Abstract
Polycystic ovary syndrome (PCOS) is a common endocrinopathy affecting 46XX individuals of reproductive age. Cardinal features of PCOS include hyperandrogenism, irregular periods, and insulin resistance. Pathogenesis is unclear but likely involves hypothalamic, pituitary, or ovarian abnormalities leading to increased androgen production. In addition, alternative insulin signaling pathways are activated to preserve ovarian sensitivity to insulin while other “classical” tissues (e.g. liver, adipose, muscle) are insulin resistant. Treatment targets specific symptoms and the most common regimens include weight loss, metformin, oral contraceptives, anti-androgen compounds, and fertility treatments. Observations of individuals with gene mutations affecting androgen metabolism suggest that androgens may influence the development of gender identity. We reviewed studies exploring the relationship between gender identity and PCOS to further elucidate this relationship. Rates of PCOS in hormone-naïve transmasculine (TM) individuals appear to be higher than in the general population as cited by small, early studies using convenience samples and inconsistent criteria for PCOS. A more recent, larger study using established guidelines for PCOS did not show this to be true. Further, other studies show that although PCOS patients are less likely to identify with a traditional feminine gender scheme compared to age-matched peers, the prevalence of gender incongruence in PCOS patients is not higher than in the general population. Larger systematic studies with control groups using modern diagnostic criteria for both PCOS and gender incongruence are needed to clarify the relationship between PCOS and gender identity.
Keywords: Polycystic Ovary Syndrome, PCOS, gender identity, gender dysphoria, gender incongruence
Introduction
Individuals with gender incongruence have been present throughout history. In the last several decades, due to greater societal acceptance and increased medical insurance coverage, more transgender individuals are seeking medical care. Hormone treatment comprises a key part of the transition process for many transgender individuals. As knowledge in this field evolves, questions arise about the role of hormones in gender identity and expression.
Observations of individuals with gene mutations affecting androgen metabolism suggest a possible relationship between hormone levels and gender identity. For example, in 5-alpha-reductase 2 deficiency, there is reduced conversion of testosterone to the more active hormone dihydrotestosterone [1]. Imperato-McGinley et al. described a cohort of 46XY individuals with this disorder, most of whom were born with female or ambiguous external genitalia (but male internal urogenital tract), leading many to be reared as girls [2]. After the onset of puberty, virilization occurs (the phallus enlarges and testes descend). Seventeen out of eighteen of the children reared as girls in Imperato-McGinley’s cohort changed to male identity after puberty [2]. In another genetic condition, 21-hydroxylase deficiency, precursors in the adrenal steroidogenesis pathway are shunted towards androgen production (Figure 1). Excess androgen exposure leads to ambiguous genitalia at birth in 46XX individuals which are associated with variations in gender roles and behavior [3,4]. These findings will be discussed in more detail later.
Figure 1.
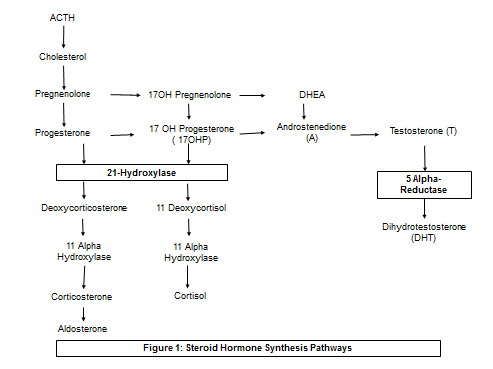
Steroid Hormone Synthesis Pathways. 21-Hydroxylase deficiency causes accumulation of 17-OH-P and androgens (androstenedione and testosterone), which can lead to virilization at birth. Gender-related behavior in cis-women with CAH may differ from unaffected individuals [3]. 5-alpha-reductase deficiency blocks production of DHT. This block is in part overcome at puberty – 46XY patients undergo masculinization and gender identity shifts to male even in individuals reared as females [20].
In this review, we explore the relationship between polycystic ovary syndrome (PCOS) and gender identity and expression. PCOS is a common endocrine disorder of 46XX reproductive-age individuals who were assigned female at birth (AFAB). Most PCOS individuals have clinical and/or biochemical hyperandrogenism. Because androgens may affect gender identity as discussed earlier, we explored the PCOS literature which examined a potential relationship between PCOS and gender incongruence.
We first discuss the pathogenesis of PCOS, its symptoms and treatment. We then examine the literature which addresses the prevalence of PCOS in individuals with gender incongruence and conversely, the prevalence of gender incongruence in those with PCOS. It is important to screen for PCOS because it is associated with metabolic and fertility issues. We finally attempt to draw some conclusions regarding the relationship between PCOS and gender incongruence and propose future areas of investigation into this important topic.
