Abstract
The thyroid is not necessary to sustain life. However, thyroid hormones (TH) strongly affect the human body. Functioning of the thyroid gland affects the reproductive capabilities of women and men, as well as fertilization and maintaining a pregnancy. For the synthesis of TH, hydrogen peroxide (H2O2) is necessary. From the chemical point of view, TH is a reactive oxygen species (ROS) and serves as an oxidative stress (OS) promoter. H2O2 concentration in the thyroid gland is much higher than in other tissues. Therefore, the thyroid is highly exposed to OS. 8-oxo-7,8-dihydro-2′-deoxyguanosine (8-oxodG) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) are DNA lesions resulting from ROS action onto guanine moiety. Due to their abundance, they are recognized as biomarkers of OS. As thyroid function is correlated with the level of OS, 8-oxodG and 8-OHdG has been taken under consideration. Studies correlate the oxidative DNA damage with various thyroid diseases (TD) such as Hashimoto’s thyroiditis (HT), Graves’ disease (GD), and thyroid cancer. Human sexual function and fertility are also affected by OS and TD. Hypothyroidism and hyperthyroidism diagnosed in pregnant women have a negative effect on pregnancy as it may increase the risk of miscarriage or fetus mortality. In the case of TD in the mother, fetal health is also at risk – neurodevelopment and cognitive function of the child may be impaired in its future life. This review presents thyroid function in the context of TD during pregnancy. The authors introduce OS and describe oxidative DNA lesions as a crucial marker of thyroid pathologies.
Keywords: thyroid diseases, oxidative stress, pregnancy, deoxyguanosine, DNA damage, thyroid hormones
Introduction
Over the past decade, increase in the incidence of TD, including thyroid cancer, has been noted [1]. TD along with gestational diabetes are the most frequently diagnosed endocrinopathies during pregnancy [2]. World Health Organization’s research (1994-2006) estimated that about 31% of the world population has insufficient iodine (I-) intake [3]. Iodine deficiency (ID) is a common problem in many regions of the world where it concerns a significant part of the population (30% in Southeast Asia, 42% in Africa, 47.2% in the East Mediterranean region and 52% in Europe) [4]. The situation is different in the US. The estimated average I- intake (138-353 mcg/day) fulfills demand for I- in the US population [5]. Insufficient I- level causes e.g. hypothyroidism – a condition in which not enough TH is produced for maintaining optimal body function [6]. The incidence of diagnosed hypothyroidism reaches 5.3% in the European population, while in the US it is 0.3% for clinical hypothyroidism and about 4% for subclinical form [7,8]. Incidence of overt hyperthyroidism in the US and Europe is 0.5% and 0.7%, respectively. However, data show that 1 in 20 people in US, including 1 in 8 women, will develop TD [9].
I- is used by the thyroid to synthesize TH. Its deficiency may result in the hypothyroidism and negative effects on reproductive functions, pregnancy, lactation, and may impact the fetus. The American Thyroid Association (ATA), American Endocrine Society (AES), and European Thyroid Association (ETA) recommended thyrotropin (TSH) ranges for pregnant women (Table 1). The guidelines are similar for the US and Europe. However, clinical trials from China and India show that ethnicity has an impact on reference values, which are higher than Western guidelines [10-13].
Table 1. Reference values for TSH level in pregnant women depending on the trimester.
| Trimester | ATAa [10] | ETAb [11] | China [12] | India [13] |
| I | < 2.50 mU/L | < 2.50 mU/L | < 4.51 mIU/L | < 5.00 μiu/mL |
| II | < 3.00 mU/L | < 3.00 mU/L | < 4.50 mIU/L | < 5.78 μiu/mL |
| III | < 3.00 mU/L | < 3.50 mU/L | < 4.54 mIU/L | < 5.70 μiu/mL |
aAmerican Thyroid Association, bEuropean Thyroid Association
In the case of maternal TD, developmental disorders of the nervous system in the child may occur and cognitive functions may be weakened (e.g. congenital ID syndrome) during the offspring’s life. In addition, there is a higher risk of miscarriage, stillbirth, mortality, and impaired somatic development [14,15]. The negative effects of TD on the fetus have been confirmed in China in over 1000 pregnant women and their children [16]. The study shows that maternal hypo- or hyperthyroxinemia increases risk of fetal loss, congenital circulation system malformations, poor vision development, and neurodevelopmental delay. Therefore, it is important to control I- levels, especially in pregnant and lactating women [17]. Table 2 presents recommended I- intake for US and Europe [18,19].
Table 2. Table 2. I- intake recommendation for US and Europe.
| Age | US (mcg/day) | Europe (mcg/day) |
| 0-6 months | 110 (AIa) | - |
| 7-11 months | - | 70 (AI) |
| 7-12 months | 130 (AI) | - |
| 1-3 years | 90 (RDAb) | 90 (AI) |
| 4-6 years | - | 90 (AI) |
| 4-8 years | 90 (RDA) | - |
| 7-10 years | - | 90 (AI) |
| 9-13 years | 120 (RDA) | - |
| 11-14 years | - | 120 (AI) |
| 13-18 years | 150 (RDA) | - |
| 15-17 years | - | 130 (AI) |
| Adult (18+ years) | 150 (RDA) | 150 (AI) |
| Pregnancy (all ages) | 220 (RDA) | 200 (AI) |
| Lactation (all ages) | 290 (RDA) | 200 (AI) |
aadequate intake, brecommended dietary allowances
TD are divided according to its underactive and overactive physiological status [20]. The two most common autoimmune thyroid diseases (AITD) are HT, a thyroid-degrading inflammation associated with hypothyroidism, and GD, associated with hyperthyroidism and gland enlargement [21]. AITD are associated with an increase of OS level. 8-oxodG and 8-OHdG are markers of OS due to its frequent formation. Therefore, they are worth considering as diagnostic markers in the field of TD [22-24].
Proper thyroid function is also important for female and male fertility. In women, hypothyroidism causes changes in menstrual cycle length and bleeding, reduces the likelihood of conception, and negatively affects the miscarriage rate [25]. The topic of female fertility is discussed further in the text. As for men, AITD can cause a decrease in semen quality, sexual behavior, and impotence disorders. Studies indicate that sperm density, morphology, and motility were unsatisfactory in patients with hyperthyroidism [26]. Hyperthyroidism is also associated with a decrease in testosterone/estradiol ratio which contributes to libido disorders [27]. Moreover, hypothyroidism appears to be correlated with fewer spermatozoa and their reduced motility, which can significantly affect fertility as the female body may even reject impaired semen [25,28-30]. AITD are also associated with male sexual dysfunctions – sexual coldness, erectile dysfunction, or premature ejaculation. According to a study from 2008, 84% of patients diagnosed with hypothyroidism have problems with sexual function [25,26,31].
Clearly, poor quality of sperm may affect the success of conception and possibility of pregnancy. Furthermore, it is worth considering that DNA damage such as 8-oxodG (which form more often in AITD) may be present also in sperm’s genetic material. It impairs the quality of sperm and carries the potential of passing on mutated DNA onto a child.
In the first part of this review, we present the thyroid gland, its hormones and autoimmune diseases. Next, we describe alterations in thyroid functions during pregnancy and its influence on fertility. The second part focuses on introducing the concept of OS, oxidative DNA damage and its correlation with TH in order to discuss the influence of OS biomarkers (8-oxodG and 8-OHdG) on the TD in pregnant women and to discuss other environmental factors that may impact rate of repair of those lesions in DNA.
Thyroid and its Hormones
Thyroid Gland Development
The thyroid is the earliest developing gland in the fetus. It appears as an epithelial proliferation in the floor of the pharynx at the base of the tongue in the embryo (weeks 3-4 of gestation) [32]. Next, it migrates to the base of the neck. During migration, the thyroid remains connected to the tongue by a narrow canal (thyroglossal duct). At the end of week 5, the thyroglossal duct degenerates. Over the following 2 weeks the detached thyroid reaches its final location. Although not much is known about the pathways of the proper thyroid development, defects at this stage lead to complex health problems (e.g. thyroid dysgenesis or reduced TH sensitivity) [32]. The thyroid gland is not crucial for survival, but its absence or hypothyroidism during fetal and neonatal life can cause severe intellectual disabilities and dwarfism in the child [33].
Thyroid Hormones
TH affect functioning of the reproductive system and conception in humans – especially in women. TH modulate the action of other hormones such as estrogen, prolactin, and gonadotrophin-releasing hormone, which impact women’s fertility and the possibility of fertilization or termination of pregnancy [34]. TH are small molecules formed by the linkage of two threonine molecules on the surface of thyroglobulin (TG). After iodination they obtain tri- or tetra-iodo versions of the hormone [35]. Triiodothyronine (T3) and thyroxine (T4) regulate metabolism and act through receptors located in the placenta, uterus, and ovaries. Approximate secretion of TH is: T4 – 80 mcg/day, T3 – 4 mcg/day, rT3 – 2 mcg/day [33].
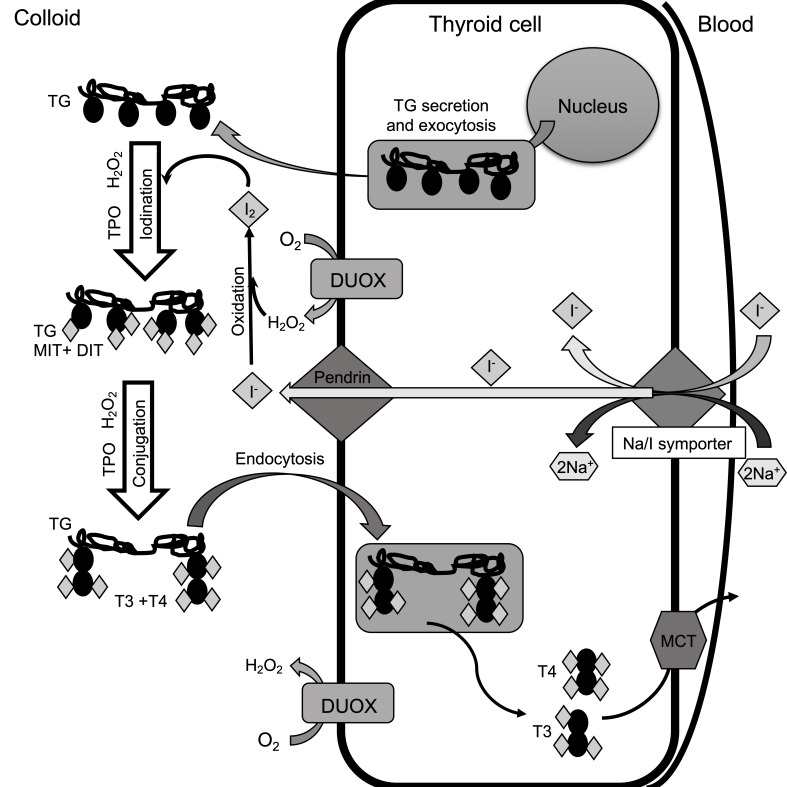
TG, which is crucial for the synthesis of TH, accounts for approximately 50% of the protein content of the thyroid gland and contains up to 1% of I- [36]. Moreover, it is a storage of the inactive forms of TH and I- within the follicular lumen of a thyrocyte [37]. About 70% of I- binds to Tyr residues in TG which results in inactive precursors formation (monoiodothyronine (MIT) and diiodothyronine (DIT)) (Figure 1) [38]. MIT and DIT are subsequently coupled to form T3 [39]. Each TG molecule forms approximately 10 molecules of TH.
