 |
| xiv | Ovcar-4 | ovarian | 2.14 |
| xv | TK-10 | renal | 3.86 |
| xvi | UACC-257 | melanoma | >10 |
| xvii | T-47D | breast | >10 |
>10 means value is greater than 10 μM. Values are average of a biological duplicate.
Conclusions
We have synthesized a library of compounds containing the tetrahydro-3H-pyrazolo[4,3-f]quinoline core, using the Povarov multicomponent reaction. These compounds, synthesized in only a single-flask operation, potently inhibited NCI-60 cancer cell lines at sub-micromolar concentrations. The tetrahydro-3H-pyrazolo[4,3-f]quinoline-containing compounds represent one of the most potent anticancer agents synthesized via Povarov reported to date. This work adds to literature examples whereby multicomponent reactions have been used to make libraries that contain potent anticancer agents with nanomolar activities.10,38−41
Experimental Section
MDA-MB-231 cell line was a kind gift from Professor Camarillo’s lab (Purdue University). The cells were cultured using Dulbecco’s modified Eagle’s medium (DMEM) (Corning), supplemented with 10% fetal bovine serum (FBS) (Atlanta Biologicals), 1× glutaMAX (Gibco), and 1× penicillin/streptomycin (Corning) at 37 °C with 5% CO2. Cells were seeded at 1.0 × 104 cells/mL in 96-well plates and incubated for up to 24 h. Cells were then treated with 1 μM of different compounds for 72 h in triplicates. The CellTiter-Blue Cell Viability Assay (Promega) was then added to the cells and incubated for 3 h before reading following the manufacturers’ recommendations.
General Synthetic Considerations
All of the reagents and solvents were purchased from commercial sources and used as received, unless otherwise stated. The 1H and 13C nuclear magnetic resonance (NMR) spectra were obtained in methanol-d4 or dimethyl sulfoxide (DMSO)-d6 as solvents using a 500 MHz spectrometer using tetramethylsilane as an internal standard. Chemical shifts reported in parts per million (δ ppm) downfield. 1H NMR data reported as follows: chemical shift (δ ppm) (multiplicity, coupling constant (Hz), integration). Multiplicities are reported as follows: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, or combinations thereof. High-resolution mass spectra (HRMS) were recorded using the electron spray ionization (ESI) technique and as a time-of-flight (TOF) mass analyzer. All of the synthesized compounds were characterized using 1H, 13C NMR, and HRMS.
General Procedure for the Multicomponent Reaction
Amine (1 mmol) and aldehyde (1 mmol) in 4 mL of 1,1,1,3,3,3-hexafluoro-2-propanol stirred for 2 h at 80 °C. After that, alkene (2 mmol) and 10 mol % scandium(III) trifluoromethanesulfonate were added to the reaction mixture at ambient temperature. The reaction mixture was continued to stir for another 8–12 h at room temperature. After completion, the reaction mixture was concentrated and purified by silica gel chromatography (hexanes/ethyl acetate 95:5 to 50:50) or dichloromethane/methanol (99:01 to 95:05) to give the desired cyclized compound.
7-(3-(Trifluoromethyl)-1H-pyrazol-4-yl)-6,7,7a,8,9,10,11,11a-octahydro 3H-8,11-methanopyrazolo[4,3-a]phenanthridine (1)
Off-white solid (168 mg, 45%). 1H NMR (500 MHz, DMSO-d6) δ 7.85 (s, 1H), 7.63 (s, 1H), 7.11 (d, J = 8.7 Hz, 1H), 6.79 (dd, J = 8.7, 1.8 Hz, 1H), 5.21 (s, 1H), 4.08–3.96 (m, 1H), 2.90 (d, J = 8.5 Hz, 1H), 2.68 (d, J = 3.9 Hz, 1H), 2.11 (t, J = 7.8 Hz, 1H), 2.04–1.98 (m, 1H), 1.66–1.58 (m, 2H), 1.58–1.48 (m, 2H), 1.29–1.20 (m, 1H), and 1.03–0.94 (m, 1H); 13C NMR (126 MHz, DMSO-d6) δ 139.7, 138.3, 136.0, 131.4, 129.6, 124.1, 123.2, 121.8 (q = 269.64 Hz), 118.5, 114.6, 108.5, 51.7, 49.9, 43.1, 42.4, 40.9, 34.2, 29.8, and 29.4. HRMS (ESI) m/z calcd for C19H19F3N5 [M + H]+ 374.1593, found 374.1586.
7-(1H-Pyrazol-4-yl)-6,7,7a,8,9,10,11,11a-octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridine (2)
Pale yellow solid (158 mg, 52%). 1H NMR (500 MHz, DMSO-d6) δ 7.83 (s, 1H), 7.49 (s, 2H), 7.08 (d, J = 8.6 Hz, 1H), 6.78 (d, J = 8.7 Hz, 1H), 6.04 (t, J = 6.6 Hz, 1H), 5.24 (s, 1H), 3.93 (d, J = 7.1 Hz, 1H), 3.92–3.80 (m, 2H), 2.87 (d, J = 8.6 Hz, 1H), 2.66 (d, J = 4.0 Hz, 1H), 2.17–2.05 (m, 2H), 1.66–1.58 (m, 2H), 1.58–1.42 (m, 2H), 1.34–1.23 (m, 1H), 0.96 (d, J = 9.7 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 140.0, 137.9, 135.8, 131.3, 126.5, 125.2, 123.3, 118.7, 114.8, 108.2, 51.2, 51.0, 43.2, 42.5, 41.0, 34.4, 29.8, and 29.6; HRMS (ESI) m/z calcd for C18H20N5 [M + H]+ 306.1719, found 306.1714.
