Abstract
Introduction:
Using the TMN classification alone to predict survival in patients with gastric cancer has certain limitations, we conducted this study was to develop an effective nomogram based on aspartate aminotransferase/alanine aminotransferase (AST/ALT) ratio to predict overall survival (OS) in surgically treated gastric cancer.
Methods:
we retrospectively analyzed 190 cases of gastric cancer and used Cox regression analysis to identify the significant prognostic factors for OS in patients with resectable gastric cancer. The predictive accuracy of nomogram was assessed using a calibration plot, concordance index (C-index) and decision curve. This was then compared with a traditional TNM staging system. Based on the total points (TPS) by nomogram, we further divided patients into different risk groups.
Results:
multivariate analysis of the entire cohort revealed that independent risk factors for survival were age, clinical stage and AST/ALT ratio, which were entered then into the nomogram. The calibration curve for the probability of OS showed that the nomogram-based predictions were in good agreement with actual observations. Additionally, the C-index of the established nomogram for predicting OS had a superior discrimination power compared to the TNM staging system [0.794 (95% CI: 0.749-0.839) vs 0.730 (95% CI: 0.688-0.772), p < 0.05]. Decision curve also demonstrated that the nomogram was better than the TNM staging system. Based on TPS of the nomogram, we further subdivided the study cohort into 3 groups including low risk (TPS ≤ 158), middle risk (158 < TPS ≤ 188) and high risk (TPS > 188) categories. The differences in OS rate were significant among the groups.
Conclusion:
the established nomogram is associated with a more accurate prognostic prediction for individual patients with resectable gastric cancer.
Keywords: nomogram, gastric cancer, AST/ALT ratio, prognosis, classifier
Introduction
Gastric cancer (GC), or stomach cancer, is one of the most common malignant cancers and the second leading cause of cancer-related death around the world, particularly in East Asia.1,2 The most common type of GC is adenocarcinoma (GA). In the United States alone, there are over 26,000 new gastric cancer cases diagnosed and 10,730 deaths from GC yearly.3 Surgery represents the primary treatment for patients with resectable gastric cancer. Early gastric cancer is associated with a more favorable long-term survival when treated with curative surgical resection, compared to patients with advanced disease. However, most GC patients are diagnosed at advanced stages, which requires multimodality therapy. Several factors, including poor early detection, recurrence and metastasis, lead to the low overall survival (OS) rate of GC.4 At present, TNM staging has been considered the main factor to predict the prognosis of gastric cancer.5,6 However, the outcomes of GC patients at the same disease stage might be completely different. Therefore, identification of other ways to increase the predictive prognostic accuracy in GC patients is essential.
Numerous studies had reported that common serum examinations, including evaluation of AST and ALT, are prognostic factors of GC patients. In particular, AST and ALT, which are common liver function tests, and serum AST and ALT, can effectively predict outcomes in patients with hepatocellular carcinoma, renal cell carcinoma and breast cancer.7-9 In addition, serum AST/ALT ratio (SLR) or ALT/AST ratio (LSR) had been used as biomarker to assess other diseases, including liver cirrhosis, insulin resistance, and alcoholic liver disease.10-13
Recent studies have indicated increasing evidence that nomogram combined with common serum examinations can predict prognosis more accurately across variety of tumors, including lung cancer, colorectal cancer, and hepatocellular carcinoma.14-16 Additionally, our pervious study has reported that LSR is a prognostic factor of GC patients, and patients with lower LSR have a greater risk of death compared to those with higher LSR.17 We conducted this study was to establish a prognostic nomogram for resectable GC based on SLR and clinicopathological parameters, as well as to evaluate whether this model can allow a more accurate prediction of GC patients.
Materials and Methods
Collection of Patient Information
We retrospectively analyzed a cohort of 190 patients with gastric cancer, which was comprised of 132 males and 58 females. The average age of the cohort was 56 years, with a range of 23-79 years. Patients included in the cohort underwent gastrectomy at Sun Yat-sen University Cancer Center from January 2008 to December 2009. Subjects were included in the study if they met the 4 inclusion criteria. First, the pathological results were confirmed by 2 observers, and the histological subtypes were identified. Only cases of gastric cancer of adenocarcinoma type were included in the study. Second, included patients did not take anti-inflammatory drugs within 1 week of surgery and underwent radical resection. Third, patients with gastric adenocarcinoma were included only if they were not treated with radiation or chemotherapy prior to surgery. Fourth, patients did not have any other cancers except for gastric adenocarcinoma.
Patients with the following conditions were excluded in the study including a history of inflammatory disease that may modify AST and ALT levels, the presence of multiple stomach tumors, bacterial or viral infection, and fever of unknown origin. Clinicopathologic parameters of each patient were collected, including age, gender, smoking history, family history, tumor status, serous infiltration, the ABO blood group, pathologic TNM stage, postoperative chemotherapy regimen, AST, ALT, SLR, CRP, ALB, and CRP/ALB. Clinical stage was assessed according to the seventh edition of the Union for International Cancer Control (UICC).18 Patients were followed up either in clinic or by telephone. The date from surgery to death or to January 2017 was considered as survival time.
All serological results were collected within 7 days after surgery. The serum levels of AST, ALT, CRP, and ALB were measured by an Automatic Biochemical Analyzer (Hitachi 7600, Japan), according to the manufacturer’s instructions.