Figure 1.
Synthesis of TH. TG is synthesized in the endoplasmic reticulum and secreted into the colloid in the process of exocytosis. The active transport of I- to follicular cells is carried out by sodium-iodide TSH-dependent symporter protein. The Na/I symporter draws 2 Na+ and 1 I- into the cell. I- are secreted into the colloid by the pendrin transporter. I-, which is necessary for TH biosynthesis, is oxidized by thyroid peroxidase (TPO) in the presence of high concentrations H2O2 and forms molecular iodine (I2). It reacts with Tyr residues in organification reaction leading to MIT and DIT coupling. They subsequently conjugate to form T4 with 2xDIT combination and T3 with MIT+DIT combination. These reactions also need TPO and H2O2. H2O2 is necessary for TPO action and is formed in the colloid through NADPH oxidases (DUOX subfamily). Next, TG is transported by endocytosis into the cell where it undergoes proteolysis and releases T3 and T4. TG is “recycled” and used for further TH synthesis. The TH are transported from the cell to the blood by the monocarboxylate transporter (MCT).
T4 is deiodinated by iodothyronine deiodinases type 1-3 (DIO1-DIO3) [40-42]. DIO3, present in placenta and neurons, deactivates TH through conversion of T4 to reverse triiodothyronine (rT3) and T3 to diiodothyronine (T2) [43,44]. Secretion of TH is regulated by TSH, which is regulated by thyrotropin-releasing hormone (TRH) [45]. It binds to its receptor (TRHR) at the basolateral membrane of the follicular cells, which results in stimulation of cell growth and TH synthesis [38]. RT3 is associated with elevated activity of DIO3 or impaired detoxication mechanisms. Increased level of rT3 occurs in e.g. euthyroid sick syndrome, heart failures, and liver cirrhosis [44,46].
Most TH are bound to thyroxine-binding globulin (TBG, 70%), thyroxin-binding prealbumin, (TBPA, 10%) and thyroxine-binding albumin (TBA, 15%), which prevent their urinary loss [47]. Hormones bind to intracellular thyroid hormone receptors (TR-α1, TR-α2, TR-β1, and TR-β2), modulate DNA transcription, and interact with enzymes within the cell membrane or cytoplasm [48]. To some extent, the thyroid gland produces T3 directly. In the follicular lumen, Tyr residues become iodinated, which requires H2O2. At high TSH levels, the TRHR couples Gq/11 protein, activating the phospholipase C-dependent inositol phosphate Ca2+/diacylglycerol pathway. It causes overgeneration of H2O2 and subsequent TG iodination [38,49].
The fetal hypothalamus and pituitary start to secrete TRH and TSH at week 11. By weeks 18-20, T4 reaches a clinically significant level [50]. Fetal T3 remains low (<15 ng/dL) until week 30 and increases at full-term (50 ng/dL). The fetus needs proper level of TH in order to avoid neurodevelopmental disorders which arise due to maternal hypothyroidism. One of the factors limiting the synthesis of TH is I- level, which is also essential for healthy neurodevelopment [51]. For proper I- supply, the T4:T3 ratio in TG is 7:1 [36]. The optimal I- intake for adults is 150 mcg/day – it ensures proper TH synthesis (Table 2) [52].
Thyroid Diseases
There are three major groups of TD [7,53]. Two of them are associated with hyper-/hypothyroidism and the third group is parathyroid disorders. AITD are mostly defined by HT and GD, especially due to their connections to genetic alterations [54]. AITD are separated into two clinical categories: (1) if goiters are present, it is understood as HT, (2) if the thyroid is atrophic, and does not present goiters, it is atrophic thyroiditis and also refers to GD [55]. Moreover, postpartum thyroiditis may be diagnosed within the first year after giving birth [56]. Postpartum thyroiditis is an autoimmune disorder in euthyroid women with thyrotoxicosis caused by high amounts of TH. It usually goes through a hypothyroid phase and eventually disappears within one year. TD is considered a growing health problem in developing countries. There is evidence that TD may be also triggered by environmental factors and threaten women’s health and fetus development. In the time of aging society, it is even more important to protect future mothers and provide them with the best possible care and accurate and early diagnosis, as the health of mothers affects offspring and therefore, future generations.
Alterations in Thyroid Functions During Pregnancy
Thyroid gland function influences conception and pregnancy. Changes in the physiology of a woman’s body during gestation have an impact on the morphology and function of the thyroid gland. Therefore, daily I- requirements (Table 2) are higher during and after pregnancy due to increased renal I- excretion, increased TH production, significant I- requirement of a fetus, and I- secretion into breast milk [57]. Pregnancy impacts thyroid secretory functions so diagnostic standards for healthy adults do not apply to pregnant women.
The estrogen and chorionic gonadotropin (hCG) interact with iodothyronines and TSH secretion. HCG is secreted by the placenta from the first weeks of pregnancy. Due to the structural similarity to TSH, hCG influences thyroid activity – increases T4 and decreases TSH secretion. Hence, a spike of T3 and T4 levels occurs. The concentration of hCG in the blood peaks at about weeks 8-11 of pregnancy, then decreases and reaches a plateau around week 20.
During pregnancy production of TH increases. TBG, a TH binding protein, is the main repository for TH and regulates the total level of thyroid hormones and the level of free TH – not bound to proteins. TBG level increases from the first weeks of pregnancy about 2-3-fold and reaches a plateau about halfway through gestation. Estrogen levels in the blood are positively correlated with TBG [58]. Increased TBG levels contribute to a drop of free TH and increase of the overall level of TH in the blood [58]. The increase in TBG is gradual and stops during the second trimester. At the same time TH show an increase of approximately 50%. Moreover, hCG causes an increase of free T3 and T4 levels, which induces TSH decrease. The action of placental DIOs contributes to the increased demand for TH. DIO3 is involved in the transformation of T4 to rT3 and T3 to T2. It protects the fetus from overexposure to maternal TH by inhibiting T4 activation and T3 deactivation. DIO2 catalyzes the transformation of T4 to T3 and provides the optimal level of T3 for fetal development. Both enzymes are produced from the beginning of pregnancy and their activity decreases with time.
At the beginning of the second trimester, the secretory activity of the placenta decreases and settles at a certain level. The level of free TH decreases, while TSH concentration increases [58]. The secretory functions of the thyroid gland change significantly as pregnancy progresses (Table 3). Due to these fluctuations the reference values of hormones differ from the values for healthy adults [28,59].
Table 3. Changes in TH depending on the trimester.
| Hormone | I trimester | II trimester | III trimester |
| TSHa | - - - | - - | - |
| T3b | ++ | +++ | +++ |
| T4c | ++ | +++ | +++ |
| fT3d | - | - - | - - |
| fT4e | - | - - | - - |
| TBGf | ++ | +++ | +++ |
| hCGg | +/++ | ++ | ++ |
athyrotropin, btriiodothyronine, cthyroxine, dfree triiodothyronine, efree thyroxine, fthyroxine-binding globulin, gchorionic gonadotropin, + increased level, - decreased level
The fetal thyroid begins to work between weeks 12-20 of pregnancy. Until then, TH must come from the mother and I- must be transported from the mother throughout pregnancy [60]. TH levels differ on the maternal and fetal side of the placenta. T3 is lower (4.34 ng/g in fetus and 5.93 ng/g in mother) and T4 is higher (67.72 ng/g in fetus and 44.96 ng/g in mother) on the fetal side [61]. I- levels on the fetal side of the placenta are lower than on the mother’s side (0.45 μg/g and 1.38 μg/g, respectively). In pregnant women, the I- level in placenta and urine is associated, however this is not observed for fetal I- placental level [61].
Pregnancy Immunosuppression and its Effect on the Thyroid Gland
Antigenic compatibility between the mother and the fetus is is rarely 100%. To prevent rejection of pregnancy, the functions of the immune system change in women – immune responses are suppressed. It was shown already in 1997 in clinical studies where the number of immune cells in pregnant women have decreased, but postpartum it has risen even above pre-pregnancy level [62]. This phenomenon is called pregnancy immunosuppression and its effect on the course of AITD is confirmed [63]. Over 400,000 Danish women were tested during the year before and two years after pregnancy. Results show that the incidence of hyperthyroidism in the first trimester increased (compared to before pregnancy) and then decreased to its lowest in the third trimester [63].
In the case of GD, alleviation of the course of hyperthyroidism is observed after the first trimester. The attack of the immune system on thyroid antigens is weakened and the disease stabilizes. However, after delivery, the immune processes regain activity, which may cause the AITD relapse [64].
After birth, the immunosuppressive effect of pregnancy subsides and the incidence of hyperthyroidism increases due to the rebound of the immune system [63,65]. The occurrence of hyperthyroidism is the highest within 7-9 months after delivery (more than 5 times higher than before delivery and 10 times higher than during the third trimester). Similar study confirms the reduction in the incidence of illness during pregnancy with a minimum in the third trimester (3 times lower than in the period before pregnancy), and a maximum in the period of 4-6 months after delivery (about 4 times higher than before pregnancy and over 12 times higher than during the minimum maturity) [66]. Therefore, endocrine control during and after pregnancy is crucial in patients with a risk of AITD or already diagnosed prior to conception.