2-(6,7,7a,8,9,10,11,11a-Octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridin-7-yl)thiazole (3)
Pale yellow solid (111 mg, 36%). 1H NMR (500 MHz, methanol-d4) δ 7.88 (s, 1H), 7.63 (dd, J = 3.4, 1.2 Hz, 1H), 7.34 (dd, J = 3.3, 1.2 Hz, 1H), 7.20 (d, J = 8.7 Hz, 1H), 6.83 (dd, J = 8.8, 1.2 Hz, 1H), 4.60 (d, J = 4.5 Hz, 1H), 3.06 (d, J = 8.7 Hz, 1H), 2.61 (d, J = 4.3 Hz, 1H), 2.43 (dd, J = 8.7, 4.5 Hz, 1H), 2.36–2.29 (m, 1H), 1.83–1.75 (m, 1H), 1.75–1.68 (m, 1H), 1.65–1.53 (m, 2H), 1.45–1.35 (m, 1H), and 1.14–1.04 (m, 1H); 13C NMR (126 MHz, methanol-d4) δ 176.5, 140.5, 137.6, 136.2, 131.0, 122.6, 119.2, 119.1, 116.0, 108.3, 57.7, 50.1, 44.7, 42.1, 41.6, 34.0, 29.8, and 28.5. HRMS (ESI) m/z calcd for C18H19N4S [M + H]+ 323.1330, found 323.1323.
7-(1H-Imidazol-4-yl)-6,7,7a,8,9,10,11,11a-octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridine (4)
Off-white solid (149 mg, 49%). 1H NMR (500 MHz, methanol-d4) δ 7.89 (s, 1H), 7.73 (s, 1H), 7.15 (d, J = 8.7 Hz, 1H), 6.89 (s, 1H), 6.80 (d, J = 8.7 Hz, 1H), 4.10 (d, J = 7.1 Hz, 1H), 2.94 (d, J = 8.6 Hz, 1H), 2.70 (d, J = 4.1 Hz, 1H), 2.34 (t, J = 7.8 Hz, 1H), 2.15 (d, J = 3.8 Hz, 1H), 1.75–1.62 (m, 2H), 1.60–1.46 (m, 2H), 1.35–1.29 (m, 1H), 1.01 (d, J = 9.9 Hz, 1H); 13C NMR (126 MHz, methanol-d4) δ 140.2, 138.7, 136.5, 134.3, 131.0, 122.7, 121.6, 119.1, 116.0, 108.2, 53.1, 48.8, 43.3, 42.2, 41.0, 33.7, 29.1. HRMS (ESI) m/z calcd for C18H20N5 [M + H]+ 306.1719, found 306.1714.
7-(1-Methyl-3-(trifluoromethyl)-1H-pyrazol-4-yl)-6,7,7a,8,9,10,11,11a-octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridine (5)
Off-white solid (144 mg, 37%). 1H NMR (500 MHz, methanol-d4) δ 7.94 (s, 1H), 7.45 (s, 1H), 7.19 (d, J = 8.7 Hz, 1H), 6.80 (d, J = 8.7 Hz, 1H), 4.18 (d, J = 6.7 Hz, 1H), 3.81 (s, 3H), 3.04 (d, J = 8.6 Hz, 1H), 2.76 (d, J = 4.2 Hz, 1H), 2.20 (t, J = 7.6 Hz, 1H), 2.11 (d, J = 3.8 Hz, 1H), 1.78–1.66 (m, 2H), 1.65–1.52 (m, 2H), 1.41–1.29 (m, 1H), and 1.15–0.89 (m, 1H); 13C NMR (126 MHz, methanol-d4) δ 138.7, 138.4, 136.4, 131.2, 130.9, 125.1 (q, J = 269.64 Hz), 124.6, 122.7, 118.9, 115.9, 108.1, 51.3, 50.0, 43.5, 42.3, 41.2, 38.1, 33.6, 29.2, and 29.1. HRMS (ESI) m/z calcd for C20H21F3N5 [M + H]+ 388.1749, found 388.1742.
7-(4-(Trifluoromethyl)phenyl)-6,7,7a,8,9,10,11,11a-octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridine (6)
Off-white solid (161 mg, 42%). 1H NMR (500 MHz, DMSO-d6) δ 8.01 (s, 1H), 7.86 (s, 1H), 7.67 (d, J = 8.2 Hz, 2H), 7.62 (d, J = 8.2 Hz, 2H), 7.13 (d, J = 8.7 Hz, 1H), 6.81 (d, J = 8.7 Hz, 1H), 5.57 (s, 1H), 5.19–5.05 (m, 1H), 3.94 (d, J = 8.2 Hz, 1H), 2.88 (d, J = 8.5 Hz, 1H), 2.77 (d, J = 3.9 Hz, 1H), 2.10 (t, J = 8.4 Hz, 1H), 2.03 (d, J = 4.1 Hz, 1H), 1.70 (d, J = 9.8 Hz, 1H), 1.61 (dt, J = 12.7, 4.6 Hz, 1H), 1.50 (dtd, J = 12.0, 7.8, 3.8 Hz, 2H), 1.23–1.11 (m, 1H), and 1.02 (d, J = 9.8 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 150.4, 139.7, 136.0, 131.5, 129.0, 128.2 (q, J = 31.5 Hz), 125.9, 125.5, 125.4, 123.9 (q, J = 286.06 Hz), 123.7, 123.2, 118.5, 114.2, 108.6, 67.7 (quintet, J = 31.5 Hz), 59.7, 51.3, 42.7, 42.6, 34.2, 30.1, and 29.0; HRMS (ESI) m/z calcd for C22H21F3N3 [M + H]+ 384.1688, found 384.1681.
7-(3-Fluoropyridin-4-yl)-6,7,7a,8,9,10,11,11a-octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridine (7)
Light brown solid (177 mg, 53%). 1H NMR (500 MHz, DMSO-d6) δ 8.51 (s, 1H), 8.30 (d, J = 5.0 Hz, 1H), 7.85 (s, 1H), 7.36 (t, J = 5.7 Hz, 1H), 7.15 (d, J = 8.7 Hz, 1H), 6.80 (d, J = 8.7 Hz, 1H), 5.59 (s, 1H), 4.29 (d, J = 6.8 Hz, 1H), 2.91 (d, J = 8.5 Hz, 1H), 2.74–2.63 (m, 1H), 2.12 (t, J = 7.8 Hz, 1H), 2.06 (d, J = 3.4 Hz, 1H), 1.68–1.57 (m, 2H), 1.55–1.44 (m, 2H), 1.27–1.16 (m, 1H), and 1.02 (d, J = 9.8 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 158.7 (J = 254.52 Hz), 146.4, 141.1, 139.3, 138.1, 136.0, 131.4, 123.6, 123.2, 118.3, 114.2, 108.9, 51.7, 50.2, 43.3, 42.3, 41.1, 34.3, 29.7, and 29.4. HRMS (ESI) m/z calcd for C20H20FN4 [M + H]+ 335.1672, found 335.1667.
