Abstract
Objective:
Test the extent to which pregnancy per- and polyfluoroalkyl substance (PFAS) concentrations were associated with gestational weight gain and postpartum weight changes.
Methods:
We studied 1614 women recruited 1999-2002 in the Project Viva cohort with pregnancy plasma concentrations of six PFAS, including perfluorooctanesulfonic acid (PFOS), perfluorooctanoic acid (PFOA), and 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid (EtFOSAA). We defined gestational weight gain as the difference between last pregnancy weight and pre-pregnancy weight; 1-year postpartum weight retention as the difference between 1-year postpartum weight and pre-pregnancy weight; and 3-year postpartum weight change as the difference between 3-years postpartum weight and pre-pregnancy weight.
Results:
During pregnancy, women gained 0.37 kg (95%CI: 0.11, 0.62) more weight per doubling of EtFOSAA. At 1-year postpartum, women retained 0.55 kg (95%CI: 0.07, 1.04) more weight per doubling of PFOA. At 3-years postpartum, women gained 0.91 kg (95%CI: 0.25, 1.56) more weight per doubling in PFOA. Findings were similar after adjustment for all PFAS. Other PFAS were not associated with weight changes. Postpartum associations were stronger among women with higher pre-pregnancy BMI. Models were adjusted for demographics.
Conclusions:
Pregnancy PFAS were associated with greater gestational weight gain, weight retention, and weight gain years after pregnancy.
Keywords: PFAS, environmental exposure, gestational weight gain, postpartum weight retention, weight gain
Introduction
Excessive gestational weight gain and postpartum weight retention can have short- and long-term health effects in women. In the short-term, excessive gestational weight gain is linked to preterm birth, Cesarean section delivery [1], postpartum weight retention, and weight gain [2 3]. In the long-term, these factors are associated with adverse cardiometabolic health [2–4]. Though an increasing body of evidence links environmental chemical exposures to weight gain and greater adiposity in non-pregnant populations [5], few studies have evaluated the extent to which chemical exposures may affect maternal weight change during and after pregnancy.
Per- and polyfluoroalkyl substances (PFAS) are environmental chemicals with potentially obesogenic effects that have been manufactured since the 1950s [6 7]. PFAS are used in a variety of products, including food packaging, stain- and water-resistant fabrics, personal care products, and cooking equipment [8–10]. U.S. adults are primarily exposed through ingestion of contaminated food, water, and indoor dust [11]. PFOS and PFOA have multi-year half-lives in humans [12]. Despite a voluntary U.S. phase-out of PFOS and PFOA beginning in 2000 [13], PFOS and PFOA continue to be ubiquitously detected in the U.S. population [14].
A large body of evidence links prenatal PFAS exposure to weight gain and adiposity in children [15–18], but evidence for PFAS exposure and weight gain in adults is mixed. In nonpregnant adults, prospective studies from the Diabetes Prevention Program Outcomes Study (DPPOS) and the Preventing Overweight Using Novel Dietary Strategies (POUNDS Lost) clinical trial reported positive associations of some PFAS with weight change [19 20], though several cross-sectional studies reported inverse or null associations [21–23]. Three studies have evaluated PFAS and weight gain during pregnancy; all reported a positive association only among women with normal pre-pregnancy body mass index (BMI) [24–26]. Previous studies have not evaluated associations between PFAS and weight changes following pregnancy, a time of rapid metabolic change.
Because PFAS exposure might be modifiable and may affect weight gain, it is important to understand the association between PFAS and weight gain in pregnancy and postpartum. In this study, we used longitudinal data [27] to evaluate associations of first trimester PFAS plasma concentrations with gestational weight gain, 1-year postpartum weight retention, and 3-year postpartum weight change. We hypothesized that higher PFAS plasma concentrations would be associated with higher gestational weight gain, greater 1-year postpartum weight retention, and more weight gain at 3 years postpartum.
Methods
Project Viva is a prospective pre-birth cohort that recruited women between 1999–2002 at their first prenatal visit from Atrius Harvard Vanguard Medical Associates, a multi-specialty group practice in eastern Massachusetts [27]. Women were English-speaking, pregnant with a single fetus <22 weeks’ gestation at recruitment, and planned to deliver in eastern Massachusetts. All participants provided written informed consent, and the human subjects committee of Harvard Pilgrim Health Care approved all procedures. Participants completed study visits in the first and second trimesters, at delivery, and at three years postpartum, and completed interviews and questionnaires at each visit. They also completed mailed questionnaires at 1 and 2 years after delivery. Twenty-eight women participated in Project Viva for two pregnancies; we limited the analysis dataset to the first eligible pregnancy for each participant. Of 2100 live births, we excluded pregnancies without plasma PFAS measurements (n=472), as well as those with prepregnancy type 1 or type 2 diabetes (n=14), leaving 1614 participants at baseline.
We collected blood samples in early pregnancy (median 9.7 weeks; range 4.8-21.4 weeks) and stored plasma in non-PFAS-containing cryovials in liquid nitrogen freezers at ≤ - 130°C until analysis. We quantified plasma concentrations of seven PFAS using online solid-phase extraction coupled with isotope dilution high-performance liquid chromatography-tandem mass spectrometry at the Centers for Disease Control and Prevention (CDC). The PFAS were: perfluorooctanoic acid (PFOA), perfluorooctane sulfonic acid (PFOS), perfluorononanoic acid (PFNA), perfluorohexane sulfonic acid (PFHxS), perfluorodecanoic acid (PFDA), 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid (EtFOSAA), and 2-(A-methyl-perfluorooctane sulfonamido) acetic acid (MeFOSAA). Limits of detection (LOD) were 0.2 ng/mL for PFOS and 0.1 ng/mL for all other PFAS. All PFAS except PFDA were detected in over 98% of samples. PFDA was detected in 45.1% of samples; we excluded it because of relatively low detection frequency. We imputed values below the LOD (<1% samples) using LOD/√2. The analysis of deidentified samples at the CDC laboratory did not constitute engagement in human subjects research.
Participants self-reported pre-pregnancy weight at enrollment. We previously found self-reported and clinically measured pre-pregnancy weight to be highly correlated (r=0.997) among a validation subset of participants (n=343) [28], We used clinically measured weights (median 13; range 3-28) to calculate weight gain during pregnancy. Pregnancy days were counted from date of last menstrual period. We defined first trimester weight gain as the difference in weight between pregnancy day 91 and pre-pregnancy, second trimester weight gain as the difference in weight between pregnancy day 182 and pregnancy day 91, and third trimester weight gain as the difference between the final clinically measured weight during pregnancy (within 4 weeks of delivery) and weight at pregnancy day 182. To estimate weight at pregnancy days 91 and 182, we used linear interpolation between the two weight measurements closest to the dates [3]. We calculated trimester-specific rate of gestational weight gain (kg/wk) by dividing weight gain by number of weeks in each trimester. We defined total gestational weight gain as the difference between the last clinically measured weight during pregnancy (within 4 weeks of delivery) and pre-pregnancy weight, and total rate of gestational weight gain by dividing total gestational weight gain by the total weeks of pregnancy. We also categorized adequacy of gestational weight according to the Institute of Medicine 2009 guidelines, which vary by pre-pregnancy BMI [29].