Impact of Thyroid Dysfunction on Female Fertility
Hyperthyroidism affects the level of sex hormones in women. In patients with thyroxicosis, estrogen levels may increase up to 3-fold. Levels of testosterone, androstenedione, and luteinizing hormone also increase. Moreover, menstruation disorders are the most common symptom of hyperthyroidism. They are associated with elevated total T4 levels and diagnosed 2.5 times more often than in healthy women. In patients with hypothyroidism, the concentration of total testosterone and estradiol in the blood decreases, while their unbound fractions increase. Disorders of blood coagulation factors and platelet function may also occur, with changes in the length of the cycle and the intensity of bleeding. Menstrual cycle disorders are the most common symptom in hypothyroidism [25,67,68].
In addition, TH may directly impact conception and pregnancy. Quintino-Moro et al. show that infertility occurs in more than 52% of patients with the diagnosis of GD and 47% with HT [25,67]. Infertility is caused mainly by hormonal imbalance e.g. increased prolactin level. It impacts gonadotropin release and subsequently leads to decreased capacity of corpus luteum. Problems with fertilization may also result in ovulation disorders [69]. Furthermore, recent clinical studies show that untreated hypothyroidism (with TSH >4 mIU/L) increases the risk of miscarriage [70,71]. Interestingly, after bringing women to the state of euthyrosis, birth rates improve [72,73].
Obviously, decreased function of women’s reproductive systems, such as menstrual disorders, impedes conception rate. In the context of human reproduction, it is of high importance that women have healthy thyroids or properly managed AITD in order to successfully plan and start a family. Therefore, in the next section we focus on the interesting aspect connected to thyroid function – oxidative stress and its markers. It is believed that this perspective may improve early diagnosis options and maybe even contribute to new therapeutic approaches.
Influence of Oxidative Stress on Thyroid
Oxidative Stress and Oxidative DNA Damage Formation
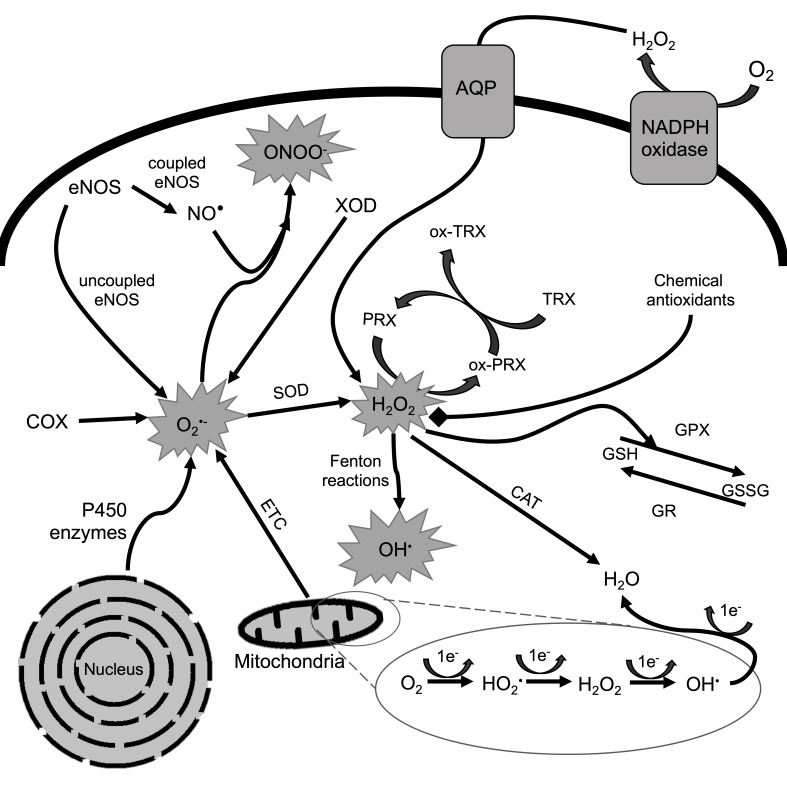
In a healthy cell, there is an oxidative balance between ROS production and their neutralization by the cell (redox homeostasis), which, when disrupted generates OS [74]. ROS include: superoxide anion radical (O2•-), hydroperoxyl radical (HO2•), hydroxyl radical (•OH), singlet oxygen (1O2), ozone (O3), H2O2, nitric oxide (NO•),and peroxinitrite (ONOO-), where •OH is the most reactive and interacts with proteins, lipids and nucleic acids [75,76]. About 90% of ROS is derived from mitochondrial oxidative phosphorylation (OXPHOS) activity through electron leakage in electron transport chain (ETC) [77-79]. In ETC, about 5% of O2 is transformed to H2O2 (Figure 2) [76].
Figure 2.
Mechanism of ROS production and neutralization in eukaryotic cells. Redox homeostasis of eukaryotic cell is presented. ROS are generated in the cell by enzymatic systems which include endothelial NOS, cyclooxygenase (COX), xanthine oxidase (XOD), P450 enzymes and mitochondrial ETC. eNOS is involved in the generation of O2•- and its transformation into ONOO- with the participation of NO•. COX, XOD and P450 enzymes also generate peroxide anion. In the VI complex of OXPHOS, a sequential univalent reduction of O2 occurs, which during the exchange of 4 electrons is reduced to H2O. However, about 5% of O2 is transformed to H2O2 in a process of electron leakage when only 2 electron reduction occurs. Moreover, extracellular H2O2 may enter the cell through membrane transporters e.g. aquaporins (AQP). H2O2 in specific conditions is transformed to •OH through Fenton reactions. •OH is highly reactive and potentially mutagenic to the cell. Antioxidant defense systems include SOD, TRX, GPX, PRX and chemical antioxidants (e.g. vitamins (C, A, E), β-carotene, GSH). Chemical antioxidants inhibit reaction cascades of ROS formation. Enzymatic antioxidants transform ROS into inactive molecules, e.g. H2O. GSH is treated as a non-enzymatic antioxidant as it is a substrate for the GPX. It reduces H2O2 and oxidizes GSH to form glutathione disulfide (GSSG). GSSG is then reduced to GSH by GR. CAT reduces H2O2 directly to H2O. PRX is another enzyme reducing reactive H2O2 molecules. PRX becomes its oxidized form (ox-PRX), reducing H2O2 to H2O. PRX is regenerated by TRX which becomes oxidized in this process (ox-TRX).
The effects of ROS include modifications within the genome [80]. Oxidative DNA lesions induced by ROS manifest as nucleobases and/or sugar fragments modification, single and double strand breaks, apurinic/apyrimidinic sites, modified purine/pyrimidine/sugars, structurally mutated bases, deleted and/or translocated chromosome fragments, and DNA-protein cross-links [81]. About 100 oxidative DNA lesions are now identified, which constitute the largest group of all lesion types [82].
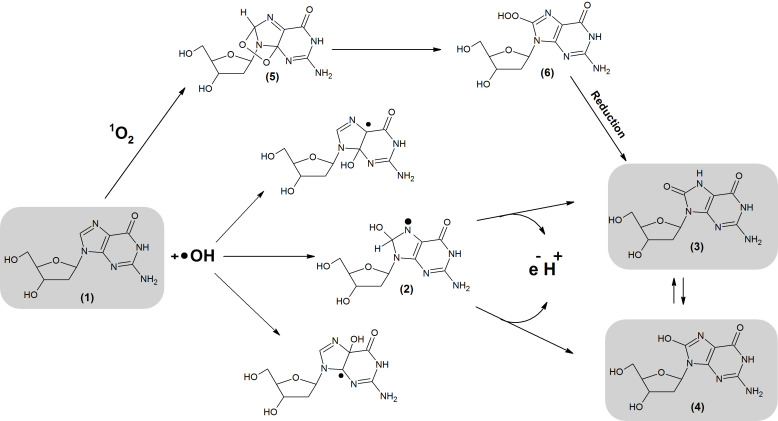
Due to the lowest redox potential, the most susceptible to OS is guanine (G) [83]. The most frequent lesions are 8-oxodG and 8-OHdG [84,85]. They are generated through interaction between 1O2 or •OH and G or 2′-deoxyguanosine (dG) (Figure 3). 8-oxodG is one of the most abundant lesions, which occurs 105 times per day/per cell [83,86].
Figure 3.
Formation of 8-oxodG and 8-OHdG. The lesions are generated through interaction between 1O2 or •OH and G or dG. As a result of •OH addition, different radical adducts are formed (1). Subsequently, electron abstraction generates 8-OHdG which undergoes keto-enol tautomerism forming an oxidized product: 8-oxodG. 8-oxodG and 8-OHdG lesions can also be generated by cycloaddition of 1O2 into the imidazole ring of dG. This reaction generates (5) which is later rearranged into (6). (1) 2′-deoxyguanosine, (2) 8-Hydroxy-7,8-dihydro-2′-deoxyguanosyl radical, (3) 8-oxodG, (4) 8-OHdG, (5) 4,8-endoperoxide-2′-deoxyguanosine, (6) 8-hydroperoxy-2′-deoxyguanosie.
Those lesions are corrected by the base excision repair (BER) system. The standard process consists of: recognition and excision of damaged base by glycosylases, filling in the nucleotide gap by polymerases, and strand ligation [87]. From glycosylases described in mammals, oxoguanine glycosylase 1 (OGG1) and formamidopyrimidine DNA glycosylase (Fpg) excise oxidative G lesions [88]. OGG1 is a primary BER bifunctional glycosylase, which removes 8-oxodG. However, OGG1 is susceptible to polymorphisms and may not always detect a damaged base. Therefore, 8-oxodG may form mis-pairs with adenine (A) leading to transversion (G:::C → T::A) or transition (G:::C → A::T) mutations. It makes G lesions highly mutagenic, especially when such mutations accumulate in the DNA strands in close proximity to one another [89]. MutY DNA glycosylase (MUTYH) is able to recognize and remove A opposite 8-oxodG and G [88,90]. OGG1 and MUTYH play crucial roles in normal cell functioning under OS and prevent development of pathological states [90].