Our use of the term “gender incongruence” draws from the descriptions provided by the Diagnostic and Statistical Manual of Mental Disorders (DSM) and International Classification of Diseases (ICD) as a condition in which one’s experienced or expressed gender is persistently incongruous with their gender assigned at birth. It is important to acknowledge, of course, that this condition is not a mental disorder or a disease. In addition, the DSM and ICD nomenclature has changed with each new edition – the condition was termed “transsexualism” and “gender identity disorder of childhood” in the ICD-10 and “gender identity disorder” in the DSM-IV [5,6]. In the DSM-V (the most recent edition), it is termed “gender dysphoria” [7]. In the upcoming ICD-11, it will be named “gender incongruence” and will be moved out of the “mental and behavioral disorders” chapter into the “sexual health” chapter in an effort to destigmatize gender variance [8]. The definitions for this condition have also evolved, though the DSM-IV and V both stipulate that clinical distress or impairment in functioning must be associated with the incongruence between the expressed and natal gender [5,7]. In this review we will use the term “gender incongruence” for general discussion. When addressing individual studies, we will cite the nomenclature used by the authors.
PCOS: Diagnostic Criteria and Clinical Manifestations
PCOS affects 5% to 20% of 46XX reproductive-age individuals [9]. Most consensus definitions characterize PCOS as a disorder of chronic anovulation and either clinical or biochemical hyperandrogenism with exclusion of other conditions with similar phenotypes (e.g. 21-hydroxylase deficiency, androgen secreting tumors, Cushing’s syndrome, etc.).
Three sets of diagnostic criteria of PCOS exist. They were proposed by the National Institutes of Health (NIH) in 1990, Rotterdam Consensus conference in 2003, and Androgen Excess and PCOS Society (AES) in 2009. NIH criteria require the presence of menstrual abnormalities (oligo- or anovulation) and either clinical or biochemical hyperandrogenism of ovarian origin [10]. Rotterdam criteria require the presence of two out of the three following features once other causes of hyperandrogenism have been excluded: oligo- or amenorrhea, clinical or biochemical hyperandrogenism, and polycystic ovary morphology on ultrasound [11]. AES criteria define PCOS as the presence of clinical and/or biochemical hyperandrogenism, ovarian dysfunction (oligo-anovulation and/or polycystic ovarian morphology on ultrasound), and the exclusion of other disorders [12].
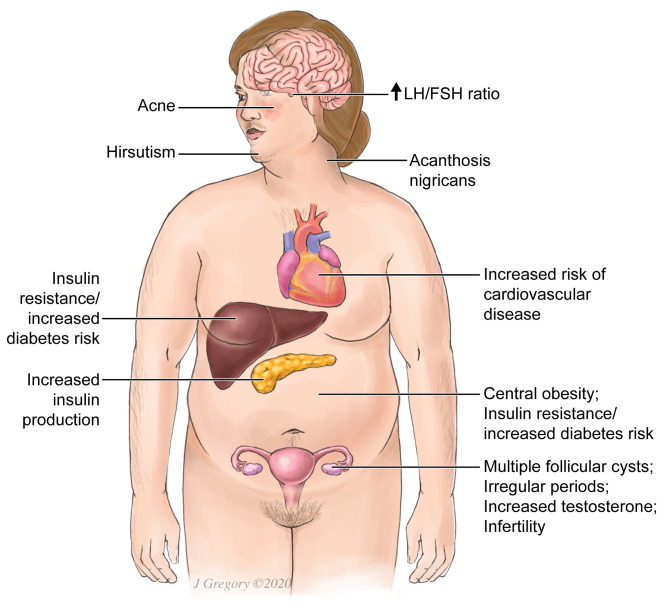
Clinical manifestations vary among individuals with PCOS (Figure 2). While not all individuals with PCOS have biochemical hyperandrogenism, the majority do. Recent cohort studies examining individuals who have PCOS by the most widely used criteria (Rotterdam 2003) estimate the prevalence of hyperandrogenemia to be 46% to 85%, with most studies citing rates of >50% [13-17]. Clinical signs of hyperandrogenism include hirsutism, seborrhea, acne, and alopecia. Evidence of virilization, such as clitoromegaly, may be present in some cases. In addition, individuals may have chronic anovulation, presenting with infertility or menstrual irregularities such as oligo/amenorrhea or dysfunctional uterine bleeding.
PCOS is also associated with insulin resistance (commonly manifesting as acanthosis nigricans), impaired glucose tolerance, increased risk of type 2 diabetes mellitus, dyslipidemia, and visceral obesity (Figure 2) [18,19]. Additionally, increased risk of cardiovascular disease and endometrial carcinoma has been observed in those with PCOS [20,21].
Figure 2.
PCOS Symptoms. Physical signs of PCOS include obesity (disproportionately central), acne, hirsutism, acanthosis nigricans (hyperpigmentation in the skin folds which is a manifestation of insulin resistance). PCOS is also associated with irregular periods and increased risk of diabetes, cardiovascular disease and infertility. Multiple follicular cysts may be seen on ovarian ultrasound in PCOS.
Genetics and Hormonal Abnormalities in PCOS
PCOS appears to be a complex genetic syndrome in which comorbid conditions and environmental factors interact with genetic variants. Over 100 genes have been examined as potential candidates for a role in the pathogenesis of PCOS but none has been shown to be the primary driver of this condition [22].
