Abstract
Breast cancer continues to be the most lethal cancer type in women and one of the most diagnosed. Understanding Breast cancer receptor status is one of the most vital processes for determining treatment options. One type of breast cancer, human epidermal growth factor receptor 2 (HER2) positive, has approved receptor-based therapies including trastuzumab and pertuzumab that can significantly increase the likelihood of survival. Current methods to determine HER2 status include biopsies with immunohistochemical staining and/or fluorescence in situ hybridization. However, positron emission tomography (PET) imaging techniques using 89Zr-trastuzumab or 89Zr-pertuzumab are currently in clinical trials for a non-invasive, full body diagnostic approach. Although the antibodies have strong specificity to the HER2 positive lesions, challenges involving long post-injection time for imaging due to the blood circulation of the antibodies and matching of long-live isotopes leading to increased dose to the patient leave opportunities for alternative PET imaging probes. Peptides have been shown to allow for shorter injection-to-imaging time and can be used with shorter lived isotopes. HER2 specific peptides under development will help improve the diagnosis and potentially therapy options for HER2 positive breast cancer. Peptides showing specificity for HER2 could start widespread development of molecular imaging techniques for HER2 positive cancers.
Keywords: HER2, PET, peptide, imaging
Introduction
Breast cancer is both one of the most commonly diagnosed and lethal cancer types in women. According to the American Cancer Society, 15% of all cancer deaths in women are breast cancer.1 This translates to about 1 in every 8 women being diagnosed with breast cancer during their lifetime.2,3 Breast cancers can be divided into multiple categories, but commonly breast cancer is categorized by receptor expression or lack-there-of. The common sub-types for breast cancer are: estrogen receptor (ER) and/or progesterone receptor (PR) expressing, human epidermal growth factor 2 receptor (HER2) expressing, or triple negative breast cancer (TNBC) which does not express any of the receptors.3 Each cancer sub-type has unique challenges related to diagnosis and treatment. However, as is the case with all cancers, breast cancer greatly benefits from early detection before its growth into widespread disease. In particular, it is critical to have early diagnosis of the HER2 positive subtype of breast cancer in order to implement effective targeted therapies.
The Human Epidermal Growth Factor Receptor 2 is a 185 kDa protein that is a member of the HER family that has 4 structurally similar members (HER1-4).4,5 The activation of each family member relies on the homodimerization with the same family member or heterodimerization with another member of the family after a ligand binds to the specific receptor. Although HER2 does not have a known natural ligand, it is the preferred heterodimerization partner for other family receptors and has been shown to have increased potency of downstream signaling compared to the other HER family members.2,6,7 The downstream effects of the activation include the MAPK and PI3K pathways which lead to cell proliferation, growth, and anti-apoptosis.5
However, while many cells express a baseline level of the HER2, its overexpression in cancer leads to increased rates of growth and metastasis and overall worse prognosis. HER2 overexpression is observed in 15% to 25% of all breast cancers and the overexpression of this receptor can be up to 2 million times more than the normal basal level expression.6 Thus, the overexpression of the receptor makes it both a diagnostic marker and a favorable target for therapy. The ability to accurately detect and quantify the amount of HER2 expression allows for identification of patients that would benefit most from HER2 targeted therapy. Current diagnosis typically involves both immunohistochemistry and fluorescence in situ hybridization which measure elevated presence of both the protein and the gene expression respectively. Although this is the standard technique for the diagnosis of HER2 positive breast cancer, there are some challenges. This type of diagnosis involves the need for biopsies of all suspected lesions which can be difficult, painful or even impossible depending on the location.7 However, the use of HER2 positron emission tomography (PET) can allow for a full-body non-invasive diagnostic option to aid in the detection of the HER2 status.
PET imaging of HER2 positive breast cancer in the clinical trial setting currently involves the use of trastuzumab (Herceptin®) or pertuzumab (Perjeta®), both monoclonal antibodies, to detect HER2 protein expression.8 The use of antibodies for imaging may also be useful for monitoring the effectiveness of the treatment of HER2 directed therapy in patients receiving the treatment.9 Each antibody is labeled with 89Zr, which has a 3.27 day half-life, which matches the biological half-life of the antibody and allows for circulation time required to reduce the activity in the blood pool to obtain a clear image.10 Currently in phase II clinical trials, the 89Zr radiolabeled antibody is injected and requires a 4-7 day post-injection imaging time.10,11 The long imaging time points, due to the circulation time of the antibody, can create logistic challenges for both patients and facilities. To pair with the biological half-life and optimal scanning time for the antibodies, longer-lived isotopes must be used which increases the amount of radiation exposure to both the patients and the personnel involved in the synthesis of the radiopharmaceuticals. Thus, although high specificity can be obtained with this method, there are still improvements that can be made to decrease injection-to-imaging time which can potentially be achieved with smaller peptide-based agents.
Peptides have many favorable characteristics suitable for the development of imaging agents including higher tissue penetration, faster circulation time in the blood, and ease of synthesis commonly involving solid-phase peptide synthesis.12,13 Peptides have been used in PET imaging for multiple applications, but the most successful has been the development of [68 Ga]-DOTATATE (NETSPOT) for imaging of somatostatin receptor type 2 (SSTR2) expression in neuroendocrine tumors. This agent is able to accurately and efficiently detect SSTR2 positive tumors in patients without the need for a biopsy.14 HER2 expressing tumors could benefit from the use of peptides which could lead to quicker imaging times, opening the door for faster and more accurate treatments depending on HER2 expression levels.
A number of peptides have shown promise in detection of HER2 expression in breast cancer. These peptides have been discovered by a collection of different methods including phage display, one-bead-one-compound (OBOC) discovery, and antibody-based peptide development. Phage display has been the most common technique used to discover HER2 specific peptides along with common binding motifs within the library of peptides.15 While the majority of the studies reported thus far have used SPECT as an imaging modality, these peptides specific for HER2 have great potential for the development of PET imaging strategies and could enable further development of effective methods of HER2 detection.