Statistical Analysis
Statistical analyses were performed using SPSS software, version 21.0 (SPSS Inc., Chicago, IL, USA). Nomogram for evaluation of possible prognostic factors associated with OS were established using R software (version 3.6.1). The cut-off values of AST, ALT, CRP and ALB were estimated by median. Additionally, the following cut-off values for continuous variables were obtained using the X-tile program: age (51 years), tumor size (4.5 cm) and SLR (1.24).19 The Kaplan-Meier curves were used to calculate survival rate, and the log-rank test was used to compare survival rates between groups. The Cox proportional hazards regression was used for multivariate analysis. All variables that met statistical significance (p < 0.05) in the multivariable model were utilized to develop a dynamic prediction nomogram model. The performance of the prediction nomogram model was evaluated by concordance index (C-index) and decision curve. The differences were considered statistically significant when p < 0.05.
Results
Clinicopathologic Characteristics
Overall, 190 patients with gastric cancer who underwent surgical resection were enrolled in this study. The detailed clinical characteristics of each patient are presented in Table 1. The cohort included 132 males (69.5%) and 58 females (30.5%). In particular, 58 (30.5%) patients had a history of smoking and 18 (9.5%) had a family history of cancer. The number of early stage and advanced stage patients were 34 (17.9%) and 156 (82.1%), respectively. Lymph node metastasis was confirmed pathologically in 133 (70.0%) patients. Only 16 (8.4%) patients had distant metastasis. Finally, all patients had adenocarcinoma (AC).
Table 1.
Clinical and Laboratory Characteristics of 190 Patients Associated With Overall Survival (OS).
| Characteristics | No. of patients | OS (Months) Mean (95% CI) | p-Value |
|---|---|---|---|
| Age(years) | 0.022 | ||
| ≤51 | 63 | 70.76 (63.31-78.21) | |
| >51 | 127 | 58.97 (52.70-65.23) | |
| Gender | 0.429 | ||
| Female | 58 | 60.46 (51.39-69.53) | |
| Male | 132 | 63.66 (57.87-69.44) | |
| Family History | 0.287 | ||
| Yes | 18 | 62.22 (56.94-67.51) | |
| No | 171 | 68.48 (55.05-81.90) | |
| Smoking Behavior | 0.250 | ||
| Yes | 58 | 67.09 (58.61-75.57) | |
| No | 132 | 61.26 (55.25-67.26) | |
| Tumor status | <0.001 | ||
| T1/T2 | 34 | 89.13 (86.82-91.41) | |
| T3/T4 | 156 | 57.09 (51.59-62.58) | |
| Lymph node metastasis | <0.001 | ||
| Yes | 133 | 53.57 (47.36-59.77) | |
| No | 57 | 84.40 (80.87-87.94) | |
| Distant metastases | <0.001 | ||
| Yes | 16 | 33.06 (19.26-46.85) | |
| No | 174 | 65.69 (60.66-70.72) | |
| Clinical stage | <0.001 | ||
| I | 22 | 86.65 (83.46-89.85) | |
| II | 46 | 85.78 (81.48-90.08) | |
| III | 81 | 58.85 (51.51-66.18) | |
| IV | 41 | 31.14 (21.90-40.37) | |
| Serous infiltration | <0.001 | ||
| S0/S1 | 60 | 75.24 (68.54-81.94) | |
| S2/S3 | 130 | 56.24 (49.91-62.56) | |
| Postoperative chemotherapy | 0.077 | ||
| Yes | 60 | 42.59 (35.82-49.37) | |
| No | 130 | 50.33 (45.67-55.00) | |
| Maximum tumor diameter(cm) | <0.001 | ||
| ≤4.5 | 87 | 56.52 (51.19-61.86) | |
| >4.5 | 103 | 40.59 (35.46-45.73) | |
| Tumor differentiation | 0.922 | ||
| poorly | 173 | 47.32 (43.34-51.31) | |
| moderately | 17 | 53.65 (38.19-69.12) | |
| Tumor Location | 0.369 | ||
| Upper | 39 | 47.60 (39.13-56.07) | |
| Middle | 45 | 43.54 (36.13-50.95) | |
| Lower | 106 | 49.90 (44.49-55.30) | |
| AST(U/L) | 0.143 | ||
| ≤28 | 99 | 66.64 (59.87-73.40) | |
| >28 | 91 | 57.43 (50.65-64.21) | |
| ALT(U/L) | 0.552 | ||
| ≤25 | 97 | 64.90 (57.94-71.85) | |
| >25 | 93 | 60.40 (53.59-67.21) | |
| SLR(AST/ALT) | 0.028 | ||
| ≤1.24 | 135 | 66.72 (61.05-72.39) | |
| >1.24 | 55 | 52.55 (43.55-61.55) | |
| CRP(mg/L) | 0.804 | ||
| ≤68.48 | 94 | 63.93 (56.95-70.91) | |
| >68.48 | 96 | 61.36 (54.43-68.30) | |
| ALB(g/L) | 0.024 | ||
| ≤34 | 94 | 57.49 (50.21-64.76) | |
| >34 | 96 | 67.92 (61.49-74.34) | |
| CRP/ALB | 0.616 | ||
| ≤1.15 | 94 | 64.66 (57.71-71.60) | |
| >1.15 | 96 | 60.65 (53.69-67.60) | |
| Blood type | 0.283 | ||
| A | 50 | 58.01 (48.19-67.82) | |
| B | 52 | 59.97 (50.92-69.02) | |
| AB | 11 | 48.09 (31.35-64.83) | |
| O | 77 | 68.22 (60.88-75.55) |
OS, overall survival; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SLR: AST/ALT ratio; ALB, albumin; CRP, C-reaction protein.
