Abstract
Testicular tumors account for 1-2% of all tumors in men, with 95% of these being germ cell tumors. Paraneoplastic limbic encephalitis is a rare sequela of testicular tumors associated with anti-Ma2 and KLH11 antibodies. The most effective treatment for paraneoplastic limbic encephalitis is treatment of the primary malignancy. We report a 41-year-old male that presented to the emergency department with episodic alteration of consciousness and memory disturbances. Negative neurologic evaluation and imaging led to concern for a paraneoplastic process from a distant malignancy. CT imaging revealed an enlarged, necrotic para-aortic lymph node and subsequent ultrasound demonstrated a right-sided testicular mass. Right radical orchiectomy was performed. Microscopically, the mass consisted of mixed respiratory epithelium, gastrointestinal glands, and squamous epithelium with keratinization consistent with a post-pubertal testicular teratoma with associated in situ germ cell neoplasia. Resection of the para-aortic mass revealed large anaplastic cells with epithelioid features, nuclear pleomorphism and frequent mitoses. Immunostaining was positive for Pan-Keratin and OCT4, consistent with poorly differentiated embryonal carcinoma. Resection of the primary and metastatic disease, as well as treatment with corticosteroids, resulted in resolution of the encephalitis. This presentation of severe neurological disturbances in the setting of a metastatic mixed non-seminomatous germ cell tumor represents a rare presentation of paraneoplastic limbic encephalitis.
Keywords: Paraneoplastic Limbic Encephalitis, Embryonal Carcinoma, Testicular Teratoma, Germ Cell Carcinoma in situ
Introduction
Testicular tumors account for 1-2% of tumors in men [1]. This percentage increases significantly in young men, with testicular tumors the most common tumor in men age 15-44 [2]. Of these tumors, 95% are germ cell tumors [1,3]. Germ cell tumors are split into seminoma and non-seminoma. Roughly 50% of testicular germ cell carcinoma patients have a non-seminoma comprised of different histological elements which can include embryonal carcinoma, teratoma, yolk sac tumor, and choriocarcinoma [4]. The remaining 5% consists of sex cord stromal tumors, mixed germ cell and stromal tumors, and others. The main risk factor for the development of testicular cancer is cryptorchidism [5]. Fortunately, with effective treatment the overall 5-year survival rate for testicular cancer is 97% [3].
Intra-tumoral heterogeneity has been documented in non-seminomatous germ cell tumors (NSGCT) of the testis [6]. Different histologic subtypes can be easy identified due to embryonic components, such as embryonal carcinoma and teratoma, or extra-embryonal components, including yolk sac and choriocarcinoma [6]. Combinations involving embryonal, embryonal + seminoma + teratoma, and embryonal + yolk sac seminoma + teratoma (EYST) have also been described [7]. The heterogeneity seen in testicular cancers can also be seen in metastasis, wherein precursor non-seminomatous tumors can have a different subtype than their distant metastases. These metastases are likely derived directly from germ cell lines not identified in the primary tumor [4]. However, these presentations are exceedingly rare. An analysis of 703 NSGCT revealed only mixed subtypes involving mixed yolk-sac, mixed choriocarcinoma, and mixed yolk sac-seminoma [6]. Further, the presence of distant metastasis of different cancer type from the tumor of origin, while described, is uniquely rare.
Paraneoplastic limbic encephalitis is a rare sequela of malignancy that presents with personality changes, visual disturbances, seizures, vertigo, and memory loss. This condition has been reported most with small cell lung, testicular, and breast cancer [8]. The current model for the pathogenesis of paraneoplastic encephalitis involves activation of dendritic cells by tumor antigens. These dendritic cells in turn activate cytotoxic CD8+ T cells, which are then responsible for autoimmune destruction of the limbic system [9,10]. In testicular cancer, this syndrome has been shown to be associated with anti-Ma2 and KLHL11 antibodies [9,11]. The full function of Ma2 has not been elucidated, but it is believed to be involved in regulation of apoptosis [10] while KLHL11 is related to protein ubiquitination [11]. The most effective treatment for paraneoplastic limbic encephalitis is treatment of the primary malignancy [9].