KCCYSL-Based Peptides
One of the most studied peptides specific for HER2 is the KCCYSL peptide or peptides with KCCYSL incorporated into the sequence. The peptide was discovered by a random 6 amino acid peptide bacteriophage display library and showed the highest frequency in phages that were bound to biotinylated extracellular domain erbB-2.16 Karasseva et al. found the KCCYSL sequence in over 75% of their clones, signifying strong affinity for HER2. It is believed that the oxidized state of the CCY motif in this peptide mimics the structure of EGF-like domain of common erbB ligands.16 This peptide has been evaluated in multiple studies both in vitro and in vivo. The 6-mer KCCYSL peptide has been modified with chelators to bind imaging isotopes along with linkers to prevent steric hindrance during binding. A prime example of this application was the synthesis and evaluation of an 111In-DOTA(GSG)-KCCYSL peptide for SPECT imaging of HER2 positive tumors.17 The GSG amino acids were incorporated into the peptide to act as a spacer to prevent the large DOTA chelator from interfering with binding to HER2. One of the first studies investigated using fluorescence assays to determine the equilibrium constant (Kd), seen in Figure 1, of the KCCYSL peptide and alanine substituted peptide variants to determine how the removal of each amino acid would affect its binding to recombinant erbB-2-extracellular domain. The Kd of the original KCCYSL peptide was 295 nM, and the largest difference in Kd values were seen in the KCCASL, ACCYSL, and the KCCYSA peptides with 710 nM, 932 nM, and then complete loss of binding altogether, respectively. Smaller changes in Kd were seen in the KAAYSL, KACYSL, and KCAYSL peptides; 305, 344, and 387 nM respectively.17 This established that KCCYSL was the optimal peptide for additional studies and showed the importance of specific positions of the amino acids in the peptide.
Figure 1.
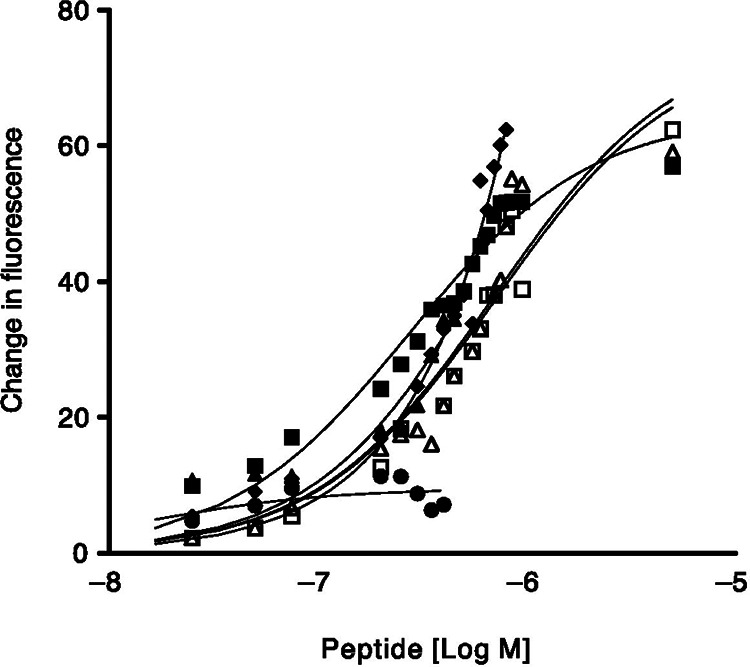
Fluorescence quenching of multiple peptides with varying concentrations were evaluated with recombinant erbB-2-ECD to determine Kd values. □ , KCCYSL, Kd = 295 ± 56 nmol/L; ⋄, KCCYAL, Kd = 560 ± 19 nmol/L; •, KCCYSA, Kd= no binding; ▴, PKCCYSLP, Kd = 714 ± 30 nmol/L; ▵, KCCASL, Kd = 932 ± 10 nmol/L; ˆ, ACCYSL, Kd = 714 ± 30 nmol/L. From Ref17 used with permission.
In cell binding studies, it was shown that the radiolabeled peptide, 111In-DOTA(GSG)-KCCYSL, specifically bound to MDA-MB-435 cells (HER2+) compared to the K-562 (HER2-). In the same study, Kumar et al. showed that a scrambled peptide 111In-DOTA(GSG)-KYLCSC peptide did not bind to MDA-MB-435 cells. They also showed that after 2 hours of incubation at 37 ºC, ∼11% of the 111In-DOTA(GSG)-KCCYSL peptide was internalized into the cell. In vivo studies confirmed that imaging with the radiolabeled peptide could distinguish HER2+ tumors from HER2- in SCID mice 2 hours post injection. In the biodistribution study, it was found that the 111In-DOTA(GSG)-KCCYSL peptide had the highest tumor-to-blood ratio, 5.0, at 2 hrs post injection, which was 7.4 times higher than at 15 minutes. This study confirmed the KCCYSL peptide is suitable for use as a molecular imaging probe
Different analogues of this peptide have been evaluated with either the addition of other amino acids to improve the kinetics of the peptide, the addition of a nucleus targeting moiety on the peptide to traffic the peptide into the nucleus for therapy, or the addition of a novel lytic peptide that can kill cancer cells from the cell surface.18-20 Using combinational evolution, a technique based on the principle of affinity maturation in antibodies, Larimer et al. developed peptides that included either 4 or 5 amino acids on the N-terminus and C-terminus of the KCCYSL peptide with the goal of improving its pharmacological profile including higher tumor accumulation, faster clearance, and a decrease in off target uptake.18 After phage characterization, there were 9 clones that had a cancer to epithelial cell binding ratio that was higher than of the original KCCYSL peptide by at least 50%. Out of those 9 novel peptides, 2 of them were found to have a binding affinity higher than the original peptide. Those 2 peptides were MEGPSKCCYSLALASH (1-D03) and GTKSKCCYSLRRSS (3-G03) with affinities of 236 ± 83 and 289 ± 13 nM respectively. These affinities were both significantly higher than the original peptide (351 ± 21 nM). Chemical modification involved the incorporation of a DOTA chelator to radiolabel each of the peptides with 111In for use in further studies. Radiolabeled peptides were evaluated for their specificity by the amount of total bound peptide to the HER2 positive MDA-MB-435 cells while their specificity ratio was evaluated by comparing the ratio of binding of the MDA-MB-435 to the HER2 negative 184A.1 cells. 111In-DOTA-KCCYSL had a specificity ratio of 3.49. The 111In-DOTA-1-D03 was chosen for in vivo experiments as it had both higher binding and specificity ratio (7.44) than either the 111In-DOTA-3-G03 (1.4) or the original peptide. The biodistribution of 111In-DOTA-1-D03 showed a tumor-to-blood ratio of approximately 6 after 2 hours in addition to lower levels of the compound in the blood, lung, kidney, and liver compared to the 111In-DOTA-KCCYSL peptide. The investigators also reported that the SPECT imaging showed clear images of the tumor along with specific binding. This was confirmed by blocking the uptake of the 111In-DOTA-1-D03 in the tumor with the addition of non-radiolabeled DOTA-1-D03. (Figure 2).