Oxidative Stress and Oxidative DNA Damage in Thyroid Diseases
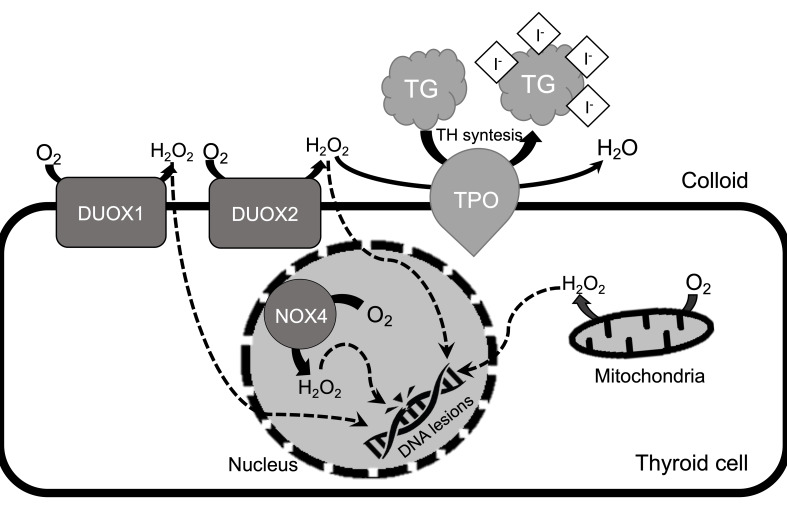
Redox homeostasis is important in the thyroid gland. H2O2, a redox signaling molecule, is produced in large amounts in thyrocytes due to its relevance in TH synthesis (Figure 4). Thyroid remains highly susceptible for oxidative damage in the case of any imbalance. Thus, it is crucial that thyroid cells maintain oxidative balance in order to prevent malignancies and disease development.
Figure 4.
Overview of oxidative DNA damage generation in thyrocytes. In the thyroid cell, TPO needs H2O2 in order to produce TH. H2O2 is generated by DUOX1 and DUOX2 which are NADPH oxidases. In the case of TD, extracellular H2O2 may cross the cellular membrane. It has a potential of damaging DNA directly or through NOX4 (NADPH oxidase 4) located in the nucleus and endoplasmic reticulum. Moreover, H2O2 generated by mitochondria may affect mitochondrial and nuclear DNA. H2O2 in specific conditions is transformed to •OH, which is highly reactive and mutagenic ROS.
TH regulate mitochondrial function and thus, the rate of oxygen metabolism. TH affect the speed of mitochondrial activity and the systemic production of ROS [79]. T3 controls the proinflammatory reactions (through induction of GPX and SOD) and reduces ROS production in mitochondria by activating the mitochondrial ATP-dependent potassium channel. Moreover, T3 stimulates autophagy, mitochondrial DNA repair, and creation of new mitochondria [91,92].
H2O2 is necessary for TH production, but at the same time it increases OS levels in the thyroid gland [93]. GPX, a major enzyme catalyzing the reduction of H2O2, protects thyrocytes from ROS action and modulates TH synthesis [94]. The present data are inconclusive – GPX level may be decreased or increased in patients with HT [95].
OS is considered one of the most important causes of DNA damage formation – in particular 8-oxodG and 8-OHdG [78]. While ID impairs TH synthesis, excess of I- in the body may induce OS and HT. Higher I- levels were confirmed in patients with newly diagnosed HT, but the mechanism is not clear [95]. It is assumed that H2O2 overproduction may result from NADPH oxidase overexpression and I-/Tyr interaction in TG [95].
ROS accumulation and OS levels are directly related to AITD. In HT, a positive correlation between OS markers and disease progression was demonstrated [96]. ROS accumulation appears to favor TG fragmentation, which indirectly stimulates the immune system to thyroid auto-aggression [91]. In GD, OS also increases. The NF-κB pathway, which is activated by OS, seems to play a significant role in GD’s autoimmunity [97]. The level of OS and accumulated lesions such as 8-oxodG and 8-OHdG is related to the occurrence of thyroid cancers. Therefore, intensification of O2 metabolism and decrease in DNA damage systems efficiency may have an effect on carcinogenesis.
8-oxodG and 8-OHdG as a Potential Stimulant of AITD Development During Pregnancy
DNA Oxidative Damage in Pregnancy
Sedentary lifestyle and exposure to toxins predispose to increased OS and might affect ova, sperm, and embryo development [98]. During uncomplicated gestation, OS is mainly stimulated by the mitochondria-rich placenta and purines metabolism. However, cells correctly neutralize or reduce the negative effects/products of ROS and maintain their concentration at the safe level.
ROS seem to play a pivotal role in right placenta development. Study on mice shows that 4-5 days after fertilization, the developing blastocyst produces O2•- (>8 nmol/embryo h-1) and H2O2 (ca. 4 nmol/embryo h-1). Moreover, cytochemical evidence of H2O2 entails the appearance of •OH [99,100]. In the first trimester, level of placental O2 is low – the embryo is protected from ROS which favors its development, placental angiogenesis, and cell proliferation. At the end of the first trimester, O2 levels increase due to stabilization of maternal intraplacental circulation. The possibility of normal fetal development is ensured by modulation of hypoxia-inducible factor 1α (HIF-1α) and antioxidant defense systems [101]. A study by Hung et al. shows that among healthy pregnant women, the urinary level of 8-oxodG increases in the third trimester and returns to physiological level after delivery. Moreover, other biomarkers of OS also increase (GPX and SOD) [102]. A subsequent study by Hung et al. presents that increased level of OS among pregnant women may implicate pregnancy complications [103]. Excessive OS may induce polycystic ovary syndrome, endometriosis, preeclampsia, idiopathic infertility, premature birth, recurrent pregnancy loss, and intrauterine growth restriction [104]. OS plays role in the course of pregnancy and thyroid disorders, therefore it is interesting to explore the scenario where both conditions are present simultaneously. Hence, the next sections attempt to explore this connection of oxidative stress and thyroid disease in pregnant women and also discuss factors which influence their incidence such as diet (e.g. antioxidant intake).
Oxidative Stress in Thyroid Disease and its Effect on Pregnancy
AITD are common endocrine dysfunctions during gestation which affect about 1% of all pregnant women [105]. H2O2 is a ROS and OS inducer but is also essential for TH synthesis. Studies show that H2O2 production is dependent on I- and TSH levels [106]. AITD are associated with TSH secretion, caused by ID and affect the H2O2 production in the thyroid gland. H2O2 does not react directly with genetic material but is a precursor of highly reactive compounds: 1O2 and •OH [107]. AITD are correlated with OS and its markers – 8-oxodG and 8-OHdG. These lesions are formed directly in the reaction of G moiety with •OH and 1O2 and, in the case of AITD, their accumulation is observed [24]. Hyperthyroidism is linked with overproduction of OS markers while hypothyroidism is linked with reduced availability of antioxidants [23].
Connections between AITD and the DNA damage is studied worldwide but still needs more extensive exploration. In 2013, studies assessed the level of 8-OHdG in people with TD. The results show that patients suffering from toxic multifollicular goiter, GD, and HT have a higher concentration of 8-OHdG in urine. For each disease, 8-OHdG is on average 22.26 ng/ml (5.11 ng/ml for the control) [108]. In addition, plasma 8-OHdG levels (1.23 ng/ml and 0.67 ng/ml in the study and control group, respectively) are useful markers of carcinogenic potential for multinodular goiter. Thyroid nodules occur in 68% of the general population (with 7-15% malignant) thus, testing 8-OHdG plasma levels may improve early diagnosis of thyroid cancer [24].
Impact of AITD in pregnant women on fetal OS is considered. A study from 2018 shows that subclinical form of hypothyroidism increases the level of OS in amniotic fluid – O2•- level increased from 0.1 to 0.2 nmol/106 cells. The authors suggest that diet and supplementation of antioxidants may counteract effects of OS [109]. A different study on pregnant women with clinical hypothyroidism shows that the level of O2•- in the amniotic fluid doubles (from 3.5 to 8.0 nmol/mL). Additionally, it describes a positive correlation between O2•- and a reduced body weight in women and reduced Apgar scores in newborns [110]. As suggested by those authors, environmental factors, such as diet and weight, impact the level of OS. In the case of pregnant women, diet and overall health is crucial for the mother and the fetus. However, not only as a mean to the well-nourished child and healthy women, but as it turns out also in terms of maintaining genetic integrity, preventing DNA damage formation, and DNA repair systems operation.
Impact of Diet on DNA Repair Mechanisms
DNA damage and its aftermath may cause cancer, neurodegenerative diseases, and others [111]. The accumulation of DNA lesions induce deregulation of cell functions e.g. DNA replication and transcription, proliferation, or immune response [112]. Fortunately, cells are equipped with several DNA repair mechanisms including direct repair, excision repairs, and recombination systems [113]. The fundamental mechanism for oxidative DNA damage repair is the BER system. Fragment of 1-20 nucleobases can be excised from DNA strand depending on the damage type [114].
The impact of a plant-rich diet on DNA repair mechanisms has been studied for over 20 years. While at first results were contradictory, currently it is known that a diet rich in vegetables and fruit has genoprotective properties and positive influence on DNA repair mechanisms, including BER. Clinical studies also stress the fact that poor dietary choices and subsequent problems, such as obesity, which induces ROS generation, are correlated with the thyroid and its disorders [115].
As diet provides antioxidants that restore the balance between the formation and removal of ROS it may reduce DNA damage level in the genome [116]. The most widely considered antioxidant is vitamin C. Its influence on oxidative lesions was identified for the first time in 1998 [117]. Subsequent years brought new results, where fruit and vegetable consumption and its influence on 8-oxodG, 8-OHdG, and in some cases DNA repair gene expression levels were examined [118-123]. Typically used in such studies are broccoli, kiwifruit, or vitamin C supplements. However, results were contradictory for a long time and hard to compare probably due to testing variable subject groups such as smokers, healthy people, or patients with different pathologies. However, subsequent years brought new insights and confirmed that diet rich in fruit and antioxidants protects from DNA damage and stimulates DNA repair [120,124-128]. Moreover, recent in vitro studies seem to confirm the beneficial properties of antioxidants on expression of proteins involved in DNA repair mechanisms (e.g. GPX, OGG1) [119,129-131]. Green tea is also widely considered as beneficial in the field of genome protection. Camellia sinensis increases OGG1 activity after only 7 days of regular intake. Moreover, DNA damage level decreases by 30% just after 1h from drinking the tea [129,132].
According to Lalonde’s theory, who already in 1976 determined that the overall health status of humans depend, in more than 50% on environmental factors, diet seems to be a major factor influencing our well-being [133]. These facts are especially important for women, as proper dietary choices and healthy lifestyle are crucial before, during, and after pregnancy.