Case Report
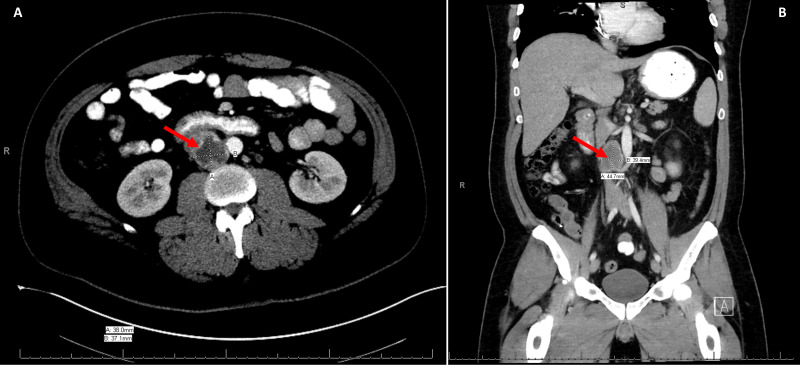
We present the case of a 41-year-old male with repeated visits to outside emergency departments over a 3-month period with a waxing and waning pattern of worsening cognitive dysfunction as well as intermittent fevers. He also complained of generalized weakness and fatigue. His initial diagnosis was viral meningitis. He had a negative workup for infectious etiologies, including negative CSF cultures, negative Lyme titers, normal ACE level. Previous lumbar puncture was notable for elevated white blood cells, elevated protein, and glucose below 50g/dL. CT scan of the abdomen at an outside hospital revealed a right pericaval lymph node measuring 3.8 x 3.7 cm with central necrosis (Figure 1). External genitalia were incompletely visualized on this scan. After transfer to our institution, the condition continued to worsen and after being admitted, the patient was unable to speak and reported weakness in all four extremities that progressed to alteration of awareness, disorientation, and difficulty speaking. Labs at this time were significant for phosphorous less than 2 mg/dL, which is known to precipitate seizures and altered mental status, however it was felt that the lower extremity shaking was not consistent with a seizure. He was placed on Keppra prior to arrival at our hospital. Based on this presentation, a CT of the head was obtained to rule out a primary central nervous system etiology. This imaging showed no acute process. Further work up with MRI could not be obtained due to a non-compatible internal pacemaker. Pacemaker analysis showed no abnormalities. Due to the para-aortic location of the mass, a 24-hour urine metanephrine was also performed to rule out a neuroendocrine tumor.
Figure 1.
Axial view (A) and coronal view (B) demonstrating 3.8 x 3.7 cm aortocaval mass.
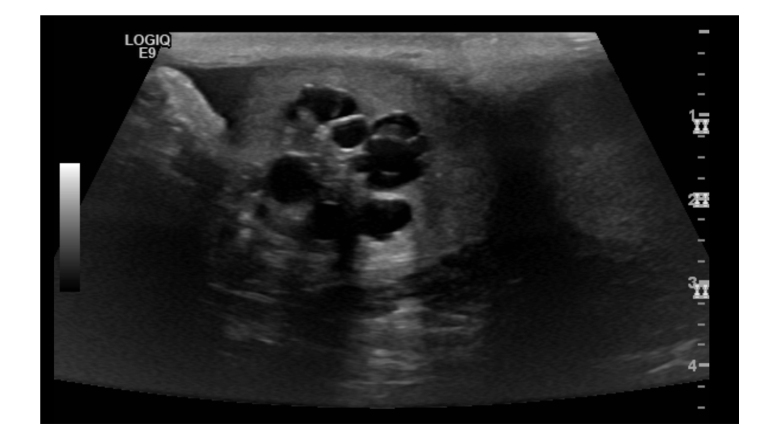
After the negative initial workup, concern for distant metastasis led to the belief current symptomatology was due to secondary to a paraneoplastic process. Specifically, the presence of retroperitoneal lymphadenopathy with necrosis raised concern for testicular origin. Sonographic evaluation of the testicles showed a right sided, ill-defined, multi-cystic 2.4 x 2.1 x 2.2cm mass (Figure 2). Further laboratory evaluation revealed an elevated B-hCG of 49.9 mIU/mL, alpha fetoprotein level of 1.67 ng/mL and normal LDH level. A right radical orchiectomy was performed.