Two main endocrine theories of PCOS have been proposed [23]. The central theory posits that there is an abnormally increased pulsatile secretion of gonadotrophin-releasing hormone (GnRH) from the hypothalamus that causes a tonically increased secretion of LH instead of the usual pulsatile pattern [24]. LH levels may also rise due to hyperandrogenism [25]. Follicle-stimulating hormone (FSH) secretion is normal or decreased due to negative feedback leading to increased LH:FSH ratio. The ovarian theory postulates that an alteration in the ovarian steroid synthesis pathway is responsible for increased androgen production. For example, dysregulation of the enzyme cytochrome P450c17-alpha, which comprises 17-hydroxylase and 17/20 lyase activities, results in increased production of testosterone precursors and ultimately increased circulating testosterone levels [26].
Insulin Resistance and Hyperinsulinemia
Insulin sensitivity is affected by many independent factors, including obesity, muscle mass, and site of body fat deposition (central vs peripheral). The etiology of insulin resistance in PCOS is unclear, although abnormalities of insulin receptor signaling have been reported [27]. Elevated circulating levels of free fatty acids (FFA) and tumor necrosis factor-α (TNF-α) have also been reported in PCOS [28,29]. Increased FFA flux into liver decreases hepatic insulin extraction and produces hyperinsulinemia. TNF-α produced by adipose tissue leads to insulin resistance and inhibition of the insulin receptor signaling cascade. Elevated serum insulin levels in PCOS result in excessive ovarian androgen production as well as ovarian growth and cyst formation [30,31].
The system of ovarian regulation by insulin, insulin-like growth factors (IGFs) and their receptors is complex [24]. Activation of alternative insulin signaling pathways via type 1 IGF receptors (possibly up-regulated by hyperinsulinemia), and peroxisome proliferator-activated receptor-gamma (PPAR-γ) activation help preserve ovarian sensitivity to insulin even in insulin resistant states [25]. The gonadotrophic activity of insulin includes direct stimulatory effects on steroidogenic enzymes, synergism with FSH and LH, and enhancement of pituitary responsiveness to GnRH. Insulin also suppresses hepatic production of sex hormone binding globulin (SHBG) leading to increased circulatory levels of free steroid hormones [32]. Additionally, insulin increases expression of PPAR-γ whose activation affects steroidogenesis [33]. These effects of insulin on ovaries can account for many PCOS features in hyper-insulinemic insulin-resistant individuals and are important in developing therapeutic strategies.
Treatment Options
There are numerous treatment modalities that should be tailored to the patient’s specific presentations and concerns. Lifestyle changes improve multiple aspects of PCOS. A 5% to 10% reduction in body weight can reduce insulin resistance, decrease circulating androgens, restore ovulation, and increase pregnancy rates [34]. Exercise, even if it does not produce this degree of weight loss, results in improved menstrual regularity, increased insulin sensitivity, and decreased hirsutism [35-37]. Insulin resistance can be reduced also by insulin sensitizers such as metformin and thiazolidinediones (TZDs). Metformin decreases hepatic gluconeogenesis and increases sensitivity of adipose tissue and muscle to insulin, resulting in reductions in insulin and androgen levels. Metformin also improves menstrual regularity, ovulation rate, and pregnancy rate [38,39]. TZDs, which activate PPAR-γ, improve insulin sensitivity. As insulin levels fall, ovulation rates improve and SHBG levels rise. Improvement in androgen level and ovulation is similar to that seen with metformin [40]. Glucagon-like peptide-1 (GLP-1) receptor agonists reduce body weight and thus increase insulin sensitivity [25].
Oral contraceptives (OCPs) regulate menstrual cycles and decrease androgen levels by inhibiting synthesis of GnRH at the hypothalamus. They also increase hepatic production of SHBG, thus decreasing circulating free androgen levels. Anti-androgen medications such as spironolactone, cyproterone acetate, finasteride, and flutamide may be used to reduce androgen levels and manifestations of hyperandrogenism [41].
Fertility options in PCOS include the partial estrogen receptor antagonist clomiphene, which interferes with the negative feedback of estrogen in the hypothalamus, resulting in increased FSH secretion leading to follicular growth. Because of improved ovulation rates and cost-effectiveness, clomiphene is frequently used for ovulation induction [42]. However, recent studies indicate that letrozole, an aromatase inhibitor which blocks peripheral conversion of androgens to estrogens, produces higher pregnancy and live-birth rates than clomiphene, making it a leading candidate for first-line treatment [43,44]. Other fertility treatment options include exogenous gonadotrophin administration, in vitro fertilization, and laparoscopic ovarian drilling [34].
Prevalence of PCOS in Transgender Patients
Prior to the publication of the 2003 Rotterdam criteria, three studies showed a wide range in prevalence (5%-91.7%) of PCOS in hormone-naïve transmasculine (TM) individuals [45-47]. Of note, reviewed studies used various terminology including “transsexual females to males” or “FTM” to describe individuals AFAB who seek gender transition using testosterone. There was no indication that gender nonbinary individuals were included. We use the abbreviation TM when referring to the studies’ cohorts. In the earliest of these studies, Futterweit et al. found that 5% of their cohort of 40 multiracial TM patients “unequivocally” had PCOS if they met the criteria of having enlarged multifollicular ovaries (seen on pathology after laparotomy) and at least one of the following two symptoms: hirsutism or oligomenorrhea (Table 1) [45]. Since these were very stringent criteria for definitively diagnosing PCOS, the authors categorized 22.5% of their subjects as “probably” having PCOS by the criteria of: “(1) a combination of hirsutism and/or oligomenorrhea with a borderline or increased plasma testosterone level and/or a plasma LH/FSH ratio >2.0; and/or (2) ultrasonographic evidence of increased ovarian volume with multiple follicle cysts.” Of note, 20% of their cohort would have met Rotterdam 2003 criteria for PCOS. The rate of hirsutism in this cohort was 30%. In a subset of 29 patients who had total testosterone measured, 31% had borderline (>80 ng/dl) or elevated (>100 ng/dl) circulating testosterone levels. On average, TM individuals had higher total testosterone levels than the control group of cis women.
