Abstract
Immobilized Candida antarctica lipase B (CALB)-catalyzed polycondensation of glycerol and sebacic acid at mild reaction conditions resulted in branched poly(glycerol sebacate) (PGS). To understand how PGS chains grow and branch, the kinetics of the CALB-catalyzed polycondensation were studied. The influence of the reaction temperature, solvent, CALB amount, and sebacic acid/glycerol feed ratio on the poly(glycerol sebacate) (PGS) molecular weight, degree of branching, and glyceridic repetitive unit distribution was also investigated. PGS architecture changes from linear to branched with the progression of the reaction, and the branching results from the simultaneous CALB-catalyzed esterification and acyl migration. For reactions performed in acetone at the temperature range from 30 to 50 °C, the apparent rate constant increases from 0.7 to 1.5 h–1, and the apparent energy of activation of 32 kJ mol–1 was estimated. The higher mass average molecular weight (16 kDa) and degree of branching (41%) were achieved using the equimolar sebacic acid/glycerol feed ratio in acetone at 40 °C with a CALB amount of 13.6 wt % and in the presence of the molecular sieves.
Introduction
Poly(glycerol sebacate) (PGS) is one of the most studied polyesters based on glycerol. This is mainly due to its biocompatibility and biodegradability, which make it suitable for biomedical applications in areas such as tissue engineering, drug delivery, and surgical sealants.1−3 PGS elastomers are nontoxic materials4,5 with mechanical properties similar to those of biological soft tissue6−8 and present in vitro and in vivo degradability7,9 and shape-memory behavior.10 They are also bioresorbable and biocompatible due to the endogenous nature of glycerol and sebacic acid, which are building blocks of glycerides and a natural intermediate metabolite of the oxidation of fatty acids, respectively.4,11−14 Conventionally, PGS elastomers are prepared in a noncatalyzed two-step process: (i) polymerization at 120 °C under an inert gas flow for 24 h and (ii) crosslinking at 120 °C under vacuum for 48 h.4,5 The PGS elastomer properties are generally modulated through the reaction parameters, such as reaction time and temperature.2,7,15 However, few studies have addressed the relationship between the PGS structure and properties.5 Given the harsh polymerization conditions, a fraction of glycerol is lost through evaporation, leading to significant deviations between the predicted and the experimental PGS properties.5,7 Furthermore, PGS for biomedical applications must be prepared in the absence of conventional catalysts due to their biological toxicity.1 In this condition, the reaction is slow, and to prevent gelation, only oligomers are formed in the polymerization step.7,16
Alternatively, three strategies have been developed for the synthesis of glycerol-based polyesters under milder reaction conditions: (i) ring-opening polymerization of diglycidyl sebacate prepared by the reaction of sebacoyl chloride and glycidol with sebacic acid catalyzed by tetrabutylammonium bromide in dioxane at 95 °C;17,18 (ii) polycondensation between glycerol and sebacoyl chloride catalyzed by the diarylborinic acid catalyst in tetrahydrofuran in the presence of N,N-diisopropylethylamine at 70 °C;19 and (iii) lipase-catalyzed polycondensation between glycerol and diacids/diesters performed both in bulk as well as in solution using solvents such as tetrahydrofuran and biphenyl ether in the temperature range from 40 to 90 °C.20−31 Among these strategies, lipase-catalyzed polycondensation seems to be the lower toxic and most sustainable and environmentally friendly alternative for the preparation of linear and branched polyesters based on glycerol.32
The enzyme Candida antarctica lipase B (CALB) is commercially available in a form supported on acrylic resin, and it is reported to be “the most used enzyme catalyst in both academia and industry”.33 This lipase is mainly used for (trans)esterifications, amidations, and hydrolysis reactions in the synthesis of surfactants, monomers, polymers, optically pure compounds, biolubricants, glycerides, and biodiesel.33 CALB is an sn-1,3-specific lipase with higher reactivity toward primary hydroxyls than secondary ones.34−36 It is capable of hindering the crosslinking of polyesters prepared from glycerol and diacids/diesters, even at a relatively high molecular weight, and of catalyzing one-pot polymerization at mild reaction conditions.27,29 Moreover, its specificity and reactivity could be modulated through the control of reaction parameters such as temperature22,25,31 and solvent.37,38 These characteristics make CALB suitable as a catalyst for the preparation of PGS polymers.1Table S1 (Supporting Information) summarizes the main reports about the synthesis of glycerol-based polyesters via CALB-catalyzed polymerization and the structural features of the polyesters. Poly(glycerol adipate) is the most studied glycerol-based polyesters prepared via CALB-catalyzed polymerization. Generally, the reaction is conducted in the tetrahydrofuran solution at the temperature range from 40 to 70 °C using divinyl adipate as a precursor.20,24,25,30,31 To the best of our knowledge, only Uyama et al.25,39 and Lang et al.40 reported the CALB-catalyzed synthesis of PSG, both performed reactions in a solvent-free medium.
Despite CALB’s positional specificity, the esterification of glycerol secondary hydroxyls occurs to some degree with the formation of trisubstituted glyceridic units and branching of the glycerol-based polyester.20−31,40,41 The structures of these polyesters are generally composed of mono-, di-, and trisubstituted glyceridic repetitive units that are structurally similar to mono-, di-, and triglycerides.30 Similarly, the esterification of secondary hydroxyls and the formation of mono-, di-, and triglycerides using Mucor miehei, an sn-1,3-specific lipase, as a catalyst for the synthesis of lipids from glycerol and fatty acids have been reported.42 The formation of triglycerides is due to a side reaction, named acyl migration (A.M.), which occurs in parallel to esterification. Acyl migration is an intramolecular reaction that allows the exchange of the acyl ester group among the adjacent hydroxyl groups, and it is the main reason for the esterification of secondary hydroxyls of glycerol, giving rise to triglycerides.42,43 As reported by You et al.,17,18 the control of the branching of PGS is an important strategy for the improvement of PGS elastomer mechanical properties. So far, the relationship among CALB selectivity and acyl migration causing branching during polycondensation reactions between glycerol and dicarboxylic acids/diesters has not been reported.