Conclusions and Outlook
AITD, as a growing health problem of developing countries, became widely investigated in relation to pregnancy and early fetus development as a crucial part of human procreation. Increasing numbers of studies indicate a connection between ROS overgeneration and the regulation of the immune system during pregnancy. During uncomplicated gestation ROS levels are higher, mainly due to mitochondria-rich placenta [99]. OS is an important factor in predicting complications during pregnancy. It may cause dysfunction in cells and lead to a generation of DNA lesions such as 8-oxodG and 8-OHdG [102]. Elevated level of these lesions during pregnancy may indicate pathological states e.g. AITD. These disorders may cause complications for mother and fetus. Current evidence shows that patients with GD and HT have elevated levels of 8-OHdG in urine samples [108]. Considering novel data level of urine 8-OHdG can be an important biomarker of pregnancy complications, including those concerning thyroid. Moreover, increased level of ROS during gestation stimulates dysfunction of the endocrine system. This might induce doubling of O2•- levelsin the amniotic fluid or provoke reduced body weight among mothers and reduced Apgar scores in newborns [110]. Further studies in the field of redox biology are highly demanded. ROS formation and identification of possible cut-off values cannot be yet precisely monitored. If possible, this would help to predict negative consequence of ROS generation and develop personalized treatments.
As 8-oxodG and 8-OHdG are connected with AITD, studies should continue to determine their levels in various pathologies. Oxidative lesions may also indicate the condition of pregnant women and fetuses, but more data is needed concerning correlation of newborn health and level of oxidative DNA damage in the mother. In order to improve the care of pregnant mothers, future studies should focus on deepening knowledge about endocrine system dysfunction during pregnancy and possible cut-off values of 8-OHdG and 8-oxodG for later clinical application. We believe it would help to better understand the etiology of AITD, select high-risk patients, and avoid passing on risk of health complications onto a child. Therefore, the oxidative DNA damage as a potential AITD biomarker are worth exploring in order to advance personalized treatment options and early diagnosis, especially for pregnant women.
Furthermore, AITD have a direct impact on human reproductive capacity [25,27]. Apart from sex hormone disorders, men experience sexual dysfunction, such as impotence or frigidity [26,31]. In women, AITD result mostly in menstrual disorders as well as limited fertility and/or ability to maintain pregnancy [67,70,71]. Information presented in this review allows us to assume that different kind of DNA lesions may be the missing link between AITD and complications of pregnancy, including miscarriages. Therefore, we believe that studies should be undertaken to examine how cellular systems of DNA damage control impact pregnant women with AITD and the condition of the fetus.
As mentioned in the previous section, nutrients and antioxidant intake influence to some extent oxidative DNA lesion levels and efficiency of BER mechanism, which in turn may impact all reproductive processes – female and male fertility, conception, pregnancy, and even proper development of the fetus. Recently, more studies confirm a connection between the diet and cellular capacity to prevent and/or repair DNA lesions, including 8-oxodG. Antioxidants present in e.g. broccoli or green tea, are important factors in maintaining cellular redox homeostasis, hence, increasing the defense capabilities of cells.
All species are not supposed to be immortal (long living). The main evolutionary goal is to sustain life, reproduce, and pass on the genetic material to ensure the survival of the entire species. In this context, DNA is the most important particle of life and any damage or impairment to its integrity may have severe consequences for the survival of the species. Therefore, it is of high importance that the genome remains intact or properly repaired by specialized systems, so the whole organism (human being) may serve its purpose in evolution.
Referring to Lalonde’s theory once more, if 50% of our health depends on environmental factors, and the major factor in everyday life is the diet, we have a vast possibility to influence our own well-being. Due to the fact that both pregnancy and AITD are related to increased DNA damage formation, a healthy plant-rich diet should be integral part of the strategy to protect our organism from destructive influence of OS and oxidative DNA damage. It especially concerns pregnant women with higher risk or already diagnosed AITD [134].
Acknowledgments
This work is supported by the Medical University of Lodz (503/3-045-02/503-31-001-19-00) and by the National Science Center, Poland (grant No. 2016/23/B/NZ7/03367).
Glossary
- 8-oxodG
8-oxo-7,8-dihydro-2′-deoxyguanosine
- 8-OHdG
8-hydroxy-2′-deoxyguanosine
- AES
American Endocrine Society
- AITD
autoimmune thyroid diseases
- ATA
American Thyroid Association
- BER
base excision repair system
- CAT
catalase
- DIO2
iodothyronine deiodinase type 2
- DIO3
iodothyronine deiodinase type 3
- DIT
diiodothyronine
- ETA
European Thyroid Association
- ETC
electron transport chain
- G
guanine
- dG
2′-deoxyguanosine
- GD
Graves’ disease
- GPX
glutathione peroxidase
- GR
glutathione reductase
- GSH
glutathione
- H2O2
hydrogen peroxide
- hCG
chorionic gonadotropin
- HO2•
hydroperoxyl radical
- HT
Hashimoto’s thyroiditis
- I-
iodine
- 131I
iodine-131
- ID
iodine deficiency
- MIT
monoiodothyronine
- MUTYH
MutY DNA glycosylase
- NO•
nitric oxide
- NOS
nitric oxide synthase
- O2•-
superoxide anion radical
- 1O2
singlet oxygen
- OGG1
oxoguanine glycosylase 1
- •OH
hydroxyl radical
- ONOO-
peroxinitrite
- OS
oxidative stress
- OXPHOS
oxidative phosphorylation
- PRX
peroxiredoxin
- ROS
reactive oxygen species
- RTH
Refetoff syndrome thyroid hormone resistance
- SOD
superoxide dismutase
- T2
diiodothyronine, 3,3′-diiodothyronine
- T3
triiodothyronine, 3,5,3′-triiodothyronine
- rT3
reverse triiodothyronine
- T4
thyroxine, 3,5,3′,5′-tetraiodothyronine
- TBG
thyroxine-binding globulin
- TD
thyroid diseases
- TG
thyroglobulin
- TH
thyroid hormones
- TPO
thyroid peroxidase
- TR
thyroid hormone receptor
- TRH
thyrotropin-releasing hormone
- TRX
thioredoxin
- TSH
thyrotropin thyroid-stimulating hormone
- TRHR
TSH receptor
- Tyr
tyrosine
Author Contributions
KH: 50% Conception and design, data collection and analysis, writing, review and editing. ORCID iD: https://orcid.org/0000-0003-1709-5897. KB: 10% Conception and design, writing, review and editing. ORCID iD: https://orcid.org/0000-0003-3772-8540. SU: 10% Conception and design, data collection and analysis, writing, review and editing. ORCID iD: https://orcid.org/0000-0003-1159-7167. MS: 10% Conception and design, data collection and analysis, writing, review and editing. ORCID iD: https://orcid.org/0000-0002-5680-6594. BTK: 20% Conception and design, review and editing, study supervision. ORCID iD: https://orcid.org/0000-0001-6922-7834.
References
- Perros P. A decade of thyroidology. Hormones (Athens). 2018. December;17(4):491–5. 10.1007/s42000-018-0068-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong LL, Liu H, Liu LH. Relationship between hypothyroidism and the incidence of gestational diabetes: A meta-analysis. Taiwan J Obstet Gynecol. 2016. April;55(2):171–5. 10.1016/j.tjog.2016.02.004 [DOI] [PubMed] [Google Scholar]
- WHO Assessment of the iodine deficiency disorders and monitoring their elimination. Geneva: WHO; 2007. pp. 1–107. [Google Scholar]
- Li M, Eastman CJ. The changing epidemiology of iodine deficiency. Nat Rev Endocrinol. 2012. April;8(7):434–40. 10.1038/nrendo.2012.43 [DOI] [PubMed] [Google Scholar]
- Murray CW, Egan SK, Kim H, Beru N, Bolger PM. US food and drug administration’s total diet study: dietary intake of perchlorate and iodine. J Expo Sci Environ Epidemiol. 2008. November;18(6):571–80. 10.1038/sj.jes.7500648 [DOI] [PubMed] [Google Scholar]
- Chaker L, Bianco AC, Jonklaas J, Peeters RP. Hypothyroidism. Lancet. 2017. September;390(10101):1550–62. 10.1016/S0140-6736(17)30703-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor PN, Albrecht D, Scholz A, Gutierrez-Buey G, Lazarus JH, Dayan CM, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018. May;14(5):301–16. 10.1038/nrendo.2018.18 [DOI] [PubMed] [Google Scholar]
- Merson J. Hypothyroidism. JAAPA. 2018. December;31(12):43–4. 10.1097/01.JAA.0000547758.46299.d9 [DOI] [PubMed] [Google Scholar]
- Maniakas A, Davies L, Zafereo ME. Thyroid Disease Around the World. Otolaryngol Clin North Am. 2018. June;51(3):631–42. 10.1016/j.otc.2018.01.014 [DOI] [PubMed] [Google Scholar]
- Alexander EK, Pearce EN, Brent GA, Brown RS, Chen H, Dosiou C, et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid. 2017. March;27(3):315–89. 10.1089/thy.2016.0457 [DOI] [PubMed] [Google Scholar]
- Lazarus J, Brown RS, Daumerie C, Hubalewska-Dydejczyk A, Negro R, Vaidya B. 2014 European thyroid association guidelines for the management of subclinical hypothyroidism in pregnancy and in children. Eur Thyroid J. 2014. June;3(2):76–94. 10.1159/000362597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan YQ, Dong ZL, Dong L, Wang FR, Yang XM, Jin XY, et al. Trimester- and method-specific reference intervals for thyroid tests in pregnant Chinese women: methodology, euthyroid definition and iodine status can influence the setting of reference intervals. Clin Endocrinol (Oxf). 2011. February;74(2):262–9. 10.1111/j.1365-2265.2010.03910.x [DOI] [PubMed] [Google Scholar]
- Marwaha RK, Chopra S, Gopalakrishnan S, Sharma B, Kanwar RS, Sastry A, et al. Establishment of reference range for thyroid hormones in normal pregnant Indian women. BJOG. 2008. April;115(5):602–6. 10.1111/j.1471-0528.2008.01673.x [DOI] [PubMed] [Google Scholar]
- Dahl L, Wik Markhus M, Sanchez PV, Moe V, Smith L, Meltzer HM, et al. Iodine deficiency in a study population of norwegian pregnant women—results from the little in Norway study (LiN). Nutrients. 2018. April;10(4):1–14. 10.3390/nu10040513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhry H, Nasrullah M. Iodine consumption and cognitive performance: confirmation of adequate consumption. Food Sci Nutr. 2018. June;6(6):1341–51. 10.1002/fsn3.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su PY, Huang K, Hao JH, Xu YQ, Yan SQ, Li T, et al. Maternal thyroid function in the first twenty weeks of pregnancy and subsequent fetal and infant development: a prospective population-based cohort study in China. J Clin Endocrinol Metab. 2011. October;96(10):3234–41. 10.1210/jc.2011-0274 [DOI] [PubMed] [Google Scholar]
- Delshad H, Azizi F. Iodine nutrition in pregnant and breastfeeding women: sufficiency, deficiency, and supplementation. Hormones (Athens). 2020. June;19(2):179–86. 10.1007/s42000-019-00160-2 [DOI] [PubMed] [Google Scholar]
- Dietary Reference Intakes for Vitamin A. Vitamin K Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. Washington (DC). US: National Academies Press; 2001. [PubMed] [Google Scholar]
- EFSA Dietary Reference Values for nutrients Summary report. EFSA Support Publ. 2017;14(12).