Association of Post-Surgical Serum SLR Levels With Clinical Characteristics
The median survival time of 190 patients was 40.1 months (range 6.8-73.4 months). The 1-, 3-, and 5-year survival rate were 90.5%, 66.8%, and 63.2%, respectively. Table 2 shows the clinical parameters of 190 patients and the correlation between postoperative SLR levels. SLR was associated with maximum tumor diameter (cm) (p = 0.047), ALT (p < 0.001), ALB (p = 0.045) and overall survival (p = 0.042). Compared to patients with better prognosis, patients with poor outcomes had higher postoperative SLR (p = 0.042). No correlation was found between SLR and age, gender, family history, smoking behavior, clinical stage, lymph node metastasis, distant metastases, serous infiltration, tumor location, tumor differentiation, postoperative chemotherapy, tumor location, CRP, and blood type.
Table 2.
Correlation Between AST/ALT and Clinicopathological Variables of Gastric Cancer Patients.
| Characteristics | No of patients | AST/ALT ratio | ||
|---|---|---|---|---|
| ≤1.24 | >1.24 | p a | ||
| Patients | 190 | 135 | 55 | |
| Age(years) | ||||
| ≤51 | 63 | 49 (77.8%) | 14 (22.2%) | 0.150 |
| >51 | 127 | 86 (67.7%) | 41 (32.3%) | |
| Gender | ||||
| Female | 58 | 40 (69.0%) | 18 (31.0%) | 0.674 |
| Male | 132 | 95 (72.0%) | 37 (28.0%) | |
| Family History | ||||
| Yes | 18 | 12 (66.7%) | 6 (33.3%) | 0.678 |
| No | 171 | 122 (71.3%) | 49 (28.7%) | |
| Smoking Behavior | ||||
| Yes | 58 | 46 (79.3%) | 12 (20.7%) | 0.096 |
| No | 132 | 89 (67.4%) | 43 (32.6%) | |
| Tumor status | ||||
| T1/T2 | 34 | 27 (79.4%) | 7 (20.6%) | 0.236 |
| T3/T4 | 156 | 108 (69.2%) | 48 (30.8%) | |
| Lymph node metastasis | ||||
| Yes | 133 | 91 (68.4%) | 42 (31.6%) | 0.222 |
| No | 57 | 44 (77.2%) | 13 (22.8%) | |
| Distant metastases | ||||
| Yes | 16 | 10 (62.5%) | 6 (37.5%) | 0.431 |
| No | 174 | 125 (71.8%) | 49 (28.2%) | |
| Clinical stage | ||||
| I | 22 | 19 (86.4%) | 3 (13.6%) | 0.292 |
| II | 46 | 32 (69.6%) | 14 (30.5%) | |
| III | 81 | 58 (71.6%) | 23 (28.4%) | |
| IV | 41 | 26 (63.4%) | 15 (36.6%) | |
| Serous infiltration | ||||
| S0/S1 | 67 | 48 (71.6%) | 19 (28.4%) | 0.895 |
| S2/S3 | 123 | 87 (70.7%) | 36 (29.3%) | |
| Postoperative chemotherapy | ||||
| Yes | 60 | 40 (66.7%) | 20 (33.3%) | 0.365 |
| No | 130 | 95 (73.1%) | 35 (26.9%) | |
| Maximum tumor diameter(cm) | ||||
| ≤4.5 | 87 | 68 (50.3%) | 19 (34.5%) | 0.047* |
| >4.5 | 103 | 67 (49.6%) | 36 (65.4%) | |
| Tumor differentiation | ||||
| poorly |
173 | 126 (93.3%) | 47 (85.4%) | 0.084 |
| moderately |
17 | 9 (6.6%) | 8 (14.5%) | |
| Tumor Location | ||||
| Upper | 39 | 23 (17.0%) | 16 (29.0%) | 0.143 |
| Middle | 45 | 35 (25.9%) | 10 (18.1%) | |
| Lower | 105 | 77 (57.0%) | 29 (52.7%) | |
| AST(U/L) | ||||
| ≤28 | 99 | 69 (69.7%) | 30 (30.3%) | 0.667 |
| >28 | 91 | 66 (72.5%) | 25 (27.5%) | |
| ALT(U/L) | ||||
| ≤25 | 97 | 51 (52.6%) | 46 (47.4%) | <0.001* |
| >25 | 93 | 84 (90.3%) | 9 (9.7%) | |
| CRP(mg/L) | ||||
| ≤68.48 | 94 | 64 (68.1%) | 30 (31.9%) | 0.372 |
| >68.48 | 96 | 71 (74.0%) | 25 (26.0%) | |
| ALB(g/L) | ||||
| ≤34 | 94 | 61 (64.9%) | 33 (35.1%) | 0.045* |
| >34 | 96 | 74 (77.1%) | 22 (22.9%) | |
| CRP/ALB | ||||
| ≤1.15 | 94 | 65 (69.1%) | 29 (30.9%) | 0.576 |
| >1.15 | 96 | 70 (72.9%) | 26 (27.1%) | |
| Blood type | ||||
| A | 50 | 36 (72.0%) | 14 (28.0%) | 0.929 |
| B | 52 | 38 (73.1%) | 14 (26.9%) | |
| AB | 11 | 7 (63.6%) | 4 (36.4%) | |
| O | 77 | 54 (70.1%) | 23 (28.9%) | |
| Overall survival | ||||
| Alive | 118 | 90 (76.3%) | 28 (23.7%) | 0.042* |
| Death | 72 | 45 (62.5%) | 27 (37.5%) | |
Note: aUsing Chi-squared test, *p < 0.05 was considered statistically significant.
