Abstract
Fenclorim is a commercial herbicide safener with fungicidal activity used for chloroacetanilide herbicides, which might be suitable as a lead compound for screening novel fungicides. However, little has been reported so far on the structure–activity relationship of fungicidal activities of fenclorim or its analogues. Here, a series of 4-chloro-6-substituted phenoxy-2-phenylpyrimidine derivatives was synthesized by a substructure splicing route using fenclorim as a lead compound. The structures of synthesized derivatives were characterized by 1H NMR, 13C NMR, and HRMS. Their fungicidal and herbicide safening activities were then evaluated. The results revealed that compound 11 had the best fungicidal activity against Sclerotinia sclerotiorum and Thanatephorus cucumeris, which was better than that of the control pyrimethanil. Moreover, compounds 3, 5, and 25 exhibited excellent safening activities against fresh weight, plant height, and root length, respectively. Such activities were significantly improved when compared to fenclorim. In summary, these findings look promising for the preparation of new fungicides and herbicide safeners based on the structure of fenclorim.
1. Introduction
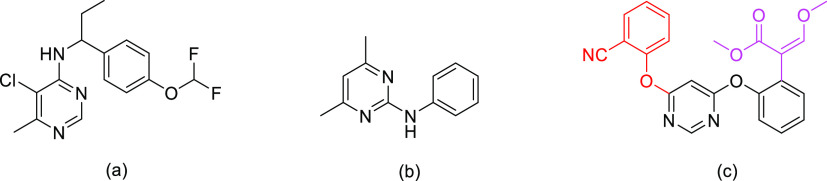
Pyrimidine derivatives are well known for their pharmacological activities, including antiviral,1 anticancer,2−4 anti-inflammatory,5 acaricidal,6 insecticidal,7 herbicidal,8 herbicide safener,9 and even fungicidal activities.10−12 Commonly used commercial pyrimidine fungicides include diflumetorim (Figure 1a), pyrimethanil (Figure 1b), and azoxystrobin (Figure 1c). These molecules can be employed for efficient control over plant pathogenic fungi, such as ascomycetes, basidiomycetes, and deuteromycetes, often found in vegetables, fruits, and grain crops.10,13−17 However, the widespread application of some commercial pyrimidine fungicide agents led to the development of resistance.18−20 For example, the destructive plant pathogen Phytophthora capsici has developed great resistance to azoxystrobin in southern China.21 Hence, the design and development of novel pyrimidine derivatives with promising fungicidal activities are required.
Figure 1.
Chemical structures of (a) diflumetorim, (b) pyrimethanil, and (c) azoxystrobin.
Azoxystrobin, which is a famous synthetic methoxyacrylate fungicide, was first discovered by Imperial Chemical Industries (ICI).22 Azoxystrobin binds to the Q0 site of the cytochrome bc1 enzyme complex to block electron transfer and freeze adenosine triphosphate production, causing the mitochondrial respiration of pathogenic fungi to be hindered.23 Azoxystrobin is primarily composed of two substituted phenoxy moieties, a pyrimidinyl moiety and a methyl (E)-β-methoxyacrylate moiety. The reported structure–activity relationship (SAR) of fungicidal activities of azoxystrobin indicates that a methoxyacrylate moiety (Figure 1c, in pink) and a phenoxy moiety at the sixth position on the pyrimidine ring in azoxystrobin (Figure 1c, in red) are active substructures.24,25 The methoxyacrylate moiety in azoxystrobin is often used as an active group in designing novel fungicides, while the phenoxy moiety is not.26−30
Herbicides are observed to cause phytotoxicity toward crops when used for controlling weeds under field conditions.31 Herbicide safeners are compounds that selectively protect crops from herbicide damage without reducing the herbicidal efficiency on target weed species.32,33 In 1970, the Gulf Oil Company developed the first commercialized herbicide safener, 1,8-naphthalic anhydride, to protect maize from thiocarbamate herbicide injury.34 After that, about 20 commercial herbicide safeners, for example, dichlormid, benoxacar, cloquintocet-mexyl, and flurazole, have been launched in the market to protect crops.18 The main safener mechanism could be ascribed to the enhancement of the detoxifying enzymes [e.g., glutathione S-transferase (GST), cytochrome P450 oxidases, UDP-glucuronosyltransferases, and peroxidase].35−37
Among all commercial herbicide safeners, fenclorim (4,6-dichloro-2-phenyl-pyrimidine) is a pyrimidine-type herbicide safener used for chloroacetanilide herbicides.38,39 When fenclorim is soaked with rice seeds in pre-sowing applications or combination with chloroacetanilide herbicides on rice seedlings, it improves the tolerance of rice seedlings to chloroacetanilide herbicides. Fenclorim detoxifies herbicides by improving the GST expression, catalyzing the conjugation of chloroacetanilide herbicides with glutathione in rice.40,41 We previously evaluated the fungicide activity of fenclorim and proved it could be used as a fungicide-lead compound as this compound showed certain fungicide activity toward fungi such as Sclerotinia sclerotiorum, Fusarium oxysporum, Fusarium graminearum, and Thanatephorus cucumeris.42,43 However, to the best of our knowledge, only a few reports have so far described the SAR of fungicidal activities of fenclorim or its analogues, indicating that more studies are needed.44 Besides, it should be noted that these research studies revealed that strategic structural modifications of the chemical skeleton of fenclorim at the position of chlorine atom on the pyrimidine ring can yield novel molecules with new interesting properties.45
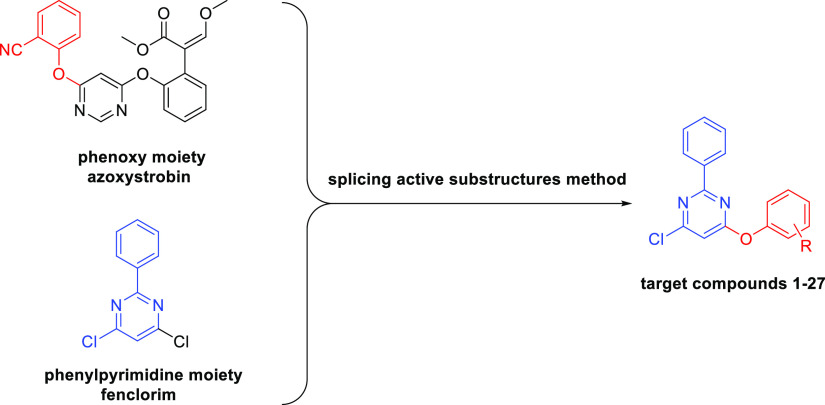
The splicing active substructures method (a combination of the active groups in two different high active compounds to construct a new structure with potential biological activities) is commonly used for the preparation of novel pesticides.46−51 Hence, to screen for fungicides with high activities, a series of 4-chloro-6-phenoxy-2-phenylpyrimidine analogues was synthesized in this study by combining the phenoxy group in azoxystrobin and phenylpyrimidine moieties in fenclorim via this method (Scheme 1). The structures of the synthesized compounds were identified by 1H NMR, 13C NMR, and high-resolution mass spectroscopy (HRMS). The fungicidal activities of synthesized analogues were then evaluated, and pyrimethanil, a pyrimidine-type fungicide, was used as a positive control. Since these compounds showed similar structures as fenclorim, the herbicide safening activities were also tested. The data looked promising and would provide guidance for the discovery of novel fungicides and herbicide safeners based on fenclorim structure.
Scheme 1. Design Strategy of Target Compounds 1–27.