Figure 2.
SPECT images of SCID mice bearing MDA-MB-435 (HER2+) tumors. a) 2 hour post injection images of purified 111In-DOTA-1-D03 b) Image with pre-injected cold DOTA-1-D03 15 minutes prior to 111In- DOTA-1-D03 injection at 2 hour post-injection. From Ref18 used with permission.
Similar studies were reported by 2 other groups which involved the addition of other sequences to the KCCYSL peptide including a nuclear targeting peptide sequence and a lytic cell-membrane sequence. The approaches of both of these studies were to use the KCCYSL peptide as the targeting moiety, but build upon the target specificity for therapeutic potential.19,20 These studies did not evaluate the ability of the KCCYSL peptide to bind to HER2 but showed that this targeting peptide could be used for delivery of an additional component. The addition of the nuclear localization sequence peptide (NLP) to the KCCYSL peptide aimed to enable targeting of HER2 using the KCCYSL sequence followed by transportation to the nucleus with the NLP portion.19 The overall goal would be to attach an Auger emitting isotope to the peptide so that it can locally kill circulating breast cancer cells and spare the normal surrounding tissue from excess radiation. It was confirmed that the cells could be targeted with the KCCYSL peptide as fluorescent confocal microscopic imaging showed fluorescence in the cytoplasm of HER2+ cells and not HER2- cells. However, they did not see any trafficking of the fluorescence into the nucleus of any cell line. Despite the lack of nuclear localization, the specificity of the peptide was confirmed with the HER2+ cell lines displaying fluorescence inside the cytoplasm. A second study evaluated the KCCYSL peptide’s ability to target HER2+ cells and deliver a lytic peptide sequence. This study by Kawamoto et al showed the lytic peptide can disrupt the cancer cell membranes, killing up to 80% of cancer cells in just 15 minutes.20 They also were able to show that between 11 cell lines, the 6 HER2 overexpressing cancer cell lines had increased cell toxicity over 5 non-HER2 expressing cancer cell lines. These studies showed that the KCCYSL peptide could help deliver additional agents to HER2 expressing cells.
The KCCYSL peptide shows great promise as a platform for the development of targeting agents for HER2. Radiolabeled versions of this peptide have been shown to have suitable imaging capabilities with SPECT with the potential for development into a PET imaging agent. Its ability to target HER2 with either the NLP or lytic peptide additions also show that it may be modified with therapeutic characteristics while retaining specificity to HER2. Imaging agents based on this peptide may also be used to monitor treatment for HER2 positive patients since it has a different binding site than trastuzumab so using this peptide as an imaging agent wouldn’t interfere with the trastuzumab treatment.16 The KCCYSL peptide is currently the gold standard for peptide-based HER2 targeting.
LTVSPWY-Based Peptides
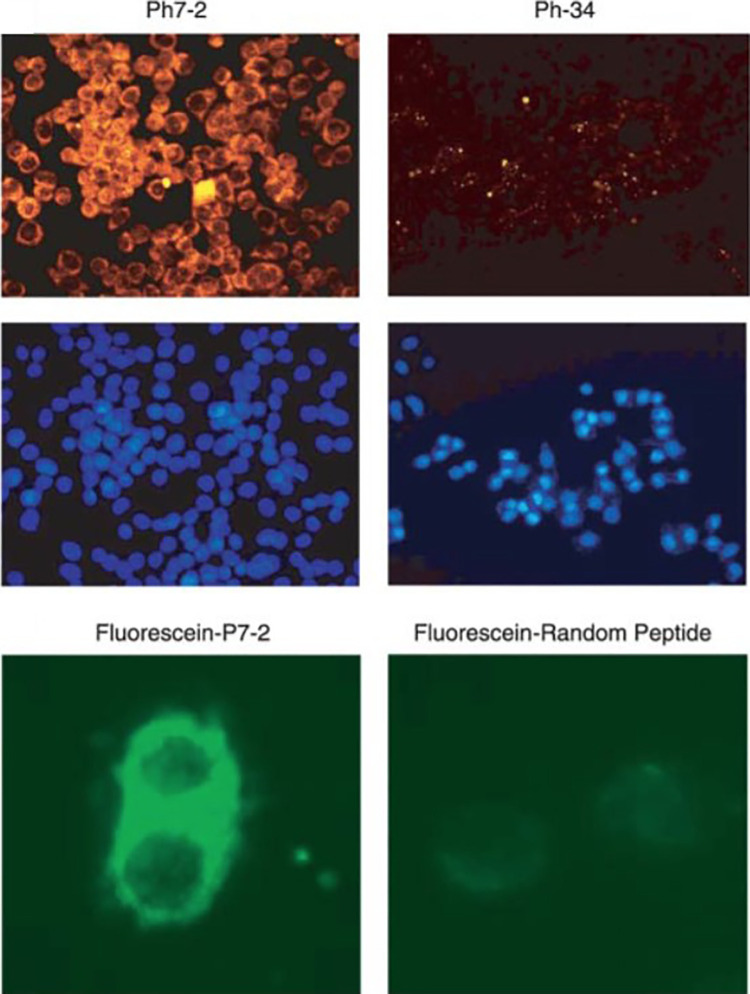
A common approach to identify targeting peptides uses phage display as described above for the KCCYSL peptide. Shadidi et al. used another phage display biopanning procedure that resulted in the discovery of the LTVSPWY core peptide sequence.21 Their method involved using HER2 positive SKBR3 cells as an “affinity matrix” to determine if any phages from a 7- and 12-mer library would have strong association with the cell line. Of all of the phages that were tested, 80% reacted with the SKBR3 cells, however, the highest number of cells were stained with the Ph7-2 phage, which was later discovered to be the LTVSPWY peptide. The investigators then aimed to determine if the peptide could selectively target cancer cells and if the peptide could be internalized by the cells for the potential for therapeutic targeting. Strong and selective binding to the SKBR3 cells was illustrated using immunofluorescence studies, Figure 3, where the Ph7-2 peptide yielded strong cell staining, as opposed to studies with a non-binding negative control peptide which showed much lower signal.