At one year postpartum, participants self-reported weight on a mailed questionnaire. We defined 1-year postpartum weight retention as the difference in weight between 1-year postpartum and pre-pregnancy. At three years postpartum, research assistants (RAs) weighed participants (fully clothed without shoes) using a Seca scale and recorded weights in kilograms to two decimal places. Women also self-reported weight on a questionnaire. The correlation between self-reported and RA-measured 3-year postpartum weight among participants with both measurements was 0.99; self-reported weights were approximately 0.77 kg lighter than RA-measured weight (women with higher pre-pregnancy BMIs underreported their weight slightly more). For participants with a self-reported weight but no RA-measured weight (79 of 883 [5.6%] participants), we predicted the participant’s RA-measured weight (RA-measured weight (kg) = −0.93341 + 0.99627*self-reported weight (kg) + 0.07942*BMI (kg/m2)). We defined 3-year postpartum weight change as the difference between weight at 3-years postpartum (using the 3-year RA measured weight, or predicted weight for participants with only self-reported weight [described above]) and pre-pregnancy weight.
We set 1-year postpartum weight values to missing if the participant was pregnant at the assessment or had another baby since the index pregnancy (n=96). We set 3-year postpartum weight values to missing if the participant was pregnant at the assessment or if she had been pregnant within the last 6 months (n=192). Sample sizes in adjusted models were n=1568 for gestational weight gain, n=860 for 1-year postpartum weight retention, and n=871 for 3-year postpartum weight change.
Statistical analysis
We examined the association of early pregnancy PFAS plasma concentrations with gestational weight gain (continuous and categorical), 1-year postpartum weight retention (continuous and categorical), and 3-year postpartum weight change (continuous). We log2-transformed PFAS plasma concentrations and present effect estimates per doubling of PFAS concentrations.
At recruitment, participants reported maternal race/ethnicity, age, education, parity, marital status, household income, and smoking status. We calculated pre-pregnancy BMI using self-reported pre-pregnancy weight and height (weight (kg)/height(m)2). We adjusted all models for age, continuous pre-pregnancy BMI, marital status (married or cohabitating versus not), race/ethnicity (black, white, or other), education (college graduate or more versus less than college graduate), annual household income (>$70,000 versus ≤$70,000), smoking (current, former, or never), and parity (0, 1, or >1). We chose covariates a priori as likely confounders based on prior literature [30 31], Because previous studies have indicated that the association between PFAS and gestational weight gain is modified by pre-pregnancy BMI [24 25], we also tested multiplicative interactions for categorical pre-pregnancy BMI (<25.0, 25.0-<30.0, ≥ 30.0 kg/m2) for all outcomes.
We used multivariable linear regression to evaluate the associations of plasma PFAS concentrations with trimester-specific and total gestational weight gain. We used multinomial regression to estimate the odds of inadequate and excessive gestational weight gain.
We used multivariable linear regression to evaluate the associations of PFAS concentrations with 1-year postpartum weight retention and 3-year postpartum weight change. We used logistic regression to estimate odds of substantial 1-year postpartum weight retention (defined as retaining ≥ 5 kg at 1-year postpartum, based on a cutoff commonly used in prior literature as well as prior analyses in this cohort) [32],
In 1- and 3-year postpartum models, we used stabilized inverse probability (IP) of censoring weights to correct for non-random loss to follow-up resulting in potential selection bias [33], We weighted participants who were not lost to follow-up based on their demographic and clinical characteristics to ensure that the analytic population continued to represent the baseline population. We calculated IP weights separately for each PFAS model.
Because some participants (12%) were missing covariates, we used chained equations in SAS PROC MI to multiply impute missing covariates. We generated fifty imputed datasets and combined them to generate results. Analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Because PFAS occur in the environment as correlated, complex mixtures, modeling the effects of exposure to one PFAS at a time may not accurately characterize each chemical’s independent effect. As a complement to traditional modeling approaches, we additionally used Bayesian Kernel Machine Regression (BKMR) in a singly imputed dataset. This method assesses the effects of exposure to both the overall PFAS mixture, as well as each individual chemical in the mixture, taking into account correlations and interactions. The BKMR model is: Yi = h (PFOSi, PFOAi, PFHxSi, PFNAi, EtFOSAAi, MeFOSAAi) +αXi+ εi, where h(PFAS) is a flexible Gaussian kernel function and X=X1, …, Xn are n potential confounders [34], BKMR analyses were conducted using a single imputation without variable selection in R version 3.5.1.
Sensitivity analyses
To ensure gestational weight gain occurred after the exposure measurement, we modeled associations with gestational weight gain excluding the first trimester. Also, to ensure any observed association with gestational weight gain was not due to effects on gestational length, we modeled the average rate of gestational weight gain over the whole pregnancy. To evaluate confounding from other PFAS, we included all PFAS in a single model. Finally, we repeated analyses without IP weights.
Results
Most participants in the eligible population (n=1614) were white (68%) and between 25 and 35 years old (63%). The majority had pre-pregnancy BMI <25.0 kg/m2 (62%), were married or cohabitating (91%), never smoked (68%), were college educated (64%), and had household incomes >$70,000 per year (58%) (Table 1). Compared to those excluded, the subset of the cohort eligible for this analysis (described above) was more likely to be white (68% versus 60%). Imputations did not affect the distribution of covariates in the study population (Supplemental Table S1). Spearman correlation coefficients between the six PFAS ranged from 0.19 to 0.71; most correlations were between 0.2 and 0.5 (Table 2).
Table 1.