2. Results and Discussion
2.1. Synthesis and Characterization of Target Compounds 1–27
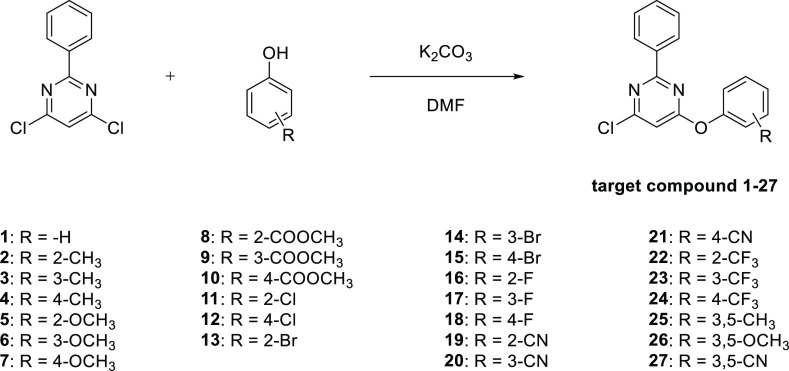
Target compounds 1–27 were prepared by the synthetic route outlined in Scheme 2. Compounds 1–27 were synthesized in N,N-dimethylformamide (DMF) by one-pot reactions of fenclorim with substituted phenols in 32–75% yields in the presence of K2CO3 as a base.52
Scheme 2. Synthesis Route of Target Compounds 1–27.
The chemical structures of target compounds were characterized by 1H NMR, 13C NMR, and HRMS spectroscopies. Here, compound 11 was selected as the model compound. The 1H NMR spectrum of compound 11 clearly showed the hydrogen proton of pyrimidine ring as a singlet at 6.84 ppm along with protons of the phenyl ring and phenoxy group as triplets at 8.21 and 8.18 ppm and multiplicities at 7.52–7.55, 7.35–7.48, and 7.29–7.32 ppm. The 13C NMR spectrum of compound 11 showed the resonance of the pyrimidine ring at 104.8, 162.2, 164.8, and 169.5 ppm, while those of the phenyl ring and phenoxy group were recorded at 123.8–148.3 ppm. Moreover, HRMS data of compound 11 agreed well with the calculated data based on chemical formula.
2.2. Crystal Structure of Compound 11
The chemical structure of the representative compound 11 was further identified by X-ray single crystallography. Selected and refined crystal data of compound 11 are listed in Table S1. The crystal structure and crystal packing diagrams of compound 11 are presented in Figures 2 and S1, and selected molecular structure parameters are provided in Table S2.
Figure 2.
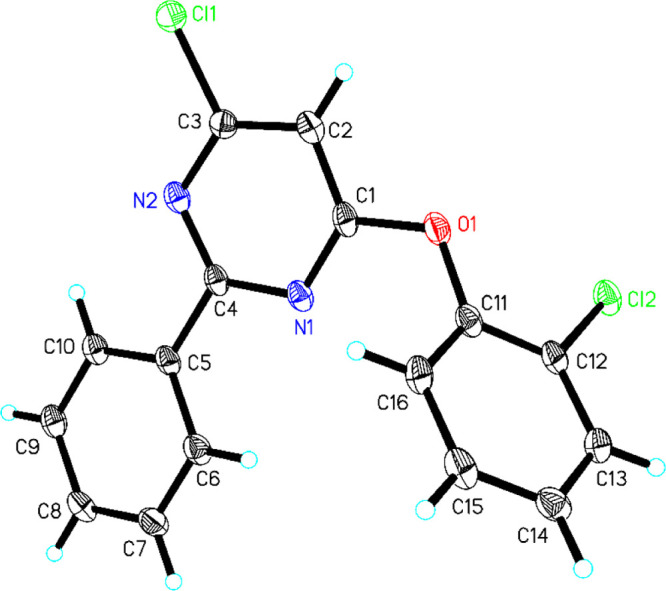
Crystal structure of compound 11.
As shown in Figure 2, the skeleton of 6-aryloxy-4-chloro-2-phenylpyrimidines was constructed by two benzene rings and a chlorine-substituted pyrimidine ring. Substituted benzene ring connected to pyrimidine through an oxygen atom (Figure 2). Furthermore, the bonds N(2)–C(3) (1.328(6) Å), N(1)–C(4) (1.351(6) Å), N(2)–C(4) (1.339(7) Å), and N(1)–C(1) (1.321(6) Å) in the pyrimidine ring were similar to bonds N–C (1.307–1.366 Å) in pyrimidine reported previously.53−55 The bonds in two phenyl rings, such as C(5)–C(10) (1.397(7) Å), C(6)–C(7) (1.383(7) Å), C(5)–C(6) (1.393(7) Å), C(11)–C(12) (1.383(7) Å), C(12)–C(13) (1.387(7) Å), and C(13)–C(14) (1.380(7) Å) were also similar to the reported C–C bonds in phenyl (1.359–1.399 Å).56−58 The chloro-substituted benzene ring was connected to the phenylpyrimidine ring through an oxygen atom with a C(1)–O(1)–C(11) bond angle of 119.3(4)°.
2.3. Evaluation of Fungicidal Activities
S. sclerotiorum, T. cucumeris, F. graminearum, and F. oxysporum are pervasive plant pathogenic fungal species, which cause great damage to the production of crop plants.59−62 Hence, we investigated the fungicidal activities of target compounds 1–27 toward these fungi in primary screen (50 mg/L) and pyrimethanil, a pyrimidine-type fungicide for controlling fungus such as S. sclerotiorum,42,63 was used as a positive control, and the results are listed in Table 1. It was found that the fungicidal activities of target compounds against S. sclerotiorum (89–97%) and T. cucumeris (50–89%) were better than those against F. graminearu (7–42%) and F. oxysporum (7–60%). All target compounds 1–27 showed excellent activities against S. sclerotiorum, comparable to the positive controls fenclorim and pyrimethanil. The target compounds displayed lower fungicidal activities against T. cucumeris when compared to that of pyrimethanil in the primary screen test but exhibited higher fungicidal activities compared to that of fenclorim. In particular, compound 1 with no substitution in the phenoxy group showed the best fungicidal activity against T. cucumeris (89%). Compounds 1–7, 11–13, 15–17, 18–19, and 22 exhibited similar patterns of fungicidal activities against F. oxysporum when compared to their fungicidal activities against F. graminearu. Among all compounds, 1 and 16 had the highest activities against F. graminearu (42%) and F. oxysporum (60%), respectively. Fenclorim exhibited poor activity against F. oxysporum (26%), and pyrimethanil did not show good activity against F. graminearu (27%) and F. oxysporum (23%).
Table 1. Fungicidal Activities of Target Compounds 1–27 against Four Pathogenic Bacteria (50 mg/L)a.
| inhibitory
rate (%) |
|||||
|---|---|---|---|---|---|
| compd | R | S. sclerotiorum | T. cucumeris | F. oxysporum | F. gaminearu |
| 1 | –H | 95 ± 0 | 89 ± 2 | 42 ± 2 | 48 ± 1 |
| 2 | 2-CH3 | 93 ± 1 | 84 ± 1 | 26 ± 2 | 46 ± 1 |
| 3 | 3-CH3 | 89 ± 2 | 83 ± 1 | 11 ± 2 | 35 ± 3 |
| 4 | 4-CH3 | 95 ± 1 | 78 ± 0 | 11 ± 2 | 44 ± 1 |
| 5 | 2-OCH3 | 92 ± 1 | 80 ± 2 | 26 ± 1 | 40 ± 0 |
| 6 | 3-OCH3 | 96 ± 1 | 74 ± 0 | 15 ± 2 | 27 ± 1 |
| 7 | 4-OCH3 | 95 ± 0 | 75 ± 8 | 25 ± 2 | 32 ± 1 |
| 8 | 2-COOCH3 | 89 ± 1 | 70 ± 1 | 9 ± 2 | 19 ± 1 |
| 9 | 3-COOCH3 | 94 ± 1 | 66 ± 4 | 10 ± 1 | 15 ± 2 |
| 10 | 4-COOCH3 | 93 ± 0 | 54 ± 2 | 20 ± 4 | 11 ± 3 |
| 11 | 2-Cl | 96 ± 0 | 85 ± 2 | 27 ± 2 | 44 ± 3 |
| 12 | 4-Cl | 92 ± 1 | 71 ± 2 | 19 ± 3 | 25 ± 0 |
| 13 | 2-Br | 93 ± 0 | 81 ± 1 | 25 ± 1 | 35 ± 1 |
| 14 | 3-Br | 95 ± 0 | 74 ± 2 | 16 ± 1 | 23 ± 2 |
| 15 | 4-Br | 94 ± 0 | 77 ± 2 | 7 ± 2 | 27 ± 1 |
| 16 | 2-F | 95 ± 1 | 86 ± 2 | 22 ± 2 | 60 ± 1 |
| 17 | 3-F | 97 ± 1 | 84 ± 1 | 21 ± 1 | 45 ± 1 |
| 18 | 4-F | 95 ± 1 | 86 ± 1 | 18 ± 1 | 37 ± 2 |
| 19 | 2-CN | 96 ± 1 | 71 ± 3 | 15 ± 3 | 25 ± 2 |
| 20 | 3-CN | 93 ± 1 | 50 ± 1 | 16 ± 1 | 12 ± 3 |
| 21 | 4-CN | 95 ± 1 | 52 ± 3 | 17 ± 3 | 7 ± 1 |
| 22 | 2-CF3 | 95 ± 1 | 76 ± 1 | 20 ± 1 | 38 ± 0 |
| 23 | 3-CF3 | 95 ± 1 | 65 ± 2 | 14 ± 2 | 19 ± 3 |
| 24 | 4-CF3 | 95 ± 1 | 69 ± 3 | 11 ± 3 | 14 ± 1 |
| 25 | 3,5-CH3 | 94 ± 0 | 67 ± 2 | 14 ± 3 | 17 ± 3 |
| 26 | 3,5-OCH3 | 95 ± 1 | 61 ± 3 | 13 ± 4 | 19 ± 1 |
| 27 | 3,5-CN | 95 ± 1 | 61 ± 3 | 20 ± 1 | 11 ± 1 |
| fenclorim | 88 ± 1 | 40 ± 0 | 26 ± 1 | 75 ± 2 | |
| pyrimethanil | 91 ± 3 | 98 ± 0 | 27 ± 0 | 23 ± 0 | |
S. sclerotiorum means Sclerotinia sclerotiorum; T. cucumeris means Thanatephorus cucumeris; F. graminearum means Fusarium graminearum. F. oxysporum means Fusarium oxysporum. The experiments were repeated three times to ensure better reproducibility.