Univariate and Multivariate Analyses of Factors Associated With Patient Prognosis
Univariate analysis showed that postoperative SLR levels were associated with OS (p = 0.034), as well as other variables including age (p = 0.017), tumor status (p < 0.001), lymph node metastases (p < 0.001), distant metastases (p = 0.003), clinical stage (p < 0.001), serous infiltration (p < 0.001), maximum tumor diameter (p < 0.001), and albumin (p = 0.024) (Table 3). Moreover, multivariate analysis using the Cox proportional hazard model indicated that age (HR = 2.282, 95% CI: 1.253-4.155, p = 0.007), clinical stage (HR = 2.286, 95% CI: 1.145-4.565, p = 0.019) and SLR (HR = 1.758, 95% CI: 1.078-2.868, p = 0.024) were independent prognostic factors of OS for GC patients.
Table 3.
Univariate and Multivariate COX Regression Analyses for Overall Survival in Patients With Gastric Cancer.
| Variables | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | CI | p | HR | CI | P | |
| Age | 1.874 | 1.087-3.229 | 0.017 | 2.282 | 1.253-4.155 | 0.007 |
| Gender | 0.822 | 0.506-1.336 | 0.434 | – | – | – |
| Family History | 0.613 | 0.247-1.522 | 0.258 | – | – | – |
| Smoking Behavior | 0.732 | 0.429-1.249 | 0.241 | – | – | – |
| Tumor status | 20.187 | 2.803-145.358 | < 0.001 | 4.202 | 0.530-33.335 | 0.174 |
| Lymph node metastasis | 8.318 | 3.347-20.676 | < 0.001 | 1.424 | 0.922-2.200 | 0.111 |
| Distant metastases | 3.079 | 1.614-5.872 | 0.003 | 1.000 | 0.473-2.115 | 1.424 |
| Clinical stage | 3.565 | 2.541-5.001 | < 0.001 | 2.286 | 1.145-4.565 | 0.019 |
| Serous infiltration | 2.982 | 1.634-5.441 | < 0.001 | 1.076 | 0.562-2.060 | 0.826 |
| Postoperative chemotherapy | 1.534 | 0.955-1.534 | 0.077 | – | – | – |
| Maximum tumor diameter(cm) | 2.784 | 1.660-4.669 | < 0.001 | 1.303 | 0.755-2.248 | 0.341 |
| Tumor differentiation | 1.040 | 0.476-2.228 | 0.922 | – | – | – |
| Tumor Location | 0.975 | 0.688-1.380 | 0.602 | – | – | – |
| AST(U/L) | 1.414 | 0.888-2.251 | 0.143 | – | – | – |
| ALT(U/L) | 1.151 | 0.724-1.828 | 0.552 | – | – | – |
| SLR(AST/ALT) | 1.699 | 1.054-2.738 | 0.034 | 1.758 | 1.078-2.868 | 0.024 |
| CRP(mg/L) | 1.06 | 0.668-1.684 | 0.804 | – | – | – |