Table 1. Summary of Studies Addressing the Relationship between PCOS and Gender Identity.
| Study [Reference #] | Country | Cohort size (n) | Criteria for PCOS Diagnosis | Findings |
| Futterweit et al. 1986 [45] | United States | 40 | “Unequivocal”: Multifollicular ovaries on pathology plus 1 of 2: hirsutism or oligomenorrhea. “Probable”: “(1) a combination of hirsutism and/or oligomenorrhea with a borderline or increased plasma testosterone level and/or a plasma LH/FSH ratio >2.0; and/or (2) ultrasonographic evidence of increased ovarian volume with multiple follicle cysts.” | PCOS rate 5% by “unequivocal” criteria in TM. PCOS rate 22.5% by “probable” criteria in TM |
| Balen et al. 1993 [46] | Britain | 16 | Hyperandrogenism, U.S. criteria, Irregular Periods | PCOS rate 43.8% in TM |
| Bosinski et al. 1997 [47] | Germany | 12 | Rotterdam 2003 | PCOS rate 91.7% in TM |
| Baba et al. 2007 [48] | Japan | 69 | Rotterdam 2003 | PCOS rate 58% in TM |
| Mueller et al. 2008 [49] | Germany | 61 | NIH 1990, Rotterdam 2003 | PCOS rate 11.5% - 14.8% in TM, not different from controls |
| Cesta et al. 2016 [56] | Sweden | 24,385 | ICD-9 or ICD-10 | Prevalence of Gender Identity Disorder NOT higher in PCOS compared to controls |
A subsequent study in a group of 16 British TM patients found a PCOS prevalence of 43.8% using the criteria of irregular periods, hyperandrogenemia, and polycystic ovary morphology on ultrasound [46]. Fifty percent of these individuals exhibited hirsutism. In a 1997 German study, 83% of TM patients had hyperandrogenemia and 11 out of 12 met PCOS criteria defined as having two of the following signs/symptoms: irregular periods, hyperandrogenemia, and polycystic ovarian ultrasound appearance [47]. However, after cosyntropin stimulation testing, 50% (n=6) of the TM patients were found to have non-classical congenital adrenal hyperplasia (NCCAH), a condition which causes hyperandrogenism and irregular periods. This raises the consideration that the prevalence of PCOS in earlier studies may have been inflated by the presence of undiagnosed mimicking conditions such as NCCAH.
In a 2007 study of 69 Japanese TM transgender patients prior to gender affirming treatment, 58% had PCOS by Rotterdam criteria and 39% had hyperandrogenemia [48]. In this study, while patients with PCOS were more likely to have elevated androgen levels (as expected) than those who did not have PCOS, hyperandrogenemia was also more prevalent in obese vs lean TM patients. When stratified by BMI, however, TM patients with PCOS still had higher androgen levels than those without PCOS, confirming that PCOS is associated with hyperandrogenemia regardless of fat mass.
A more recent study from Germany explored the prevalence of PCOS (diagnosed by both the NIH 1990 and Rotterdam 2003 criteria) in 61 hormone-naïve TM individuals compared to 94 cis women without known medical disorders [49]. Vaginal ultrasound to assess for polycystic ovarian morphology was obtained in both groups. Hyperandrogenism was defined as a modified Ferriman Galwey score of ≥ 6 or elevated circulating free testosterone ≥ 0.028 nmol/liter. The Ferriman Galwey method is a semiquantitative assessment of hair growth in which higher grades indicate denser hair in more areas of the body [50]. Participants were deemed anovulatory if their menstrual cycles were longer than 35 days or if mid-luteal phase progesterone was ≤ 4 ng/mL. Other androgen excess disorders such as NCCAH, androgen-secreting neoplasms, and hyperandrogenic insulin-resistant acanthosis nigricans were excluded (ACTH stimulation test and computed tomography of the adrenal glands were used to rule out the first two conditions). No difference was found in PCOS rates between TM individuals and controls – 11.5% in TM individuals vs 9.6% in controls by NIH criteria (p=0.9) and 14.8% vs 12.8% by Rotterdam criteria (p=0.9). TM individuals did have a greater prevalence of hyperandrogenemia compared to controls (44.3% vs 20.3%, p=0.002) but there was no difference in hirsutism.
Thus, although earlier studies found relatively high rates of PCOS in several international TM populations, diagnostic criteria for PCOS varied and the presence of other androgen excess disorders may have caused overestimation of PCOS prevalence. A more recent study using two widely accepted criteria for PCOS, rigorous testing for PCOS components, and evaluations which ruled out similar disorders did not show a higher rate of PCOS in TM people. These studies, however, did show high rates of hyperandrogenism in TM individuals.