Figure 3.
Fluorescence imaging of a HER2 positive (pH7-2) and negative (Ph-34) phage. Phage uptake with epifluorescene microscope (top) and Hoechst staining for cell nuclei (middle) SKBR3 cell staining with fluorescein-P7-2 peptide and fluorescein-random peptide (bottom). From Ref21 used with permission.
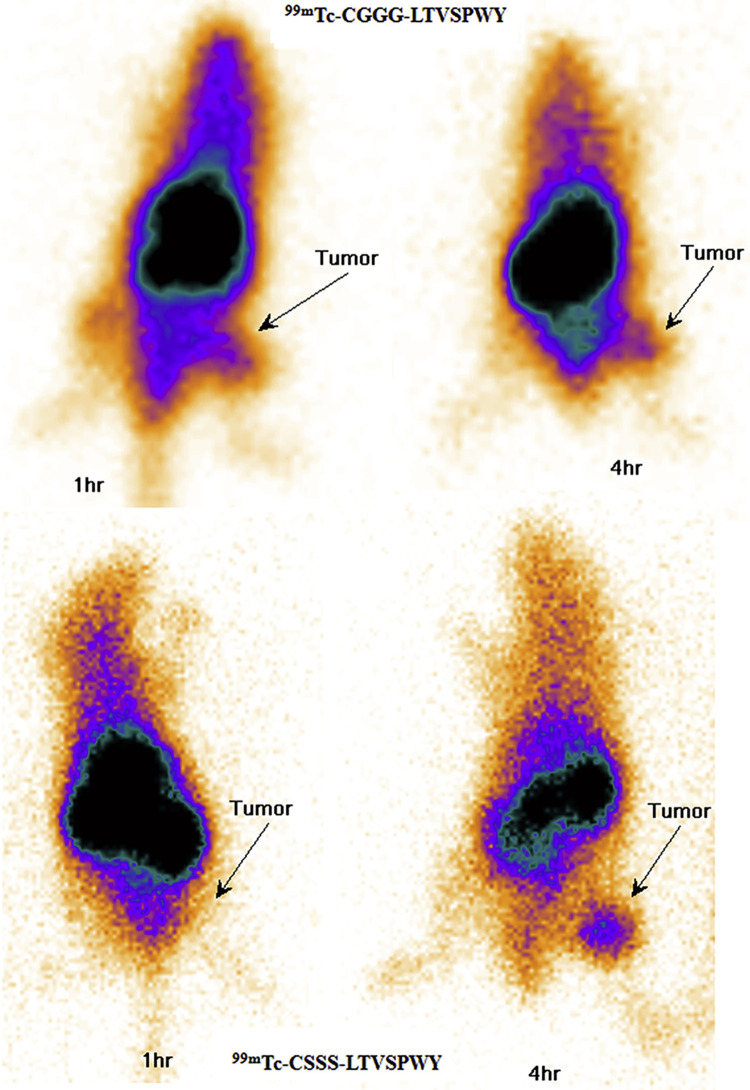
Another notable experiment illustrated the receptor specific binding for this peptide. First, to prove that the Ph7-2 phage was expressing the LTVSPWY peptide, a chemically synthesized LTVSPWY peptide was administered to SKBR3 cells and was shown to inhibit the binding of the Ph7-2 phage in a dose dependent manner. Shadidi et al. also found that the Ph7-2 phage displaying LTVSPWY peptide had the highest binding to breast cancer cells (with SKBR3 and T47D being the 2 highest), compared to normal, non-cancerous cells by using flow cytometry. In a similar fashion, Sabahnoo et al showed that the LTVSPWY core peptide bound specifically to HER2.22 This study involved the evaluation of 2 peptides with the core LTVSPWY and cysteine-based ligands (CGGG or CSSS) which served as the chelator for 99mTc. The 99mTc-CGGG-LTVSPWY and 99mTc-CSSS-LTVSPWY peptides were evaluated for stability at 37 ºC in both serum and PBS (representing their shelf life values). The 99mTc-CGGG-LTVSPWY was 75% intact after 24 hours in PBS and 92% intact in serum after 4 hours. For 99mTc-CSSS-LTVSPWY, those values were 65% and 80% in PBS and in serum, respectively. Both peptides showed significantly higher binding to SKOV-3 (HER2+) cells compared to A549 and MCF-7 (HER2-) cells. Sabahnoo et al. also reported competitive binding using trastuzumab that confirmed that the peptides had similar binding sites to trastuzumab ( Figure 4). Kd values determined by saturation assay were 4.3 ± 0.8 nM and 33.9 ± 9.7 nM for 99mTc-CGGG-LTVSPWY and 99mTc-CSSS-LTVSPWY respectively. In in vivo studies, mice bearing SKOV-3 xenografts were injected with each peptide to determine the tumor %ID/g at 1 and 4 hours (Figure 5). The 99mTc-CGGG-LTVSPWY had a tumor %ID/g of 3.84 ± 2.5 and 2.44 ± 1.1%ID/g at 1 and 4 hours respectively. The 99mTc-CSSS-LTVSPWY peptide had similar values of 4.98 ± 4.8 and 2.26 ± 2.1%ID/g respectively at the same time points. Similar to other peptide-based agents, both peptides showed high kidney signal. The 99mTc-CGGG-LTVSPWY peptide had slightly higher uptake in multiple organs including the liver, spleen, and also the lungs, than 99mTc-CSSS-LTVSPWY. Imaging studies with both peptides visualized HER2 positive tumors on the flank of nude mice at 4 hours, consistent with the promising in vitro binding studies.