Based on the analysis of the experimental results, the SAR for fungicidal activities of target compounds were determined as follows: (1) the target compounds with substituents on phenoxy moiety exhibited similar excellent activities against S. sclerotiorum, indicating that insertion of phenoxy moiety into the structure of fenclorim might be beneficial for the enhancement of fungicidal activities against S. sclerotiorum; (2) substituents on phenoxy moiety were generally more active against T. cucumeris at ortho-position than at para- and meta-positions. With the exception of chlorine atoms, methyl and methoxycarbonyl-substituted compounds, activity against T. cucumeris was lower with substituents at meta-position (6, 14, 17, 20, and 23); (3) comparing compounds 2–7 and 16–24, electron-donating substituents (methyl and methoxy groups) on phenoxy moiety were more active at ortho-position than para- and meta-positions against F. graminearu and F. oxysporum. On the other hand, when the substituent groups were fluorine atoms and cyano and trifluoromethyl groups, the activity against F. graminearu showed the following pattern: ortho-position > meta-position > para-position. (4) Comparing compounds 25–27, there was no significant difference in the fungicidal activities against all four pathogenic bacteria between monosubstituted and disubstituted compounds.
To further investigate the fungicidal activities of the as-prepared compounds, median effective concentrations (EC50 values) of the control and the compounds with high activities (inhibitory rate > 80%) against S. slerotiorum and T. cucumeris, which were chosen as representative plant pathogens, were compared, and the data are listed in Tables 2 and 3. As shown in Table 2, the tested compounds (1–27 and pyrimethanil) displayed moderate to excellent fungicidal activity with EC50 values varying from 0.93 to 33.17 mg/L. Compound 2, 4, 6, 11, 17, and 19 had better fungicidal activity against S. sclerotiorum than that of both fenclorin and commercial fungicide pyrimethanil, and compound 11 exhibited the best fungicidal activity. In general, ortho substitution is better than meta and para: except for ester and fluorine miniseries where meta is better than para and ortho.
Table 2. Median Effective Concentrations (EC50 Values) of Compound 1–27 and Pyrimethanil with High Activities against S. sclerotioruma.
| compd | regression equation | EC50 (mg/L) | R2 |
|---|---|---|---|
| 1 | y = 4.7129 + 1.3468x | 1.63 | 0.9658 |
| 2 | y = 4.4548 + 1.1389x | 2.93 | 0.9459 |
| 3 | y = 3.2423 + 1.7133x | 10.61 | 0.9887 |
| 4 | y = 4.6088 + 1.6642x | 1.72 | 0.9684 |
| 5 | y = 3.4569 + 1.9498x | 6.19 | 0.9837 |
| 6 | y = 3.7257 + 3.6264x | 2.25 | 0.8736 |
| 7 | y = 3.4406 + 2.3434x | 4.63 | 0.9921 |
| 8 | y = 2.6144 + 2.3831x | 10.02 | 0.9671 |
| 9 | y = 3.3492 + 2.0168x | 6.58 | 0.9926 |
| 10 | y = 3.0060 + 2.2181x | 7.92 | 0.9954 |
| 11 | y = 5.0418 + 1.3366x | 0.93 | 0.9841 |
| 12 | y = 3.3324 + 1.9899x | 6.89 | 0.9935 |
| 13 | y = 3.0490 + 2.1184x | 8.34 | 0.9851 |
| 14 | y = 0.1807 + 4.4782x | 11.92 | 0.9316 |
| 15 | y = 1.4425 + 5.3152x | 16.30 | 0.9556 |
| 16 | y = 1.4142 + 4.8817x | 20.60 | 0.9666 |
| 17 | y = 3.9169 + 3.5435x | 2.02 | 0.8825 |
| 18 | y = 0.3326 + 4.1711x | 13.15 | 0.9096 |
| 19 | y = 3.7449 + 4.7318x | 1.84 | 0.9544 |
| 20 | y = 3.1576 + 2.3194x | 6.23 | 0.9820 |
| 21 | y = −1.3728 + 5.4696x | 14.63 | 0.9370 |
| 22 | y = 0.2663 + 4.3309x | 12.39 | 0.9189 |
| 23 | y = 0.2400 + 4.0280x | 15.20 | 0.9194 |
| 24 | y = 1.8941 + 4.5332x | 33.17 | 0.8941 |
| 25 | y = −1.4069 + 5.1238x | 17.80 | 0.9576 |
| 26 | y = −2.0993 + 5.1383x | 24.08 | 0.8887 |
| 27 | y = −0.1213 + 5.3025x | 9.24 | 0.9613 |
| Fenclorim | y = 3.3612 + 1.1608x | 25.81 | 0.0945 |
| Pyrimethanil | y = 4.5148 + 1.2897x | 2.38 | 0.9721 |
The experiments were carried out three times to ensure better reproducibility. To obtain E[C50 values, data of fungicidal activities were statistically analyzed by the SPSS 22.0 software package.
Table 3. Median Effective Concentrations (EC50 Values) of Compound 1–3, 11, 13, 16–18, and Pyrimethanil with High Activities against T. cucumerisa.
| compd | regression equation | EC50 (mg/L) | R2 |
|---|---|---|---|
| 1 | y = −1.3229 + 5.5673x | 13.67 | 0.9233 |
| 2 | y = 2.5735 + 2.4618x | 9.68 | 0.9269 |
| 3 | y = 1.3842 + 5.5297x | 14.27 | 0.9370 |
| 5 | y = 0.3677 + 3.9391x | 15.00 | 0.9019 |
| 11 | y = 4.3517 + 1.2403x | 3.33 | 0.9631 |
| 13 | y = 2.0194 + 5.0056x | 25.25 | 0.8824 |
| 16 | y = 3.8307 + 1.5370x | 5.76 | 0.9830 |
| 17 | y = 2.8427 + 2.0571x | 11.19 | 0.9864 |
| 18 | y = 4.3211 + 1.2048x | 3.66 | 0.9862 |
| pyrimethanil | y = 4.0854 + 1.3827x | 4.59 | 0.9534 |
The experiments were carried out three times to ensure better reproducibility. To obtain EC50 values, data of fungicidal activities were statistically analyzed by SPSS 22.0 software package.