Self-Perception and Gender Identity in PCOS patients
We also reviewed the literature to determine self-perceptions of gender roles, femininity, and the prevalence of gender incongruence in PCOS patients given that many have biochemical and clinical hyperandrogenism. A study which used the AES criteria for PCOS did not find a difference in gender roles between adolescents with PCOS and cis female peers without PCOS [51]. Another small study showed that individuals AFAB who self-identified as being diagnosed with PCOS recalled less feminine behavior and less gender conformity in childhood compared to controls but no difference as adolescents and adults [52]. Qualitative studies report an alteration of the female gender scheme in adults with PCOS [53-55]. These studies examined self-reported personality traits, attitudes, social roles, and behaviors associated with symptoms of PCOS. They did not assess for the presence of gender incongruence/dysphoria, which is a distinct entity. The current DSM-V criteria for “gender dysphoria” specifies a marked incongruence between one’s experienced/expressed gender and natal gender of 6 months or more in duration, associated with clinically significant distress or impairment in functioning [7].
When the prevalence of “gender identity disorder” was studied in PCOS patients, there was no difference compared to control women after adjusting for comorbidity with any psychiatric disorder [56]. In this large Swedish population study (24,385 PCOS patients), subjects were included if they had a diagnosis of PCOS by ICD-9 or 10 and did not have a diagnosis of mimicking conditions such as congenital adrenal hyperplasia (CAH), adrenal tumor, or Cushing’s syndrome. Because participant selection was made based on ICD coding, it is possible that some participants diagnosed with PCOS may have been misclassified since entering a diagnostic code depends on the judgement of the clinical provider. The mode and vigor of work-up for conditions that present similarly to PCOS may have varied in this population, leading to false positive diagnosis of PCOS. Additionally, it is possible that patients who did in fact have PCOS did not have the proper coding in their medical chart, leading to exclusion from the study.
Comparison to Another Disorder of Androgen Excess – Congenital Adrenal Hyperplasia
Congenital adrenal hyperplasia (CAH) is another condition in which 46XX individuals can be exposed to high levels of androgens. In this disorder, precursors for cortisol and aldosterone are shunted towards androgen-synthesizing pathways due to enzyme deficiencies (Figure 1). Androgen exposure occurs in utero, often leading to genital virilization present at birth, whereas in PCOS hyperandrogenemia occurs after the onset of puberty. However, similar to PCOS, studies suggest that gender roles and behavior in 46XX patients with CAH may differ from those in the general 46XX population. Studies prior to the 1990s reported that in 46XX children with classical CAH, behaviors shift from female-typical towards male-typical in terms of juvenile play, clothing and style preference, athletic activity, career interest, and adult sexual behavior [57,58]. Satisfaction with the female sex role was higher in control children AFAB than in 46XX children with CAH [57]. However, those in the latter group who thought it was preferable to be a boy did not believe that they were assigned to the wrong gender. In a more recent study exploring gender development as a function of CAH severity, there was a greater degree of self-reported male-typical self-perception and behavior in 46XX individuals as the severity of the condition increased [3].
In a 2003 study in which individuals AFAB who were 18 years old or younger took part in a gender identity interview, 46XX children with CAH scored in between “control girls” and “tomboys” on the spectrum from complete female-typical identity to complete male-typical identity [4]. Age was not correlated with gender identity in this study. The “tomboy” group was recruited through newspaper articles asking parents who perceive their daughters to be tomboys to enroll them in the study (investigators did not provide a definition of “tomboy” to parents). A 2014 study found that compared to control children AFAB, 46XX children with CAH were more likely to show cross-gender identification as assessed by questions derived from part A of the diagnostic criteria for “gender identity disorder” in the DSM-IV-TR [59]. This trend was independent of gender role behavior. It is important to note that this study was not designed to diagnose gender incongruence in children and did not assess part D of the DSM-IV criteria for “gender identity disorder” which requires clinically significant distress or impairment in functioning (retained in the current DSM-V as part B) [7].
Conclusions and Outlook
PCOS is a syndrome characterized by clinical or biochemical hyperandrogenism, chronic anovulation manifesting as irregular periods, and polycystic ovaries. Many individuals with PCOS exhibit insulin resistance and hyperinsulinemia of unknown etiology, often related to obesity. A variety of symptom-driven therapeutic regimens is available. Future research may allow improvement of therapy by identifying specific molecular variants in individual patients.
The cardinal features of PCOS – hyperandrogenism (either clinical or biochemical) and irregular periods – may affect individuals’ perceptions of their femininity, social roles, and behavior but current evidence does not clearly indicate that PCOS is associated with gender incongruence. Older studies using small convenience samples showed that hormone-naïve TM people had a higher prevalence of PCOS and hyperandrogenism than the general population. However, the diagnostic criteria for PCOS were not uniform in these studies and the presence of other androgen excess disorders may have inflated the rates of PCOS. A more recent, larger study using established criteria for diagnosing PCOS did not show higher PCOS prevalence in TM individuals. In addition, although PCOS patients are less likely to identify with a traditional feminine gender scheme compared to age-matched peers, the prevalence of gender incongruence in PCOS patients is not higher than in the general population. Moderate androgen excess early in development (as seen in the genetic disorder CAH) appears to be associated with a higher risk of male-typical preferences and behavior, which is likely independent of its association with gender identity. Larger, systemic studies with control groups using modern diagnostic criteria for gender incongruence are needed to examine the association of early and late androgen exposure on gender identity. CAH has been shown to be a good model of early androgen exposure. To identify patients with late androgen exposure, it is likely feasible to first identify PCOS patients and then recruit only those who have biochemical hyperandrogenism. Gender incongruence can then be studied in a cohort of people assigned female at birth who have elevated circulating androgen levels.