Therefore, this work concerns the synthesis of PGS via CALB-catalyzed polycondensation between glycerol and sebacic acid at mild reaction conditions, a kinetic study, and an understanding of the mechanism of the polymer chain growth and branching. The influence of the solvent, temperature, CALB amount, and sebacic acid/glycerol molar feed ratio on the polymer molecular weight, glyceridic repetitive unit distribution, and the degree of branching (DB) was investigated by gel permeation chromatography (GPC) and 1H NMR spectroscopy. The temporal evaluation of the PGS structure sheds light on some insights into the chain growth behavior of polyesters based on glycerol prepared by CALB catalysis and the role of both CALB selectivity and acyl migration in branch formation. Overall, these results could assist the development of strategies toward polymer architecture control.
Experimental Section
Materials
Sebacic acid (99.0%, Sigma-Aldrich), lipase acrylic resin—CALB (lipase B from C. antarctica, ≥5000 U g–1, Sigma-Aldrich), and deuterated acetone (99.9% atom D, Sigma-Aldrich) were used without any treatment. Molecular sieves, 5 Å (spheres, 4–8 mesh, Sigma-Aldrich), were exhaustively washed with distilled water and dried in an oven at 400 °C for 4 h. Glycerol (≥99.5%, Sigma-Aldrich), tert-butanol (99.5%, Acros Organics), acetone (≥99.5%, LabSynth), and acetonitrile (≥99.5%, LabSynth) were kept under molecular sieves. Tetrahydrofuran (≥99.0%, Vetec) was dried using metallic sodium and benzophenone under reflux and argon flow; this was followed by distillation and storage in a closed bottle with molecular sieves.
CALB-Catalyzed Polycondensation
In a typical polycondensation procedure, 5.0 mmol (1.01 g) of sebacic acid, 5.0 mmol (0.46 g) of glycerol, 200 mg of CALB (13.6 wt % relative to monomers), 2.0 g of 5 Å molecular sieves (a 2-fold excess based on molecular sieves water sorption capability of ca. 20 wt %), and 5 mL of solvent (tetrahydrofuran, acetone, tert-butanol, or acetonitrile) were put in a 30 mL capped glass flask. The reaction was maintained under gentle magnetic stirring (150 rpm) at a desirable temperature (30, 40, 50, 60, or 70 °C) for 24 h. For the kinetic studies, aliquots of 100 μL were taken after 2, 4, 6, 8, 10, 12, and 24 h reaction times, dried under vacuum, and kept at −20 °C for posterior analyses. After 24 h, the reaction medium was filtered and washed with acetone. The solvent was removed by distillation under reduced pressure. The residue was dried under vacuum and kept at −20 °C. Polymer purification by precipitation was not performed before analyses. PGS is a colorless to a yellowish polymer with an oily to a sticky gel consistency, depending on the molecular weight.
Instrumental Methods
The number average molecular weight (Mn), mass average molecular weight (Mw), and molecular weight dispersity (Đ = Mw/Mn) of PGS were determined by gel permeation chromatography (GPC) performed on a Viscotek GPCmax VE2001 instrument equipped with a Viscotek TGuard 10 × 4.6 mm2 guard column and three Shodex KF-806M columns kept at 40 °C and using tetrahydrofuran as an eluent at a flow rate of 1.0 mL min–1. The detection was performed using a Viscotek VE3580 refractive index detector. PGS solutions in tetrahydrofuran were prepared at a concentration of 5.0 mg mL–1 and filtered in poly(tetrafluoroethylene) filters (0.45 μm) before analysis. Polystyrene (PS) standards (Viscotek, Malvern) with the molecular weight from 1050 to 3 800 000 g mol–1 were used to determine the relative molecular weight of the PGS. The software OmniSEC. 4.6.2 (Viscotec, Malvern) was used for data collection and processing.
Infrared (IR) spectroscopy analysis of PGS was performed using Agilent Cary 360 Fourier transform infrared (FTIR) equipment operating in an attenuated total reflectance mode, spectral range from 400 to 4000 cm–1, resolution of 4 cm–1, and 64 scans.
Proton nuclear magnetic resonance (1H NMR) analyses were performed on a Bruker Avance 400 MHz spectrometer operating at 25 °C, 2.0 s acquisition time, 1.0 s recycle delay, a spectra width of 8012 Hz, 16 scans, 32k points, and a free induction decay (FID) resolution of 0.49 Hz. Carbon nuclear magnetic resonance (13C NMR) analyses were performed on a Bruker Avance 500 MHz spectrometer operating at 25 °C, 1.0 s acquisition time, 60 s recycle delay, a spectra width of 32 894 Hz, 920 scans, 64k points, and an FID resolution of 1.0 Hz without the nuclear Overhauser enhancement. Analyses of multiplicity editing proton–carbon heteronuclear single quantum coherence spectroscopy (1H–13C HSQC) were performed on a Bruker Avance 400 MHz spectrometer operating at 25 °C using the pulse program hsqcedetgp, with proton and carbon acquisition parameters of 0.24 and 0.01 s acquisition time, spectra width of 4244 and 19 120 Hz, 2k and 0.5k points, FID resolution of 4.15 and 74.7 Hz, respectively, 1.0 s recycle delay, and 16 scans. Analyses of proton homonuclear correlation spectroscopy (1H–1H COSY) were performed on a Bruker Avance 400 MHz spectrometer operating at 25 °C using the pulse program cosygpqf, with acquisition parameters for each proton of 0.24 and 0.06 s acquisition time, 1.0 s recycle delay, a spectra width of 4244 Hz, 2k and 0.5k points, FID resolution of 4.1 and 16.6 Hz, and eight scans. PGS solutions in (CD3)2CO were used, and the chemical shifts (δ) in parts per million (ppm) were assigned to the residual solvent proton and carbon of the (CD3)2CO at δH = 2.05 ppm and δC = 206.26 ppm, respectively.