Figure 4.
Evaluation of both 99mTc-CGGG-LTVSPWY and 99mTc-CSSSLTVSPWY binding to SKOV-3 cells. A) 99mTc-CGGG-LTVSPWY with CGGG-LTVSPWY block at 2 h at 37 ºC B) 99mTc-CSSS-LTVSPWY with CGGG-LTVSPWY block at 2 h at 37 ºC C) 99mTc-CGGG-LTVSPWY blocking with various antibodies D) 99mTc-CSSS-LTVSPWY blocking with various antibodies. From Ref22 used with permission.
Figure 5.
Imaging of SKOV-3 tumors with 99mTc-CGGG-LTVSPWY and 99mTc-CSSSLTVSPWYpeptides at 1 h and 4 h respectively. From Ref22 used with permission.
There have been a variety of recent publications that have been evaluating the same core peptide with the addition of a new chelator and linker system, 99mTc-HYNIC-(Ser)3-LTVPWY. The reported Kd value for this peptide was 9.7 ± 2.0 nM from Shahsavari et al. and 2.6 ± 0.5 nM from Aligholikhamseh et al.23,24 Ardakani et al, conducted an in depth analysis of the pharmacokinetic and toxicity profiles of the peptide and found that there were no morphologic alterations in the liver, kidneys, or spleen and that hematology parameters remained normal in the mice.25 Aligholikhamseh et al. showed the peptide could specifically target HER2+ tumors including SKOV-3 ovarian cancer at 4 hours post-injection while Shahsavari et al. showed that after just 1 hour post-injection, U-87 MG glioma based tumors could be visualized.24
The LTVSPWY core peptide has been evaluated for its ability to selectively bind to HER2. It was also shown that the peptide was stable for multiple hours to allow for extended times for imaging or delivery to other sites. Along with the low Kd values, the small size of the peptide would allow for easier tissue penetration and potentially less immunologic response than full antibodies. However, compared to the KCCYSL peptide, the similar binding site to trastuzumab would potentially make it more difficult to monitor trastuzumab treatment with this agent. Both peptides do share similar size and have the ability to be modified for radiolabeling and HER2 targeting and thus the LTVSPWY peptide should be evaluated in future studies.
FCGDFYACYMDV-Based Peptides
The anti-HER2/neu Peptide (AHNP) is a 1.5 kDa peptide developed in 2000 by Park et al. and similarly to the LTVSPWY peptide, binds to the same site as the monoclonal antibody trastuzumab.26 The peptide was developed by analyzing the amino acid sequences of CD3 loops of trastuzumab and other antibodies which commonly bind to antigens. The AHNP has an amino acid sequence of FCGDFYACYMDV and was reported to have a binding affinity for HER2 of 300 nM using recombinant HER2 measured by surface plasma resonance. The investigators reported that the AHNP was specific for HER2 but whole cell in vitro data was not reported. In vivo studies with athymic nude mice with T6-17 transfected fibroblast tumors that express human HER2 were shown to have decreased tumor formation and tumor growth showing biological activity of the peptide. This is an important characteristic which is different than the previous discussed peptides. The ability of the peptide to change biological activity means the peptide may be unsuitable as a true radiotracer for HER2 breast cancer imaging. However, several studies have focused on using this peptide as an imaging agent.
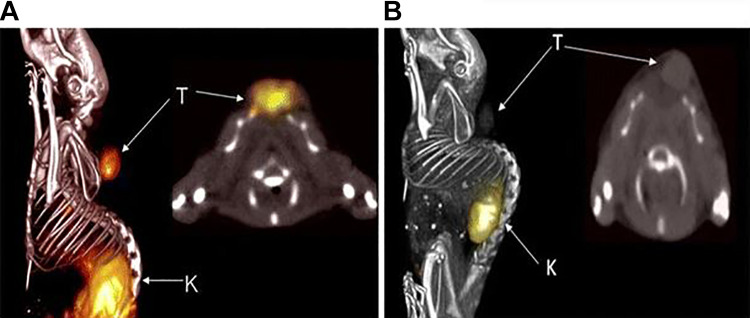
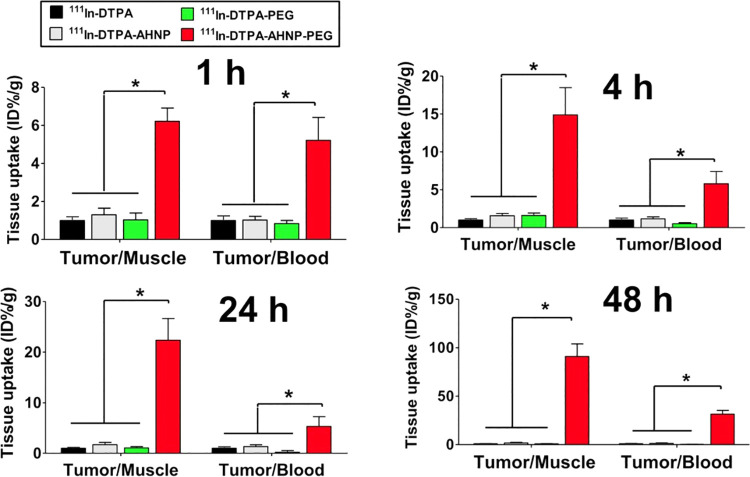
An example of this approach using AHNP to detect HER2 expressing tumors was the use of 111In-DTPA-AHNP-PEG for potential imaging of HER2 positive gastric cancer patients.27 Using flow cytometric analysis with NCI-N87 (High HER2) and MKN45 (Low HER2) cells, fluorescein isothiocyanate (FITC) labeled peptides showed significantly higher binding to NCI-N87 cells over the MKN45 and the signal was significantly lower after being blocked with non-FTIC conjugated AHNP-PEG. Similar results were obtained in cell binding studies with fluorescent imaging with NCI-N87 cells showing fluorescence on the cell membrane which was absent on the MKN45 cells. Fluorescent imaging in vivo showed binding to the NCI-N87 tumors but not the MKN45 tumors, confirming the specificity of the peptide to HER2. The corresponding radiopharmaceutical, 111In-DTPA-AHNP-PEG, was evaluated at 1, 4, 24, 48 hours for imaging and biodistribution in tumor xenograft mice. Tumors could be clearly identified at all of the time points along with at least a 4-fold ratio over the non-targeted control, 111In-DTPA. There were favorable tumor-to-blood ratios of approximately 6:1 at 1 hour and 4 hours and tumor-to-muscle of 6:1 and 14:1 at the same time points respectively, as shown in Figure 6.