Acknowledgments
We thank Jill Gregory for her assistance in creating Figure 2.
Glossary
- AFAB
Assigned female at birth
- CAH
Congenital adrenal hyperplasia
- FSH
Follicle-stimulating hormone
- ICD
International Classification of Diseases
- LH
Luteinizing hormone
- NCCAH
Nonclassic congenital adrenal hyperplasia
- PCOS
Polycystic ovary syndrome
- TM
Transmasculine
Funding sources
ML: Northwell Health Walk Clinical Care Innovations Grants, Katz Foundation. LP: Gerald J. Friedman Foundation.
References
- Imperato-McGinley J, Guerrero L, Gautier T, German JL, Peterson RE. Steroid 5α-reductase deficiency in man. An inherited form of male pseudohermaphroditism. Birth Defects Orig Artic Ser. 1975;11(4):91–103. [PubMed] [Google Scholar]
- Imperato-McGinley J, Peterson RE, Gautier T, Sturla E. Androgens and the evolution of male-gender identity among male pseudohermaphrodites with 5α-reductase deficiency. N Engl J Med. 1979. May;300(22):1233–7. [DOI] [PubMed] [Google Scholar]
- Meyer-Bahlburg HF, Dolezal C, Baker SW, Ehrhardt AA, New MI. Gender development in women with congenital adrenal hyperplasia as a function of disorder severity. Arch Sex Behav. 2006. December;35(6):667–84. [DOI] [PubMed] [Google Scholar]
- Berenbaum SA, Bailey JM. Effects on gender identity of prenatal androgens and genital appearance: evidence from girls with congenital adrenal hyperplasia. J Clin Endocrinol Metab. 2003. March;88(3):1102–6. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. (DSM-IV). Washington, D.C.; 1994. 873 p. [Google Scholar]
- World Health Organization ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision, 2nd Ed. Geneva; 2004. [Google Scholar]
- American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-5). 5th ed. 2013. 991 p. [DOI] [PubMed] [Google Scholar]
- WHO/Europe | WHO/Europe brief – Transgender health in the context of ICD-11 [Internet]. 2019. [cited 2020 May 1]. Available from: http://www.euro.who.int/en/health-topics/health-determinants/gender/gender-definitions/whoeurope-brief-transgender-health-in-the-context-of-icd-11
- Ding T, Hardiman PJ, Petersen I, Wang FF, Qu F, Baio G. The prevalence of polycystic ovary syndrome in reproductive-aged women of different ethnicity: a systematic review and meta-analysis. Oncotarget. 2017. July;8(56):96351–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lujan ME, Chizen DR, Pierson RA. Diagnostic criteria for polycystic ovary syndrome: pitfalls and controversies. J Obstet Gynaecol Can. 2008. August;30(8):671–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauser BC, Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004. January;81(1):19–25. [DOI] [PubMed] [Google Scholar]
- Azziz R, Carmina E, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Futterweit W, et al. Task Force on the Phenotype of the Polycystic Ovary Syndrome of The Androgen Excess and PCOS Society The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertil Steril. 2009. February;91(2):456–88. [DOI] [PubMed] [Google Scholar]
- Leerasiri P, Wongwananuruk T, Indhavivadhana S, Techatraisak K, Rattanachaiyanont M, Angsuwathana S. Correlation of clinical and biochemical hyperandrogenism in Thai women with polycystic ovary syndrome. J Obstet Gynaecol Res. 2016. June;42(6):678–83. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wang J, Shen S, Liu J, Sun J, Gu T, et al. Association of Androgen Excess with Glucose Intolerance in Women with Polycystic Ovary Syndrome. Biomed Res Int. 2018. March;2018:6869705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katulski K, Czyzyk A, Podkowa N, Podfigurna-Stopa A, Ignaszak N, Paczkowska K, et al. Clinical and hormonal features of women with polycystic ovary syndrome living in rural and urban areas. Ann Agric Environ Med. 2017. September;24(3):522–6. [DOI] [PubMed] [Google Scholar]
- Li L, Chen X, He Z, Zhao X, Huang L, Yang D. Clinical and metabolic features of polycystic ovary syndrome among Chinese adolescents. J Pediatr Adolesc Gynecol. 2012. December;25(6):390–5. [DOI] [PubMed] [Google Scholar]
- Liou TH, Yang JH, Hsieh CH, Lee CY, Hsu CS, Hsu MI. Clinical and biochemical presentations of polycystic ovary syndrome among obese and nonobese women. Fertil Steril. 2009. December;92(6):1960–5. [DOI] [PubMed] [Google Scholar]
- Dunaif A. Hyperandrogenic anovulation (PCOS): a unique disorder of insulin action associated with an increased risk of non-insulin-dependent diabetes mellitus. Am J Med. 1995. January;98(1A SUPPL. 1):33S–9S. [DOI] [PubMed] [Google Scholar]
- Liao EP, Poretsky L. Polycystic Ovary Syndrome and Its Metabolic Complications. In: Obesity and Diabetes. 2007. p. 255–76.