The software TopSpin version 3.6.1 (Bruker) was used for NMR spectra processing, signal assignments, integration, and deconvolution. The signal integration was performed using the integration tool with baseline correction. The deconvolution of signals was performed by defining peak positions and fitting the curve with Gaussian and Lorentzian functions.
Results and Discussion
Structural Characterization of PGS
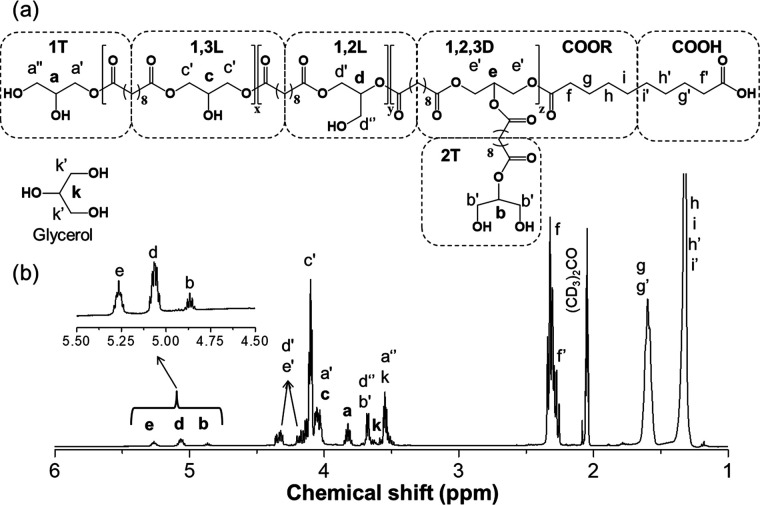
The IR spectrum of PGS (Figure S1) reveals the presence of bands centered at 3440, 1735, 1060, and 1090 cm–1, which are related to hydroxyl, carbonyl ester, and primary and secondary alcohol stretching vibrations, respectively. These bands prove the formation of polyester with primary and secondary hydroxyl pendant groups along the polymer chains.22,31 The PGS is branched and composed of the terminal (1T and 2T), linear (1,3L and 1,2L), and dendritic (1,2,3D) glyceridic repetitive units, Figure 1a. The 1H NMR spectrum (Figure 1b) of PGS shows the characteristic signals of the sebacic acid/ester in the chemical shift region from 1.00 to 2.50 ppm, overlapped signals in the region from 3.30 to 4.50 ppm related to methine and methylene protons of glycerol and glyceridic repetitive units, and signals in the chemical shift range from 4.70 to 5.40 ppm related to methine protons of glyceridic repetitive units with secondary hydroxyl esterified (2T, 1,2L, and 1,2,3D).5,19,22,44,45
Figure 1.
(a) Substitution pattern of PGS composed of the terminal (1T and 2T), linear (1,3L and 1,2L), and dendritic (1,2,3D) glyceridic units as indicated by the (b) 1H NMR spectrum in (CD3)2CO.
The 13C NMR analysis (Figure S2) was also performed. The signals of glycerol and glyceridic repetitive units, in the chemical shift range from 55 to 80 ppm, were not overlapped; their assignments have been well established in the literature.19,22,27,29,44,45 However, these signals are of very low intensity and, when performing a quantitative analysis without the nuclear Overhauser enhancement, the signal intensity is even lower and some signals could not be detected if the number of scans and the runtime were not high enough, a problem that does not take place in the 1H NMR spectra.22 Thus, HSQC and COSY analyses (Figures S3 and S4, respectively) were performed to obtain a precise and detailed assignment of the signals in the 1H NMR spectrum. Data from the one-dimensional (1D) and two-dimensional (2D) NMR analyses show that the methine protons a (3.83 ppm), b (4.89 ppm), d (5.09 ppm), and e (5.29 ppm) of 1T, 2T, 1,2L, and 1,2,3D glyceridic units, respectively, present signals in the 1H NMR spectrum free of overlapping. Therefore, the areas of these signals were used to determine the average molar fraction (xi) of each glyceridic species (i) in the reaction medium, by normalizing it by the carbonyl signal area (f + f′). The molar fractions of the nonreacted glycerol and the 1,3L units were calculated using eqs S1–S3. An example of the evolution of 1H NMR spectrum signals during the CALB-catalyzed polycondensation of sebacic acid and glycerol can be found in Figure S5. The carboxylic acid conversion (pCOOH) was estimated by the relative areas of the signals f and f′ determined by curve-fitting of the overlapped signals (Figure S6 and eq S4, Supporting Information). The relative molar fraction of each glyceridic unit (yi) in the PGS chain was determined from the average molar fraction of each glyceridic species in the reaction medium (eq S5). The degree of branching (DB) was calculated as proposed by Frey et al.46 using the relative molar fraction of each glyceridic unit, and the number average degree of polymerization (Dpn) of PGS was calculated based on Flory47 and Frey46 models for linear and branched polymers (eq S6). The number average molecular weight (Mn) and the average number of each glyceridic unit per PGS chain (ni) (eqs S7 and S8, respectively) were also estimated from Dpn. The degree of substitution (DS) of the glycerol and the hydroxyl conversion (pOH) were calculated from the average molar fraction of glyceridic units and DS values using eqs S9 and S10 (Supporting Information), respectively. The average molar fractions of each glyceridic unit estimated from 1H NMR and quantitative 13C NMR spectra were very close, as can be seen in Table S2, Supporting Information. Therefore, 1H NMR analysis is a valuable tool to follow the evolution of the polymer structure. It provides an estimate of the polymer number average molecular weight and the type of glyceridic units incorporated in the polymer over time. The overall results from GPC and 1H NMR are summarized in Tables S3–S6 as a function of the reaction time, temperature, and solvent, and they will be discussed below.