7-(Thiophen-2-yl)-6,7,7a,8,9,10,11,11a-octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridine (8)
Pale yellow solid (150 mg, 47%). 1H NMR (500 MHz, methanol-d4) δ 8.26 (d, J = 1.1 Hz, 1H), 7.51 (dd, J = 5.2, 1.2 Hz, 1H), 7.47 (dt, J = 8.8, 1.1 Hz, 1H), 7.32 (dd, J = 3.6, 1.3 Hz, 1H), 7.21 (d, J = 8.8 Hz, 1H), 7.11 (dd, J = 5.2, 3.5 Hz, 1H), 4.63 (d, J = 10.3 Hz, 1H), 3.33–3.31 (m, 1H), 3.17–3.04 (m, 1H), 2.63 (ddd, J = 10.0, 8.5, 1.2 Hz, 1H), 2.22–2.14 (m, 1H), 1.90–1.78 (m, 1H), 1.77–1.66 (m, 2H), 1.60 (dt, J = 10.5, 1.9 Hz, 1H), 1.49–1.38 (m, 1H), and 1.27–1.16 (m, 1H); 13C NMR (126 MHz, methanol-d4) δ 140.7, 139.5, 132.7, 128.3, 127.9, 126.8, 126.5, 124.4, 122.0, 120.0, 109.4, 57.3, 49.5, 43.1, 39.8, 33.2, 29.4, and 28.2. HRMS (ESI) m/z calcd for C19H20N3S [M + H]+ 322.1378, found 322.1371.
5-(6,7,7a,8,9,10,11,11a-Octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridin-7-yl)pyridin-2-amine (9)
Pale yellow solid (53 mg, 16%). 1H NMR (500 MHz, methanol-d4) δ 7.95 (d, J = 1.1 Hz, 1H), 7.89 (d, J = 2.4 Hz, 1H), 7.58 (dd, J = 8.6, 2.4 Hz, 1H), 7.18 (d, J = 8.7 Hz, 1H), 6.84 (d, J = 8.7 Hz, 1H), 6.60 (d, J = 8.6 Hz, 1H), 3.72 (d, J = 9.3 Hz, 1H), 2.94 (dd, J = 21.9, 6.3 Hz, 2H), 2.17 (d, J = 8.9 Hz, 1H), 2.04 (d, J = 4.2 Hz, 1H), 1.78–1.70 (m, 2H), 1.65–1.56 (m, 2H), 1.28 (tdd, J = 11.0, 5.6, 2.3 Hz, 1H), and 1.11 (dt, J = 9.9, 1.5 Hz, 1H); 13C NMR (126 MHz, methanol-d4) δ 158.6, 146.5, 145.3, 139.6, 137.7, 128.8, 122.7, 119.0, 115.5, 109.1, 108.1, 58.1, 51.0, 42.9, 42.2, 39.6, 33.2, 29.7, and 28.3. HRMS (ESI) m/z calcd for C20H22N5 [M + H]+ 332.1875, found 332.1870.
2-Fluoro-5-(-6,7,7a,8,9,10,11,11a-octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridin-7-yl)benzonitrile (10)
Off-white solid (154 mg, 43%). 1H NMR (500 MHz, DMSO-d6) δ 7.94 (dd, J = 6.4, 2.3 Hz, 1H), 7.85 (d, J = 1.3 Hz, 1H), 7.83–7.75 (m, 1H), 7.46 (t, J = 9.0 Hz, 1H), 7.12 (d, J = 8.6 Hz, 1H), 6.78 (d, J = 8.7 Hz, 1H), 5.51 (s, 1H), 3.93 (dd, J = 8.0, 1.4 Hz, 1H), 2.88 (d, J = 8.5 Hz, 1H), 2.74 (d, J = 3.9 Hz, 1H), 2.10 (t, J = 8.3 Hz, 1H), 2.01 (d, J = 4.0 Hz, 1H), 1.73–1.58 (m, 2H), 1.55–1.41 (m, 2H), 1.31–1.16 (m, 1H), and 1.01 (d, J = 9.8 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 162.9 (J = 255.78Hz), 143.4, 139.6, 136.0, 135.7, 133.4, 131.5, 123.2, 118.4, 116.8, (18.19Hz), 114.6, 114.3, 108.6, 100.1(15.12), 58.5, 51.0, 42.7, 42.6, 34.3, 30.1, and 29.0. HRMS (ESI) m/z calcd for C22H20FN4 [M + H]+ 359.1672, found 359.1665.
4-(-6,7,7a,8,9,10,11,11a-Octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridin-7-yl)phenol (11)
Off-white solid (151 mg, 45%). 1H NMR (500 MHz, DMSO-d6) δ 9.26 (s, 1H), 7.85 (s, 1H), 7.19 (d, J = 8.5 Hz, 2H), 7.08 (d, J = 8.7 Hz, 1H), 6.82 (d, J = 8.7 Hz, 1H), 6.70 (d, J = 8.5 Hz, 2H), 5.20 (s, 1H), 3.64 (d, J = 9.0 Hz, 1H), 2.85–2.74 (m, 2H), 2.05–1.93 (m, 2H), 1.77–1.58 (m, 2H), 1.55–1.44 (m, 2H), 1.23–1.09 (m, 1H), and 0.99 (d, J = 9.7 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 156.8, 140.6, 135.9, 131.5, 129.2, 123.3, 118.6, 115.3,. 9, 108.3, 59.9, 52.0, 43.0, 42.0, 34.0, 30.4, and 28.8. HRMS (ESI) m/z calcd for C21H22N3O [M + H]+ 332.1763, found 332.1755.