Figure 2.
Ultrasound of the right testicle demonstrating a 2.4 x 2.1 x 2.2 cm multi-cystic mass. Units of depth in cm may be found to the right of the image.
Gross pathology obtained from the right radical orchiectomy showed a 2.1 x 1.8 x 1.6 cm tan-white, ill defined, soft, multicystic lesion in the medial and inferior pole of the testis, without hemorrhage or necrosis. Histologic evaluation revealed mixed respiratory epithelium, gastrointestinal glands, and squamous epithelium with keratinization consistent with a post-pubertal testicular teratoma with associated germ cell neoplasia in situ (Figures 3, 4).
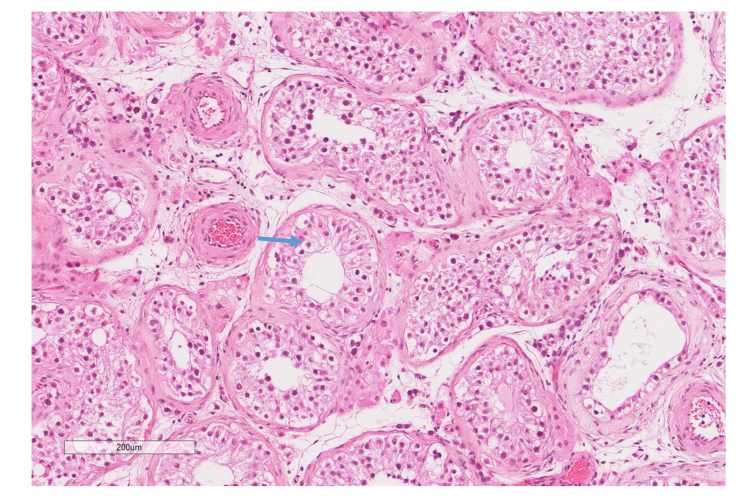
Figure 3.
H&E stain (10X) of the right testicular mass showing large atypical round cells, angulated and prominent nucleoli, and clear cytoplasm filling the seminiferous tubules. Blue arrow indicates tumor cells. This was identified as a germ cell carcinoma in situ.
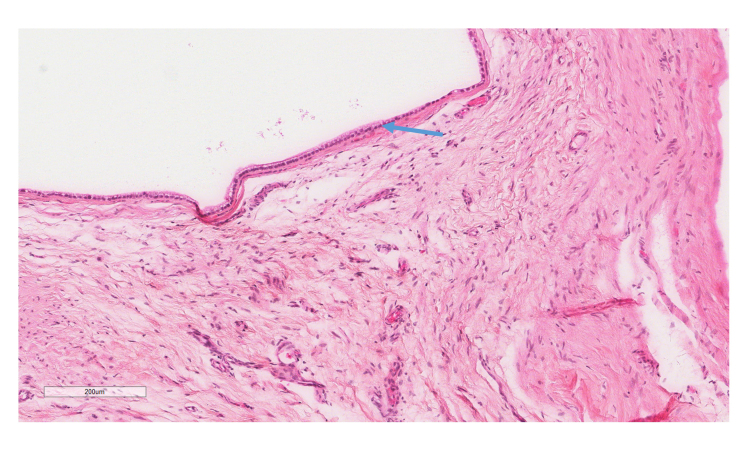
Figure 4.
H&E stain (10X) of the right testicular mass showing a cystically dilated area lined by respiratory-type epithelium with abundant intervening stroma. Blue arrow denotes the respiratory-type epithelium. This was identified as a testicular teratoma.