All target compounds with high activity against S. sclerotiorum demonstrated nearly complete inhibition of S. sclerotiorum based on the results of the preliminary screening. Moreover, considering the EC50 test results, some of these compounds showed stronger fungicidal activity than pyrimethanil. These indicated that they might be potential candidates for controlling S. sclerotiorum and useful lead compounds to design new fungicides. Hence, more compounds of this series will be synthesized, and their EC50 values against S. sclerotiorum will be tested to be used for building a quantitative SAR model in the future together with those reported in this article.
Also, compounds 1–3, 11, 13, 16–18, and pyrimethanil exhibited moderate to good fungicidal activities against T. cucumeris with EC50 values ranging from 3.33 to 25.25 mg/L (Table 3). Compounds 11 and 18 showed better fungicidal activities than pyrimethanil, and moreover, the fungicidal activities of compound 11 against S. slerotiorum and T. cucumeris were the best of the series, thus being the most interesting compound of the series. The results of fungicidal activities above also revealed that a combination of substituted phenoxy groups with phenylpyrimidine moiety in fenclorim may form novel pyrimidine-type fungicides with improved activities against the tested plant pathogens than commercial fungicide pyrimethanil.
2.4. Evaluation of Herbicide Safening Activities
The effects of target compounds 1–27 (1 mg/L) on the growth of rice plant height, root length, and fresh weight were evaluated, and the data are depicted in Table 4. Note that the results were represented as the relative value of plant height, root length, and fresh weight. Compared to nontreated controls, the relative changes in growth indices of compounds 1–27 varied from 92 to 99% for plant height, 91–100% for root length, and 92–100% for fresh weight. For security testing of rice plants, these data indicated that compounds 1–27 had very low inhibitory effects on the growth of rice seedlings.
Table 4. Effect of Target Compounds 1–27 on the Growth in Rice Plant Heights, Root Lengths, and Fresh Weight at a Concentration of 1 mg/La.
| safening
effect (% of non-treated control) |
||||
|---|---|---|---|---|
| compd | R | plant height | root length | fresh weight |
| 1 | –H | 97 ± 1 | 99 ± 2 | 97 ± 1 |
| 2 | 2-CH3 | 98 ± 1 | 98 ± 1 | 99 ± 1 |
| 3 | 3-CH3 | 98 ± 1 | 94 ± 2 | 98 ± 1 |
| 4 | 4-CH3 | 93 ± 1 | 96 ± 2 | 95 ± 2 |
| 5 | 2-OCH3 | 99 ± 1 | 99 ± 2 | 99 ± 0 |
| 6 | 3-OCH3 | 96 ± 2 | 98 ± 1 | 92 ± 2 |
| 7 | 4-OCH3 | 97 ± 1 | 99 ± 0 | 98 ± 1 |
| 8 | 2-CO2CH3 | 97 ± 2 | 99 ± 1 | 97 ± 2 |
| 9 | 3-CO2CH3 | 96 ± 1 | 100 ± 1 | 99 ± 1 |
| 10 | 4-CO2CH3 | 98 ± 1 | 98 ± 0 | 97 ± 1 |
| 11 | 2-Cl | 99 ± 0 | 98 ± 1 | 94 ± 2 |
| 12 | 4-Cl | 99 ± 0 | 98 ± 1 | 98 ± 1 |
| 13 | 2-Br | 99 ± 0 | 94 ± 3 | 98 ± 1 |
| 14 | 3-Br | 96 ± 1 | 93 ± 2 | 96 ± 1 |
| 15 | 4-Br | 92 ± 2 | 96 ± 1 | 94 ± 1 |
| 16 | 2-F | 98 ± 1 | 98 ± 2 | 98 ± 1 |
| 17 | 3-F | 97 ± 1 | 96 ± 2 | 100 ± 0 |
| 18 | 4-F | 94 ± 3 | 92 ± 2 | 99 ± 1 |
| 19 | 2-CN | 96 ± 2 | 95 ± 2 | 99 ± 1 |
| 20 | 3-CN | 99 ± 0 | 100 ± 0 | 95 ± 1 |
| 21 | 4-CN | 99 ± 1 | 99 ± 3 | 94 ± 0 |
| 22 | 2-CF3 | 99 ± 0 | 99 ± 0 | 94 ± 2 |
| 23 | 3-CF3 | 94 ± 3 | 91 ± 2 | 93 ± 2 |
| 24 | 4-CF3 | 93 ± 2 | 97 ± 1 | 95 ± 1 |
| 25 | 3-CH3, 5-CH3 | 99 ± 0 | 95 ± 1 | 98 ± 2 |
| 26 | 3-OCH3, 5-OCH3 | 98 ± 1 | 98 ± 2 | 98 ± 1 |
| 27 | 3-CN, 5-CN | 98 ± 2 | 94 ± 2 | 97 ± 1 |
All experiments were performed in triplicate.
The herbicide safening activities of target compounds 1–27 were also evaluated due to their similar structures as that of fenclorim. Table 5 compares the relative values of plant height, root length, and fresh weight. The biological activities indicated that the growth of height (51%), root length (48%), and fresh weight (65%) of the rice plant was significantly inhibited by metolachlor (M, 0.25 μM). By combining target compounds 1–27 with M, the rice plant injury from M was alleviated. The relative values of plant height under combined treatment with compounds 1–27 and M varied from 60 to 92%, where the highest value (92% for non-treated control) was recorded with combined treatment of compounds 5 (R = 2-OCH3) and M, followed by M and then compound 8 (84%). The relative values of root length under the combined treatment of compounds 1–27 and M ranged from 62 to 97%. Compounds 1–3, 6, 10–11, 13, 15, and 25–27 exhibited better or similar activities when compared to the control fenclorim (85%). Compound 25 with no substitution displayed the best activity on root length (97%). The relative values of fresh weight for combined treatment of compounds 1–27 were in the range of 81–97%, where compound 3 (97%) displayed the highest safener activity that was significantly larger than that of fenclorim (89%).
Table 5. Herbicide Safening Effect of 7-Day-Old Rice Seedlings Treated with Metolachlor on Plant Height, Root Length, and Fresh Weighta.
| safening
effect (% of non-treated control) |
|||
|---|---|---|---|
| compd | plant height | root length | fresh weight |
| M | 51 ± 1 | 48 ± 2 | 65 ± 1 |
| 1 + M | 78 ± 1 | 97 ± 0 | 93 ± 1 |
| 2 + M | 67 ± 1 | 87 ± 1 | 87 ± 2 |
| 3 + M | 68 ± 1 | 82 ± 2 | 97 ± 0 |
| 4 + M | 68 ± 0 | 76 ± 2 | 82 ± 1 |
| 5 + M | 92 ± 1 | 69 ± 3 | 88 ± 3 |
| 6 + M | 72 ± 0 | 94 ± 2 | 86 ± 2 |
| 7 + M | 79 ± 2 | 74 ± 1 | 81 ± 1 |
| 8 + M | 84 ± 2 | 77 ± 2 | 93 ± 2 |
| 9 + M | 74 ± 2 | 77 ± 1 | 87 ± 2 |
| 10 + M | 73 ± 2 | 92 ± 2 | 83 ± 2 |
| 11 + M | 72 ± 1 | 89 ± 1 | 91 ± 2 |
| 12 + M | 72 ± 1 | 65 ± 1 | 93 ± 2 |
| 13 + M | 74 ± 2 | 88±±2 | 90 ± 1 |
| 14 + M | 73 ± 1 | 62 ± 2 | 90 ± 2 |
| 15 + M | 72 ± 1 | 91 ± 2 | 92 ± 1 |
| 16 + M | 71 ± 1 | 66 ± 1 | 81 ± 0 |
| 17 + M | 69 ± 2 | 56 ± 2 | 84 ± 2 |
| 18 + M | 69 ± 2 | 62 ± 1 | 85 ± 2 |
| 19 + M | 75 ± 1 | 67 ± 1 | 93 ± 2 |
| 20 + M | 62 ± 1 | 76 ± 2 | 86 ± 4 |
| 21 + M | 60 ± 0 | 64 ± 3 | 93 ± 2 |
| 22 + M | 64 ± 1 | 72 ± 1 | 92 ± 2 |
| 23 + M | 63 ± 1 | 70 ± 2 | 86 ± 1 |
| 24 + M | 63 ± 3 | 71 ± 0 | 86 ± 2 |
| 25 + M | 76 ± 1 | 97 ± 1 | 93 ± 1 |
| 26 + M | 67 ± 2 | 84 ± 1 | 84 ± 1 |
| 27 + M | 71 ± 0 | 83 ± 2 | 89 ± 2 |
| F + M | 85 ± 1 | 85 ± 2 | 88 ± 1 |
All experiments were performed in triplicates. M: 0.25 μM metolachlor. 1–27 + M: combined treatment of 1 mg/L compounds 1–27 and 0.25 μM metolachlor. F + M: combined treatment of 1 mg/L fenclorim and 0.25 μM metolachlor.