| Albumin(g/L) | 0.584 | 0.365-0.936 | 0.024 | 0.953 | 0.565-1.608 | 0.858 |
| CRP/ALB ratio | 1.126 | 0.709-1.788 | 0.616 | – | – | – |
| Blood type | 0.849 | 0.706-1.020 | 0.084 | – | – | – |
p < 0.05, statistically significant. CI = confidence interval; HR = hazard ratio.
The Nomogram for the Prediction of OS
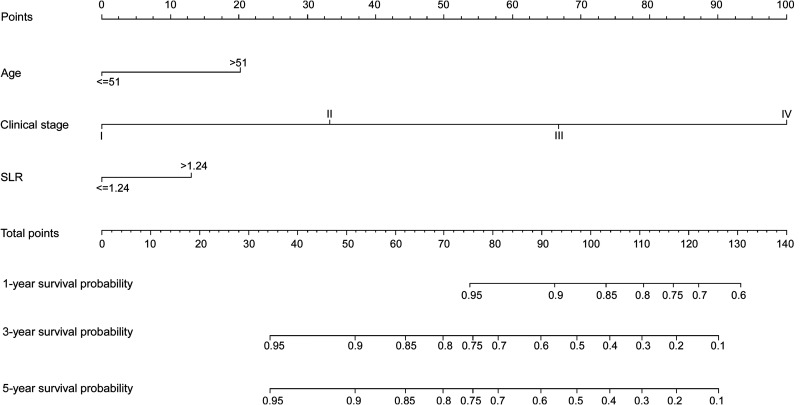
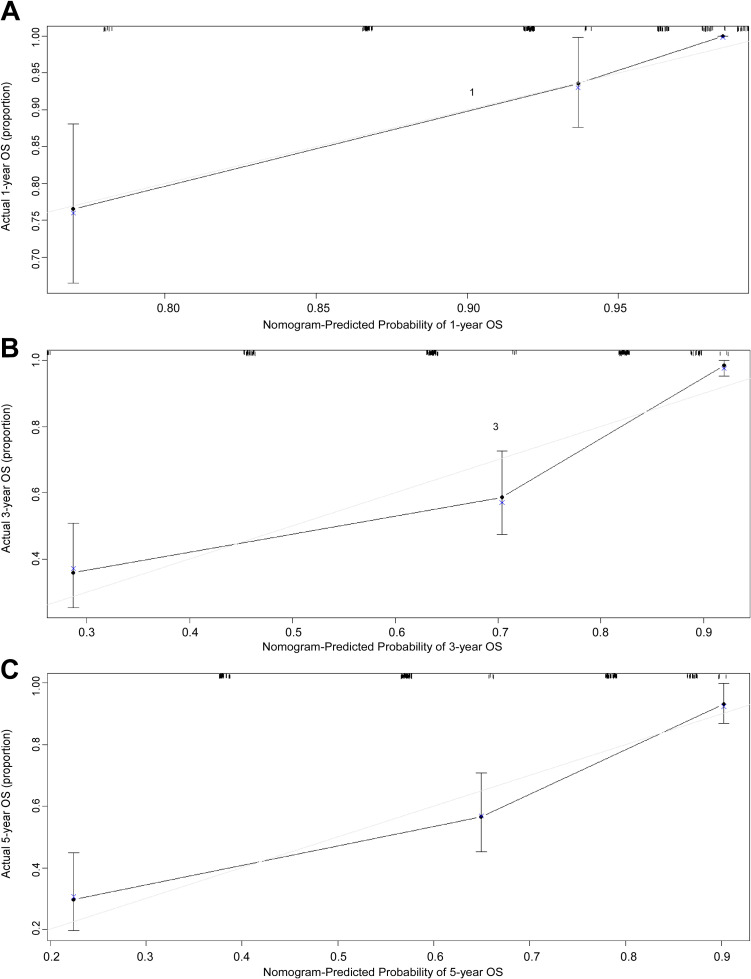
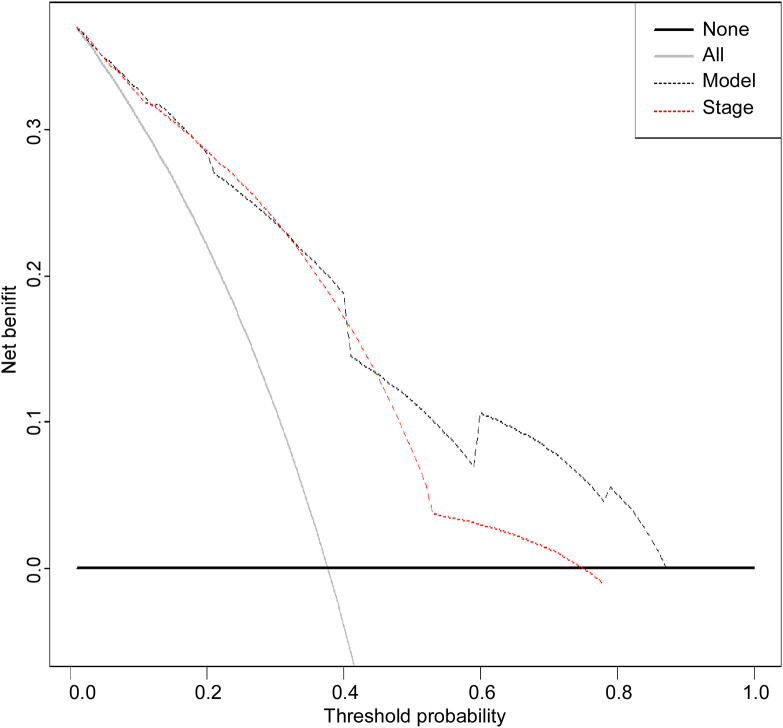
According to multivariate Cox regression model, age > 51, advanced clinical stage, and SLR > 1.24 are poor prognostic factors for OS. Therefore, a nomogram containing age, clinical stage, and SLR was established to predict OS of GC patients (Figure 1). Overall survival at 1, 3, and 5 years was calculated based on addition of the risk scores of age, TNM stage, and SLR. The nomogram model achieved a C-index of 0.794 (95% CI: 0.749-0.839), which was higher than the C-index of the TNM staging system (0.730; 95% CI: 0.688-0.772; p < 0.05). Additionally, the predictive accuracies for OS for GC between the nomogram and the TNM system were compared by calculating the Harrell’s C-index (Table 4). As confirmed by the calibration curve of postoperative 1-year, 3-year and 5-year survival, predictions established in Nomogram best matched actual observations (Figure 2) The results of the decision curve analysis at 5 years are presented in Figure 3. Compared to the traditional TNM staging system, the established nomogram model has a higher overall net benefit across a wide range of threshold probabilities.
Figure 1.
Nomogram convey the results of prognostic models using age, clinical stage and SLR characteristics predict OS. The nomogram was used summing the points identified on the points scale for each variable. The total points projected on the bottom scales indicate the probability of 1-, 3- and 5-year survival.
Table 4.
The C-Index of Nomogram Model and TNM Stage for Prediction of OS.
| Variables | C-index (95% CI) | p |
|---|---|---|
| Nomogram Model | 0.794 (0.749-0.839) | |
| TNM stage | 0.730 (0.688-0.772) | |
| Nomogram Model vs TNM stage | < 0.05 |
C-index = concordance index; CI = confidence interval.
Figure 2.