- Legro RS. Polycystic ovary syndrome and cardiovascular disease: a premature association? Endocr Rev. 2003. June;24(3):302–12. [DOI] [PubMed] [Google Scholar]
- Hardiman P, Pillay OC, Atiomo W. Polycystic ovary syndrome and endometrial carcinoma. Lancet. 2003. May;361(9371):1810–2. [DOI] [PubMed] [Google Scholar]
- Unluturk U, Harmanci A, Kocaefe C, Yildiz BO. The genetic basis of the polycystic ovary syndrome: A literature review including discussion of PPAR-γ. PPAR Res. 2007;2007:49109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretsky L, Cataldo NA, Rosenwaks Z, Giudice LC. The insulin-related ovarian regulatory system in health and disease. Endocr Rev. 1999. August;20(4):535–82. [DOI] [PubMed] [Google Scholar]
- Zumoff B, Freeman R, Coupey S, Saenger P, Markowitz M, Kream J. A chronobiologic abnormality in luteinizing hormone secretion in teenage girls with the polycystic-ovary syndrome. N Engl J Med. 1983. November;309(20):1206–9. [DOI] [PubMed] [Google Scholar]
- Sood M, Zweig SB, Tolentino MC, Strizhevsky M, Poretsky L. Polycystic ovary syndrome. In: Principles of Diabetes Mellitus: Third Edition. 2017. p. 659–77.
- Qin KN, Rosenfield RL. Role of cytochrome P450c17 in polycystic ovary syndrome. Mol Cell Endocrinol. 1998. October;145(1-2):111–21. [DOI] [PubMed] [Google Scholar]
- Dunaif A, Xia J, Book CB, Schenker E, Tang Z. Excessive insulin receptor serine phosphorylation in cultured fibroblasts and in skeletal muscle. A potential mechanism for insulin resistance in the polycystic ovary syndrome. J Clin Invest. 1995. August;96(2):801–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holte J, Bergh T, Berne C, Lithell H. Serum lipoprotein lipid profile in women with the polycystic ovary syndrome: relation to anthropometric, endocrine and metabolic variables. Clin Endocrinol (Oxf). 1994. October;41(4):463–71. [DOI] [PubMed] [Google Scholar]
- Escobar-Morreale HF, Calvo RM, Sancho J, San Millán JL. TNF-α and hyperandrogenism: a clinical, biochemical, and molecular genetic study. J Clin Endocrinol Metab. 2001. August;86(8):3761–7. [DOI] [PubMed] [Google Scholar]
- Poretsky L, Clemons J, Bogovich K. Hyperinsulinemia and human chorionic gonadotropin synergistically promote the growth of ovarian follicular cysts in rats. Metabolism. 1992. August;41(8):903–10. [DOI] [PubMed] [Google Scholar]
- Poretsky L. On the paradox of insulin-induced hyperandrogenism in insulin-resistant states. Endocr Rev. 1991. February;12(1):3–13. [DOI] [PubMed] [Google Scholar]
- Plymate SR, Matej LA, Jones RE, Friedl KE. Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab. 1988. September;67(3):460–4. [DOI] [PubMed] [Google Scholar]
- Seto-Young D, Paliou M, Schlosser J, Avtanski D, Park A, Patel P, et al. Direct thiazolidinedione action in the human ovary: insulin-independent and insulin-sensitizing effects on steroidogenesis and insulin-like growth factor binding protein-1 production. J Clin Endocrinol Metab. 2005. November;90(11):6099–105. [DOI] [PubMed] [Google Scholar]
- Patel SM, Nestler JE. Fertility in polycystic ovary syndrome. Endocrinol Metab Clin North Am. 2006. March;35(1):137–55. [DOI] [PubMed] [Google Scholar]
- Tiwari N, Pasrija S, Jain S. Randomised controlled trial to study the efficacy of exercise with and without metformin on women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2019. March;234:149–54. [DOI] [PubMed] [Google Scholar]
- Halama A, Aye MM, Dargham SR, Kulinski M, Suhre K, Atkin SL. Metabolomics of dynamic changes in insulin resistance before and after exercise in PCOS. Front Endocrinol (Lausanne). 2019. February;10(FEB):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepto N, Hiam D, Gibson-Helm M, Cassar S, Harrison CL, Hutchison SK, et al. Exercise and insulin resistance in PCOS: muscle insulin signalling and fibrosis. Endocr Connect. 2020. March;EC-19-0551.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghetti P, Castello R, Negri C, Tosi F, Perrone F, Caputo M, et al. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 2000. January;85(1):139–46. [DOI] [PubMed] [Google Scholar]
- Legro RS, Barnhart HX, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Cooperative Multicenter Reproductive Medicine Network Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007. February;356(6):551–66. [DOI] [PubMed] [Google Scholar]
- Glintborg D, Andersen M. Thiazolinedione treatment in PCOS—an update. Gynecol Endocrinol. 2010. November;26(11):791–803. [DOI] [PubMed] [Google Scholar]