Effect of Solvent and Temperature on Polymerization and the PGS Structure
Except for the presence of the immobilized enzyme beads and molecular sieves, the reaction medium was homogeneous and clear at the beginning of the reaction in tetrahydrofuran. When acetone, acetonitrile, or tert-butanol was used as a solvent, a cloudy mixture was initially observed due to the lower solubility of sebacic acid in these solvents. However, after approximately 30 min, the reaction media became clear due to sebacic acid consumption by esterification. The carboxylic acid and hydroxyl conversion (pCOOH and pOH, respectively), the number average molecular weight, the mass average molecular weight, the molecular weight dispersity, the degree of branching, and the relative molar fraction of each glyceridic unit in the PGS chains are presented in Table 1 for polycondensation reactions carried out in different solvents for 24 h at temperatures from 30 to 70 °C.
Table 1. Structural Parameters of PGS Synthesized Using 13.6 wt % of CALB in Different Reaction Conditions for 24 h.
|
yi (%)a |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| solvent | T (°C) | pCOOHa | pOHa | Mn (kDa)a | Mw (kDa)b | Đb | DB (%)a | 1T | 2T | 1,3L | 1,2L | 1,2,3D |
| tetrahydrofuran | 40 | 0.92 | 0.64 | 3.2 | 10 | 5.6 | 38 | 24 | 2 | 39 | 18 | 17 |
| 50 | 0.91 | 0.64 | 3.0 | 9 | 5.4 | 41 | 23 | 3 | 34 | 18 | 18 | |
| 60 | 0.87 | 0.61 | 1.9 | 7 | 4.6 | 34 | 26 | 3 | 40 | 16 | 15 | |
| tert-butanol | 40 | 0.76 | 0.57 | 1.0 | 2 | 2.1 | 25 | 34 | 4 | 35 | 18 | 9 |
| 50 | 0.79 | 0.57 | 1.1 | 3 | 2.0 | 24 | 31 | 4 | 39 | 17 | 9 | |
| 60 | 0.83 | 0.59 | 1.4 | 4 | 2.7 | 28 | 28 | 4 | 40 | 17 | 11 | |
| 70 | 0.83 | 0.59 | 1.4 | 4 | 2.7 | 28 | 27 | 4 | 41 | 17 | 11 | |
| acetone | 30 | 0.94 | 0.64 | 3.8 | 13 | 6.5 | 34 | 21 | 2 | 47 | 15 | 16 |
| 40 | 0.97 | 0.66 | 9.4 | 16 | 6.8 | 41 | 19 | 3 | 41 | 17 | 20 | |
| 50 | 0.94 | 0.65 | 4.9 | 15 | 6.7 | 42 | 21 | 3 | 39 | 17 | 20 | |
| acetonitrile | 40 | 0.82 | 0.59 | 1.4 | 3 | 3.1 | 33 | 32 | 3 | 35 | 16 | 13 |
| 50 | 0.84 | 0.60 | 1.4 | 4 | 3.1 | 38 | 28 | 3 | 42 | 15 | 12 | |
| 60 | 0.95 | 0.61 | 2.7 | 5 | 3.4 | 31 | 26 | 3 | 42 | 16 | 13 | |
Determined by 1H NMR.
Determined by GPC.
The molar fraction of glyceridic units per PGS chain is similar regardless of the solvent and temperature. The relative molar fraction of glyceridic units with esterified primary hydroxyls (1T and 1,3L) was higher compared to those with an esterified secondary hydroxyl (2T and 1,2L), showing higher CALB reactivity toward glycerol primary hydroxyls. The highest carboxylic acid and hydroxyl conversions were observed for reactions performed in tetrahydrofuran and acetone, for which pCOOH > 90% and pOH > 60%, indicating that these solvents are suitable for CALB-catalyzed polycondensation reactions. The solvent selection guide CHEM2148 classifies acetone, tert-butanol, and acetonitrile as “recommended” solvents. On the other hand, tetrahydrofuran is classified as “problematic”. PGS with a higher molecular weight was obtained using acetone at 40 °C. In this condition, branched PGS with Mn, Mw, and DB up to 9.4 kDa, 16 kDa, and 41%, respectively, was formed. In the reactions performed in tetrahydrofuran at 40 °C, PGS molecular weights are smaller (Mn = 3.2 kDa and Mw = 10 kDa), with DB = 38%. For comparison, it has been reported that PGS molecular weights in the range of Mn = 4.6–6.5 kDa and Mw = 17.0–23.0 kDa were obtained using the conventional noncatalyzed process.49,50 Moreover, Uyama et al.25 reported that PGS with Mw = 19.0 kDa and y1,2,3D = 16% was prepared by the CALB-catalyzed polycondensation of vinyl sebacate and glycerol in bulk at 60 °C in an inert atmosphere for 8 h, and Lang et al.40 reported PGS with Mw = 63.9 kDa (Đ = 16.9) by performing the CALB-catalyzed polycondensation of sebacic acid and glycerol in bulk at 90 °C under reduced pressure for 47 h. Therefore, our results demonstrated that CALB-catalyzed polycondensation between sebacic acid and glycerol using a low hazard acetone as a solvent, molecular sieves, and mild reaction conditions in a closed system is suitable for the preparation of PGS. Also, higher molecular weights were obtained from this process than by performing the reaction in tetrahydrofuran, and the molecular weights were similar to the results from using the conventional process or vinyl sebacate as a precursor.
The general rule for biocatalyzed reactions in organic solvents is that there is a decrease in biocatalyst activity with decreasing solvent log P (P = octanol/water partition coefficient).51 This is due to the distortions of the enzyme structure caused by the interactions of the solvent molecules with the catalytic site or by solvent interacting and pulling out the essential water molecules that stabilize the enzyme. For esterification, the further release of these water molecules to the reaction medium could lead to hydrolysis reactions.51−54 However, as observed in Table 1, a decrease in biocatalyst activity with decreasing solvent log P was not followed. This was independent of the temperature, and the increase in the molecular weight followed the order: acetone (log P = −0.23) > tetrahydrofuran (log P = 0.49) > acetonitrile (log P = −0.33) > tert-butanol (log P = 0.35). Therefore, the effect of solvent on the enzyme structure and its activity may not be the main factor responsible for the different PGS molecular weights. A possible explanation for the observed tendency could be polymer–solvent interactions. If the polymer–solvent interactions are not favorable during polymer chain growth, the polymer coil will contract or possibly even form aggregates (as will be shown further), which, in turn, will result in a decrease in the availability of polymer reactive groups to proceed toward polymerization.