On the basis of the experimental results, the SAR for safening activities of these compounds were as follows: (1) combining phenoxy group in azoxystrobin and phenylpyrimidine moieties in fenclorim decreased the safening effect on plant height of rice seedlings, with the exception of 5 with 2-OCH3 moiety; (2) comparing 2, 6, 10–11, 13, 15, and 25, it was indicated that introducing the methyl moiety, methoxyl moiety, methoxycarbonyl moiety, chlorine atom, and 3,5-dimethyl moiety might enhance the safening effect on root length of rice seedlings. However, an explicit relationship was not found between the positions of the substituents and the safening effect on root length; (3) bromine atoms on the phenoxy moiety were equal or more active than fluorine atoms for the safening effect on root length. (4) The hydrogen atoms on the phenoxy ring were superior to all electron-donating groups and electron-withdrawing groups, with the exception of the 3,5-dimethyl moiety. (5) No significant differences were observed in the herbicide safening activities between the monosubstituted and disubstituted compounds.
3. Conclusions
A series of 6-aryloxy-4-chloro-2-phenylpyrimidines was successfully synthesized by a one-pot and splicing active substructures method. The structures of the synthesized compounds were confirmed by NMR, HRMS, and X-ray crystal structure analyses. Their fungicidal and herbicide safening activities were evaluated. The data revealed that compound 11 possessed the best fungicidal activity against S. sclerotiorum and T. cucumeris, indicating potent activity when compared to the control fenclorin and pyrimethanil. On the other hand, compound 5 exhibited excellent safener activity on plant height, which was significantly higher than that of control fenclorim. Compound 25 displayed the best herbicide safener activity on root length. Compound 3 exhibited the highest safener activity on fresh weight, which was significantly larger than that of fenclorim. Hence, incorporation of the phenoxy group in azoxystrobin and phenylpyrimidine moieties in fenclorim could lead to the formation of novel compounds with high safener herbicide and fungicidal activities.
4. Experimental Section
4.1. Materials and Characterization
Fenclorim (purity 98%), substituted phenols (purity 97–99%), and anhydrous potassium carbonate (purity 99%) were provided by Jilin Chinese Academy of Sciences-Yanshen Technology Co., Ltd. (Jilin, China). DMF, petroleum ether (PE), and dichloromethane (DCM) were obtained from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China) and used as received without further purification. The melting points were measured by a Hanon MP100 automatic melting point apparatus on open capillary tubes (Jinan Hanon Instruments Co., Ltd., Jinan, China). 1H NMR and 13C NMR spectra of target compounds were obtained on a Varian Mercury-Plus 300 spectrometer (Varian, Inc., Salt Lake, USA) operating at 300 MHz (1H NMR) and 75 MHz (13C NMR), respectively, or a Bruker AVANCE-500 spectrometer (Bruker Optics, Ettlingen, BW, Germany) operating at 500 MHz (1H NMR) and 125 MHz (13C NMR). High-resolution mass spectral data were recorded on an FTICR-MS Varian 7.0 T FTICR-MS instrument (Varian IonSpec, Lake Forest, USA). X-ray single-crystal structure data were collected on a Rigaku SuperNova, Dual, Cu at zero, AtlasS2 diffractometer (Agilent, CA, USA).
4.2. Synthesis of Compounds 1–27
A modified procedure was used for the synthesis of target compounds 1–27 (Scheme 2).52 First, fenclorim (4.4 mmol, 1.00 g) and anhydrous K2CO3 (8.8 mmol, 1.22 g) were dissolved in DMF (30 mL). Substituted phenols (4.4 mmol) were added in a dropwise manner into the mixed solution and then stirred at 60 °C for 4 h followed by cooling down, pouring into water, and filtering to yield crude products as residue. The products were further purified by column chromatography with DCM/PE (1/10) to obtain pure target compounds 1–27.
Physical and chemical properties, 1H NMR, 13C NMR, and HRMS data, 1H NMR and 13C NMR spectra of 1–27 (Figures S2–S55) can be found in the Supporting Information.
4.3. Crystal Structure Analysis
Compound 11 was first recrystallized from ethanol to obtain a suitable colorless single crystal. X-ray single-crystal structure data of compound 11 was then collected on a Rigaku SuperNova, Dual, Cu at zero, AtlasS2 diffractometer (Agilent, CA, USA) equipped with Mo Kα radiation (λ = 1.54184 Å) at 100.00(10) K. The data processing was accomplished with SHELXL program. Using Olex2,64 the crystal structure of compound 11 was directly solved by ShelXT structure solution program and refined with ShelXL refinement package by means of least squares method.65,66 Selected crystallographic data of compound 11 are listed in Table S1, and some molecular structure parameters are described in Table S2. The crystallographic structural data of compound 11 were deposited at the Cambridge Crystallographic Data Centre (CCDC) under CCDC number of 1966186. These data can be accessed free of charge at https://www.ccdc.cam.ac.uk/structures or by application to CCDC, 12 Union Road, Cambridge CB2 1EZ, United Kingdom (Tel: +44-1223-336408; Fax: +44-1223-336033; E-mail: deposit@ccdc.cam.ac.uk).
4.4. Evaluation of Fungicidal Activity
The fungicidal activities of compounds 1–27 were tested in vitro against four fungus species by mycelium growth rate method reported in the literature.67 The employed fungus species were S. sclerotiorum, T. cucumeris, F. graminearum, and F. oxysporum. The solutions were prepared by dissolving 10 mg of compounds 1–27 in 1 mL of dimethyl sulfoxide (DMSO) to yield a concentration of 10 mg/mL. The solutions were then mixed with 199 mL potato dextrose agar. Next, media containing compounds 1–27 at concentrations of 50 mg/L for initial screening were poured into sterile Petri dishes (d = 9 cm) followed by cooling down. Mycelia disks of 0.5 cm diameter were then inoculated on the center of the Petri dishes at 25 °C for 2 days. DMSO without any other compounds was employed as the non-treated control. The treated hypha diameter was measured using a cross bracketing method, and commercial fungicide pyrimethanil and fenclorim were used as positive controls. The inhibition rate of compounds 1–27 against fungi was calculated according to eq 1
| 1 |
when C0 is the colony diameter of control and C is the colony diameter of the treated sample. All experiments in this work were repeated three times, and the bioassay results were the average of three replicates. The test concentrations used for calculating EC50 values were 25, 12.5, 6.25, 3.13, and 1.56 mg/L, respectively. All statistical analyses were performed by the SPSS 22.0 software package (IBM, NY, USA). The EC50 values were calculated using log-probit analysis.