4-(6,7,7a,8,9,10,11,11a-Octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridin-7-yl)benzamide (12)
Off-white solid (122 mg, 34%). 1H NMR (500 MHz, DMSO-d6) δ 7.89 (s, 1H), 7.85 (s, 1H), 7.80 (d, J = 8.0 Hz, 2H), 7.46 (d, J = 8.0 Hz, 2H), 7.28 (s, 1H), 7.10 (d, J = 8.6 Hz, 1H), 6.81 (d, J = 8.8 Hz, 1H), 5.44 (s, 1H), 3.87 (d, J = 8.2 Hz, 1H), 2.86 (d, J = 8.6 Hz, 1H), 2.77 (s, 1H), 2.11–2.08 (m, 1H), 2.03 (s, 1H), 1.70 (d, J = 9.9 Hz, 1H), 1.64–1.59 (m, 1H), 1.54–1.46 (m, 2H), 1.21–1.15 (m, 1H), and 1.04–1.00 (m, 1H); 13C NMR (126 MHz, DMSO-d6) δ 169.0, 149.8, 140.9, 136.7, 134.3, 132.3, 128.8, 128.6, 124.1, 119.3, 114.9, 109.3, 60.7, 52.3, 43.6, 43.3, 35.0, 31.0, and 29.8. HRMS (ESI) m/z calcd for C22H23N4O [M + H]+ 359.1872, found 359.1865.
7-(3-Cyclopropyl-1-methyl-1H-pyrazol-4-yl)-6,7,7a,8,9,10,11,11a-octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridine (13)
Light brown solid (166 mg, 46%).1H NMR (500 MHz, DMSO-d6) δ 7.86 (s, 1H), 7.35 (s, 1H), 7.09 (d, J = 8.6 Hz, 1H), 6.81 (d, J = 8.7 Hz, 1H), 5.09 (s, 1H), 3.90 (d, J = 8.4 Hz, 1H), 3.63 (s, 2H), 2.85 (d, J = 8.5 Hz, 1H), 2.77 (d, J = 3.8 Hz, 1H), 2.11 (t, J = 8.5 Hz, 1H), 2.07 (d, J = 3.9 Hz, 1H), 1.90 (tt, J = 8.2, 5.1 Hz, 1H), 1.69–1.64 (m, 1H), 1.61 (dt, J = 10.7, 3.6 Hz, 1H), 1.52 (tdd, J = 13.3, 8.0, 3.8 Hz, 2H), 1.21 (ddd, J = 13.1, 9.8, 6.7 Hz, 1H), 1.02–0.96 (m, 1H), 0.83–0.69 (m, 4H).13C NMR (126 MHz, DMSO-d6) δ 150.6, 140.5, 135.9, 131.5, 129.7, 123.4, 123.3, 118.6, 114.3, 108.3, 51.8, 51.0, 42.8, 42.4, 38.6, 34.1, 30.2, 29.2, 7.9, 7.8, and 7.7. HRMS (ESI) m/z calcd for C22H26N5 [M + H]+ 360.2188, found 360.2182.
3-Fluoro-4-(6,7,7a,8,9,10,11,11a-octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridin-7-yl)phenol (14)
Off-white solid (209 mg, 66%). 1H NMR (500 MHz, DMSO-d6) δ 9.76 (s, 1H), 7.85 (s, 1H), 7.24 (t, J = 8.6 Hz, 1H), 7.10 (d, J = 8.7 Hz, 1H), 6.81 (d, J = 8.7 Hz, 1H), 6.57 (dd, J = 8.5, 2.4 Hz, 1H), 6.52 (dd, J = 12.2, 2.4 Hz, 1H), 5.24 (s, 1H), 4.02 (d, J = 8.7 Hz, 1H), 2.84 (d, J = 8.5 Hz, 1H), 2.78 (s, 1H), 2.06 (t, J = 8.6 Hz, 1H), 1.97 (s, 1H), 1.71–1.57 (m, 2H), 1.51 (td, J = 10.8, 4.0 Hz, 2H), 1.24–1.12 (m, 1H), and 1.05–0.98 (m, 1H); 13C NMR (126 MHz, DMSO-d6) δ 161.9, 160.0 (J = 253.26 Hz), 140.4, 135.9, 131.5, 129.8, 123.3, 122.2, 118.5, 112.1, 108.4, 102.5, 102.3, 52.1, 51.4, 42.9, 42.3, 34.1, 30.3, and 28.9. HRMS (ESI) m/z calcd for C21H21FN3O [M + H]+ 350.1669, found 350.1663.
7-(3-Methoxypyridin-4-yl)-6,7,7a,8,9,10,11,11a-octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridine (15)
Pale yellow solid (169 mg, 47%). 1H NMR (500 MHz, DMSO-d6) δ 8.56 (s, 1H), 8.33 (d, J = 5.6 Hz, 1H), 7.90 (s, 1H), 7.59 (d, J = 5.6 Hz, 1H), 7.21 (d, J = 8.7 Hz, 1H), 6.88 (d, J = 8.7 Hz, 1H), 4.52 (d, J = 5.7 Hz, 1H), 4.03 (s, 3H), 2.96 (d, J = 8.6 Hz, 1H), 2.63 (s, 1H), 2.22 (t, J = 7.2 Hz, 1H), 2.11 (d, J = 3.4 Hz, 1H), 1.65–1.56 (m, 2H), 1.52 (dt, J = 8.8, 5.2 Hz, 2H), 1.30–1.21 (m, 1H), and 1.01 (d, J = 9.7 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 154.8, 137.3, 137.3, 137.2, 136.6, 131.4, 128.2, 124.0, 123.0, 123.0, 118.4, 109.3, 57.8, 52.2, 48.7, 44.3, 42.1, 41.8, 34.5, 29.8, and 29.4. HRMS (ESI) m/z calcd for C21H23N4O [M + H]+ 347.1872, found 347.1866.