Figure 6.
Comparison of tumor-to-muscle (T/M) and tumor-to-blood (T/B) ratios among 111In-DTPA, 111In-DTPA-AHNP, 111In-DTPA-PEG, and 111In-DTPA-AHNP-PEG injected mice after 1, 4, 24, and 48 h injection. From Ref27 used with permission.
Other Peptides
There are other potential peptides that show promise for the development of HER2 imaging agents. One peptide that has been evaluated as a SPECT imaging agent is the H6F (YLFFVFER) peptide in the form of 99mTc-HYNIC-H6F. The H6F peptide was discovered in a one-bead one-compound combination library for HER2 protein and was shown to have high binding and selectivity by the use of fluorescence staining and flow cytometry.28 The peptide was tested both in vitro and in vivo using 99mTc-HYNIC-H6F for HER2 targeting and tumor imaging. Fluorescent imaging with FITC-HYNIC-H6F showed close to 100% of MDA-MB-453 cells with only 5% to the MDA-MB-231 cells bound to the fluorescent peptide. These results were also very similar to FITC labeled trastuzumab with the same cell lines. For the SPECT imaging, MDA-MB-453 tumors were implanted in female mice and images of the 99mTc-HYNIC-H6F peptide were taken at 30 min, 1 hr, and 2 hrs. The tumors were clearly seen at all time points with a %ID/g of 2.47 ± 0.12, 0.66 ± 0.24, and 0.21 ± 0.05 at these time points respectively.
The A9 peptide was synthesized based on the design of the trastuzumab binding region and was synthesized with the chelator DTPA for labeling with 111In, to be used as a potential HER2 imaging peptide.29 The peptide was shown to be internalized into the BT474 cell via HER2 by mass spectrometry analysis using peptides that were bound to biotin and subsequently found within the cell lysate. Peaks associated with the peptide were found consistently within the cells. Other cell binding studies showed that the peptide had 2 potential binding sites, one with an affinity of 4.9 nM and the other of 103 nM. This strong data shows that the study of this peptide in animal models is warranted.
Another large study produced multiple peptides using the one-bead one-compound method and found 72 novel peptides that had between them 3 similar binding motifs that favored HER2 binding.30 Two of the most promising peptides which had the lowest binding free energy were CDTFPYLGWWNPNEYRY and CKTIYYLGYYNPNEYRY. 99.7% and 98% of SKBR3 (HER2+) cells showed uptake of the CDTFPYLGWWNPNEYRY and CKTIYYLGYYNPNEYRY peptides while only 3% of 293A (HER2−) cells showed uptake of each peptide, showing selectivity for HER2. Both peptides also showed the ability to detect tumors in vivo using fluorescence imaging.
Another study involved the synthesis of a “hybrid peptide” that combined 2 different HER2 specific peptides to form 1 larger peptide.31 This peptide was labeled to 99mTc by the GGC linker similar to as discussed above.22 An interesting finding was that they observed a Kd value of 50.0 ± 14 nM for the peptide in a strictly HER2+ cell line (SKBR3), but in ER+/HER+ cell lines (MCF7 and T47D) the Kd values were much lower, 158 ± 25 and 169 ± 22 nM respectively. Imaging studies with the labeled peptide, 99mTc-GGCAKIFGSLAFLKCCYSL, in nude mice bearing HER2+ SKBR3 tumors showed 2.81 ± 0.79 and 1.22 ± 0.25%ID/g at 1 hr and 4 hr, respectively. Lastly, Rahmanian et al developed the radiolabeled 99mTc-tricine-HYNIC-SSS-GE11 peptide (YHWYGYTPQNVI) that was previously discovered by phage display and showed binding to both HER2 and EGFR.32,33 Through the radiolabeling modification, the peptide had a Kd value of 73 ± 14 nM to HER2. Cell binding studies showed that the peptide had preferred HER2 binding to SKOV-3 cells 9 times higher compared to high EGFR, low HER2 expressing A549 cells and 10 times higher to low EGFR, low HER2 MCF-7 cells. This group was also able to confirm the specificity of the peptide for HER2 by cell-blocking studies using trastuzumab as a blocking agent.34 Tumor-to-muscle ratios of 2.4 and 3.4 at 1 hour and 4 hours, respectively were obtaining in SKOV-3 tumor bearing mice.