- Reincke M, Lehnert H. [Thyroid diseases - an update]. Internist (Berl). 2018. July;59(7):641–3. 10.1007/s00108-018-0437-y [DOI] [PubMed] [Google Scholar]
- Banga JP, Schott M. Autoimmune Thyroid Diseases. Horm Metab Res. 2018. December;50(12):837–9. 10.1055/a-0799-5068 [DOI] [PubMed] [Google Scholar]
- Young O, Crotty T, O’Connell R, O’Sullivan J, Curran AJ. Levels of oxidative damage and lipid peroxidation in thyroid neoplasia. Head Neck. 2010. June;32(6):750–6. [DOI] [PubMed] [Google Scholar]
- Mancini A, Di Segni C, Raimondo S, Olivieri G, Silvestrini A, Meucci E, Currò D. Thyroid Hormones, Oxidative Stress, and Inflammation. Mediators Inflamm. 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donmez-Altuntas H, Bayram F, Bitgen N, Ata S, Hamurcu Z, Baskol G. Increased Chromosomal and Oxidative DNA Damage in Patients with Multinodular Goiter and Their Association with Cancer. Int J Endocrinol. 2017;2017:2907281. 10.1155/2017/2907281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassas GE, Markou KB. The impact of thyroid diseases starting from birth on reproductive function. Hormones (Athens). 2019. December;18(4):365–81. 10.1007/s42000-019-00156-y [DOI] [PubMed] [Google Scholar]
- Krassas GE, Tziomalos K, Papadopoulou F, Pontikides N, Perros P. Erectile dysfunction in patients with hyper- and hypothyroidism: how common and should we treat? J Clin Endocrinol Metab. 2008. May;93(5):1815–9. 10.1210/jc.2007-2259 [DOI] [PubMed] [Google Scholar]
- Meikle AW. The interrelationships between thyroid dysfunction and hypogonadism in men and boys. Thyroid. 2004;14 Suppl 1:S17–25. 10.1089/105072504323024552 [DOI] [PubMed] [Google Scholar]
- Gaberšček S, Zaletel K. Thyroid physiology and autoimmunity in pregnancy and after delivery. Expert Rev Clin Immunol. 2011. September;7(5):697–706. 10.1586/eci.11.42 [DOI] [PubMed] [Google Scholar]
- Kumar Sharma M, Parchwani D, Maheria P, Upadhyah A. Relationship Between Thyroid Profile and Semen Quality. Natl J Community Med. 2012;3:250–8. [Google Scholar]
- García-Vázquez FA, Gadea J, Matás C, Holt WV. Importance of sperm morphology during sperm transport and fertilization in mammals. Asian J Androl. 2016. Nov-Dec;18(6):844–50. 10.4103/1008-682X.186880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carani C, Isidori AM, Granata A, Carosa E, Maggi M, Lenzi A, et al. Multicenter study on the prevalence of sexual symptoms in male hypo- and hyperthyroid patients. J Clin Endocrinol Metab. 2005. December;90(12):6472–9. 10.1210/jc.2005-1135 [DOI] [PubMed] [Google Scholar]
- Luster M, Duntas LH, Wartofsky L. The Thyroid and its Diseases - A Comprehensive Guide for the Clinician. 1st ed. Springer; 2019. 10.1007/978-3-319-72102-6 [DOI] [Google Scholar]
- Barret KE, Barman SM, Brooks HL, Yan J. Ganong’s Review of Medical Physiology. McGraw-Hill Education; 2019. [Google Scholar]
- Silva JF, Ocarino NM, Serakides R. Thyroid hormones and female reproduction. Biol Reprod. 2018. November;99(5):907–21. [DOI] [PubMed] [Google Scholar]
- Zhou A, Wei Z, Read RJ, Carrell RW. Structural mechanism for the carriage and release of thyroxine in the blood. Proc Natl Acad Sci USA. 2006. September;103(36):13321–6. 10.1073/pnas.0604080103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodwell VW, Kennelly PJ, Bender DA, Botham KM, Weil PA. Harper’s Illustrated Biochemistry. McGraw-Hill Education; 2018. [Google Scholar]
- Fountoulakis S, Philippou G, Tsatsoulis A. The role of iodine in the evolution of thyroid disease in Greece: from endemic goiter to thyroid autoimmunity. Hormones (Athens). 2007. Jan-Mar;6(1):25–35. [PubMed] [Google Scholar]
- Soundarrajan M, Kopp PA. Thyroid Hormone Biosynthesis and Physiology In: Eaton J. Thyroid Disease and Reproduction. Cham: Springer; 2019. 10.1007/978-3-319-99079-8_1 [DOI] [Google Scholar]
- van de Graaf SA, Ris-Stalpers C, Pauws E, Mendive FM, Targovnik HM, de Vijlder JJ. Up to date with human thyroglobulin. J Endocrinol. 2001. August;170(2):307–21. 10.1677/joe.0.1700307 [DOI] [PubMed] [Google Scholar]
- Popławski P, Wiśniewski JR, Rijntjes E, Richards K, Rybicka B, Köhrle J, et al. Restoration of type 1 iodothyronine deiodinase expression in renal cancer cells downregulates oncoproteins and affects key metabolic pathways as well as anti-oxidative system. PLoS One. 2017. December;12(12):e0190179. 10.1371/journal.pone.0190179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakane Y, Kanamoto N, Yamauchi I, Tagami T, Morita Y, Miura M, et al. Regulation of type 1 iodothyronine deiodinase by LXRα. PLoS One. 2017. June;12(6):e0179213. 10.1371/journal.pone.0179213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento BP, Bocco BM, Fernandes GW, Fonseca TL, McAninch EA, Cardoso CV, et al. Induction of type 2 iodothyronine deiodinase after status epilepticus modifies hippocampal gene expression in male mice. Endocrinology. 2018. August;159(8):3090–104. 10.1210/en.2018-00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura K, Takeda M, Yamashita JK, Shiojima I, Toyoda N. Type 3 iodothyronine deiodinase is expressed in human induced pluripotent stem cell derived cardiomyocytes. Life Sci. 2018. June;203:276–81. 10.1016/j.lfs.2018.04.037 [DOI] [PubMed] [Google Scholar]
- Lee S, Farwell AP. Euthyroid sick syndrome. Compr Physiol. 2016. March;6(2):1071–80. 10.1002/cphy.c150017 [DOI] [PubMed] [Google Scholar]
- Benvenga S, Tuccari G, Ieni A, Vita R. Thyroid Gland: Anatomy and Physiology 2nd edition. In: Encyclopedia of Endocrine Diseases. Elsevier Inc. 2018. 382–390.
- Peeters RP, Wouters PJ, van Toor H, Kaptein E, Visser TJ, Van den Berghe G. Serum 3,3′,5′-triiodothyronine (rT3) and 3,5,3′-triiodothyronine/rT3 are prognostic markers in critically ill patients and are associated with postmortem tissue deiodinase activities. J Clin Endocrinol Metab. 2005. August;90(8):4559–65. 10.1210/jc.2005-0535 [DOI] [PubMed] [Google Scholar]
- Ahad F, Ganie SA. Iodine, Iodine metabolism and Iodine deficiency disorders revisited. Indian J Endocrinol Metab. 2010. January;14(1):13–7. [PMC free article] [PubMed] [Google Scholar]
- Visser WE, Friesema EC, Jansen J, Visser TJ. Thyroid hormone transport by monocarboxylate transporters. Best Pract Res Clin Endocrinol Metab. 2007. June;21(2):223–36. 10.1016/j.beem.2007.03.008 [DOI] [PubMed] [Google Scholar]
- Schomburg L, Köhrle J. On the importance of selenium and iodine metabolism for thyroid hormone biosynthesis and human health. Mol Nutr Food Res. 2008. November;52(11):1235–46. 10.1002/mnfr.200700465 [DOI] [PubMed] [Google Scholar]
- Elzouki AY. Textbook of clinical pediatrics. 2nd edition. Harfi HA, Nazer HM, Stapleton FB, Oh W, Whitley RJ, editors. Springer. 2012. https://doi.org/ 10.1007/978-3-642-02202-9 [DOI]
- Skeaff SA. Iodine deficiency in pregnancy: the effect on neurodevelopment in the child. Nutrients. 2011. February;3(2):265–73. 10.3390/nu3020265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panel E, Nda A. Scientific Opinion on nutrient requirements and dietary intakes of infants and young children in the European Union. EFSA J. 2013;11(10):1–103. [Google Scholar]
- Rao SD. Epidemiology of parathyroid disorders. Best Pract Res Clin Endocrinol Metab. 2018. December;32(6):773–80. 10.1016/j.beem.2018.12.003 [DOI] [PubMed] [Google Scholar]
- McGrogan A, Seaman HE, Wright JW, de Vries CS. The incidence of autoimmune thyroid disease: a systematic review of the literature. Clin Endocrinol (Oxf). 2008. November;69(5):687–96. 10.1111/j.1365-2265.2008.03338.x [DOI] [PubMed] [Google Scholar]
- Cui Z, Wang Z, Liu X, Cai Y, Xu X, Yang T. Establishment of clinical diagnosis model of Graves’ disease and Hashimoto’s thyroiditis. J Transl Med. 2019. January;17(1):11. 10.1186/s12967-018-1765-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groër MW. Postpartum thyroiditis. Expert Rev Obstet Gynecol. 2008;3(2):239–44. 10.1586/17474108.3.2.239 [DOI] [Google Scholar]
- Fuse Y, Shishiba Y, Irie M. Gestational changes of thyroid function and urinary iodine in thyroid antibody-negative Japanese women. Endocr J. 2013;60(9):1095–106. 10.1507/endocrj.EJ13-0184 [DOI] [PubMed] [Google Scholar]
- Moleti M, Trimarchi F, Vermiglio F. Thyroid physiology in pregnancy. Endocr Pract. 2014. June;20(6):589–96. 10.4158/EP13341.RA [DOI] [PubMed] [Google Scholar]
- Miranda A, Sousa N. Maternal hormonal milieu influence on fetal brain development. Brain Behav. 2018. January;8(2):e00920. 10.1002/brb3.920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco I, Bath SC, Rayman MP. Iodine as essential nutrient during the first 1000 days of life. Nutrients. 2018. March;10(3):1–16. 10.3390/nu10030290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng S, Li C, Xie X, Zhang X, Wang D, Lu X, et al. Divergence of Iodine and Thyroid Hormones in the Fetal and Maternal Parts of Human-Term Placenta. Biol Trace Elem Res. 2020. May;195(1):27–38. 10.1007/s12011-019-01834-z [DOI] [PubMed] [Google Scholar]