Baseline demographics (imputed to n=1614)1
| Characteristic | Study population n(%) |
|---|---|
| Age at enrollment | |
| Mean (SD) age (years) | 31.8 (5.2) |
| <25 years | 153 (9.5) |
| 25 - <30 years | 348 (21.6) |
| 30 - <35 years | 665 (41.2) |
| ≥ 35 years | 448 (27.8) |
| Year of enrollment | |
| 1999 | 405 (25.1) |
| 2000 | 597 (37.0) |
| 2001 | 559 (34.6) |
| 2002 | 53 (3.3) |
| Pre-pregnancy body mass index | |
| Median (IQR) BMI (kg/m2) | 23.6(21.2 - 27.5) |
| <25.0 kg/m2 | 993 (61.5) |
| 25.0 - <30.0 kg/m2 | 359 (22.3) |
| ≥ 30.0 kg/m2 | 262 (16.2) |
| Race/ethnicity | |
| White | 1104 (68.4) |
| Black | 254 (15.7) |
| Other | 256 (15.8) |
| Parity | |
| 0 | 794 (49.2) |
| 1 | 559 (34.6) |
| >1 | 261 (16.2) |
| Education | |
| < College graduate | 574 (35.6) |
| College graduate or more | 1040 (64.4) |
| Married or cohabitating | |
| No | 143 (8.9) |
| Yes | 1471 (91.1) |
| Smoking status | |
| Smoked during pregnancy | 212 (13.2) |
| Former | 300 (18.6) |
| Never | 1101 (68.2) |
| Annual household income | |
| ≤ $70,000/year | 687 (42.5) |
| >$70,000/year | 927 (57.5) |
Calculated from all imputations and Ns are rounded to the nearest integer; values may not sum to 1614.
Table 2.
PFAS plasma concentrations (ng/mL) and Spearman correlations between PFASs measured in Project Viva participants (n=1614)
| PFAS | % >LOD | Geometric mean | 25% | median | 75% | 95% |
|---|---|---|---|---|---|---|
| PFOS | 99.8 | 25.4 | 18.8 | 25.7 | 34.9 | 57.3 |
| PFOA | 100 | 5.7 | 4.2 | 5.9 | 7.9 | 12.1 |
| PFHxS | 99.3 | 2.5 | 1.6 | 2.5 | 3.8 | 9.1 |
| PFNA | 98.6 | 0.7 | 0.5 | 0.7 | 0.9 | 1.5 |
| EtFOSAA | 99.7 | 1.2 | 0.7 | 1.2 | 1.9 | 4.3 |
| MeFOSAA | 100 | 1.9 | 1.3 | 1.9 | 3.2 | 5.6 |
| Correlation coefficients | ||||||
| PFOS | PFOA | PFHxS | PFNA | EtFOSAA | MeFOSAA | |
| PFOS | 1.00 | |||||
| PFOA | 0.71 | 1.00 | ||||
| PFHxS | 0.50 | 0.52 | 1.00 | |||
| PFNA | 0.60 | 0.52 | 0.42 | 1.00 | ||
| EtFOSAA | 0.52 | 0.39 | 0.21 | 0.19 | 1.00 | |
| MeFOSAA | 0.40 | 0.37 | 0.23 | 0.23 | 0.40 | 1.00 |
LOD: limit of detection (0.2 ng/mL for PFOS and 0.1 ng/mL for all other PFAS chemicals). Concentrations below the limit of detection were imputed as LOD/√2).
PFOA: perfluorooctanoic acid; PFOS: perfluorooctanesulfonic acid; PFNA: perfluorononanoic acid; PFHxS: perfluorohexane sulfonic acid; EtFOSAA: 2-(N-ethyl-perfluorooctane sulfonamido) acetic acid; MEFOSAA: 2-(N-methyl-perfluorooctane sulfonamido) acetic acid
Gestational weight gain