The calibration curves for predicting patient OS at 1 year (A), 3 years (B) and 5 years (C) in the primary cohort. Nomogram model-predicted OS is plotted on the x-axis; actual OS is plotted on the y-axis. The reference line is 45 degree and indicates perfect calibration.
Figure 3.
Decision curve analysis for 5-year survival predictions. In the decision curve analysis, the y-axis indicates net benefit. The straight line represents the assumption that all patients will die, and the horizontal line represents the assumption that no patients will die.
Risk Stratification of OS
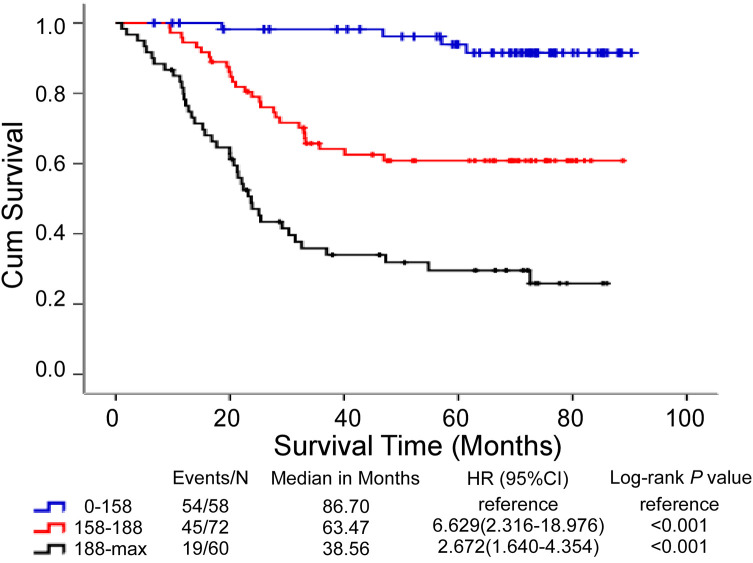
According to Nomogram’s predictors, GC patients were divided into 3 subgroups by total score including the low-risk group (score: 0-158), middle-risk group (score: 158-188), and high-risk group (score: ≥188) (Table 5). The results suggested that GC patients with a higher score corresponded to worse prognoses. The low-risk group have survival probabilities of 100%, 98.2% and 94.8% for 1, 3 and 5 years, respectively. The middle-risk group have survival probabilities of 94.4%, 65.3% and 62.5% for 1, 3 and 5 years, respectively. The high-risk group have the lowest probability of survival with 76.7%, 38.3%, and 33.3% for 1, 3 and 5 years, respectively. Then, a Kaplan-Meier curve was plotted based on the cutoff values. The median OS of low-risk, middle-risk, and high-risk groups were 86.70, 63.47 and 38.56 months, respectively. Survival outcomes for these 3 risk groups were effectively distinguished by this stratification (p < 0.001 in Figure 4).
Table 5.
Point Assignment and Prognostic Score of the Nomogram Model.
| Variable and prognostic score | Score | Estimated 1-year OS (%) | Estimated 3-year OS (%) | Estimated 5-year OS (%) |
|---|---|---|---|---|
| Age group points | ||||
| ≤51 | 0 | |||
| >51 | 20 | |||
| Stage group points | ||||
| I | 0 | |||
| II | 33 | |||
| III | 67 | |||
| IV | 100 | |||
| SLR group points | ||||
| ≤1.24 | 0 | |||
| >1.24 | 13 | |||
| Total prognostic Score | ||||
| 0-158 | 100 | 98.2 | 94.8 | |
| 158-188 | 94.4 | 65.3 | 62.5 | |
| ≥188 | 76.7 | 38.3 | 33.3 |
Figure 4.
Kaplan-Meier curve of GC patients OS for 3 groups based on the predictor from the nomogram model.
Discussion
GC is a highly aggressive cancer with high incidence and mortality rate worldwide.20 H. Pylori infection, inflammation and other factors may increase risk of GC development.21-23 The survival of individual GC patients within the same disease stage is remarkably heterogeneous. Currently, surgical resection remains the best treatment for patients with GC. However, patients that undergo gastrectomy often experience recurrence or metastasis within 5 years.24 Thus, it is important to identify independent prognostic factors that can help optimize postoperative treatments. Nomogram is a statistical prediction tool that generates a numerical probability of death and cancer recurrence. It incorporates all prognostic factors to estimate the survival outcome for cancer patients.25,26 A growing number of studies have shown that, compared to the traditional staging system, some nomograms are more accurately able to predict survival.14,27 In a previous study, we found that LSR is an independent prognostic factor for GC patients, and patients with higher LSR have better survival compared to those with lower LSR.17 However, the study did not comprehensively evaluate the significance of serum aminotransferase and clinicopathological parameters in GC patients.
In this study, our results indicate that the clinical values of AST, ALB and overall survival are associated with SLR levels. We used univariate and multivariate analysis to determine age, clinical stage and SLR as independent prognostic factors for surgically treated GC patients. We evaluated the prognostic power of SLR in GC patients, and established an effective predictive nomogram model for GC patients. The nomogram model included age, stage status, and SLR. The C-index of the nomogram model predicted OS with an accuracy of 0.794 (95% CI: 0.749-0.839). Interestingly, it had a better accuracy than the current TNM classification system, which was 0.730 (95% CI: 0.688-0.772). In addition, the nomogram model had a higher overall net benefit than the TNM staging system at 5 years. To our knowledge, this is the first study providing a nomogram based on SLR and clinical characteristics to predict the survival of resectable GC patients. According to the nomogram model scores, GC patients were divided into 3 risk groups. Each group had distinct survival outcomes, with the high-risk group having the shortest OS of the 3 risk groups. Such a nomogram model provided clinicians a consistent and reliable tool to predict outcomes in patients with GC after gastrectomy.