- Watanabe M, Iwatani Y, Kaneda T, Hidaka Y, Mitsuda N, Morimoto Y, et al. Changes in T, B, and NK lymphocyte subsets during and after normal pregnancy. Am J Reprod Immunol. 1997. May;37(5):368–77. 10.1111/j.1600-0897.1997.tb00246.x [DOI] [PubMed] [Google Scholar]
- Andersen SL, Olsen J, Carlé A, Laurberg P. Hyperthyroidism incidence fluctuates widely in and around pregnancy and is at variance with some other autoimmune diseases: a Danish population-based study. J Clin Endocrinol Metab. 2015. March;100(3):1164–71. 10.1210/jc.2014-3588 [DOI] [PubMed] [Google Scholar]
- Niewold TB, Mehta-Lee S. When pregnancy tames the wolf. J Exp Med. 2019. May;216(5):1012–3. 10.1084/jem.20190378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhaim Rochester D, Davies TF. Increased risk of Graves’ disease after pregnancy. Thyroid. 2005. November;15(11):1287–90. 10.1089/thy.2005.15.1287 [DOI] [PubMed] [Google Scholar]
- Andersen SL, Carlé A, Olsen J, Laurberg P. Hypothyroidism incidence in and around pregnancy: a Danish nationwide study. Eur J Endocrinol. 2016. November;175(5):387–93. 10.1530/EJE-16-0446 [DOI] [PubMed] [Google Scholar]
- Quintino-Moro A, Zantut-Wittmann DE, Tambascia M, MacHado HDC, Fernandes A. High prevalence of infertility among women with Graves’ disease and Hashimoto’s thyroiditis. Int J Endocrinol. 2014;2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krassas GE, Pontikides N, Kaltsas T, Papadopoulou P, Paunkovic J, Paunkovic N, et al. Disturbances of menstruation in hypothyroidism. Clin Endocrinol (Oxf). 1999. May;50(5):655–9. 10.1046/j.1365-2265.1999.00719.x [DOI] [PubMed] [Google Scholar]
- Unuane D, Velkeniers B. Impact of thyroid disease on fertility and assisted conception. Best Pract Res Clin Endocrinol Metab. 2020. January;101378:101378. 10.1016/j.beem.2020.101378 [DOI] [PubMed] [Google Scholar]
- Pfeifer S, Butts S, Dumesic D, Fossum G, Goldberg J, Gracia C, et al. Practice Committee of the American Society for Reproductive Medicine . Subclinical hypothyroidism in the infertile female population: a guideline. Fertil Steril. 2015. September;104(3):545–53. 10.1016/j.fertnstert.2015.05.028 [DOI] [PubMed] [Google Scholar]
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ. Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol. 2009. June;160(6):985–91. 10.1530/EJE-08-0953 [DOI] [PubMed] [Google Scholar]
- Liu H, Shan Z, Li C, Mao J, Xie X, Wang W, et al. Maternal subclinical hypothyroidism, thyroid autoimmunity, and the risk of miscarriage: a prospective cohort study. Thyroid. 2014. November;24(11):1642–9. 10.1089/thy.2014.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Pan X, Teng W, Shan Z. Patients with subclinical hypothyroidism before 20 weeks of pregnancy have a higher risk of miscarriage: A systematic review and meta-analysis. PLoS One. 2017. April;12(4):e0175708. 10.1371/journal.pone.0175708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aledo JC. Life-history constraints on the mechanisms that control the rate of ROS production. Curr Genomics. 2014. June;15(3):217–30. 10.2174/1389202915666140515230615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes Silva A. The Association between Physical Exercise and Reactive Oxygen Species (ROS) Production. J Sports Med Doping Stud. 2015;05(01):1–7. 10.4172/2161-0673.1000152 [DOI] [Google Scholar]
- Karwowski BT. 5′8-cyklo-deoksyadenozyna. Podwójne uszkodzenie w obrębie pojedynczego nukleozydu/nukleotydu. Wiad Chem. 2010;64:1013–48. [Google Scholar]
- Jastroch M, Divakaruni AS, Mookerjee S, Treberg JR, Brand MD. Mitochondrial proton and electron leaks. Essays Biochem. 2010;47:53–67. 10.1042/bse0470053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameziane El Hassani R, Buffet C, Leboulleux S, Dupuy C. Oxidative stress in thyroid carcinomas: biological and clinical significance. Endocr Relat Cancer. 2019. March;26(3):R131–43. 10.1530/ERC-18-0476 [DOI] [PubMed] [Google Scholar]
- Zambrano A, García-Carpizo V, Gallardo ME, Villamuera R, Gómez-Ferrería MA, Pascual A, et al. The thyroid hormone receptor β induces DNA damage and premature senescence. J Cell Biol. 2014. January;204(1):129–46. 10.1083/jcb.201305084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das KC, White CW. Redox systems of the cell: possible links and implications. Proc Natl Acad Sci USA. 2002. July;99(15):9617–8. 10.1073/pnas.162369199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitaki Z, Hellweg CE, Georgakilas AG, Ravanat JL. Stress-induced DNA damage biomarkers: applications and limitations. Front Chem. 2015. June;3(June):35. 10.3389/fchem.2015.00035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet J, Wagner JR. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol. 2013. February;5(2):a012559. 10.1101/cshperspect.a012559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba X, Boldogh I. 8-Oxoguanine DNA glycosylase 1: beyond repair of the oxidatively modified base lesions. Redox Biol. 2018. April;14(14):669–78. 10.1016/j.redox.2017.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valavanidis A, Vlachogianni T, Fiotakis C. 8-hydroxy-2′ -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis. J Environ Sci Health Part C Environ Carcinog Ecotoxicol Rev. 2009. April;27(2):120–39. 10.1080/10590500902885684 [DOI] [PubMed] [Google Scholar]
- Urbaniak SK, Boguszewska K, Szewczuk M, Kaźmierczak-Barańska J, Karwowski BT. 8-Oxo-7,8-Dihydro-2′-Deoxyguanosine (8-oxodG) and 8-Hydroxy-2′-Deoxyguanosine (8-OHdG) as a Potential Biomarker for Gestational Diabetes Mellitus (GDM) Development. Molecules. 2020. January;25(1):1–13. 10.3390/molecules25010202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara M, Jiang L, Akatsuka S, Suyama M, Toyokuni S. Genome-wide profiling of 8-oxoguanine reveals its association with spatial positioning in nucleus. DNA Res. 2014. December;21(6):603–12. 10.1093/dnares/dsu023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izumi T, Mellon I. Base Excision Repair and Nucleotide Excision Repair. Genome Stability: From Virus to Human Application. Elsevier Inc.; 2016. pp. 275–302. [Google Scholar]
- Krokan HE, Bjørås M. Base excision repair. Cold Spring Harb Perspect Biol. 2013. April;5(4):a012583. 10.1101/cshperspect.a012583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunniffe S, Walker A, Stabler R, O’Neill P, Lomax ME. Increased mutability and decreased repairability of a three-lesion clustered DNA-damaged site comprised of an AP site and bi-stranded 8-oxoG lesions. Int J Radiat Biol. 2014. June;90(6):468–79. 10.3109/09553002.2014.899449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira AH, da Silva AE, de Oliveira IM, Henriques JA, Agnez-Lima LF. MutY-glycosylase: an overview on mutagenesis and activities beyond the GO system. Mutat Res. 2014. November;769:119–31. 10.1016/j.mrfmmm.2014.08.002 [DOI] [PubMed] [Google Scholar]
- Gheorghiu ML, Badiu C. Selenium involvement in mitochondrial function in thyroid disorders. Hormones (Athens). 2020. March;19(1):25–30. 10.1007/s42000-020-00173-2 [DOI] [PubMed] [Google Scholar]
- Forini F, Nicolini G, Kusmic C, Iervasi G. Protective effects of euthyroidism restoration on mitochondria function and quality control in cardiac pathophysiology. Int J Mol Sci. 2019. July;20(14):1–20. 10.3390/ijms20143377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansourian AR. Metabolic pathways of tetraidothyronine and triidothyronine production by thyroid gland: a review of articles. Pak J Biol Sci. 2011. January;14(1):1–12. 10.3923/pjbs.2011.1.12 [DOI] [PubMed] [Google Scholar]
- Jiao Y, Wang Y, Guo S, Wang G. Glutathione peroxidases as oncotargets. Oncotarget. 2017. August;8(45):80093–102. 10.18632/oncotarget.20278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rostami R, Aghasi MR, Mohammadi A, Nourooz-Zadeh J. Enhanced oxidative stress in Hashimoto’s thyroiditis: inter-relationships to biomarkers of thyroid function. Clin Biochem. 2013. March;46(4-5):308–12. 10.1016/j.clinbiochem.2012.11.021 [DOI] [PubMed] [Google Scholar]
- Ates I, Arikan MF, Altay M, Yilmaz FM, Yilmaz N, Berker D, et al. The effect of oxidative stress on the progression of Hashimoto’s thyroiditis. Arch Physiol Biochem. 2018. October;124(4):351–6. 10.1080/13813455.2017.1408660 [DOI] [PubMed] [Google Scholar]
- Žarković M. The role of oxidative stress on the pathogenesis of Graves’ disease. J Thyroid Res. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bak A, Roszkowski K. Oxidative stress in pregnant women. Arch Perinat Med. 2013;19(3):150–5. [Google Scholar]
- Lappas M, Hiden U, Desoye G, Froehlich J, Hauguel-de Mouzon S, Jawerbaum A. The role of oxidative stress in the pathophysiology of gestational diabetes mellitus. Antioxid Redox Signal. 2011. December;15(12):3061–100. 10.1089/ars.2010.3765 [DOI] [PubMed] [Google Scholar]