Repeat CT scan of the abdomen and pelvis showed continuous enlargement of the right peri-aortic lymph node, now measuring 5.6 cm in greatest dimension, as well as a new left peri-aortic node measuring 2.2 cm in the largest dimension. After multidisciplinary discussion, given the likelihood of a paraneoplastic syndrome that would likely worsen with up front chemotherapy, the decision was made to proceed with a primary retroperitoneal lymph node dissection. The large aortocaval mass was adherent to the inferior vena cava (IVC), but no invasion was noted. Measurement of the extirpated mass was noted to be 7 x 6 x 4.5 cm in size cm in the largest diameter. A complete bilateral template retroperitoneal lymph node dissection was performed extending inferiorly from the renal veins to the bifurcation of the common iliac arteries and to the ureters bilaterally.
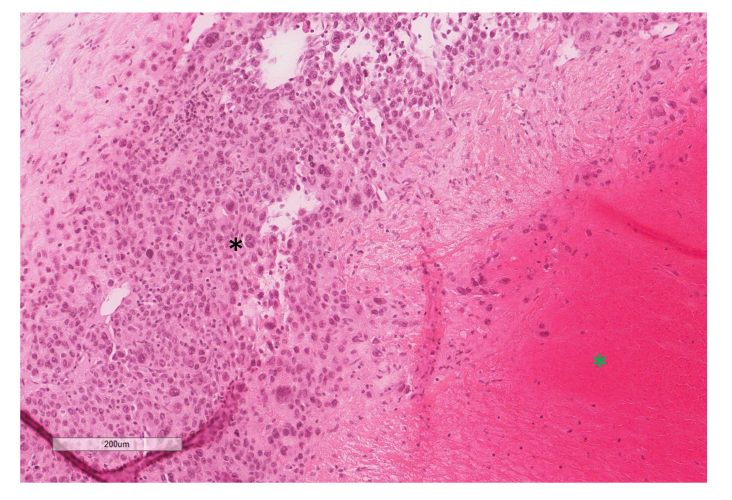
On gross pathology, the aortocaval mass was well encapsulated with hemorrhagic and necrotic cut surfaces. Microscopic evaluation displayed large anaplastic cells with epithelioid features, nuclear pleomorphism and frequent mitoses. Giant cells with granular cytoplasm were also present. One node measuring 1.5 x 1.0 x 1.0 cm was positive for malignancy. Immunostaining of the mass and positive lymph node displayed positive staining for Pan-Keratin and OCT4, and negative staining for CD30, S-100, Desmin, pan-melanoma, and SOX2. The microscopic evaluation and immunostaining combination led to the diagnosis of poorly differentiated embryonal carcinoma (Figure 5).
Figure 5.
H&E stain (10X) of the aortocaval mass displaying poorly differentiated, pleomorphic cells with prominent nucleoli (black star) and extensive necrosis (green star). This was identified as metastatic embryonal carcinoma.
The patient’s neurological symptoms were managed with methylprednisolone, and Keppra was continued for seizure prophylaxis. Following resection of the aortocaval mass, the patient recovered well, and the diplopia and paralysis resolved. He was managed post-operatively with Bleomycin-Etoposide-Cisplatin (BEP) therapy. At the time of publication, he had received three cycles of BEP and has shown no evidence of tumor recurrence.
Discussion
We report the unique case of a patient with post-pubertal teratoma and germ cell carcinoma in situ as with concurrent para-aortic poorly differentiated embryonal carcinoma that presented with paraneoplastic limbic encephalitis. The presentation of vertigo, diplopia, and paralysis with a negative neurological work-up should prompt evaluation for malignancy. While the most common cause of paraneoplastic limbic encephalitis is small cell cancer of the lung, physicians should consider testicular origin following negative thoracic work-up [8].