7-(1H-Indazol-5-yl)-6,7,7a,8,9,10,11,11a-octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridine (16)
Off-white solid (120 mg, 33%). 1H NMR (500 MHz, DMSO-d6) δ 8.01 (t, J = 1.3 Hz, 1H), 7.87 (s, 1H), 7.74 (s, 1H), 7.50 (d, J = 8.7 Hz, 1H), 7.44 (dd, J = 8.6, 1.6 Hz, 1H), 7.11 (d, J = 8.7 Hz, 1H), 6.84 (d, J = 8.8 Hz, 1H), 5.36 (s, 1H), 3.87 (d, J = 8.9 Hz, 1H), 2.86 (d, J = 8.6 Hz, 1H), 2.83 (s, 1H), 2.13 (t, J = 8.8 Hz, 1H), 2.02 (s, 1H), 1.73 (d, J = 9.8 Hz, 1H), 1.65–1.55 (m, 1H), 1.55–1.42 (m, 2H), 1.19–1.09 (m, 1H), and 1.02 (d, J = 9.8 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 140.5, 139.8, 137.5, 135.9, 133.8, 131.5, 126.8, 123.4, 123.1, 119.6, 118.6, 113.9, 110.3, 108.4, 60.6, 52.0, 43.0, 42.1, 34.1, 30.4, and 28.8. HRMS (ESI) m/z calcd for C22H22N5 [M + H]+ 356.1875, found 356.1869.
7-(1H-Indazol-6-yl)-6,7,7a,8,9,10,11,11a-octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridine (17)
Off-white solid (133 mg, 37%). 1H NMR (500 MHz, DMSO-d6) δ 8.00 (s, 1H), 7.86 (s, 1H), 7.69 (d, J = 8.3 Hz, 1H), 7.49 (s, 1H), 7.19 (dd, J = 8.4, 1.4 Hz, 1H), 7.11 (d, J = 8.7 Hz, 1H), 6.83 (d, J = 8.7 Hz, 1H), 5.44 (s, 1H), 3.94 (d, J = 8.3 Hz, 1H), 2.88 (d, J = 8.6 Hz, 1H), 2.83–2.74 (m, 1H), 2.15 (t, J = 8.5 Hz, 1H), 2.07 (d, J = 4.1 Hz, 1H), 1.74 (d, J = 9.8 Hz, 1H), 1.67–1.56 (m, 1H), 1.57–1.39 (m, 2H), 1.25–1.10 (m, 1H), and 1.03 (d, J = 9.7 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 143.9, 140.4, 140.3, 135.9, 133.6, 131.5, 123.3, 122.5, 121.3, 120.5, 118.5, 114.0, 109.0, 108.4, 60.5, 51.8, 42.9, 42.5, 34.2, 30.2, and 29.0. HRMS (ESI) m/z calcd for C22H22N5 [M + H]+ 356.1875, found 356.1870.
5-(6,7,7a,8,9,10,11,11a-Octahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridin-7-yl)-1H-indazol-3-amine (HSD1787)
Compound 10 (0.3 mmol) was treated with hydrazine monohydrate (2.5 equiv) in ethanol (3 mL) at reflux for 4–8 h. After that completion reaction was concentrated and purified by silica gel chromatography (dichloromethane/methanol (99:01 to 95:05)). Off-white solid (94 mg, 85%). 1H NMR (500 MHz, DMSO-d6) δ 11.29 (s, 1H), 7.88 (s, 1H), 7.75 (d, J = 1.6 Hz, 1H), 7.32 (dd, J = 8.6, 1.6 Hz, 1H), 7.19 (d, J = 8.5 Hz, 1H), 7.10 (d, J = 8.7 Hz, 1H), 6.85 (d, J = 8.7 Hz, 1H), 5.29 (s, 3H), 3.74 (d, J = 9.6 Hz, 1H), 2.86 (dd, J = 15.4, 6.2 Hz, 2H), 2.11 (t, J = 9.1 Hz, 1H), 2.03 (d, J = 4.2 Hz, 1H), 1.71 (d, J = 9.8 Hz, 1H), 1.64 (tt, J = 12.3, 4.4 Hz, 1H), 1.56–1.44 (m, 2H), 1.18–1.09 (m, 1H), and 1.04 (d, J = 9.7 Hz, 1H);13C NMR (126 MHz, DMSO-d6) δ 149.6, 141.6, 140.8, 135.9, 134.5, 131.6, 126.9, 123.4, 119.7, 118.6, 114.3, 113.7, 109.5, 108.3, 61.0, 52.3, 43.2, 41.7, 39.3, 34.0, 30.6, and 28.6; HRMS (ESI) m/z calcd for C22H23N6 [M + H]+ 371.1984, found 371.1977.
5-(8,9,10,11-Tetrahydro-3H-8,11-methanopyrazolo[4,3-a]phenanthridin-7-yl)-1H-indazol-3-amine (18)
Synthesized by oxidation of HSD1787. To a reaction mixture of compound HSD1787 (0.3 mmol) dissolved in acetonitrile (5 mL), DDQ was added (1.5 equiv) and reaction stirred for 8 h at room temperature. After completion, reaction mixture was diluted with ethyl acetate and washed with the aqueous solution of sodium hydroxide followed by washing with brine. Organic layer was concentrated and purified by silica gel column chromatography. Brown solid (49 mg, 45%). 1H NMR (500 MHz, DMSO-d6) δ 11.53 (s, 1H), 8.68 (s, 1H), 7.87 (d, J = 9.6 Hz, 1H), 7.84–7.74 (m, 2H), 7.37 (d, J = 8.6 Hz, 1H), 5.52 (s, 2H), 4.34 (s, 1H), 3.86 (d, J = 3.3 Hz, 1H), 2.27–2.11 (m, 2H), 1.84 (d, J = 8.8 Hz, 1H), 1.69 (d, J = 8.7 Hz, 1H), 1.47–1.32 (m, 1H), and 1.32–1.20 (m, 1H); 13C NMR (126 MHz, DMSO-d6) δ 152.4, 150.4, 144.9, 141.6, 139.5, 138.0, 134.9, 129.9, 129.5, 127.8, 121.3, 116.3, 115.9, 114.7, 114.4, 109.6, 49.9, 43.4, 42.6, 27.1, and 25.5. HRMS (ESI) m/z calcd for C22H19N6 [M + H]+ 367.1671, found 367.1666.