The potential mechanism of how the established nomogram based on SLR can predict patient prognosis can be explained as follows. First, the liver is the main organ involved in metabolism, secretion, and immunity,28,29 and serum AST and ALT activity have long been used as inflammatory markers to evaluate the functional status of liver.30,31 Lin et al. reported that LSR is associated with a strong risk of hepatic steatosis in patients with chronic HCV infction.32 AST and ALT levels reflect hepatic inflammation, which is often related to cancer development.33 Numerous studies had shown that the presence of inflammatory response is linked to poor survival in many cancers.14,34 Moreover, it has been reported that there is increased production of reactive oxygen species (ROS) and DNA damage in patients with liver injury. ROS includes hydrogen peroxide (H2O2), and superoxide and hydroxyl free radicals. Meanwhile, ROS is considered as a toxic product of cellular metabolism, and it has been shown to modify protein activity for cell growth and survival.35,36 DNA damage can lead cell to apoptosis, which is an essential process for programmed cell death in multicellular organisms.37
The established nomogram could predict survival more precisely for resectable GC patients. However, there are still several limitations. First, our study lacks a validation cohort. Second, our study was retrospective, and therefore, we could not avoid potential biases. Third, this is a single-center study with a small sample size of 190 patients. Therefore, our results need to be further verified by multi-center studies that use greater sample sizes. Despite these limitations, this model provided an effective tool for predicting OS of GC patients, and can be helpful when making individualized treatment decision for patients.
Conclusions
In conclusion, we established a nomogram that incorporated age, clinical stage and SLR to predict survival of patients with resected GC. The established nomogram shows better discriminatory ability compared to traditional TNM classification. This model is a simple, precise and easy-to-use scoring system for clinicians to estimate the survival of GC patients.
Acknowledgments
We thank the staff at the Director of Clinical Laboratories, Sun Yat-sen University Cancer Center for providing support on research conditions in this study.
Authors’ Note: Linfang Li, Qiuyao Zeng, Ning Xue, Shan Xing and Shulin Chen contributed equally to this work. This study was approved by the Clinical Research Ethics Committee of the Sun Yat-sen University Cancer Center, and all patients provided written informed consent at the first visit to our center. The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit (RDD) public platform (www.researchdata.org.cn) with the approval RDD number as RDDB2019000518.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Grants No. 81472008 from the National Natural Science Foundation of China.
ORCID iDs: Linfang Li, MD  https://orcid.org/0000-0002-2336-9885
https://orcid.org/0000-0002-2336-9885
Shulin Chen, MD  https://orcid.org/0000-0003-0816-2664
https://orcid.org/0000-0003-0816-2664
References
- 1. Yamaoka Y, Kato M, Asaka M. Geographic differences in gastric cancer incidence can be explained by differences between Helicobacter pylori strains. Intern Med. 2008;47(12):1077–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Takahashi T, Saikawa Y, Kitagawa Y. Gastric cancer: current status of diagnosis and treatment. Cancers (Basel). 2013;5(1):48–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. [DOI] [PubMed] [Google Scholar]
- 4. Noh SH, Park SR, Yang HK, et al. Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(12):1389–1396. [DOI] [PubMed] [Google Scholar]
- 5. Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–4393. [DOI] [PubMed] [Google Scholar]
- 6. Fujitani K, Kurokawa Y, Takeno A, et al. Time to initiation or duration of S-1 adjuvant chemotherapy; which really impacts on survival in stage II and III gastric cancer? Gastric Cancer. 2018;21(3):446–452. [DOI] [PubMed] [Google Scholar]
- 7. Zhang JP, Wang HB, Lin YH, et al. Lactate dehydrogenase is an important prognostic indicator for hepatocellular carcinoma after partial hepatectomy. Transl Oncol. 2015;8(6):497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ishihara H, Kondo T, Yoshida K, et al. Evaluation of preoperative aspartate transaminase/alanine transaminase ratio as an independent predictive biomarker in patients with metastatic renal cell carcinoma undergoing cytoreductive nephrectomy: a propensity score matching study. Clin Genitourin Cancer. 2017;15(5):598–604. [DOI] [PubMed] [Google Scholar]
- 9. Thornburg JM, Nelson KK, Clem BF, et al. Targeting aspartate aminotransferase in breast cancer. Breast Cancer Res. 2008;10(5):R84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bezan A, Mrsic E, Krieger D, et al. The preoperative AST/ALT (De Ritis) ratio represents a poor prognostic factor in a cohort of patients with nonmetastatic renal cell carcinoma. J Urol. 2015;194(1):30–35. [DOI] [PubMed] [Google Scholar]
- 11. Nyblom H, Bjornsson E, Simren M, Aldenborg F, Almer S, Olsson R. The AST/ALT ratio as an indicator of cirrhosis in patients with PBC. Liver Int. 2006;26(7):840–845. [DOI] [PubMed] [Google Scholar]