As presented in Table 1 for reactions performed in tert-butanol and acetonitrile, the molecular weight tends to slightly increase as the temperature increases in the range from 40 to 70 °C. However, for acetone and tetrahydrofuran, an increase in the temperature of above 50 °C leads to a decrease in the molecular weight. This phenomenon is due to CALB thermal deactivation, as reported by Distel et al.55 for the CALB-catalyzed polymerization of ethyl-S-lactate in tetrahydrofuran and chloroform and by Taresco et al.31 for the polycondensation of glycerol and vinyl adipate in tetrahydrofuran at temperatures above 50 °C. It is worthwhile to observe that using apolar solvents or by performing reactions in bulk, the decrease of CALB activity at temperatures of around 50–60 °C was not observed, and successful polymerizations in these media at temperatures up to 90 °C have been reported (Table S1).40,56−58
Effect of the Enzyme Amount and Sebacic Acid/Glycerol Molar Feed Ratio on Polymerization and the PGS Structure
For reactions performed in acetone at 40 °C for 24 h, the increase in the enzyme amount from 3.4 to 13.6 wt % leads to an increase in Mn, Mw, Đ, and DB from 1.4 to 9.4 kDa, 3 to 16 kDa, 2.1 to 6.8, and 17 to 41%, respectively (Table 2). These results agree with the literature that CALB catalyzes esterification reactions according to the kinetic model Ping-Pong Bi-Bi, for which the reaction rate is proportional to the enzyme amount.59,60 The highest enzyme concentration of 13.6 wt % used in this work aimed to achieve higher conversion in shorter reaction times. For the synthesis of glycerol-based polyester, the CALB amount of 10 wt % is generally employed in both solvent-free and in solution reactions.22,23,27,29 However, Gameiro et al.41 reported the use of a CALB amount of 25 wt % for the enzymatic synthesis of poly(glycerol succinate) in a supercritical carbon dioxide solution and the formation of polymers with Mn up to 3.5 kDa. Uyama et al.25 reported the use of a CALB amount of 15 wt % for the synthesis of PGS with Mw = 19.0 kDa and y1,2,3D = 16% from vinyl sebacate in bulk at 60 °C for 8 h. These PGS molecular weight and the fraction of the dendritic unit are similar to those achieved by us when using a CALB amount of 13.6 wt % (Mw = 16 kDa and y1,2,3D = 20%). Although, these results were achieved in shorter reaction times than our experiments due to the higher reactivity of vinyl sebacate compared to sebacic acid.
Table 2. Structural Parameters of PGS Prepared Using Different Amounts of Enzyme and Sebacic Acid/Glycerol Feed Ratios in Acetone at 40 °C for 24 h.
|
yi (%)a |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [enzyme] (wt %) | molar feed ratio | Mn (kDa)a | Mw (kDa)b | Đb (Da) | DB (%) | 1T | 2T | 1,3L | 1,2L | 1,2,3D |
| 13.6 | 1.0/1.0 | 9.4 | 16 | 6.8 | 41 | 19 | 3 | 41 | 17 | 20 |
| 10.2 | 1.0/1.0 | 5.4 | 11 | 5.8 | 41 | 22 | 2 | 40 | 16 | 20 |
| 6.8 | 1.0/1.0 | 3.7 | 13 | 4.5 | 36 | 22 | 2 | 44 | 15 | 16 |
| 3.4 | 1.0/1.0 | 1.4 | 3 | 2.1 | 17 | 30 | 2 | 53 | 9 | 6 |
| 13.6 | 1.5/1.0 | n.d.c | 11 | 4.8 | 56 | 8 | 0 | 44 | 12 | 36 |
| 13.6 | 1.2/1.0 | n.d.c | 8 | 4.0 | 54 | 15 | 1 | 37 | 16 | 31 |
| 13.6 | 1.0/1.2 | n.d.c | 7 | 3.1 | 28 | 31 | 3 | 39 | 16 | 11 |
| 13.6 | 1.0/1.5 | n.d.c | 3 | 1.7 | 17 | 44 | 6 | 33 | 13 | 5 |
Determined by 1H NMR.
Determined by GPC.
n.d. = not determined.
PGS with a higher molecular weight was obtained in acetone at 40 °C in 24 h using an equimolar feed ratio sebacic acid/glycerol. Deviation from this molar ratio leads to a decrease in the molecular weight (Table 2). These results indicate that glycerol tends to act as a diol in the CALB-catalyzed polycondensation. This is in agreement with Yang et al.29 and Rao et al.,22 who reported that CALB is capable of hindering crosslinking, and deviation from equimolarity between the monomers leads to a decrease in the molecular weight of poly(oleic diacid-co-glycerol) and poly(glycerol suberate), respectively. The high PGS molecular weight dispersity values (Đ > 2.0) observed in Table 2 may be explained by the difference in CALB reactivity toward primary and secondary hydroxyl, which leads to randomly branched polymers composed of the terminal, linear, and dendritic units instead of the formation of uniformly “perfect” dendrimers composed of only terminal and dendritic units. This structural randomness of PGS is associated with the high Đ value, as reported by Kulshrestha et al.27 for the terpolymer of adipic acid, 1,8-octanediol, and glycerol synthesized via CALB catalysis. Rao et al.22 have reported that the architecture of poly(glycerol suberate) can be changed from linear (Đ ≈ 2.0) to irregular like-dendrimers (Đ > 2.0) by changing the monomer feed ratio in the CALB-catalyzed polycondensation. Also, Table 2 shows that the higher the glycerol feed, the smaller is the formation of dendritic units, and more linear are the PGS chains. Furthermore, an increase in the sebacic acid feed from 1.0 up to 1.5 increased DB and decreased the average molar fraction of glyceridic end groups (y1T and y2T), whereas an increase in the glycerol feed from 1.0 up to 1.5 led to an opposite effect, a decrease in the DB and an increase in the average molar fraction of glyceridic end groups. Therefore, the change in the sebacic acid/glycerol feed ratio would be a strategy toward the control of the PGS structure by tailoring the polymer molecular weight, degree of branching, and end-group functionality.