Summary
All of the peptides discussed in this review show strong potential for continued research and development into HER2 targeting peptides for imaging or therapy. Each of the peptides with the labeling strategy used in each study and reported Kd values are given in Table 1. Despite the KCCYSL peptide having been more widely studied, the lowest reported dissociation constant was with the CGGGLTVSPWY peptide. It can be noted however that each peptide has a Kd that is significantly higher than the trastuzumab antibody (∼1 nM).22 However, the benefit for the development of these peptides includes the potentially quicker circulation time and deeper tissue penetration that would allow for a more rapid image than that with radiolabeled trastuzumab or other antibodies. The majority of the peptides were evaluated for their ability to distinguish HER2 positive from negative tumors in vivo along with biodistribution in vital organs. The 111In-DOTA-GSGKCCYSL had a tumor to blood ratio of 1.95 at 1 hour while the 111In-DOTA-MEGPSKCCYSLALASH also had a ratio of 1.95 after 1 hour.17,18 A higher tumor to blood ratio were seen in the 111In-DTPA-AHNP-PEG peptide at a ratio of ∼6.26 Another important factor that should be considered for the future peptide use is the competition with trastuzumab. For example, the 99mTc-HYNIC-H6F peptide was reported to have a binding site that was shared with trastuzumab. It can also be expected that peptides that were developed from the receptor binding portion of trastuzumab i.e. FCGDFYACYMDV and A9 peptides, would also have competitive binding with trastuzumab. Other peptides discussed did not show any competitive binding to the binding sites of trastuzumab, but it is possible that any of these peptides could compete with each other or pertuzumab. Each peptide did show selectivity to HER2, and thus more work is warranted for any of the peptides for future studies.
Table 1.
HER2 Targeted Peptides.
| Peptide | Labeling Strategy | Kd (nM) | Reference |
|---|---|---|---|
| KCCYSL | 111In-DOTA-GSG | 295 ± 56 | 16 |
| MEGPSKCCYSLALASH | 111In-DOTA | 236 ± 83 | 18 |
| GTKSKCCYSLRRSS | 111In-DOTA | 289 ± 13 | 18 |
| LTVSPWY | N/A | N/A | 21 |
| CGGGLTVSPWY | 99mTc | 4.3 ± 0.8 | 22 |
| CSSSLTVSPWY | 99mTc | 33.9 ± 9.7 | 22 |
| FCGDFYACYMDV | 111In-DTPA-peptide-PEG | 300 | 26 |
| H6F | 99mTc-HYNIC | N/A | 28 |
| A9 | 111In-DTPA | 4.9 and 103 | 29 |
| CDTFPYLGWWNPNEYRY | Cy5.5 | 18.6 | 30 |
| CKTIYYLGYYNPNEYRY | Cy5.5 | 81.2 | 30 |
| GGCAKIFGSLAFLKCCYSL | 99mTc | 50.0 ± 14 | 31 |
| YHWYGYTPQNVI | 99mTc-tricine-HYNIC-SSS | 73 ± 14 | 33 |
Future Studies
There are several considerations for the development of peptides into viable imaging or therapeutic agents. One of the main concerns that the binding affinity is generally lower for peptides as compared to antibodies. This could become a challenge during the development of these agents for clinical use. Approaches to overcome this may include the use of multimeric agents or nanoparticles which can incorporate more than 1 peptide per molecule. However, detailed imaging and biodistribution studies are required to shed light on this issue. To modify these agents for use as PET probes, future studies will require the addition of an appropriate chelator for the radiolabeling with a suitable positron-emitting isotope. Since peptides have a shorter biological half-life, shorter lived isotopes, like 68Ga, may be optimal but in certain cases, longer lived isotopes such as 43Sc or 64Cu may be required. Another potential use of HER2 imaging peptides could be to yield a quantitative readout of tumor heterogeneity which would give physicians and researchers more insight into the tumor nature and receptor status.
Conclusion
Each peptide possesses different strengths which would allow for continued research and development. With the range of peptide sizes and variable labeling strategies, adaptation is possible for newer types of imaging or therapeutic modalities i.e. PET imaging, or development into therapeutic peptides can be attained. The development of HER2 specific peptides will benefit the need for rapid diagnosis of HER2 positive patients. Each of the peptides discussed are promising candidates as new tools for imaging of HER2 positive cancers.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Maxwell Ducharme  https://orcid.org/0000-0001-9765-2029
https://orcid.org/0000-0001-9765-2029
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2. Paplomata E, O’Regan R. The PI3K/AKT/mTOR pathway in breast cancer: targets, trials and biomarkers. Ther Adv Med Oncol. 2014;6(4):154–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller KD, Nogueira L, Mariotto AB, et al. Cancer treatment and survivorship statistics, 2019. CA Cancer J Clin. 2019;69(5):363–385. [DOI] [PubMed] [Google Scholar]
- 4. Krishnamurti U, Silverman JF. HER2 in breast cancer: a review and update. Adv Anat Pathol. 2014;21(2):100–107. [DOI] [PubMed] [Google Scholar]
- 5. Tai W, Mahato R, Cheng K. The role of HER2 in cancer therapy and targeted drug delivery. J Control Release. 2010;146(3):264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gutierrez C, Schiff R. HER2: biology, detection, and clinical implications. Arch Pathol Lab Med. 2011;135(1):55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61(Suppl 2):1–13. [DOI] [PubMed] [Google Scholar]