Mean gestational weight gain was 15.7 kg (SD=5.7 kg), and 60% of participants gained excessive weight. Plasma PFAS concentrations were not associated with first trimester weight gain (which preceded blood collection for PFAS measurement for 87% of participants). However, each doubling of EtFOSAA concentrations was associated with 0.02 kg (95% CI: 0.01, 0.02) more weight gain per week in the second trimester, 0.01 kg (95% CI: 0.00, 0.02) more weight gain per week in the third trimester, and 0.37 kg (95% CI: 0.11, 0.62) more total weight gain (Figure 1; Supplemental Table S2; Table 3). Higher PFAS concentrations were associated with lower odds of inadequate (versus adequate) total gestational weight gain (PFOS adjusted odds ratio [aOR]: 0.76 [95% CI: 0.62, 0.94]; PFOA aOR: 0.76 [95% CI: 0.59, 0.98]; PFHxS aOR: 0.84 [95%CI: 0.71, 0.99]; PFNA aOR: 0.80 [95% CI: 0.65, 1.00], per doubling of PFAS), but odds of gaining excessive (versus adequate) weight in pregnancy were similar regardless of participants’ PFAS plasma concentrations. Other PFAS were not associated with gestational weight gain. Pre-pregnancy BMI did not significantly modify associations between any PFAS and gestational weight gain (Figure 2).
Figure 1.
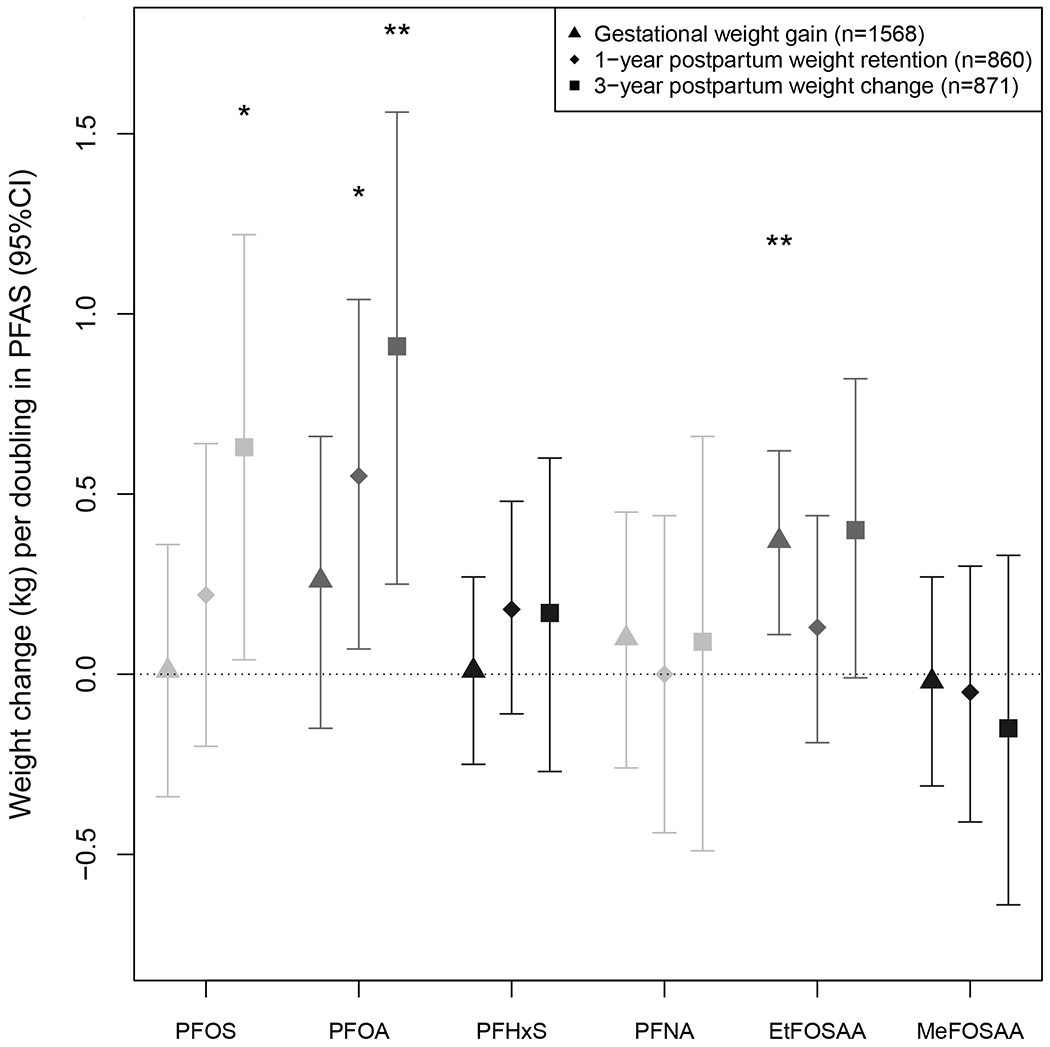
Weight change (kg) per doubling in PFAS plasma concentrations during pregnancy (gestational weight gain) and 1- and 3-years postpartum. All estimates were adjusted for age, pre-pregnancy BMI, marital status, race/ethnicity, education, household income, smoking, and parity. One-year postpartum weight retention and 3-year postpartum weight change estimates were IP-weighted to account for differential loss to follow up. Plotted values listed in Table 3. *P < 0.05; **P < 0.01.
Figure 2.
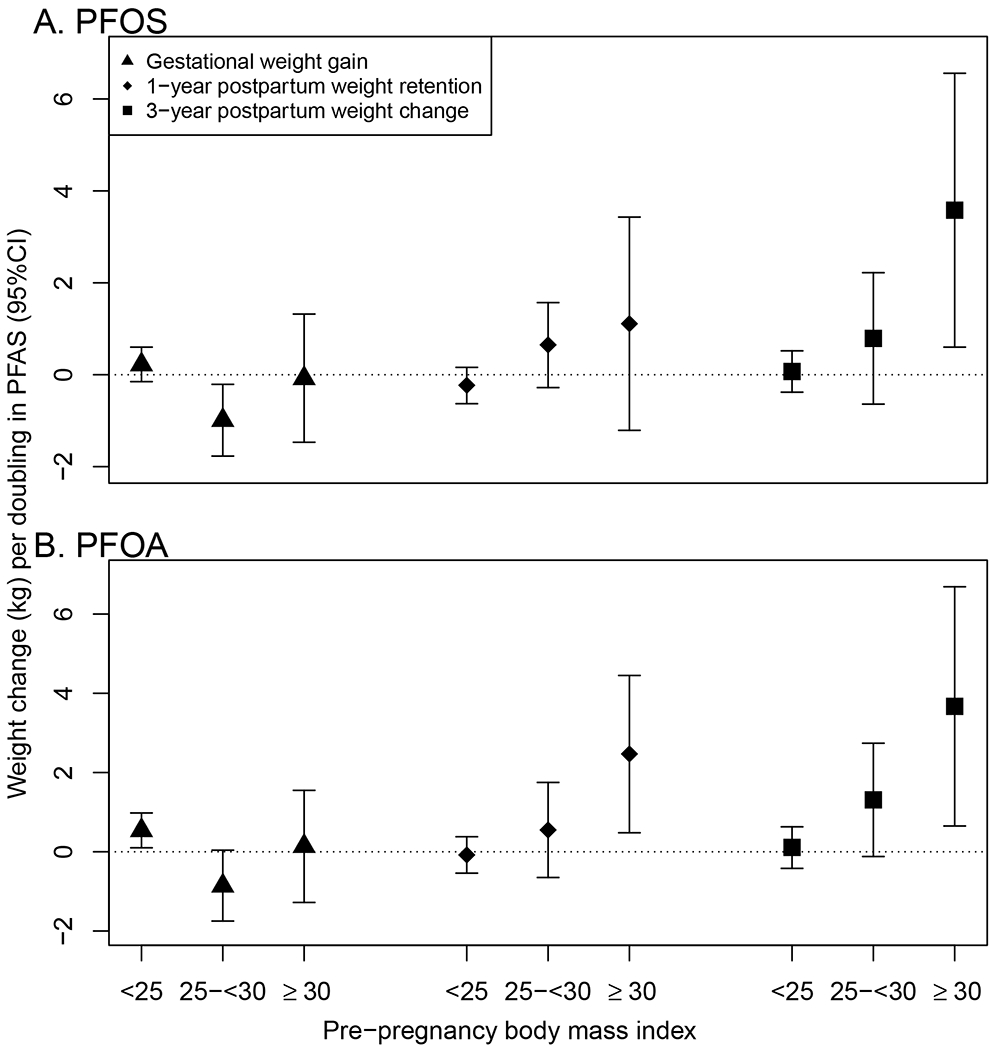
Weight change (kg) per doubling in PFAS plasma concentrations during pregnancy (gestational weight gain) and 1- and 3-years postpartum, stratified by pre-pregnancy body mass index (BMI) category. Part A. shows PFOS and part B. shows PFOA. All estimates were adjusted for age, pre-pregnancy BMI, marital status, race/ethnicity, education, household income, smoking, and parity. One-year postpartum weight retention and 3-year postpartum weight change estimates were IP-weighted to account for differential loss to follow up.
In the PFAS mixture analyses, higher EtFOSAA concentrations were associated with more total gestational weight gain, and higher PFOS concentrations were associated with less total gestational weight gain (Supplemental Figure S1, part A); EtFOSAA was significantly associated with greater total gestational weight gain when concentrations of other PFAS were above the median (Supplemental Figure S1, part D). The BKMR model also suggested a potential interaction between PFOA and EtFOSAA (Supplemental Figure S1, part B). Despite significant associations with EtFOSAA, higher concentrations of the PFAS mixture were non-significantly associated with greater gestational weight gain (Supplemental Figure S1, part C). In a follow-up linear model containing all PFAS and a PFOA x EtFOSAA product term, there was a significant inverse association between PFOS and gestational weight gain and a positive interaction (p=0.089) between PFOA and EtFOSAA (Table 3).