Evaluation of the Temporal Evolution of PGS Molecular Weight
The influence of branching on the Mn determined by 1H NMR and GPC is shown in Figure 2a,b for PGS synthesized in acetone at 30 and 40 °C, respectively.
Figure 2.
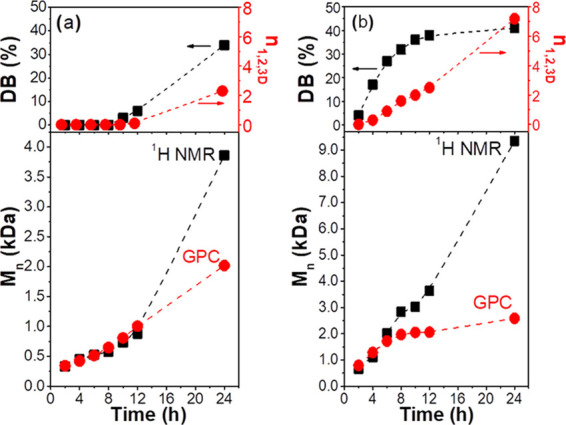
Degree of branching (DB) and the average number of dendritic units per PGS chain (n1,2,3D), determined by 1H NMR, and the number average molecular weight (Mn) as a function of reaction time as determined by 1H NMR and GPC analyses for reactions performed in acetone at (a) 30 °C and (b) 40 °C.
The Mn values determined by 1H NMR and GPC are in agreement with oligomers having Mn ≤ 1.0 kDa, and the deviations occurred when PGS became branched, i.e., when the DB value is approximately 30% and every polymer chain seems to have at least one dendritic unit (n1,2,3D ≥ 1.0). The discrepancies between Mn values determined by these techniques increase with an increase in the number of dendritic units. It is well established that GPC analysis of branched polymers provides molecular weight values smaller than the real one because branched polymers have a more dense and compact polymer coils and a smaller hydrodynamic radius (Rh) than their linear analogues. Because of this, the Mn value determined from GPC is generally smaller than the value determined by 1H NMR.27,61,62
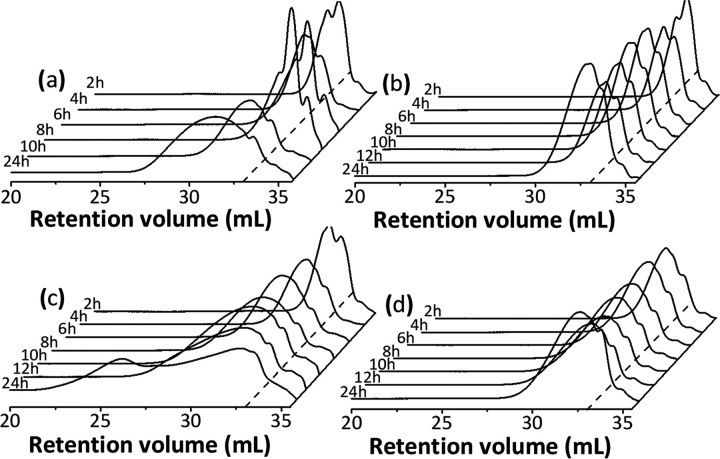
Despite the deviations of the molecular weight determined by GPC for branched polymers, this technique is a valuable tool to follow the relative molecular weight evolution and molecular weight distribution during the polymerization. Figure 3a,b,c,d illustrates the evolution of the GPC chromatograms for the reactions performed at 40 °C in tetrahydrofuran, tert-butanol, acetone, and acetonitrile, respectively.
Figure 3.
GPC chromatograms illustrating the temporal evolution of the molecular weight and molecular weight distribution of the PGS prepared in (a) tetrahydrofuran, (b) tert-butanol, (c) acetone, and (d) acetonitrile at 40 °C. The dashed lines indicate the retention volume related to the PS standard with the lowest molecular weight (1050 g mol–1) at 33 mL.
For all of the reaction conditions studied, an increase in the reaction time from 2 to 24 h led to a shift of the peaks in the chromatograms to a smaller retention volume (higher molecular weight), an enlargement of the molecular weight distribution, and the appearance of multimodal peaks, which are related to an increase in the structural complexity of randomly branched polymers due to the statistical character of chain growth. During polymer growth, the number of dendritic units increases and each dendritic unit gives rise to a new terminal unit. The high number of terminal units available during the polymerization makes possible the formation of structures with different sizes and architectures (more or less branched molecules).27,61,62 In Figure 3c, a bimodal molecular weight distribution curve with peak maxima at retention volumes of around 32.5 and 26.0 mL is observed for PGS after 24 h reaction in acetone at 40 °C. The last peak (26.0 mL), corresponding to a very high molecular weight (approximately 100 kDa according to the calibration curve using PS standards), is assigned to polymer aggregates. Because of this, it was not considered in the estimation of the mass average molecular weight and molecular weight distribution. Similar results were observed for the reaction performed in acetone at 50 °C/24 h.