2-Fluoro-5-(2,3,4a,6,9,11c-hexahydro-5H-[1,4]dioxino[2,3-c]pyrazolo[4,3-f]quinolin-5-yl)benzonitrile (S-1)
Pale yellow solid (126 mg, 36%). 1H NMR (500 MHz, DMSO-d6) δ 8.02–7.99 (m, 1H), 7.96–7.92 (m, 1H), 7.89 (d, J = 1.0 Hz, 1H), 7.53 (t, J = 9.1 Hz, 1H), 7.27 (d, J = 8.9 Hz, 1H), 6.81 (d, J = 8.8 Hz, 1H), 5.81 (s, 1H), 5.29 (d, J = 3.5 Hz, 1H), 4.65 (s, 1H), 3.92–3.84 (m, 1H), 3.59–3.48 (m, 2H), 3.38–3.35 (m, 1H), and 3.26–3.20 (m, 1H); 13C NMR (126 MHz, DMSO-d6) δ 163.1 (J = 255.7 Hz), 139.9, 139.0, 136.1, 133.4, 122.4, 122.0, 119.8, 118.3, 116.6, 116.4, 114.6, 106.7, 99.7, 72.3, 70.4, 66.3, 59.7, and 57.3. HRMS (ESI) m/z calcd for C19H16 FN4O2 [M + H]+ 351.1257, found 351.1260.
5-(2,3,4a,6,9,11c-Hexahydro-5H-[1,4]dioxino[2,3-c]pyrazolo[4,3-f]quinolin-5-yl)-1H-indazol-3-amine (19)
Prepared using same method as for HSD1787. Pale yellow solid (88 mg, 81%). 1H NMR (500 MHz, DMSO-d6) δ 11.28 (s, 1H), 7.88 (d, J = 4.5 Hz, 2H), 7.39 (dd, J = 8.6, 1.6 Hz, 1H), 7.24 (d, J = 8.7 Hz, 1H), 7.18 (d, J = 8.5 Hz, 1H), 6.85 (d, J = 8.8 Hz, 1H), 5.70 (s, 1H), 5.35 (d, J = 3.5 Hz, 1H), 5.29 (s, 2H), 4.59 (s, 1H), 3.83 (d, J = 3.6 Hz, 1H), 3.57–3.45 (m, 2H), 3.40–3.34 (m, 1H), and 3.24 (td, J = 11.4, 3.1 Hz, 1H); 13C NMR (126 MHz, DMSO-d6) δ 149.6, 141.7, 140.7, 135.5, 132.2, 130.2, 127.2, 122.3, 119.9, 118.3, 114.2, 110.3, 109.0, 106.4, 73.3, 70.8, 66.2, 59.7, and 59.1. HRMS (ESI) m/z calcd for C19H19N6O2 [M + H]+ 363.1569, found 363.1570.
Acknowledgments
The authors thank Purdue University and Purdue Institute for Drug Discovery for financial support. Funding for the single-crystal X-ray diffractometer was provided by the National Science Foundation through the Major Research Instrumentation Program under Grant no. CHE 1625543. NMR and MS data were acquired by facilities supported by NIH P30 CA023168.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03001.
The authors declare the following competing financial interest(s): H.O.S. is a co-founder of KinaRx LLC, a start-up interested in oncology drugs.
Supplementary Material
References
- a Ugi I.; Meyr R.; Fetzer U.; Steinbruckner C. Studies on isonitriles. Angew. Chem. 1959, 71, 386. [Google Scholar]; b Ugi I.; Steinbrückner C. Über ein neues Kondensations-Prinzip. Angew. Chem. 1960, 72, 267–268. 10.1002/ange.19600720709. [DOI] [Google Scholar]; c Ugi I.; Rosendahl F. K.; Bodesheim F. Isonitrile, XIII. Kondensation von primären Aminen und Ketonen mit Isonitrilen und Rhodanwasserstoffsäure. Justus Liebigs Ann. Chem. 1963, 666, 54–61. 10.1002/jlac.19636660107. [DOI] [Google Scholar]
- Puterová Z.; Krutošíková A.; Végh D. Gewald reaction: synthesis, properties and applications of substituted 2-aminothiophenes. Arkivoc 2010, 1, 209–246. [Google Scholar]
- Pedrola M.; Jorba M.; Jardas E.; Jardi F.; Ghashghaei O.; Viñas M.; Lavilla R. Multicomponent Reactions Upon the Known Drug Trimethoprim as a Source of Novel Antimicrobial Agents. Front. Chem. 2019, 7, 475 10.3389/fchem.2019.00475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantzsch A. Condensationsprodukte aus Aldehydammoniak und ketonartigen Verbindungen. Ber. Dtsch. Chem. Ges. 1881, 14, 1637–1638. 10.1002/cber.18810140214. [DOI] [Google Scholar]
- Biginelli P. Ueber Aldehyduramide des Acetessigäthers. Ber. Dtsch. Chem. Ges. 1891, 24, 1317–1319. 10.1002/cber.189102401228. [DOI] [Google Scholar]
- a Passerini M.; Simone L. Sopra gli isonitrili (I). Composto del p-isonitril-azobenzolo con acetone ed acido acetico. Gazz. Chim. Ital. 1921, 51, 126–129. [Google Scholar]; b Passerini M. Isonitriles. II. Compounds with aldehydes or with ketones and monobasic organic acids. Gazz. Chim. Ital. 1921, 51, 181–189. [Google Scholar]
- Weber L. The application of multi-component reactions in drug discovery. Curr. Med. Chem. 2002, 9, 2085–2093. 10.2174/0929867023368719. [DOI] [PubMed] [Google Scholar]
- Hulme C.; Akritopoulou-Zanze I.; Dai W. M.; Beck B.; Srivastava S.; Wang W.; Wang K.; Czarna A.; Holak T. A.; Meireles L.; Camacho C.. Multi-Component Reactions in Drug Discovery. In MCR; Springer: New York, 2009; pp 75–106. [Google Scholar]
- Slobbe P.; Ruijter E.; Orru R. V. Recent applications of multicomponent reactions in medicinal chemistry. MedChemComm 2012, 3, 1189–1218. 10.1039/c2md20089a. [DOI] [Google Scholar]
- Insuasty D.; Castillo J.; Becerra D.; Rojas H.; Abonia R. Synthesis of Biologically Active Molecules through Multicomponent Reactions. Molecules 2020, 25, 505 10.3390/molecules25030505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loev B.; Goodman M. M.; Snader K. M.; Tedeschi R.; Macko E. Hantzsch-type dihydropyridine hypotensive agents. J. Med. Chem. 1974, 17, 956–965. 10.1021/jm00255a010. [DOI] [PubMed] [Google Scholar]