4.5. Evaluation of Herbicide Safening Activity
The germination method reported in the literature was used for rice seed experiments (Oryza sativa L. ssp. Indica).68 To this end, healthy rice seeds with uniform size and full-grain were sterilized for 15 min by 5% sodium hypochlorite solution and then thoroughly washed by distilled water. They were then soaked in distilled water for 24 h at 28 °C and were germinated for 36 h in the dark in a climatic cabinet at 28 °C. The herbicide safening activities were evaluated under laboratory conditions according to the literature methods. Uniformly germinated paddy rice seedlings were selected before emergence and transplanted in 0.3% agar media containing 0.25 μM metolachlor (M), 1 mg/L of compounds 1–27, combined fenclorim (F) with 1 mg/L, and 0.25 μM M, and combined compounds 1–27 with 1 mg/L and 0.25 μM M for the primary screening test. Agar medium without compounds was used as the non-treated control. Fifty seeds were put on plates for each test and then incubated for 14 h under growth light intensity of 110–130 μE m–2 s–1 at 30 °C followed by 10 h dark photoperiod at 25 °C. The indices of plant height, root length, and fresh weight-related to herbicide safening activities were measured after 7 days. The herbicide safening effects of plant height, root length, or fresh weight relative value used for SAR analysis were calculated by eqs 2–4
| 2 |
| 3 |
| 4 |
where R1 is the relative value of plant height, Xn is the plant height foreach treatment, and X0 is the average plant height of the non-treated control. R2 represents the average relative value of root length, Yn is the root length of each treatment, and Y0 is the average root length of the non-treated control. R3 is the fresh weight of the non-treated control. Zn is the fresh weight of each treatment, and Z0 is the average fresh weight of the non-treated control. Note that all experiments were performed three times to ensure better reproducibility, and the bioassay results (the average of three replicates) were obtained according to a similar method in the reference we reported before.69 All statistical analyses were performed using the SPSS 22.0 software package (IBM, NY, USA).
4.6. Data Analysis
Data in the experiments were examined via a one-way analysis of variance, and the value of P < 0.05 was considered to be significant.
Acknowledgments
This research was funded by National Natural Science Foundation of China (no. 31772182).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsomega.0c03300.
Crystal packing diagrams of 11; selected crystallographic data of 11; selected molecular structure parameters of 11; physical and chemical properties, 1H NMR, 13C NMR, and HRMS data of 1–27; and 1H NMR and 13C NMR spectra of 1–27 (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Raić-Malić S.; Johayem A.; Ametamey S. M.; Batinac S.; De Clercq E.; Folkers G.; Scapozza L. Synthesis, 18F-radiolabelling and biological evaluations of C-6 alkylated pyrimidine nucleoside analogues. Nucleosides, Nucleotides Nucleic Acids 2004, 23, 1707–1721. 10.1081/ncn-200033914. [DOI] [PubMed] [Google Scholar]
- Abbas S. E.-S.; George R. F.; Samir E. M.; Aref M. M.; Abdel-Aziz H. A. Synthesis and anticancer activity of some pyrido[2,3-d]pyrimidine derivatives as apoptosis inducers and cyclin-dependent kinase inhibitors. Future Med. Chem. 2019, 11, 2395–2414. 10.4155/fmc-2019-0050. [DOI] [PubMed] [Google Scholar]
- Shanmugasundaram M.; Senthilvelan A.; Kore A. R. C-5 substituted pyrimidine nucleotides/nucleosides: Recent progress in synthesis, functionalization, and applications. Curr. Org. Chem. 2019, 23, 1439–1468. 10.2174/1385272823666190809124310. [DOI] [Google Scholar]
- Jarusiewicz J. A.; Jeon J. Y.; Connelly M. C.; Chen Y.; Yang L.; Baker S. D.; Guy R. K. Discovery of a diaminopyrimidine FLT3 inhibitor active against acute myeloid leukemia. ACS Omega 2017, 2, 1985–2009. 10.1021/acsomega.7b00144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F.; Zang L.; Miao X.; Jia F.; Wang J.; Zhu M.; Gong P.; Jiang N.; Zhai X. Design, synthesis and anti-inflammatory evaluation of novel pyrrolo[2,3-d]pyrimidin derivatives as potent JAK inhibitors. Bioorg. Med. Chem. 2019, 27, 4089–4100. 10.1016/j.bmc.2019.07.037. [DOI] [PubMed] [Google Scholar]
- Chai B.-S.; Liu C.-L.; Li H.-C.; Liu S.-W.; Xu Y.; Song Y.-Q.; Chang J.-B. Synthesis and acaricidal activity of strobilurin-pyrimidine derivatives. Chin. Chem. Lett. 2014, 25, 137–140. 10.1016/j.cclet.2013.10.006. [DOI] [Google Scholar]
- Liu X.-H.; Wang Q.; Sun Z.-H.; Wedge D. E.; Becnel J. J.; Estep A. S.; Tan C.-X.; Weng J.-Q. Synthesis and insecticidal activity of novel pyrimidine derivatives containing urea pharmacophore against Aedes aegypti. Pest Manage. Sci. 2017, 73, 953–959. 10.1002/ps.4370. [DOI] [PubMed] [Google Scholar]
- Zuo Y.; Wu Q.; Su S.-w.; Niu C.-w.; Xi Z.; Yang G.-F. Synthesis, Herbicidal Activity, and QSAR of Novel N-Benzothiazolyl- pyrimidine-2,4-diones as Protoporphyrinogen Oxidase Inhibitors. J. Agric. Food Chem. 2016, 64, 552–562. 10.1021/acs.jafc.5b05378. [DOI] [PubMed] [Google Scholar]
- Brazier-Hicks M.; Knight K. M.; Sellars J. D.; Steel P. G.; Edwards R. Testing a chemical series inspired by plant stress oxylipin signalling agents for herbicide safening activity. Pest Manage. Sci. 2018, 74, 828–836. 10.1002/ps.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P.; Guan A.; Xia X.; Sun X.; Wei S.; Yang J.; Wang J.; Li Z.; Lan J.; Liu C. Design, synthesis, and structure-activity relationship of new arylpyrazole pyrimidine ether derivatives as fungicides. J. Agric. Food Chem. 2019, 67, 11893–11900. 10.1021/acs.jafc.9b05185. [DOI] [PubMed] [Google Scholar]
- Sharma V.; Chitranshi N.; Agarwal A. K. Significance and biological importance of pyrimidine in the microbial world. Int. J. Med. Chem. 2014, 2014, 202784. 10.1155/2014/202784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbitskiy E. V.; Rusinov G. L.; Charushin V. N.; Chupakhin O. N. Development of new antituberculosis drugs among of 1,3- and 1,4-diazines. Highlights and perspectives. Russ. Chem. Bull. 2019, 68, 2172–2189. 10.1007/s11172-019-2686-x. [DOI] [Google Scholar]
- Yan Z.; Liu A.; Ou Y.; Li J.; Yi H.; Zhang N.; Liu M.; Huang L.; Ren J.; Liu W.; Hu A. Design, synthesis and fungicidal activity evaluation of novel pyrimidinamine derivatives containing phenyl-thiazole/oxazole moiety. Bioorg. Med. Chem. 2019, 27, 3218–3228. 10.1016/j.bmc.2019.05.029. [DOI] [PubMed] [Google Scholar]
- Aleksić M.; Stanisavljević D.; Smiljković M.; Vasiljević P.; Stevanović M.; Soković M.; Stojković D. Pyrimethanil: Between efficient fungicide against Aspergillus rot on cherry tomato and cytotoxic agent on human cell lines. Ann. Appl. Biol. 2019, 175, 228–235. 10.1111/aab.12532. [DOI] [Google Scholar]
- Shim J.-H.; Abd El-Aty A. M.; Choi J.-H.; Kang C.-A. Determination of field-incurred pyrimethanil residues in persimmon (Diospyros kaki Linn) by liquid chromatography. Biomed. Chromatogr. 2007, 21, 1279–1283. 10.1002/bmc.884. [DOI] [PubMed] [Google Scholar]
- Święciło A.; Krzepiłko A.; Michałek S. Evaluation of azoxystrobin toxicity to saprophytic fungi and radish in the early stages of growth. Ecol. Chem. Eng. A 2018, 25, 81–92. 10.2428/ecea.2018.25(1)9. [DOI] [Google Scholar]
- Zhou Y.; Wang C.; Xin F.; Han X.; Zhang J.; Sun K. Synthesis, insecticidal, fungicidal activities and structure-activity relationships of tschimganin analogs. Molecules 2018, 23, 1473. 10.3390/molecules23061473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo K.-L.; Zhao L.-X.; Wang Z.-W.; Rong S.-Z.; Zhou X.-L.; Gao S.; Fu Y.; Ye F. Design, synthesis and evaluation of novel trichloromethyl dichlorophenyl triazole derivatives as potential safener. Biomolecules 2019, 9, 438. 10.3390/biom9090438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaghefi N.; Hay F. S.; Kikkert J. R.; Pethybridge S. J. Genotypic diversity and resistance to azoxystrobin of Cercospora beticola on processing table beet in New York. Plant Dis. 2016, 100, 1466–1473. 10.1094/pdis-09-15-1014-re. [DOI] [PubMed] [Google Scholar]
- Caiazzo R.; Kim Y. K.; Xiao C. L. Occurrence and phenotypes of pyrimethanil resistance in Penicillium expansum from apple in Washington state. Plant Dis. 2014, 98, 924–928. 10.1094/pdis-07-13-0721-re. [DOI] [PubMed] [Google Scholar]
- Ma D.; Jiang J.; He L.; Cui K.; Mu W.; Liu F. Detection and characterization of Qoi-resistant Phytophthora capsici causing pepper phytophthora blight in China. Plant Dis. 2018, 102, 1725–1732. 10.1094/pdis-01-18-0197-re. [DOI] [PubMed] [Google Scholar]
- Bartlett D. W.; Clough J. M.; Godwin J. R.; Hall A. A.; Hamer M.; Parr-Dobrzanski B. The strobilurin fungicides. Pest Manage. Sci. 2002, 58, 649–662. 10.1002/ps.520. [DOI] [PubMed] [Google Scholar]
- Lurwanu Y.; Wang Y.-P.; Abdul W.; Zhan J.; Yang L.-N. Temperature-mediated plasticity regulates the adaptation of phytophthora infestans to azoxystrobin fungicide. Sustainability 2020, 12, 1188. 10.3390/su12031188. [DOI] [Google Scholar]
- Clough J. M.; Godfrey C. R. A.; Streeting I. T.; Cheetham R.; De Fraine P. J.; Bartholomew D.; Eshelby J. J.. Preparation of [(pyrimidinyloxy)phenyl]methoxy propenoates and related compounds as agrochemical fungicides. EP468695A1, 1992, p 57
- Clough J. M.; Godfrey C. R. A.; Streeting I. T.; Cheetham R.. Preparation of 2-[[(phenoxy)pyrimidinyloxy]phenyl]-3-methoxypropenoates as agrochemical fungicides. EP382375A2, 1990, p 46.