Dynamic light scattering (DLS) experiments were performed in conditions similar to those of GPC analyses to determine whether aggregation of PGS (Mn = 9.4 kDa) occurs in the tetrahydrofuran solution. The particle size distribution profile (Figure S7a) shows three particle populations in the PGS solution in tetrahydrofuran with concentrations ranging from 1 to 10 mg mL–1: (i) unimers with an Rh of 3–4 nm; (ii) aggregates with an Rh of 8–15 nm, and (iii) a second aggregate population with an Rh of 40–80 nm. A similar particle size distribution profile was reported by Murillo et al.63 for hydroxylated hyperbranched polyester in dimethylformamide, for which a population of unimers with an Rh of 2–5 nm and aggregates with an Rh of 150–500 nm was observed. Xiang et al.64 studied the self-assembly of amphiphilic hyperbranched polyurea in water and proposed a stepwise mechanism in which unimers with an Rh of 2–3 nm form micelles with an Rh of 6–14 nm. Furthermore, these micelles associate in larger aggregates with an Rh ≥ 100 nm. The interactions among hydroxylated polymeric chains were studied by Žagar and Grdadolnik,65 who reported that hydroxylated hyperbranched polyester chains interact with each other by strong hydrogen bonds among hydroxyl groups and between hydroxyl groups and carbonyl ester groups, even in the presence of water. These strong interactions between the hydroxylated polymeric chains and the mechanism of self-assembly of amphiphilic polymers in solution could explain the PGS aggregation in tetrahydrofuran as observed by DLS and the presence of a population of high molecular weight molecules in the GPC chromatograms (Figure 3c). From the DLS analyses, the critical aggregation concentration (CAC) of PGS in tetrahydrofuran was estimated to be 0.74 mg mL–1 or 7.9 × 10–5 mol L–1, by plotting the mean scattering intensity as a function of the PGS concentration (Figure S7b). PGS aggregation occurred in all solvents studied, as verified by DLS experiments. The particle size distribution curves of the PGS solution in acetone, acetonitrile, and tert-butanol can be found in Figure S8, Supporting Information.
Kinetics of CALB-Catalyzed Polycondensation
The kinetic study of the enzymatic catalyzed polycondensation was performed in four different polar solvents (tetrahydrofuran, tert-butanol, acetone, and acetonitrile) in the temperature range from 30 to 70 °C. The determination of the apparent constant rate (kapp) for the polycondensation was performed as proposed by Flory,47 assuming the following: (i) glycerol reacts as a diol and (ii) cyclization does not take place. The first assumption is supported by the CALB higher reactivity toward primary hydroxyls than the secondary ones.34−36 The second assumption is supported by the work performed by Kline et al.20 and by Godinho et al.,66 in which cyclic end groups were not observed and some degree of cyclization was only observed for tetramers, respectively, for glycerol-based polyesters prepared via CALB catalysis.
The Dpn vs t curves for the reactions performed in tetrahydrofuran, tert-butanol, acetone, and acetonitrile in the temperature range from 30 to 70 °C are presented in Figure 4a,b,c,d, respectively. The kapp was determined as the slope of the Dpn vs t curves and plotted as a function of the reaction temperature, as presented in Figure 4e.
Figure 4.
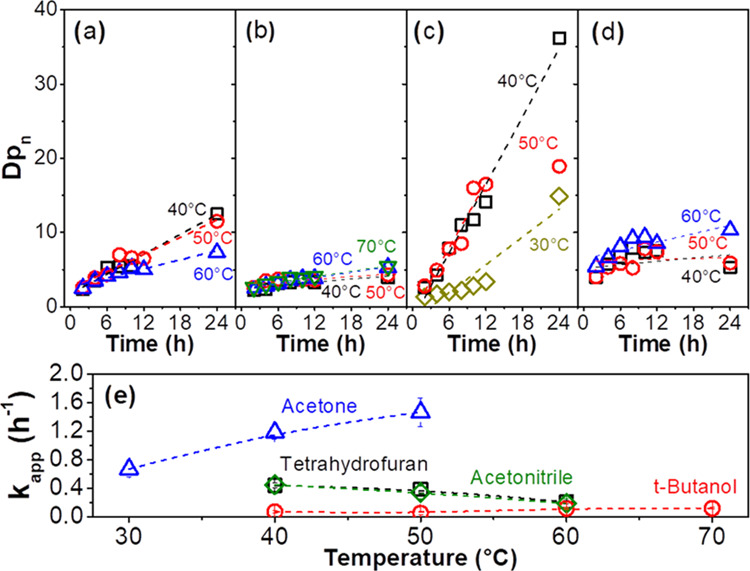
Number average degree of polymerization (Dpn) as a function of reaction time for reactions performed in (a) tetrahydrofuran, (b) tert-butanol, (c) acetone, and (d) acetonitrile. (e) Apparent rate constant (kapp) as a function of temperature.
The kapp values are similar for the reactions conducted in tetrahydrofuran and acetonitrile. A slight dependence of the reaction rate on the temperature is observed for reactions in these solvents with a decrease of the kapp from around 0.5 to 0.2 h–1 when the reaction temperature was increased from 40 to 60 °C. tert-Butanol was the worst solvent for the preparation of PGS, for which a Dpn of around 4–5 and kapp around 0.1 h–1 were achieved for reactions performed in the temperature range from 40 to 70 °C for 24 h. Using acetone as a solvent, Dpn and kapp achieved the highest values. The polycondensation performed at 30, 40, and 50 °C resulted in PGS with Dpn of 15, 36, and 19 and kapp of 0.7, 1.2, and 1.5 h–1, respectively. By constructing an Arrhenius plot (ln kapp vs T–1) from the kapp values of the reactions performed in acetone, apparent energy of activation of 32 kJ mol–1 was obtained. For the noncatalyzed polycondensation of sebacic acid and glycerol in toluene at temperatures ranging from 120 to 140 °C, Maliger et al.16 reported kapp values ranging from 0.04 to 0.13 h–1 and apparent energy of activation of 71 kJ mol–1. This indicates that CALB catalysis can increase the reaction rate of the polycondensation of sebacic acid and glycerol by approximately 5-fold and decrease the apparent energy of activation by approximately half, compared to the noncatalyzed polycondensation. The kapp of reactions performed in acetone increased by around 75% by increasing the temperature from 30 to 40 °C. However, a further temperature increase from 40 to 50 °C led to a minor increase in the kapp of just 25%. This tendency of the kapp to decrease in reactions performed in acetone, tetrahydrofuran, and acetonitrile by increasing the reaction temperature above 50–60 °C may be related to enzyme deactivation as discussed previously.
PGS Chain Growth and Branching
The temporal evolution of the number average degree of polymerization and average molar fraction of each glyceridic species (xi) in the reaction medium as a function of the time for the reactions performed in acetone at 30, 40, and 50 °C is presented in Figure 5a,b,c, respectively.
Figure 5.