- 8. Ulaner GA, Lyashchenko SK, Riedl C, et al. First-in-human human epidermal growth factor receptor 2–targeted imaging using 89Zr-Pertuzumab PET/CT: dosimetry and clinical application in patients with breast cancer. J Nucl Med. 2018;59(6):900–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dehdashti F, Wu N, Bose R, et al. Evaluation of [89 Zr] trastuzumab-PET/CT in differentiating HER2-positive from HER2-negative breast cancer. Breast Cancer Res Treat. 2018;169(3):523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Massicano AV, Marquez-Nostra BV, Lapi SE. Targeting HER2 in nuclear medicine for imaging and therapy. Mol Imaging. 2018;17:1536012117745386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Massicano AV, Lee S, Crenshaw BK, et al. Imaging of HER2 with [89Zr] pertuzumab in response to T-DM1 therapy. Cancer Biother Radio. 2019;34(4):209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fani M, Maecke H, Okarvi S. Radiolabeled peptides: valuable tools for the detection and treatment of cancer. Theranostics. 2012;2(5):481–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fosgerau K, Hoffmann T. Peptide therapeutics: current status and future directions. Drug Discov. 2015;20(1):122–128. [DOI] [PubMed] [Google Scholar]
- 14. Poeppel TD, Binse I, Petersenn S, et al. 68Ga-DOTATOC versus 68Ga-DOTATATE PET/CT in functional imaging of neuroendocrine tumors. J Nucl Med. 2011;52(12):1864–1870. [DOI] [PubMed] [Google Scholar]
- 15. Diderich P, Heinis C. Phage selection of bicyclic peptides binding Her2. Tetrahedron. 2014;70(42):7733–7739. [Google Scholar]
- 16. Karasseva NG, Glinsky VV, Chen NX, Komatireddy R, Quinn TP. Identification and characterization of peptides that bind human ErbB-2 selected from a bacteriophage display library. J Protein Chem. 2002;21(4):287–296. [DOI] [PubMed] [Google Scholar]
- 17. Kumar SR, Quinn TP, Deutscher SL. Evaluation of an 111In-radiolabeled peptide as a targeting and imaging agent for ErbB-2 receptor–expressing breast carcinomas. Clin Cancer Res. 2007;13(20):6070–6079. [DOI] [PubMed] [Google Scholar]
- 18. Larimer BM, Thomas WD, Smith GP, Deutscher SL. Affinity maturation of an ERBB2-targeted SPECT imaging peptide by in vivo phage display. Mol Imaging Biol. 2014;16(4):449–458. [DOI] [PubMed] [Google Scholar]
- 19. Cai H, Singh AN, Sun X, Peng F. Synthesis and characterization of HER2-NLP peptide conjugates targeting circulating breast cancer cells: cellular uptake and localization by fluorescent microscopic imaging. J Fluoresc. 2015;25(1):113–117. [DOI] [PubMed] [Google Scholar]
- 20. Kawamoto M, Horibe T, Kohno M, Kawakami K. HER2-targeted hybrid peptide that blocks HER2 tyrosine kinase disintegrates cancer cell membrane and inhibits tumor growth in vivo. Mol Cancer Ther. 2013;12(4):384–393. [DOI] [PubMed] [Google Scholar]
- 21. Shadidi M, Sioud M. Identification of novel carrier peptides for the specific delivery of therapeutics into cancer cells. FASEB J. 2003;17(2):256–258. [DOI] [PubMed] [Google Scholar]
- 22. Sabahnoo H, Noaparast Z, Abedi SM, Hosseinimehr SJ. New small 99mTc-labeled peptides for HER2 receptor imaging. Eur J Med Chem. 2017;127:1012–1024. [DOI] [PubMed] [Google Scholar]
- 23. Shahsavari S, Shaghaghi Z, Abedi SM, Hosseinimehr SJ. Evaluation of 99mTc-HYNIC-(ser) 3-LTVPWY peptide for glioblastoma imaging. Int J Radiat Biol. 2020;96(4):502–509. [DOI] [PubMed] [Google Scholar]
- 24. Aligholikhamseh N, Ahmadpour S, Khodadust F, Abedi SM, Hosseinimehr SJ. 99mTc-HYNIC-(Ser) 3-LTVPWY peptide bearing tricine as co-ligand for targeting and imaging of HER2 overexpression tumor. Radiochim Acta. 2018;106(7):601–609. [Google Scholar]
- 25. Ardakani JB, Amiri FT, Khorramimoghaddam A, Abbasi A, Molavipordanjani S, Hosseinimehr SJ. Preclinical pharmacokinetic, biodistribution, radiation dosimetry, and toxicity studies of 99mTc-HYNIC-(Ser) 3-LTVPWY: a novel HER2-targeted peptide radiotracer. Regul Toxicol Pharmacol. 2020;112:104591. [DOI] [PubMed] [Google Scholar]
- 26. Park B-W, Zhang H-T, Wu C, et al. Rationally designed anti-HER2/neu peptide mimetic disables p185 HER2/neu tyrosine kinases in vitro and in vivo. Nat Biotechnol. 2000;18(2):194–198. [DOI] [PubMed] [Google Scholar]
- 27. Guan S-S, Wu C-T, Chiu C-Y, et al. Polyethylene glycol-conjugated HER2-targeted peptides as a nuclear imaging probe for HER2-overexpressed gastric cancer detection in vivo. J Transl Med. 2018;16(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li L, Wu Y, Wang Z, et al. SPECT/CT imaging of the novel HER2-targeted peptide probe 99mTc-HYNIC-H6F in breast cancer mouse models. J Nucl Med. 2017;58(5):821–826. [DOI] [PubMed] [Google Scholar]
- 29. Honarvar H, Calce E, Doti N, et al. Evaluation of HER2-specific peptide ligand for its employment as radiolabeled imaging probe. Sci Rep. 2018;8(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Geng L, Wang Z, Jia X, et al. HER2 targeting peptides screening and applications in tumor imaging and drug delivery. Theranostics. 2016;6(8):1261–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okarvi SM, AlJammaz I. Development of the tumor-specific antigen-derived synthetic peptides as potential candidates for targeting breast and other possible human carcinomas. Molecules. 2019;24(17):3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li Z, Zhao R, Wu X, et al. Identification and characterization of a novel peptide ligand of epidermal growth factor receptor for targeted delivery of therapeutics. FASEB J. 2005;19(14):1978–1985. [DOI] [PubMed] [Google Scholar]
- 33. Rahmanian N, Hosseinimehr SJ, Khalaj A, Noaparast Z, Abedi SM, Sabzevari O. 99 m Tc-radiolabeled GE11-modified peptide for ovarian tumor targeting. DARU. 2017;25(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rahmanian N, Hosseinimehr SJ, Khalaj A, Noaparast Z, Abedi SM, Sabzevari O. 99 m Tc labeled HYNIC-EDDA/tricine-GE11 peptide as a successful tumor targeting agent. Med Chem Res. 2018;27(3):890–902. [Google Scholar]