- Manes C, Lai NC. Nonmitochondrial oxygen utilization by rabbit blastocysts and surface production of superoxide radicals. J Reprod Fertil. 1995. May;104(1):69–75. 10.1530/jrf.0.1040069 [DOI] [PubMed] [Google Scholar]
- Pereira AC, Martel F. Oxidative stress in pregnancy and fertility pathologies. Cell Biol Toxicol. 2014. October;30(5):301–12. 10.1007/s10565-014-9285-2 [DOI] [PubMed] [Google Scholar]
- Hung TH, Lo LM, Chiu TH, Li MJ, Yeh YL, Chen SF, et al. A longitudinal study of oxidative stress and antioxidant status in women with uncomplicated pregnancies throughout gestation. Reprod Sci. 2010. April;17(4):401–9. 10.1177/1933719109359704 [DOI] [PubMed] [Google Scholar]
- Hung TH, Chen SF, Hsieh TT, Lo LM, Li MJ, Yeh YL. The associations between labor and delivery mode and maternal and placental oxidative stress. Reprod Toxicol. 2011. February;31(2):144–50. 10.1016/j.reprotox.2010.11.009 [DOI] [PubMed] [Google Scholar]
- Wojsiat J, Korczyński J, Borowiecka M, Żbikowska HM. The role of oxidative stress in female infertility and in vitro fertilization. Postepy Hig Med Dosw. 2017. May;71(0):359–66. 10.5604/01.3001.0010.3820 [DOI] [PubMed] [Google Scholar]
- Yim CH. Update on the management of thyroid disease during pregnancy. Endocrinol Metab (Seoul). 2016. September;31(3):386–91. 10.3803/EnM.2016.31.3.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szanto I, Pusztaszeri M, Mavromati M. H2O2 Metabolism in Normal Thyroid Cells and in Thyroid Tumorigenesis: focus on NADPH Oxidases. Antioxidants. 2019. May;8(5):126. 10.3390/antiox8050126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justyna J, Czarnocka B. Oxidative DNA damage and repair in thyroid gland. Postępy Nauk Med. 2011;(11). [Google Scholar]
- Ece H, Mehmet E, Cigir BA, Yavuz D, Muammer K, Cumhur G, et al. Serum 8-OHdG and HIF-1α levels: do they affect the development of malignancy in patients with hypoactive thyroid nodules? Contemp Oncol (Pozn). 2013;17(1):51–7. 10.5114/wo.2013.33774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakovic TR, Dolicanin ZC, Djordjevic NZ. Effects of maternal subclinical hypothyroidism on amniotic fluid cells oxidative status. Reprod Toxicol. 2018. June;78:97–101. 10.1016/j.reprotox.2018.04.002 [DOI] [PubMed] [Google Scholar]
- Novakovic TR, Dolicanin ZC, Djordjevic NZ. Oxidative stress biomarkers in amniotic fluid of pregnant women with hypothyroidism. J Matern Fetal Neonatal Med. 2019. April;32(7):1105–10. 10.1080/14767058.2017.1400005 [DOI] [PubMed] [Google Scholar]
- Murata M. Inflammation and cancer. Environ Health Prev Med. 2018. October;23(1):50. 10.1186/s12199-018-0740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedelnikova OA, Redon CE, Dickey JS, Nakamura AJ, Georgakilas AG, Bonner WM. Role of oxidatively induced DNA lesions in human pathogenesis. Mutat Res. 2010. Apr-Jun;704(1-3):152–9. 10.1016/j.mrrev.2009.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee N, Walker GC. Mechanisms of DNA damage, repair, and mutagenesis. Environ Mol Mutagen. 2017. June;58(5):235–63. 10.1002/em.22087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabzinski J, Mucha B, Cuchra M, Markiewicz L, Przybylowska K, Dziki A, et al. Efficiency of Base Excision Repair of Oxidative DNA Damage and Its Impact on the Risk of Colorectal Cancer in the Polish Population. Oxid Med Cell Longev. 2016;2016:3125989. 10.1155/2016/3125989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscemi S, Massenti FM, Vasto S, Galvano F, Buscemi C, Corleo D, et al. Association of obesity and diabetes with thyroid nodules. Endocrine. 2018. May;60(2):339–47. 10.1007/s12020-017-1394-2 [DOI] [PubMed] [Google Scholar]
- Del Bo’ C, Marino M, Martini D, Tucci M, Ciappellano S, Riso P, et al. Overview of human intervention studies evaluating the impact of the mediterranean diet on markers of DNA damage. Nutrients. 2019. February;11(2):E391. 10.3390/nu11020391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke MS, Evans MD, Podmore ID, Herbert KE, Mistry N, Mistry P, et al. Novel repair action of vitamin C upon in vivo oxidative DNA damage. FEBS Lett. 1998. November;439(3):363–7. 10.1016/S0014-5793(98)01403-3 [DOI] [PubMed] [Google Scholar]
- Møller P, Vogel U, Pedersen A, Dragsted LO, Sandström B, Loft S. No effect of 600 grams fruit and vegetables per day on oxidative DNA damage and repair in healthy nonsmokers. Cancer Epidemiol Biomarkers Prev. 2003. October;12(10):1016–22. [PubMed] [Google Scholar]
- Riso P, Martini D, Møller P, Loft S, Bonacina G, Moro M, et al. DNA damage and repair activity after broccoli intake in young healthy smokers. Mutagenesis. 2010. November;25(6):595–602. 10.1093/mutage/geq045 [DOI] [PubMed] [Google Scholar]
- Bøhn SK, Myhrstad MC, Thoresen M, Holden M, Karlsen A, Tunheim SH, et al. Blood cell gene expression associated with cellular stress defense is modulated by antioxidant-rich food in a randomised controlled clinical trial of male smokers. BMC Med. 2010. September;8(1):54. 10.1186/1741-7015-8-54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins AR, Harrington V, Drew J, Melvin R. Nutritional modulation of DNA repair in a human intervention study. Carcinogenesis. 2003. March;24(3):511–5. 10.1093/carcin/24.3.511 [DOI] [PubMed] [Google Scholar]
- Prado RP, dos Santos BF, Pinto CL, de Assis KR, Salvadori DM, Ladeira MS. Influence of diet on oxidative DNA damage, uracil misincorporation and DNA repair capability. Mutagenesis. 2010. September;25(5):483–7. 10.1093/mutage/geq030 [DOI] [PubMed] [Google Scholar]
- Obtułowicz T, Swoboda M, Speina E, Gackowski D, Rozalski R, Siomek A, et al. Oxidative stress and 8-oxoguanine repair are enhanced in colon adenoma and carcinoma patients. Mutagenesis. 2010. September;25(5):463–71. 10.1093/mutage/geq028 [DOI] [PubMed] [Google Scholar]
- Brevik A, Karlsen A, Azqueta A, Tirado AE, Blomhoff R, Collins A. Both base excision repair and nucleotide excision repair in humans are influenced by nutritional factors. Cell Biochem Funct. 2011. Jan-Feb;29(1):36–42. 10.1002/cbf.1715 [DOI] [PubMed] [Google Scholar]
- van Gils CH, Bostick RM, Stern MC, Taylor JA. Differences in base excision repair capacity may modulate the effect of dietary antioxidant intake on prostate cancer risk: an example of polymorphisms in the XRCC1 gene. Cancer Epidemiol Biomarkers Prev. 2002. November;11(11):1279–84. [PubMed] [Google Scholar]
- Tarng DC, Liu TY, Huang TP. Protective effect of vitamin C on 8-hydroxy-2′-deoxyguanosine level in peripheral blood lymphocytes of chronic hemodialysis patients. Kidney Int. 2004. August;66(2):820–31. 10.1111/j.1523-1755.2004.00809.x [DOI] [PubMed] [Google Scholar]
- Slyskova J, Lorenzo Y, Karlsen A, Carlsen MH, Novosadova V, Blomhoff R, et al. Both genetic and dietary factors underlie individual differences in DNA damage levels and DNA repair capacity. DNA Repair (Amst). 2014. April;16(1):66–73. 10.1016/j.dnarep.2014.01.016 [DOI] [PubMed] [Google Scholar]
- Cooke MS, Mistry N, Ahmad J, Waller H, Langford L, Bevan RJ, et al. Deoxycytidine glyoxal: lesion induction and evidence of repair following vitamin C supplementation in vivo. Free Radic Biol Med. 2003. January;34(2):218–25. 10.1016/S0891-5849(02)01240-6 [DOI] [PubMed] [Google Scholar]
- Ho CK, Choi SW, Siu PM, Benzie IF. Effects of single dose and regular intake of green tea (Camellia sinensis) on DNA damage, DNA repair, and heme oxygenase-1 expression in a randomized controlled human supplementation study. Mol Nutr Food Res. 2014. June;58(6):1379–83. 10.1002/mnfr.201300751 [DOI] [PubMed] [Google Scholar]
- Yubero-Serrano EM, Gonzalez-Guardia L, Rangel-Zuñiga O, Delgado-Casado N, Delgado-Lista J, Perez-Martinez P, et al. Postprandial antioxidant gene expression is modified by Mediterranean diet supplemented with coenzyme Q(10) in elderly men and women. Age (Dordr). 2013. February;35(1):159–70. 10.1007/s11357-011-9331-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonucci J, Gragnani A, Trincado MM, Vincentin V, Correa SA, Ferreira LM. The role of vitamin C in the gene expression of oxidative stress markers in fibroblasts from burn patients. Acta Cir Bras. 2018. August;33(8):703–12. 10.1590/s0102-865020180080000006 [DOI] [PubMed] [Google Scholar]
- Choi SW, Yeung VT, Collins AR, Benzie IF. Redox-linked effects of green tea on DNA damage and repair, and influence of microsatellite polymorphism in HMOX-1: results of a human intervention trial. Mutagenesis. 2015. January;30(1):129–37. 10.1093/mutage/geu022 [DOI] [PubMed] [Google Scholar]
- Labelle H. A new perspective on the health of Canadians. AARN News Lett. 1976. June;32(6):1–5. [PubMed] [Google Scholar]
- Gruszka K, Kubicka K, Jonak W, Sobiech KA, Steciwko A. Preferred and undesirable products in the dietary habits of women. Adv Clin Exp Med. 2014. Jan-Feb;23(1):111–6. 10.17219/acem/37032 [DOI] [PubMed] [Google Scholar]