- a Znabet A.; Polak M. M.; Janssen E.; de Kanter F. J.; Turner N. J.; Orru R. V.; Ruijter E. A highly efficient synthesis of telaprevir by strategic use of biocatalysis and multicomponent reactions. Chem. Commun. 2010, 46, 7918–7920. 10.1039/c0cc02823a. [DOI] [PubMed] [Google Scholar]; b Rossen K.; Pye P. J.; DiMichele L. M.; Volante R. P.; Reider P. J. An efficient asymmetric hydrogenation approach to the synthesis of the Crixivan piperazine intermediate. Tetrahedron Lett. 1998, 39, 6823–6826. 10.1016/S0040-4039(98)01484-1. [DOI] [Google Scholar]
- a Desroy N.; Housseman C.; Bock X.; Joncour A.; Bienvenu N.; Cherel L.; Labeguere V.; Rondet E.; Peixoto C.; Grassot J. M.; Picolet O. Discovery of 2-[[2-Ethyl-6-[4-[2-(3-hydroxyazetidin-1-yl)-2-oxoethyl] piperazin-1-yl]-8-methylimidazo [1, 2-a] pyridin-3-yl] methylamino]-4-(4-fluorophenyl) thiazole-5-carbonitrile (GLPG1690), a first-in-class autotaxin inhibitor undergoing clinical evaluation for the treatment of idiopathic pulmonary fibrosis. J. Med. Chem. 2017, 60, 3580–3590. 10.1021/acs.jmedchem.7b00032. [DOI] [PubMed] [Google Scholar]; b Joncour A.; Desroy N.; Housseman C.; Bock X.; Bienvenu N.; Cherel L.; Labeguere V.; Peixoto C.; Annoot D.; Lepissier L.; Heiermann J.; et al. Discovery, structure–activity relationship, and binding mode of an imidazo [1, 2-a] pyridine series of autotaxin inhibitors. J. Med. Chem. 2017, 60, 7371–7392. 10.1021/acs.jmedchem.7b00647. [DOI] [PubMed] [Google Scholar]
- Ghashghaei O.; Masdeu C.; Alonso C.; Palacios F.; Lavilla R. Recent advances of the Povarov reaction in medicinal chemistry. Drug Discovery Today 2018, 29, 71–79. 10.1016/j.ddtec.2018.08.004. [DOI] [PubMed] [Google Scholar]
- Bello Forero J. S.; Jones Junior J.; da Silva F. M. The Povarov reaction as a versatile strategy for the preparation of 1, 2, 3, 4-tetrahydroquinoline derivatives: An overview. Curr. Org. Synth. 2016, 13, 157–175. 10.2174/1570179412666150706183906. [DOI] [Google Scholar]
- Muthukrishnan I.; Sridharan V.; Menéndez J. C. Progress in the Chemistry of Tetrahydroquinolines. Chem. Rev. 2019, 119, 5057–5191. 10.1021/acs.chemrev.8b00567. [DOI] [PubMed] [Google Scholar]
- Priestley E. S.; De Lucca I.; Zhou J.; Zhou J.; Saiah E.; Stanton R.; Robinson L.; Luettgen J. M.; Wei A.; Wen X.; Knabb R. M.; et al. Discovery and gram-scale synthesis of BMS-593214, a potent, selective FVIIa inhibitor. Bioorg. Med. Chem. Lett. 2013, 23, 2432–2435. 10.1016/j.bmcl.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Wong P. C.; Luettgen J. M.; Rendina A. R.; Kettner C. A.; Xin B.; Knabb R. M.; Wexler R. R.; Priestley E. S. BMS-593214, an active site-directed factor VIIa inhibitor: enzyme kinetics, antithrombotic and antihaemostatic studies. Thromb. Haemost. 2010, 104, 261–269. 10.1160/TH10-01-0025. [DOI] [PubMed] [Google Scholar]
- Powell D. A.; Batey R. A. Total synthesis of the alkaloids martinelline and martinellic acid via a hetero Diels– Alder multicomponent coupling reaction. Org. Lett. 2002, 4, 2913–2916. 10.1021/ol026293d. [DOI] [PubMed] [Google Scholar]
- Goli N.; Mainkar P. S.; Kotapalli S. S.; Tejaswini K.; Ummanni R.; Chandrasekhar S. Expanding the tetrahydroquinoline pharmacophore. Bioorg. Med. Chem. Lett. 2017, 27, 1714–1720. 10.1016/j.bmcl.2017.02.077. [DOI] [PubMed] [Google Scholar]
- Carlino L.; Christodoulou M. S.; Restelli V.; Caporuscio F.; Foschi F.; Semrau M. S.; Costanzi E.; Tinivella A.; Pinzi L.; Lo Presti L.; Battistutta R.; et al. Structure–Activity Relationships of Hexahydrocyclopenta [c] quinoline Derivatives as Allosteric Inhibitors of CDK2 and EGFR. ChemMedChem 2018, 13, 2627–2634. 10.1002/cmdc.201800687. [DOI] [PubMed] [Google Scholar]
- Thakur G. A.; Kulkarni A. R.; Deschamps J. R.; Papke R. L. Expeditious Synthesis, Enantiomeric Resolution, and Enantiomer Functional Characterization of (4-(4-Bromophenyl)-3a, 4, 5, 9b-tetrahydro-3 H-cyclopenta [c] quinoline-8-sulfonamide (4BP-TQS): An Allosteric Agonist-Positive Allosteric Modulator of α7 Nicotinic Acetylcholine Receptors. J. Med. Chem. 2013, 56, 8943–8947. 10.1021/jm401267t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann G.; Breitenbücher F.; Schuler M.; Ehrenhofer-Murray A. E. A novel sirtuin 2 (SIRT2) inhibitor with p53-dependent pro-apoptotic activity in non-small cell lung cancer. J. Biol. Chem. 2014, 289, 5208–5216. 10.1074/jbc.M113.487736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan V.; Suryavanshi P. A.; Menendez J. C. Advances in the chemistry of tetrahydroquinolines. Chem. Rev. 2011, 111, 7157–7259. 10.1021/cr100307m. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration . FDA Approves Talazoparib for gBRCAm HER2-Negative Locally Advanced or Metastatic Breast Cancer. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm623540.htm (accessed January 17, 2019).