A variety of antibodies have been associated with paraneoplastic limbic encephalitis. These can be subdivided into antibodies to intracellular antigens and to extracellular antigens. Antibodies to intracellular antigens include anti-Hu, Yo, Ma2, CRMP-5, amphiphysin, and Ri. Their extracellular counterparts include anti-NMDA, VGKC, and AChR. Of these, the anti-NMDA has been the best characterized, and has been shown to be a reliable predictor of paraneoplastic origin of diplopia and paralysis [12]. Additionally, there have been some antibodies identified that directly correlate to a testicular primary. These include anti-Ma2 [13] and, more recently, antibodies to KLHL11 [11]. The immunologic work-up of our patient was limited to anti-NMDA antibodies, which were negative. However, we recommend use of a paraneoplastic antibody panel for patients with diplopia and paralysis following a negative initial neurologic work-up. In particular, the KLH11 should be included in all patients with potential paraneoplastic limbic encephalitis as it has been shown to be positive in most cases [14]. Therefore, early use of a paraneoplastic antibody panel including KLH11 could increase the speed of diagnosis of the primary tumor in these patients.
As treatment for paraneoplastic limbic encephalitis is essentially treatment of the primary malignancy, the ideal treatment for paraneoplastic encephalitis secondary to testicular cancer is typically orchiectomy [9]. This becomes more difficult with the presence of distant metastasis. In our case, the metastasis was adjacent to the IVC, making surgical resection technically challenging. As testicular teratoma is typically less chemosensitive, and the difficulty of receiving up front chemotherapy in the setting of his limbic encephalitis, primary retroperitoneal lymph node dissection was chosen as the optimal option for primary therapy for our patient.
Tumoral heterogeneity poses a challenging obstacle in the treatment of malignancy, as it creates inherent resistance to precision medicine approaches [6]. Studies on heterogeneous tumors have been done due to a resistance to systemic therapy of metastasis where typically few testicular cancer cases show resistance [4]. Certain phenotypes of testicular cancer, including choriocarcinoma and seminoma, have been documented to respond differently clinically when they are a pure or mixed tumor [6]. In our case, chemotherapy options were limited, as embryonal carcinoma is typically chemosensitive while teratoma is chemoresistant [6]. This tumor was staged at stage IIC non-seminomatous germ cell tumor by size and pathology showing embryonal cell carcinoma. As such, upfront chemotherapy to eradicate microscopic metastatic disease followed by surgical excision of testicular cancers is the standard of care [15]. While cases have been reported of disease progression after orchiectomy [16], excision with chemotherapy is curative in most cases. As such, we recommend surgical resection of visible tumor if possible.
Conclusion
A 41-year-old male presented with primary complaints of neurological disturbance that were eventually found to be the result of paraneoplastic encephalitis. Vertigo, diplopia, and altered mental status in a young patient with no clear cause should be concerning for testicular malignancy. We recommend the use of a full paraneoplastic antibody panel in patients where there is concern for a paraneoplastic origin of neurologic findings.
Glossary
- NSGCT
non-seminomatous germ cell tumors
- EYST
embryonal carcinoma yolk sac tumor, seminoma, and teratoma
- IVC
inferior vena cava
Author Contributions
Chase J. Wehrle was the primary author of the manuscript with significant assistance from Asad Ullah and Margaret A. Sinkler. The remaining authors were involved in the care of the patient and provided specialty-specific information and advice regarding the manuscript. No funding was obtained.