- 12. Gleeson D, Jones JS, McFarlane E, et al. Severity of alcohol dependence in decompensated alcoholic liver disease: comparison with heavy drinkers without liver disease and relationship to family drinking history. Alcohol Alcohol. 2009;44(4):392–397. [DOI] [PubMed] [Google Scholar]
- 13. Kawamoto R, Kohara K, Kusunoki T, et al. Alanine aminotransferase/aspartate aminotransferase ratio is the best surrogate marker for insulin resistance in non-obese Japanese adults. Cardiovasc Diabetol. 2012;11(1):117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li Y, Jia H, Yu W, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer. 2016;139(1):220–231. [DOI] [PubMed] [Google Scholar]
- 15. Zou Q, Li J, Wu D, et al. Nomograms for pre-operative and post-operative prediction of long-term survival of patients who underwent repeat hepatectomy for recurrent hepatocellular carcinoma. Ann Surg Oncol. 2016;23(8):2618–2626. [DOI] [PubMed] [Google Scholar]
- 16. Zeng Q, Xue N, Dai D, et al. A nomogram based on inflammatory factors C-reactive protein and fibrinogen to predict the prognostic value in patients with resected non-small cell lung cancer. J Cancer. 2017;8(5):744–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen SL, Li JP, Li LF, Zeng T, He X. Elevated preoperative serum alanine aminotransferase/aspartate aminotransferase (ALT/AST) ratio is associated with better prognosis in patients undergoing curative treatment for gastric adenocarcinoma. Int J Mol Sci. 2016;17(6):911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kubota T, Ohyama S, Hiki N, Nunobe S, Yamamoto N, Yamaguchi T. Endocrine carcinoma of the stomach: clinicopathological analysis of 27 surgically treated cases in a single institute. Gastric Cancer. 2012;15(3):323–330. [DOI] [PubMed] [Google Scholar]
- 19. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004;10(21):7252–7259. [DOI] [PubMed] [Google Scholar]
- 20. Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–156. [DOI] [PubMed] [Google Scholar]
- 21. Mbulaiteye SM, Hisada M, El-Omar EM. Helicobacter pylori associated global gastric cancer burden. Front Biosci (Landmark Ed). 2009;14:1490–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li X, Fang P, Mai J, Choi ET, Wang H, Yang XF. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J Hematol Oncol. 2013;6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Talley NJ, Zinsmeister AR, Weaver A, et al. Gastric adenocarcinoma and Helicobacter pylori infection. J Natl Cancer Inst. 1991;83(22):1734–1739. [DOI] [PubMed] [Google Scholar]
- 24. Liu X, Wu Z, Lin E, et al. Systemic prognostic score and nomogram based on inflammatory, nutritional and tumor markers predict cancer-specific survival in stage II-III gastric cancer patients with adjuvant chemotherapy. Clin Nutr. 2019;38(4):1853–1860. [DOI] [PubMed] [Google Scholar]
- 25. Albert JM, Liu DD, Shen Y, et al. Nomogram to predict the benefit of radiation for older patients with breast cancer treated with conservative surgery. J Clin Oncol. 2012;30(23):2837–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Valentini V, van Stiphout RG, Lammering G, et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29(23):3163–3172. [DOI] [PubMed] [Google Scholar]
- 27. Sternberg CN. Are nomograms better than currently available stage groupings for bladder cancer? J Clin Oncol. 2006;24(24):3819–3820. [DOI] [PubMed] [Google Scholar]
- 28. Adesanoye OA, Farombi EO. Hepatoprotective effects of Vernonia amygdalina (astereaceae) in rats treated with carbon tetrachloride. Exp Toxicol Pathol. 2010;62(2):197–206. [DOI] [PubMed] [Google Scholar]
- 29. Wolf PL. Biochemical diagnosis of liver disease. Indian J Clin Biochem. 1999;14(1):59–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Frederiks WM, Vogels IM, Fronik GM. Plasma ornithine carbamyl transferase level as an indicator of ischaemic injury of rat liver. Cell Biochem Funct. 1984;2(4):217–220. [DOI] [PubMed] [Google Scholar]
- 31. Drotman RB, Lawhorn GT. Serum enzymes as indicators of chemically induced liver damage. Drug Chem Toxicol. 1978;1(2):163–171. [DOI] [PubMed] [Google Scholar]
- 32. Lin MS, Lin HS, Chung CM, et al. Serum aminotransferase ratio is independently correlated with hepatosteatosis in patients with HCV: a cross-sectional observational study. BMJ Open. 2015;5(9):e008797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Czauderna C, Castven D, Mahn FL, Marquardt JU. Context-dependent role of NF-kappaB signaling in primary liver cancer-from tumor development to therapeutic implications. Cancers (Basel). 2019;11(8):1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Toiyama Y, Inoue Y, Saigusa S, et al. C-reactive protein as predictor of recurrence in patients with rectal cancer undergoing chemoradiotherapy followed by surgery. Anticancer Res. 2013;33(11):5065–5074. [PubMed] [Google Scholar]
- 35. Concepcion Navarro M, Pilar Montilla M, Martin A, Jiménez J, Pilar Utrilla M. Free radical scavenger and antihepatotoxic activity of rosmarinus tomentosus. Planta Med. 1993;59(4):312–314. [DOI] [PubMed] [Google Scholar]
- 36. AbdulSalam SF, Thowfeik FS, Merino EJ. Excessive reactive oxygen species and exotic DNA lesions as an exploitable liability. Biochemistry. 2016;55(38):5341–5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fulda S, Gorman AM, Hori O, Samali A. Cellular stress responses: cell survival and cell death. Int J Cell Biol. 2010;2010:214074. [DOI] [PMC free article] [PubMed] [Google Scholar]