- Chen C.; Zingales S.; Wang T.; Yuan M.; Wang D.; Cai L.; Jiang Q. Synthesis and in vitro evaluation of 4-substituted furano [3, 2-c] tetrahydroquinolines as potential anti-cancer agents. J. Enzyme Inhib. Med. Chem. 2016, 31, 853–858. 10.3109/14756366.2015.1064120. [DOI] [PubMed] [Google Scholar]
- Díaz J. L.; Christmann U.; Fernandez A.; Luengo M.; Bordas M.; Enrech R.; Carro M.; Pascual R.; Burgueno J.; Merlos M.; Benet-Buchholz J.; et al. Synthesis and biological evaluation of a new series of hexahydro-2 H-pyrano [3, 2-c] quinolines as novel selective σ1 receptor ligands. J. Med. Chem. 2013, 56, 3656–3665. 10.1021/jm400181k. [DOI] [PubMed] [Google Scholar]
- Dent R.; Trudeau M.; Pritchard K. I.; Hanna W. M.; Kahn H. K.; Sawka C. A.; Lickley L. A.; Rawlinson E.; Sun P.; Narod S. A. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- Kassam F.; Enright K.; Dent R.; Dranitsaris G.; Myers J.; Flynn C.; Fralick M.; Kumar R.; Clemons M. Survival outcomes for patients with metastatic triple-negative breast cancer: implications for clinical practice and trial design. Clin. Breast Cancer 2009, 9, 29–33. 10.3816/CBC.2009.n.005. [DOI] [PubMed] [Google Scholar]
- Smith C. D.; Gavrilyuk J. I.; Lough A. J.; Batey R. A. Lewis acid catalyzed three-component hetero-Diels– Alder (Povarov) reaction of N-arylimines with strained norbornene-derived dienophiles. J. Org. Chem. 2010, 75, 702–715. 10.1021/jo9021106. [DOI] [PubMed] [Google Scholar]
- Ralbovsky J. L.; Beckett R. P.. Bridged Phenanthridines. U.S. Patent US2008214537A12008.
- Nossal G. J. V. The Walter and Eliza Hall Institute of Medical Research. Mol. Med. 1996, 2, 165–168. 10.1007/BF03401612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. J.; Green D. V.; Luscombe C. N.; Hill A. P. Getting physical in drug discovery II: the impact of chromatographic hydrophobicity measurements and aromaticity. Drug Discovery Today 2011, 16, 822–830. 10.1016/j.drudis.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Chen H.; Nilsson I.; Muresan S.; Engkvist O. Investigation of the relationship between topology and selectivity for druglike molecules. J. Med. Chem. 2010, 53, 7709–7714. 10.1021/jm1008456. [DOI] [PubMed] [Google Scholar]
- Clemons P. A.; Bodycombe N. E.; Carrinski H. A.; Wilson J. A.; Shamji A. F.; Wagner B. K.; Koehler A. N.; Schreiber S. L. Small molecules of different origins have distinct distributions of structural complexity that correlate with protein-binding profiles. Proc. Natl. Acad. Sci. U.S.A. 2010, 107, 18787–18792. 10.1073/pnas.1012741107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering F.; Bikker J.; Humblet C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 2009, 52, 6752–6756. 10.1021/jm901241e. [DOI] [PubMed] [Google Scholar]
- Schiemann K.; Bruge D.; Buchstaller H.; Finsinger D.; Staehle W.; Amendt C.; Emde U.; Zenke F.. Tetrahydroquinolines. WIPO Patent WO2006002726A12006.
- Dayal N.; Opoku-Temeng C.; Hernandez D. E.; Sooreshjani M. A.; Carter-Cooper B. A.; Lapidus R. G.; Sintim H. O. Dual FLT3/TOPK inhibitor with activity against FLT3-ITD secondary mutations potently inhibits acute myeloid leukemia cell lines. Future Med. Chem. 2018, 10, 823–835. 10.4155/fmc-2017-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opoku-Temeng C.; Dayal N.; Hernandez D. E.; Naganna N.; Sintim H. O. Tetrahydro-3 H-pyrazolo [4, 3-a] phenanthridine-based CDK inhibitor. Chem. Commun. 2018, 54, 4521–4524. 10.1039/C8CC01154K. [DOI] [PubMed] [Google Scholar]
- Opoku-Temeng C.; Dayal N.; Sooreshjani M. A.; Sintim H. O. 3H-pyrazolo [4, 3-f] quinoline haspin kinase inhibitors and anticancer properties. Bioorg. Chem. 2018, 78, 418–426. 10.1016/j.bioorg.2018.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayal N.; Mikek C. G.; Hernandez D.; Naclerio G. A.; Chu E. F. Y.; Carter-Cooper B. A.; Lapidus R. G.; Sintim H. O. Potently inhibiting cancer cell migration with novel 3H-pyrazolo [4, 3-f] quinoline boronic acid ROCK inhibitors. Eur. J. Med. Chem. 2019, 180, 449–456. 10.1016/j.ejmech.2019.06.089. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.