- Liu A.; Wang X.; Ou X.; Huang M.; Chen C.; Liu S.; Huang L.; Liu X.; Zhang C.; Zheng Y.; Ren Y.; He L.; Yao J. Synthesis and fungicidal activities of novel bis(trifluoromethyl)phenyl-based strobilurins. J. Agric. Food Chem. 2008, 56, 6562–6566. 10.1021/jf800651z. [DOI] [PubMed] [Google Scholar]
- Chen H.; Taylor J. L.; Abrams S. R. Design and synthesis of β-methoxyacrylate analogues via click chemistry and biological evaluations. Bioorg. Med. Chem. Lett. 2007, 17, 1979–1983. 10.1016/j.bmcl.2007.01.021. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Wang F.; Li H.; Yang W.; Chen Q.; Yang G. Design, synthesis, and bioevaluation of novel strobilurin derivatives. Chin. J. Chem. 2012, 30, 1999–2008. 10.1002/cjoc.201200607. [DOI] [Google Scholar]
- Guan A.-Y.; Liu C.-L.; Li M.; Zhang H.; Li Z.-N.; Li Z.-M. Design, synthesis and structure–activity relationship of novel coumarin derivatives. Pest Manage. Sci. 2011, 67, 647–655. 10.1002/ps.2103. [DOI] [PubMed] [Google Scholar]
- Hao G.-F.; Yang S.-G.; Huang W.; Wang L.; Shen Y.-Q.; Tu W.-L.; Li H.; Huang L.-S.; Wu J.-W.; Berry E.-A.; Yang G.-F. Rational design of highly potent and slow-binding cytochrome bc1 inhibitor as fungicide by computational substitution optimization. Sci. Rep. 2015, 5, 13471. 10.1038/srep13471. [DOI] [Google Scholar]
- Deng X.; Zhou Y.; Zheng W.; Bai L.; Zhou X. Dissipation dynamic and final residues of oxadiargyl in paddy fields using high-performance liquid chromatography-tandem mass spectrometry coupled with modified QuEChERS Method. Int. J. Environ. Res. Public Health 2018, 15, 1680. 10.3390/ijerph15081680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao L. X.; Yin M. L.; Wang Q. R.; Zou Y. L.; Ren T.; Gao S.; Fu Y.; Ye F. Novel thiazole phenoxypyridine derivatives protect maize from residual pesticide injury caused by ppo-inhibitor fomesafen. Biomolecules 2019, 9, 514. 10.3390/biom9100514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-Y.; Gao S.; Liu Y.-X.; Wang C.; Jiang W.; Zhao L.-X.; Fu Y.; Ye F. Design, synthesis, and biological activity of novel diazabicyclo derivatives as safeners. J. Agric. Food Chem. 2020, 68, 3403–3414. 10.1021/acs.jafc.9b07449. [DOI] [PubMed] [Google Scholar]
- Abu-Qare A. W.; Duncan H. J. Herbicide safeners: uses, limitations, metabolism, and mechanisms of action. Chemosphere 2002, 48, 965–974. 10.1016/s0045-6535(02)00185-6. [DOI] [PubMed] [Google Scholar]
- Hu L.; Yao Y.; Cai R.; Pan L.; Liu K.; Bai L. Effects of fenclorim on rice physiology, gene transcription and pretilachlor detoxification ability. BMC Plant Biol. 2020, 20, 100. 10.1186/s12870-020-2304-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannakopoulos G.; Dittgen J.; Schulte W.; Zoellner P.; Helmke H.; Lagojda A.; Edwards R. Safening activity and metabolism of the safener cyprosulfamide in maize and wheat. Pest Manage. Sci. 2020, 10.1002/ps.5801. [DOI] [PubMed] [Google Scholar]
- Ye F.; Zhai Y.; Guo K.-L.; Liu Y.-X.; Li N.; Gao S.; Zhao L.-X.; Fu Y. Safeners improve maize tolerance under herbicide toxicity stress by increasing the activity of enzymes in vivo. J. Agric. Food Chem. 2019, 67, 11568–11576. 10.1021/acs.jafc.9b03587. [DOI] [PubMed] [Google Scholar]
- Scarponi L.; Del Buono D.; Vischetti C. Effect of pretilachlor and fenclorim on carbohydrate and protein formation in relation to their persistence in rice. Pest Manage. Sci. 2005, 61, 371–376. 10.1002/ps.985. [DOI] [PubMed] [Google Scholar]
- Thiripurasundari S.; Asokan S.; Thangapandiyan S. Acute toxicity impact of herbicide pretilachlor on biochemical, haematological and ionic changes in gill, liver and kidney of a cultivable fish Labeo rohita (Hamilton). Int. J. Pharm. Biol. Sci. 2019, 9, 990–999. 10.21276/ijpbs.2019.9.2.119. [DOI] [Google Scholar]
- Brazier-Hicks M.; Evans K. M.; Cunningham O. D.; Hodgson D. R. W.; Steel P. G.; Edwards R. Catabolism of Glutathione Conjugates inArabidopsis thaliana. J. Biol. Chem. 2008, 283, 21102–21112. 10.1074/jbc.m801998200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng F.; Hatzios K. K. Purification and characterization of two glutathione S-transferase isozymes from indica-type rice involved in herbicide detoxification. Pestic. Biochem. Physiol. 2002, 72, 10–23. 10.1006/pest.2001.2580. [DOI] [Google Scholar]
- Zheng W.-N.; Zhu Z.-Y.; Deng Y.-N.; Wu Z.-C.; Zhou Y.; Zhou X.-M.; Bai L.-Y.; Deng X.-L. Synthesis, crystal structure, herbicide safening, and antifungal activity of N-(4,6-dichloropyrimidine-2-yl)benzamide. Crystals 2018, 8, 75. 10.3390/cryst8020075. [DOI] [Google Scholar]
- Xiong K.-J.; Du F.-P. Design, synthesis, crystal structure, and fungicidal activity of two fenclorim derivatives. Crystals 2020, 10, 587. 10.3390/cryst10070587. [DOI] [Google Scholar]
- Wu J.; Sun Y.-P.; Zhang P.-Z.; Yu Q.-S. Synthesis and biological activity of substituted pyrimidine derivatives. Chin. J. Org. Chem. 2004, 24, 1403–1406. [Google Scholar]
- Bai L. Y.; Deng X. L.; Zheng W. N.; Zhou X. M.; Liu S. H.. Preparation of phenylpyrimidine derivatives for pesticides safety agent. CN109942498A, 2019; p 10.