Table 3.
Change in gestational weight gain, 1-year postpartum weight retention, and 3-year postpartum weight change per doubling in plasma PFAS concentration (ng/mL) from individual PFAS models; models containing all PFAS; and models containing all PFAS as well as any product terms suggested by BKMR.
| Total gestational weight gain (kg)1 (n=1568) | |||
|---|---|---|---|
| Single PFAS | All PFAS | All PFAS, product terms | |
| PFAS | β (95% CI) | β (95% CI) | β (95% CI) |
| PFOS | 0.01 (−0.34, 0.36) | −0.52 (−1.05, 0.01) | −0.55 (−1.09, −0.02)* |
| PFOA | 0.26 (−0.15, 0.66) | 0.34 (−0.26, 0.94) | 0.28 (−0.32, 0.88) |
| PFHxS | 0.01 (−0.25, 0.27) | −0.03 (−0.32, 0.27) | −0.00 (−0.30, 0.29) |
| PFNA | 0.10 (−0.26, 0.45) | 0.15 (−0.31, 0.61) | 0.19 (−0.27, 0.65) |
| EtFOSAA | 0.37(0.11, 0.62)** | 0.52 (0.20, 0.83)** | −0.08 (−0.83, 0.68) |
| MeFOSAA | −0.02 (−0.31, 0.27) | −0.21 (−0.54, 0.12) | −0.19 (−0.52, 0.14) |
| PFOA*EtFOSAA | -- | -- | 0.23 (−0.04, 0.50) |
|
1-year postpartum weight retention (kg)2 (n=860) | |||
| Single PFAS | All PFAS | All PFAS, product terms | |
| PFAS | β (95% CI) | β (95% CI) | β (95% CI) |
| PFOS | 0.22 (−0.20, 0.64) | −0.01 (−0.60, 0.58) | 0.27 (−1.05, 1.60) |
| PFOA | 0.55 (0.07, 1.04)* | 0.84 (0.12, 1.57)* | 1.35 (−0.86, 3.57) |
| PFHxS | 0.18 (−0.11, 0.48) | 0.10 (−0.24, 0.45) | 0.10 (−0.24, 0.44) |
| PFNA | 0.00 (−0.44, 0.44) | −0.36 (−0.91, 0.18) | −0.38 (−0.93, 0.17) |
| EtFOSAA | 0.13 (−0.19, 0.44) | 0.01 (−0.37, 0.39) | 0.02 (−0.36, 0.41) |
| MeFOSAA | −0.05 (−0.41, 0.30) | −0.23 (−0.63, 0.17) | −0.24 (−0.64, 0.16) |
| PFOS*PFOA | -- | -- | −0.11 (−0.58, 0.35) |
| 3-year postpartum weight change (kg)2 (n=871) | |||
| Single PFAS | All PFAS | All PFAS, product terms3 | |
| PFAS | β (95% CI) | β (95% CI) | β (95% CI) |
| PFOS | 0.63 (0.04, 1.22)* | 0.18 (−0.63, 1.00) | 0.18 (−0.63, 1.00) |
| PFOA | 0.91 (0.25, 1.56)** | 1.33 (0.33, 2.33)** | 1.33 (0.33, 2.33)** |
| PFHxS | 0.17 (−0.27, 0.60) | −0.03 (−0.53, 0.47) | −0.03 (−0.53, 0.47) |
| PFNA | 0.09 (−0.49, 0.66) | −0.46 (−1.20, 0.28) | −0.46 (−1.20, 0.28) |
| EtFOSAA | 0.40 (−0.01, 0.82) | 0.25 (−0.27, 0.76) | 0.25 (−0.27, 0.76) |
| MeFOSAA | −0.15 (−0.64, 0.33) | −0.64 (−1.21, −0.08)* | −0.64 (−1.21, −0.08)* |
P< 0.05;
P< 0.01
Models adjusted for age, pre-pregnancy BMI, marital status, race ethnicity, education, income smoking, and parity
Models adjusted for age, pre-pregnancy BMI, marital status, race ethnicity, education, income smoking, and parity, and additionally IP weighted to account for differential loss to follow-up
Parameter estimates are identical to “All PFAS” column because BKMR models did not suggest any interactions.
One-year postpartum weight retention
Mean 1-year postpartum weight retention was 0.8 kg (SD=4.9); 13.5% of women had substantial 1-year postpartum weight retention ( ≥ 5 kg). Each doubling of PFOA was associated with 0.55 kg (95% CF 0.07, 1.04) more weight retention at 1-year postpartum (Figure 1; Table 3). Certain PFAS concentrations were associated with greater odds of retaining substantial weight at 1-year postpartum (PFOS aOR: 1.25 [95% CI: 0.94, 1.66]; PFOA aOR: 1.59 [95% CI: 1.17, 2.16]; EtFOSAA aOR: 1.10 [95% CI: 0.91, 1.34], per doubling of PFAS plasma concentrations). Other PFAS were not associated with 1-year postpartum weight retention (Table 3). Pre-pregnancy BMI significantly modified the associations of 1-year postpartum weight retention with PFOS (p=0.022) and PFOA (p=0.009); associations were stronger among women with higher prepregnancy BMI (Figure 2). For example, each doubling in PFOA was associated with retaining 0.08 kg (95%CI: −0.54, 0.38) less weight at 1-year postpartum among women with prepregnancy BMI <25.0 kg/m2, 0.55 kg (95%CI: −0.65, 1.75) more weight among women with prepregnancy BMI 25.0-<30.0 kg/m2, and 2.47 kg (95%CI: 0.48, 4.45) more weight among women with pre-pregnancy BMI ≥ 30.0 kg/m2. Applied to the observed range of PFOA concentrations, among women with pre-pregnancy BMI ≥ 30.0 kg/m2, those with the highest PFOA concentrations would retain on average about 10 kg more than those with the lowest PFOA concentrations.
In the PFAS mixture analyses, higher plasma concentrations of PFOA were associated with retaining more weight, and higher concentrations of PFNA were associated with retaining less weight, though associations were not statistically significant (Supplemental Figure S2, part A, D). BKMR models also suggested a potential interaction between PFOS and PFOA (Supplemental Figure S2, part B). Higher concentrations of the entire PFAS mixture were non-significantly associated with greater 1-year postpartum weight retention (Supplemental Figure S2, part C). A follow-up IP-weighted linear model containing all PFAS and a product term between PFHxS and PFNA confirmed the direction of associations of PFOA and PFNA seen in BKMR models (Table 3).