References
- Manecksha RP, Fitzpatrick JM. Epidemiology of testicular cancer. BJU Int. 2009. November;104(9b 9 Pt B):1329–33. 10.1111/j.1464-410X.2009.08854.x [DOI] [PubMed] [Google Scholar]
- Farmanfarma KK, Mahdavifar N, Mohammadian-Hafshejani A, Salehiniya H. Testicular cancer in the world: an epidemiological review. WCRJ. 2018;5(4):e1180. [Google Scholar]
- Baird DC, Meyers GJ, Hu JS. Testicular Cancer: diagnosis and Treatment. Am Fam Physician. 2018. February;97(4):261–8. [PubMed] [Google Scholar]
- Dorssers LC, Gillis AJ, Stoop H, van Marion R, Nieboer MM, van Riet J, et al. Molecular heterogeneity and early metastatic clone selection in testicular germ cell cancer development. Br J Cancer. 2019. February;120(4):444–52. 10.1038/s41416-019-0381-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasiou I, Deligiannis D, Katafigiotis I, Skarmoutsos I, Karaolanis G, Palla VV, et al. Synchronous Bilateral Testicular Tumors with Different Histopathology. Case Rep Urol. 2015;2015:492183. 10.1155/2015/492183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu SM, Bilen MA, Hess KR, Broaddus RR, Kopetz S, Wei C, et al. Intratumoral heterogeneity: role of differentiation in a potentially lethal phenotype of testicular cancer. Cancer. 2016. June;122(12):1836–43. 10.1002/cncr.29996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilen MA, Hess KR, Campbell MT, Wang J, Broaddus RR, Karam JA, et al. Intratumoral heterogeneity and chemoresistance in nonseminomatous germ cell tumor of the testis. Oncotarget. 2016. December;7(52):86280–9. 10.18632/oncotarget.13380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gultekin SH, Rosenfeld MR, Voltz R, Eichen J, Posner JB, Dalmau J. Paraneoplastic limbic encephalitis: neurological symptoms, immunological findings and tumour association in 50 patients. Brain. 2000. July;123(Pt 7):1481–94. 10.1093/brain/123.7.1481 [DOI] [PubMed] [Google Scholar]
- Voltz R. Paraneoplastic neurological syndromes: an update on diagnosis, pathogenesis, and therapy. Lancet Neurol. 2002. September;1(5):294–305. 10.1016/S1474-4422(02)00135-7 [DOI] [PubMed] [Google Scholar]
- Dalmau J, Graus F, Villarejo A, Posner JB, Blumenthal D, Thiessen B, et al. Clinical analysis of anti-Ma2-associated encephalitis. Brain. 2004. August;127(Pt 8):1831–44. 10.1093/brain/awh203 [DOI] [PubMed] [Google Scholar]
- ESMO (n.d.). Paraneoplastic Encephalitis in Testicular Cancer. Retrieved February 16, 2020, from https://www.esmo.org/oncology-news/Paraneoplastic-Encephalitis-in-Testicular-Cancer
- Dalmau J, Gleichman AJ, Hughes EG, Rossi JE, Peng X, Lai M, et al. Anti-NMDA-receptor encephalitis: case series and analysis of the effects of antibodies. Lancet Neurol. 2008. December;7(12):1091–8. 10.1016/S1474-4422(08)70224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenner L, Einhorn L. Ma-2 paraneoplastic encephalitis in the presence of bilateral testicular cancer: diagnostic and therapeutic approach. J Clin Oncol. 2009. August;27(23):e57–8. 10.1200/JCO.2009.23.3635 [DOI] [PubMed] [Google Scholar]
- Mandel-Brehm C, Dubey D, Kryzer TJ, O’Donovan BD, Tran B, Vazquez SE, et al. Kelch-like Protein 11 Antibodies in Seminoma-Associated Paraneoplastic Encephalitis. N Engl J Med. 2019. July;381(1):47–54. 10.1056/NEJMoa1816721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh DJ, Funt SA, Silber D, Knezevic A, Patil S, O’Donnell D, et al. Adjuvant chemotherapy with etoposide plus cisplatin for patients with pathologic stage II non-seminomatous germ cell tumors. J Clin Oncol. 2020. April;38(12):1332–7. 10.1200/JCO.19.02712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almedallah D, Alsaffar G, Al-Shabeeb G, Baarmah A, Nassim E. Paraneoplastic limbic encephalitis associated with testicular mixed germ cell tumor. Neuroimmunol Neuroinflamm. 2018;2018: 10.20517/2347-8659.2018.55 [DOI] [Google Scholar]