- Luo Y.; Zhang S.; Liu Z.-J.; Chen W.; Fu J.; Zeng Q.-F.; Zhu H.-L. Synthesis and antimicrobical evaluation of a novel class of 1,3,4-thiadiazole: Derivatives bearing 1,2,4-triazolo[1,5-a]pyrimidine moiety. Eur. J. Med. Chem. 2013, 64, 54–61. 10.1016/j.ejmech.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Sun J.; Zhou Y. Design, synthesis, and insecticidal activity of some novel diacylhydrazine and acylhydrazone derivatives. Molecules 2015, 20, 5625–5637. 10.3390/molecules20045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M.; Zhu Y.-B.; Wang M.; Khan I. A.; Liu X.-H.; Weng J.-Q. Microwave-assisted Synthesis and Antifungal Activity of Novel 1,2,4- Triazole Thioether Derivatives Containing Pyrimidine Moiety. Lett. Drug Des. Discovery 2018, 15, 347–352. 10.2174/1570180814666170602082440. [DOI] [Google Scholar]
- Lei K.; Li P.; Yang X.-F.; Wang S.-B.; Wang X.-K.; Hua X.-W.; Sun B.; Ji L.-S.; Xu X.-H. Design and synthesis of novel 4-hydroxyl-3-(2-phenoxyacetyl)-pyran-2-one derivatives for use as herbicides and evaluation of their mode of action. J. Agric. Food Chem. 2019, 67, 10489–10497. 10.1021/acs.jafc.9b03109. [DOI] [PubMed] [Google Scholar]
- Zhao L.-X.; Jiang M.-J.; Hu J.-J.; Zou Y.-L.; Cheng Y.; Ren T.; Gao S.; Fu Y.; Ye F.; Ye F. Design, synthesis, and herbicidal activity of novel diphenyl ether derivatives containing fast degrading tetrahydrophthalimide. J. Agric. Food Chem. 2020, 68, 3729–3741. 10.1021/acs.jafc.0c00947. [DOI] [PubMed] [Google Scholar]
- Fu Y.; Zhang D.; Zhang S.-Q.; Liu Y.-X.; Guo Y.-Y.; Wang M.-X.; Gao S.; Zhao L.-X.; Ye F. Discovery of N-aroyl diketone/triketone derivatives as novel 4-hydroxyphenylpyruvate dioxygenase inhibiting-based herbicides. J. Agric. Food Chem. 2019, 67, 11839–11847. 10.1021/acs.jafc.9b01412. [DOI] [PubMed] [Google Scholar]
- Bahrami K.; Targhan H. A new strategy to design a graphene oxide supported palladium complex as a new heterogeneous nanocatalyst and application in carbon-carbon and carbon-heteroatom cross-coupling reactions. Appl. Organomet. Chem. 2019, 33, e4842 10.1002/aoc.4842. [DOI] [Google Scholar]
- Mahgoub M. Y.; Elmaghraby A. M.; Harb A.-E. A.; Ferreira Da Silva J. L.; Justino G. C.; Marques M. M. Synthesis, crystal structure, and biological evaluation of fused thiazolo[3,2-α]pyrimidines as new acetylcholinesterase inhibitors. Molecules 2019, 24, 2306. 10.3390/molecules24122306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J.-T.; Gong Y.-L.; Li J.; Wang Y.; Chen Y.; Ding S.; Liu J. Synthesis, structure and biological activity of 2-[2-(4-Fluorobenzylidene)hydrazinyl]-4-(1-methyl-1H-indol-3-yl)thieno[3,2-d]pyrimidine. Chin. J. Struct. Chem. 2019, 38, 1530–1536. [Google Scholar]
- Sheikhi M.; Shahab S.; Filippovich L.; Dikusar E.; Khaleghian M. DFT investigations (geometry optimization, UV/Vis, FT-IR, NMR, HOMO-LUMO, FMO, MEP, NBO, Excited States) and the syntheses of new pyrimidine dyes. Chin. J. Struct. Chem. 2018, 08, 1201–1222. [Google Scholar]
- Lee J.-S.; Zeller M.; Warkad S. D.; Nimse S. B. Synthesis, characterization, and crystal structure of N-(3-nitrophenyl)cinnamamide. Crystals 2019, 9, 599. 10.3390/cryst9110599. [DOI] [Google Scholar]
- Deng X.-L.; Zhou X.-M.; Wang Z.-Y.; Rui C.-H.; Yang X.-L. Synthesis, crystal structure and insecticidal activity of N-(pyridin-2-ylmethyl)-1-phenyl-1,4,5,6,7,8-hexahydrocyclohepta[c]pyrazole-3-carboxamide. Chin. J. Struct. Chem. 2018, 37, 551–556. [Google Scholar]
- Zeng W.; Wang X.; Jiang J. Design and crystal structures of two new compounds fused with 3,4,5-trimethoxybenzyl group and 6,10-dioxaspiro group. Crystals 2018, 8, 146. 10.3390/cryst8040146. [DOI] [Google Scholar]
- Gao Y.; Hao J.; Li J.; Song Z.; Shang S. Structural modification of turpentine with natural chiral preservation and low-risk application prospects in crop protection. ACS Omega 2019, 4, 6392–6398. 10.1021/acsomega.9b00241. [DOI] [Google Scholar]
- Singh S. K.; Patel M. B.; Thakker B. N.; Hooda K. S.; Barad A. K. Rhizoctonia solani f.sp. Sasakii inciting banded leaf and sheath blight of maize and their management: an overview. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 2858–2866. 10.20546/ijcmas.2019.807.356. [DOI] [Google Scholar]
- Ntushelo K.; Ledwaba L. K.; Rauwane M. E.; Adebo O. A.; Njobeh P. B. The Mode of Action of Bacillus Species against Fusarium graminearum, Tools for Investigation, and Future Prospects. Toxins 2019, 11, 606. 10.3390/toxins11100606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B.; Wang J.; Liu C.; Hu J.; Tan K.; Zhao F.; Yuan M.; Zhang J.; Gai Z. Preventive effects of fluoro-substituted benzothiadiazole derivatives and chitosan oligosaccharide against the rice seedling blight induced by Fusarium oxysporum. Plants 2019, 8, 538. 10.3390/plants8120538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T.; Shang Y. K.; Shi H. X.; Mu W.; Liu F. Antifungal activity of cationic surfactants quarternary ammonium salt on Sclerotinia sclerotiorum. Chin. J. Pestic. Sci. 2008, 10, 99–104. [Google Scholar]
- Dolomanov O. V.; Bourhis L. J.; Gildea R. J.; Howard J. A. K.; Puschmann H. OLEX2: a complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. 10.1107/s0021889808042726. [DOI] [Google Scholar]
- Sheldrick G. M. Crystal structure refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. 10.1107/s2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. SHELXT- Integrated space-group and crystal-structure determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. 10.1107/s2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veignie E.; Ceballos C.; Len C.; Rafin C. Design of new antifungal dithiocarbamic esters having bio-based acrylate moiety. ACS Omega 2019, 4, 4779–4784. 10.1021/acsomega.8b03685. [DOI] [Google Scholar]
- Tang X.; Zhou X.; Wu J.; Li J.; Bai L. A novel function of sanshools: The alleviation of injury from metolachlor in rice seedlings. Pestic. Biochem. Physiol. 2014, 110, 44–49. 10.1016/j.pestbp.2014.02.006. [DOI] [PubMed] [Google Scholar]
- Deng X.; Zheng W.; Zhou X.; Bai L. The effect of salicylic acid and 20 substituted molecules on alleviating metolachlor herbicide injury in rice (Oryza sativa). Agronomy 2020, 10, 317. 10.3390/agronomy10030317. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.