Three-year postpartum weight change
On average, participants gained 2.7 kg (SD=6.7) at three years postpartum; 28% of women weighed at least 5 kg more than they had before pregnancy, and 10.5% women weighed at least 10 kg more. Certain PFAS concentrations were positively associated with greater weight gain at three years postpartum (0.63 kg [95% CI: 0.04, 1.22] more weight per doubling in PFOS, 0.91 kg [95% CI: 0.25, 1.56] more weight per doubling in PFOA, and 0.40 kg (95% CI: −0.01, 0.82) more weight per doubling in EtFOSAA) (Figure 1; Table 3). Other PFAS were not associated with 3-year weight change. Pre-pregnancy categorical BMI significantly modified the associations between 3-year postpartum weight change and PFOS (p=0.008) and PFOA (p=0.01); associations were stronger among women with higher pre-pregnancy BMI (Figure 2). For example, each doubling in PFOS was associated with gaining 0.07 kg (95% CI: −0.38, 0.52) more weight among women with pre-pregnancy BMI <25.0 kg/m2, 0.79 kg (95% CI: −0.64, 2.22) more weight among women with pre-pregnancy BMI 25.0-<30.0 kg/m2, and 3.58 kg (95% CI: 0.60, 6.56) more weight among women with pre-pregnancy BMI ≥ 30.0 kg/m2. Applied to the observed the range of PFOS concentrations, among women with pre-pregnancy BMI ≥ 30.0 kg/m2, those with high PFOS concentrations would gain on average about 15 kg more than those with low PFOS concentrations.
At three years postpartum, the PFAS mixtures analysis suggested that higher concentrations of PFOS and PFOA were associated with more weight gain, and higher concentrations PFNA and MeFOSAA were associated with less weight gain, though associations were not statistically significant (Supplemental Figure S3, part A, D). The BKMR model did not indicate any potential interactions (Supplemental Figure S3, part B). Higher concentrations of the entire PFAS mixture were not significantly associated with 3-year postpartum weight change (Supplemental Figure S3, part C). In a follow-up IP-weighted linear model containing all PFAS as linear terms, PFOA was positively associated, MeFOSAA was inversely associated, and PFOS was no longer significantly associated with three-year weight gain (Table 3).
Secondary and sensitivity analyses
Gestational weight gain models excluding first trimester weight gain, and modeling rate of total gestational weight gain, produced results similar to the primary analysis. Similarly, models including all PFAS (Table 3) and models without IP weights (not shown) were similar to the primary analysis.
Discussion
In a large cohort, early pregnancy PFAS plasma concentrations were associated with gestational weight gain, 1-year postpartum weight retention, and 3-year postpartum weight change. Specifically, EtFOSAA was associated with gestational weight gain; PFOA was associated with 1-year postpartum weight retention; and both PFOS and PFOA were associated with 3-year postpartum weight gain (though PFOS was not statistically significantly associated with 3-year postpartum weight gain after adjustment for other PFAS). We also found that associations of PFAS concentrations with postpartum weight changes were most pronounced among women who had pre-pregnancy BMI >25 kg/m2. To our knowledge, ours is the first study to report that pregnancy PFAS concentrations are associated with weight both during and after pregnancy.
Our findings of an association between plasma PFAS concentrations and gestational weight gain are broadly consistent with prior studies. Three prior studies of this association reported no overall effect of PFASs on gestational weight gain, but positive associations with gestational weight gain among women with normal pre-pregnancy BMI [24–26]. Similarly, in our study, PFOA predicted significantly higher gestational weight gain only among women with normal pre-pregnancy BMI. Unlike prior studies, EtFOSAA was significantly associated with higher gestational weight gain in our sample. EtFOSAA is an oxidation product of a compound largely found in paper and packaging protectors [35]. During the years of Viva recruitment (1999-2002), other studies detected EtFOSAA in high-fat fast food items like pizza and chicken nuggets, probably due to migration from PFAS-treated oil-resistant wrappers. Consumption of these high-fat foods may partly explain the associations we observed [36]. Though EtFOSAA has not been previously associated with weight gain or adiposity, a recent analysis reported an association between EtFOSAA concentrations and microvascular disease, suggesting EtFOSAA concentrations may affect cardiometabolic health [37].
We additionally reported positive associations of PFAS plasma concentrations with 1-year postpartum weight retention and 3-year postpartum weight change. Though no prior studies investigated these associations, the association of PFOA with 1-year postpartum weight retention is similar in magnitude to well-known risk factors. For example, we found the odds of substantial 1-year postpartum weight retention was 1.59 times greater per doubling in PFOA, which is similar to the effect estimate for walking one hour less each day at 6-months postpartum (aOR=1.52) [38], We also saw effect modification by pre-pregnancy BMI on the associations between PFAS and postpartum weight changes, with stronger associations in women with higher pre-pregnancy BMI. Because women with high pre-pregnancy BMI may be particularly susceptible, clinicians might focus PFAS exposure reduction efforts on this group.
Interestingly, we found PFOA to have a stronger effect on maternal weight with increasing time after pregnancy. Other studies of PFAS and adiposity or weight gain, including the DPPOS [20], the POUNDS Lost Trial [19], and the European Youth Heart Study [39] have also reported positive longitudinal associations between certain PFAS and weight gain. It is not clear why associations may strengthen with increasing follow-up time.
There are several potential mechanisms through which PFAS exposure may be associated with increased adiposity and weight gain. In vitro studies of PFAS show an increase in adipocyte cell number, reduced cell size, and less lipid accumulation in differentiating adipocytes [40 41], PFAS also activates PPAR-γ and ER-α in mouse and human cell lines and in vivo in mice, indicating that PFAS exposure may lead to increased adiposity and weight gain via estrogenic signaling and lipid metabolism [42–45], The association between PFAS exposure and weight gain reported here is consistent with this potential metabolic disruption pathway.
Findings from the all-PFAS and BKMR models largely reinforced findings from single-pollutant regression models. Single-PFAS, all-PFAS, and BKMR models signaled that EtFOSAA was associated with greater gestational weight gain, PFOA was associated with greater 1-year postpartum weight retention, and PFOA was associated with higher 3-year postpartum weight gain. PFOS was associated with higher 3-year postpartum weight gain in single-PFAS and BKMR models, but not in all-PFAS models. These consistent findings in single- and all-PFAS models, and qualitatively similar findings from the BKMR models, suggest that individual PFAS did not strongly confound one another’s effects. We used follow-up linear models to quantify interactions suggested by BKMR; the inclusion of product terms in these models reduced power (especially for main effects of PFAS that were part of the product term) but the direction of the associations appeared mostly similar. While the beta coefficient for EtFOSAA changed from 0.52 (0.20, 0.83) to −0.08 (−0.83, 0.68) in the model with a PFOA x EtFOSAA product term, the EtFOSAA-total gestational weight gain association conditional on PFOA was positive for all observed values of PFOA above the minimum (0.3 ng/mL). BKMR models, shown in Supplemental Figure S1B, also indicate that positive associations of EtFOSAA with total gestational weight gain are stronger at higher PFOA levels. Based on BKMR modeling, concentrations of the PFAS mixture as a whole were not significantly associated with weight change in pregnancy or postpartum, indicating that observed effects are largely due to individual PFAS.