- Board JA, Rosenberg SM, Smeltzer JS. Spironolactone and estrogen-progestin therapy for hirsutism. South Med J. 1987. April;80(4):483–6. [DOI] [PubMed] [Google Scholar]
- Usadi RS, Legro RS. Reproductive impact of polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes. 2012. December;19(6):505–11. [DOI] [PubMed] [Google Scholar]
- Legro RS, Brzyski RG, Diamond MP, Coutifaris C, Schlaff WD, Casson P, et al. NICHD Reproductive Medicine Network Letrozole versus clomiphene for infertility in the polycystic ovary syndrome. N Engl J Med. 2014. July;371(2):119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franik S, Eltrop SM, Kremer JA, Kiesel L, Farquhar C. Aromatase inhibitors (letrozole) for subfertile women with polycystic ovary syndrome. Cochrane Database Syst Rev. 2018. 5(5):CD010287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterweit W, Weiss RA, Fagerstrom RM. Endocrine evaluation of forty female-to-male transsexuals: increased frequency of polycystic ovarian disease in female transsexualism. Arch Sex Behav. 1986. February;15(1):69–78. [DOI] [PubMed] [Google Scholar]
- Balen AH, Schachter ME, Montgomery D, Reid RW, Jacobs HS. Polycystic ovaries are a common finding in untreated female to male transsexuals. Clin Endocrinol (Oxf). 1993. March;38(3):325–9. [DOI] [PubMed] [Google Scholar]
- Bosinski HA, Peter M, Bonatz G, Arndt R, Heidenreich M, Sippell WG, et al. A higher rate of hyperandrogenic disorders in female-to-male transsexuals. Psychoneuroendocrinology. 1997. July;22(5):361–80. [DOI] [PubMed] [Google Scholar]
- Baba T, Endo T, Honnma H, Kitajima Y, Hayashi T, Ikeda H, et al. Association between polycystic ovary syndrome and female-to-male transsexuality. Hum Reprod. 2007. April;22(4):1011–6. [DOI] [PubMed] [Google Scholar]
- Mueller A, Gooren LJ, Naton-Schötz S, Cupisti S, Beckmann MW, Dittrich R. Prevalence of polycystic ovary syndrome and hyperandrogenemia in female-to-male transsexuals. J Clin Endocrinol Metab. 2008. April;93(4):1408–11. [DOI] [PubMed] [Google Scholar]
- Ferriman D, Gallwey JD. Clinical assessment of body hair growth in women. J Clin Endocrinol Metab. 1961. November;21:1440–7. [DOI] [PubMed] [Google Scholar]
- Zachurzok A, Gawlik A, Nowak A, Drosdzol-Cop A, Małecka-Tendera E. Social abilities and gender roles in adolescent girls with polycystic ovary syndrome - a pilot study. Endokrynol Pol. 2014;65(3):189–94. [DOI] [PubMed] [Google Scholar]
- Manlove HA, Guillermo C, Gray PB. Do women with polycystic ovary syndrome (PCOS) report differences in sex-typed behavior as children and adolescents?: results of a pilot study. Ann Hum Biol. 2008. Nov-Dec;35(6):584–95. [DOI] [PubMed] [Google Scholar]
- Kitzinger C, Willmott J. ‘The thief of womanhood’: women’s experience of polycystic ovarian syndrome. Soc Sci Med. 2002. February;54(3):349–61. [DOI] [PubMed] [Google Scholar]
- Nasiri Amiri F, Ramezani Tehrani F, Simbar M, Mohammadpour Thamtan RA, Shiva N. Female gender scheme is disturbed by polycystic ovary syndrome: A qualitative study from Iran. Iran Red Crescent Med J. 2014. February;16(2):e12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk R, Skrzypulec V, Lew-Starowicz Z, Nowosielski K, Grabski B, Merk W. Psychological gender of patients with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2012. June;91(6):710–4. [DOI] [PubMed] [Google Scholar]
- Cesta CE, Månsson M, Palm C, Lichtenstein P, Iliadou AN, Landén M. Polycystic ovary syndrome and psychiatric disorders: co-morbidity and heritability in a nationwide Swedish cohort. Psychoneuroendocrinology. 2016. November;73:196–203. [DOI] [PubMed] [Google Scholar]
- Ehrhardt AA, Epstein R, Money J. Fetal androgens and female gender identity in the early-treated adrenogenital syndrome. Johns Hopkins Med J. 1968. March;122(3):160–7. [PubMed] [Google Scholar]
- Ehrhardt AA, Evers K, Money J. Influence of androgen and some aspects of sexually dimorphic behavior in women with the late-treated adrenogenital syndrome. Johns Hopkins Med J. 1968. September;123(3):115–22. [PubMed] [Google Scholar]
- Pasterski V, Zucker KJ, Hindmarsh PC, Hughes IA, Acerini C, Spencer D, et al. Increased Cross-Gender Identification Independent of Gender Role Behavior in Girls with Congenital Adrenal Hyperplasia: Results from a Standardized Assessment of 4- to 11-Year-Old Children. Arch Sex Behav. 2015. July;44(5):1363–75. [DOI] [PubMed] [Google Scholar]