Our analysis was subject to limitations. Self-reported pre-pregnancy and 1-year postpartum weight may be subject to desirability biases, but self-reported weights have been validated in Project Viva [38]. Also, about half of participants were lost to follow-up before 1-year postpartum; we used IP weights to counteract potential bias caused by this loss. Finally, though fast food may be a source of EtFOSAA, we did not adjust for diet.
Our study also had many strengths, including use of a high-quality prospective cohort with plasma PFAS measurements, the gold standard for measuring PFAS body burden. Additionally, we followed participants for multiple years postpartum, including in-person visits with weight measurements. Our study is among the first to model PFAS mixtures using BKMR, allowing us to separate each chemical’s effects while accounting for correlation between chemicals. Finally, PFAS concentrations measured in Project Viva are similar to those reported nationally in the same time frame [30], so findings may be generalizable to the American population.
Conclusions
To our knowledge, this is the first study to test associations between pregnancy PFAS concentrations and maternal weight change across the perinatal period, a marker for long-term cardiometabolic health. In Project Viva, we found that pregnancy concentration of EtFOSAA was associated with gestational weight gain and PFOA was associated with both 1-year postpartum weight retention and greater weight gain at three years postpartum. We observed the strongest postpartum associations among women with high pre-pregnancy BMI, suggesting that this subgroup may be especially vulnerable to long-term adverse health outcomes associated with PFAS exposure. Further research is needed to more fully understand the mechanism connecting PFAS exposure to maternal weight changes. If replicated, incorporating PFAS exposure reduction strategies into clinical care could improve maternal health by reducing substantial weight gain during the perinatal period.
Supplementary Material
Study importance:
What is already known about this subject?
Prenatal PFAS exposure is linked to greater offspring weight gain and adiposity, but evidence for an association between PFAS exposure and weight gain in adulthood is mixed.
Three studies have examined the link between PFAS and gestational weight gain; all found a positive association only among women with normal pre-pregnancy BMI.
No studies have investigated the association between pregnancy PFAS concentrations and 1 year postpartum weight retention or longer-term postpartum weight change.
What does your study add?
Pregnancy plasma concentrations of EtFOSAA were positively associated with gestational weight gain; PFOA was positively associated with gestational weight gain only among women with normal pre-pregnancy BMI.
Pregnancy concentrations of PFOA were associated with greater 1-year postpartum weight retention and more weight gain at three years postpartum.
The strongest associations between PFAS and postpartum weight change were among women with high pre-pregnancy BMI.
How might your results change the direction of research or the focus of clinical practice?
If replicated, incorporating PFAS exposure reduction strategies into clinical care could improve maternal health by reducing substantial weight gain during the perinatal period.
Acknowledgements
We thank Kayoko Kato, Tao Jia, and the late Xiaoyun Ye for technical assistance measuring PFAS concentrations. We thank Louisa H. Smith and Andrea Bellavia for R assistance.
Funding: This work was supported by the National Institutes of Health (T32-ES007069, R01-ES024765, R01-ES030101, K23-ES024803, R01-HD034568, R01-HD096032, and UH3-OD023286).
Footnotes
Disclosure: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or NIH. Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services. The authors declare no competing financial interest and no conflict of interest of any kind.
Data Sharing
Data collection instruments are publicly available. Deidentified participant data will be shared with investigators for specific analyses approved by Project Viva co-investigators. Project Viva policies can be found at https://www.hms.harvard.edu/viva/policies-for-using-our-data.pdf.
References
- 1.Goldstein RF, Abell SK, Ranasinha S, et al. Association of gestational weight gain with maternal and infant outcomes: A systematic review and meta-analysis. JAMA 2017;317(21):2207–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rooney BL, Schauberger CW. Excess pregnancy weight gain and long-term obesity: one decade later. Obstet Gynecol 2002;100:245–52. [DOI] [PubMed] [Google Scholar]
- 3.Walter JR, Perng W, Kleinman KP, Rifas-Shiman SL, Rich-Edwards JW, Oken E. Associations of trimester-specific gestational weight gain with maternal adiposity and systolic blood pressure at 3 and 7 years postpartum. Am J Obstet Gynecol 2015;212(4):499 e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kew S, Ye C, Hanley AJ, et al. Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes Care 2014;37(7):1998–2006. [DOI] [PubMed] [Google Scholar]
- 5.Darbre PD. Endocrine disruptors and obesity. Current Obesity Reports 2017;6(1):18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Petrakis D, Vassilopoulou L, Mamoulakis C, et al. Endocrine disrupotrs leading to obesity and related dieases. Int J Environ Res Public Health 2017;14(10):1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Z, Cousins IT, Schreinger M, Buck RC, Hungerbuhler K. Global emission inventories for C4-C14 perfluoroalkyl carboxylic acid (PFCA) homologues from 1951 to 2030, Part I: production and emissions from quantifiable sources. Environment International 2014;70:62–75. [DOI] [PubMed] [Google Scholar]
- 8.Kotthoff M, Müller J, Jurling H, Schlummer M, Fiedler D. Perfluoroalkyl and polyfluoroalkyl substances in consumer products. Environ Sci Pollut Res 2015;22:14546–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EPA. Basic Information on PFAS. 2018. https://www.epa.gov/pfas/basic-information-pfas.
- 10.ATSDR. Per- and polyfluoroalkyl substances (PFAS) and your health. 2018. https://www.atsdr.cdc.gov/pfas/pfas-exposure.html.
- 11.Egeghy PP, Lorber M. An assessment of the exposure of Americans to perfluorooctane sulfonate: A comparison of estimated intake with values inferred from NHANES data. Journal of Exposure Science & Environmental Epidemiology 2010;21(2):150–68. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Beesoon S, Zhu L, Martin JW. Biomonitoring of perfluoroalkyl acids in human urine and estimates of biological half-life. Environ Sci Technol 2013;47(18):10619–27. [DOI] [PubMed] [Google Scholar]
- 13.Willis J Memorandum to the Docket from Jim Willis. In: United States Environmental Protection Agency, ed., 2006. [Google Scholar]
- 14.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol 2011;45(19):8037–45. [DOI] [PubMed] [Google Scholar]
- 15.Braun JM, Chen A, Romano ME, et al. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity (Silver Spring) 2016;24(1):231–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halldorsson TI, Rytter D, Haug LS, et al. Prenatal exposure to perfluorooctanoate and risk of overweight at 20 years of age: a prospective cohort study. Environ Health Perspect 2012;120(5):668–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mora AM, Oken E, Rifas-Shiman SL, et al. Prenatal exposure to perfluoroalkyl substances and adiposity in early and mid-childhood. Environ Health Perspect 2017;125(3):467–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gyllenhammar I, Diderholm B, Gustafsson J, et al. Perfluoroalkyl acid levels in first-time mothers in relation to offspring weight gain and growth. Environ Int 2018;111:191–99. [DOI] [PubMed] [Google Scholar]
- 19.Liu G, Dhana K, Furtado JD, et al. Perfluoroalkyl substances and changes in body weight and resting metabolic rate in response to weight-loss diets: A prospective study. PLOS Medicine 2018;15(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cardenas A, Hauser R, Gold DR, et al. Association of perfluoroalkyl and polyfluoroalkyl substances with adiposity. JAMA Network Open 2018;1(4):e181493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nelson JW, Hatch EE, Webster TF. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect 2010;118(2):197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher M, Arbuckle TE, Wade M, Haines DA. Do perfluoroalkyl substances affect metabolic function and plasma lipids?--Analysis of the 2007-2009, Canadian Health Measures Survey (CHMS) Cycle 1. Environ Res 2013;121:95–103. [DOI] [PubMed] [Google Scholar]
- 23.Lin C-Y, Chen P-C, Lin Y-C, Lin L-Y. Association among serum perfluoroalkyl chemicals, glucose homeostasis and metabolic syndrome in adolescents and adults. Diabetes Care 2009;32(4):702–07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaacks LM, Boyd Barr D, Sundaram R, Grewal J, Zhang C, Buck Louis GM. Pre-pregnancy maternal exposure to persistent organic pollutants and gestational weight gain: A prospective cohort study. Int J Environ Res Public Health 2016;13(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashley-Martin J, Dodds L, Arbuckle TE, et al. Maternal and neonatal levels of perfluoroalkyl substances in relation to gestational weight gain. Int J Environ Res Public Health 2016;13(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marks KJ, Jeddy Z, Flanders WD, et al. Maternal serum concentrations of perfluoroalkyl substances during pregnancy and gestational weight gain: The Avon Longitudinal Study of Parents and Children. Reprod Toxicol 2019;90:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oken E, Baccarelli AA, Gold DR, et al. Cohort profile: Project Viva. Int J Epidemiol 2015;44(1):37–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Provenzano AM, Rifas-Shiman SL, Herring SJ, Rich-Edwards JW, Oken E. Associations of maternal material hardships during childhood and adulthood with prepregnancy weight, gestational weight gain, and postpartum weight retention. J Women’s Health 2015;24(7):563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rasmussen KM, Catalano PM, Yaktine AL. New guidelines for weight gain during pregnancy: what obstetrician/gynecologists should know. Curr Opin Obstet Gynecol 2009;21(6):521–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sagiv SK, Rifas-Shiman SL, Webster TF, et al. Sociodemographic and perinatal predictors of early pregnancy per- and polyfluoroalkyl substance (PFAS) concentrations. Environ Sci Technol 2015;49(19):11849–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brantsaeter AL, Whitworth KW, Ydersbond TA, et al. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ Int 2013;54:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiol Rev 2000;22(2):261–74. [DOI] [PubMed] [Google Scholar]
- 33.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics 2000;56(3):779–88. [DOI] [PubMed] [Google Scholar]
- 34.Bobb JF, Valeri L, Henn BC, et al. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 2015;16(3):493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buck RC, Franklin J, Berger U, et al. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manag 2011;7(4):513–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tittlemeier SA, Pepper K, Edwards L, Cao XL. Concentrations of perfluorooctanesulfonamides in Canadian Total Diet Study composite food samples collected between 1992 and 2004. J Agric Food Chem 2006;54(21):8385–89. [DOI] [PubMed] [Google Scholar]
- 37.Cardenas A, Hivert MF, Gold DR, et al. Associations of perfluoroalkyl and polyfluoroalkyl substances with incident diabetes and microvascular disease. Diabetes Care 2019:pii: dc182254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oken E, Taveras EM, Popoola FA, Rich-Edwards JW, Gillman MW. Television, walking, and diet: Associations with postpartum weight retention. Am J prev Med 2007;32(4):305–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Domazet SL, Grontved A, Timmermann AG, Nielsen F, Jensen TK. Longitudinal associations of exposure to perfluoroalkylated substances in childhood and adolescence and indicators of adiposity and glucose metabolism 6 and 12 years later: The European Youth Heart Study. Diabetes Care 2016;39:1745–51. [DOI] [PubMed] [Google Scholar]
- 40.Watkins AM, Wood CR, Lin MT, Abbott BD. The effects of perfluorinated chemicals on adipocyte differentiation in vitro. Mol Cell Endocrinol 2015;400:90–101. [DOI] [PubMed] [Google Scholar]
- 41.van den Dungen MW, Murk AJ, Kok DE, Steegenga WT. Persistent organic pollutants alter DNA methylation during human adipocyte differentiation. Toxicol In Vitro 2017;40:79–87. [DOI] [PubMed] [Google Scholar]
- 42.Shipley JM, Hurst CH, Tanaka SS, et al. trans-activation of PPARalpha and induction of PPARalpha target genes by perfluorooctane-based chemicals. Toxicol Sci 2004;80(1):151–60. [DOI] [PubMed] [Google Scholar]
- 43.Wolf CJ, Schmid JE, Lau C, Abbott BD. Activation of mouse and human peroxisome proliferator-activated receptor-alpha (PPARalpha) by perfluoroalkyl acids (PFAAs): further investigation of C4-C12 compounds. Reprod Toxicol 2012;33(4):546–51. [DOI] [PubMed] [Google Scholar]
- 44.Rosenmai AK, Taxvig C, Svingen T, et al. Fluorinated alkyl substances and technical mixtures used in food paper-packaging exhibit endocrine-related activity in vitro. Andrology 2016;4(4):662–72. [DOI] [PubMed] [Google Scholar]
- 45.Rosen MB, Das KP, Rooney J, Abbott B, Lau C, Corton JC. PPARalpha-independent transcriptional targets of perfluoroalkyl acids revealed by transcript profiling. Toxicology 2017;387:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