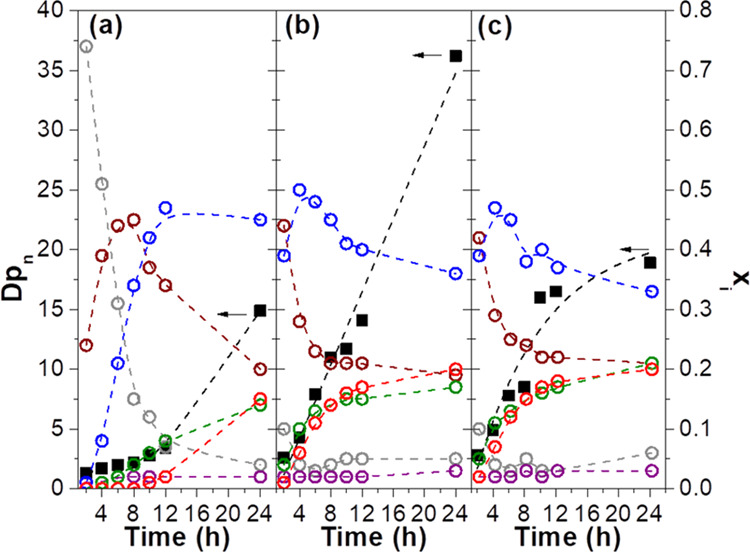
Number average degree of polymerization (Dpn, box solid) and the average molar fraction (xi) of glycerol (gray circle open) and glyceridic units 1T (maroon circle open), 2T (purple circle open), 1,3L (blue circle open), 1,2L (green circle open), and 1,2,3D (red circle open) in the reaction medium as a function of reaction time for reactions performed in acetone at (a) 30 °C, (b) 40 °C, and (c) 50 °C.
The plots in Figure 5 show that the chain growth behavior of PGS is similar to that reported by Rao et al.22 for poly(glycerol suberate) prepared by polycondensation of the diethyl suberate and glycerol via CALB catalysis, which is composed of three growth steps: (i) an initial linear growth step due to the reaction among terminal units to produce linear segments; (ii) graft growth, and (iii) dendritic growth occurring simultaneously through reactions between the terminal and linear units. The dendritic growth is characterized by a high steric hindrance that makes it difficult for the reactive groups in the middle of a polymeric chain to access the enzyme catalytic site. Therefore, the formation of 1,2,3D units probably proceeds toward the esterification of the primary hydroxyl available in the 1,2L unit.22,67 However, as shown in Figure 5, the production of the 1,2,3D units occurs simultaneously to the consumption of the 1,3L units. This leads to the following question: Does the formation of 1,2,3D units proceed through the enzymatically catalyzed esterification of the secondary hydroxyl of 1,3L units or is it due to acyl migration, which converts 1,3L units into 1,2L units followed by the CALB-catalyzed esterification of the primary hydroxyl of 1,2L units, similar to the mechanism proposed by Lortie et al.42 for the lipase-catalyzed synthesis of triolein?
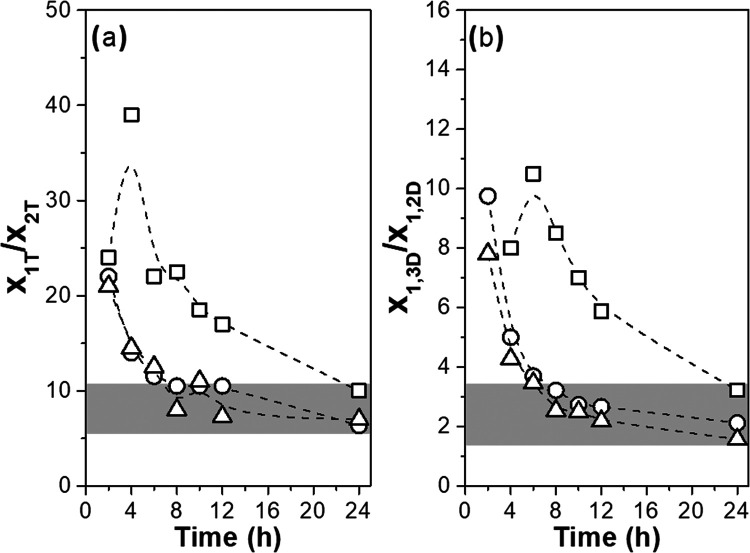
Acyl migration is frequently observed in the synthesis of glycerides via enzymatic or chemical catalysts, and its suppression is a difficult task.68,69 The rate of acyl migration depends on reaction parameters such as temperature, glyceride concentration, and solvent polarity, and it decreases with a decrease in solvent log P or temperature.42,70−75 For glycerol-based polyesters prepared using a chemical catalyst that selectively esterifies primary hydroxyls, Slavko and Taylor19 reported an increase in the 1,2L unit fraction and maintenance of the 1,2,3D unit fraction when polyesters mainly composed of 1,3L units were subjected to conditions favorable for the acyl migration reaction in the absence of the esterification catalyst. Many studies regarding acyl migration of glycerides show that at equilibrium, the molar ratio between monoglycerides esterified at primary hydroxyl and secondary hydroxyl is approximately 10, and the molar ratio for diglycerides with both primary hydroxyl esterified and with one primary and one secondary hydroxyl esterified is approximately 2.42,68,70,75,77Figure 6 presents these molar ratios as a function of time for PGS prepared in acetone at 30, 40, and 50 °C (data for other reaction conditions are presented in Figure S9).
Figure 6.
Molar ratio of glyceridic units as a function of time for reactions performed in acetone at 30 °C (□), 40 °C (○), and 50 °C (Δ): (a) x1T/x2T and (b) x1,3L/x1,2L.
Surprisingly, for all reaction conditions, the x1T/x2T and x1,3L/x1,2L ratios tend to values of around 10 and 2 at 24 h reaction, respectively. These values are reached at shorter reaction times as the temperature increases. By performing an experiment similar to that reported by Slavko and Taylor,19 a solution of PGS with different DB in tetrahydrofuran in the presence of triethylamine and molecular sieves was prepared and maintained under gentle stirring for 24 h at 40 °C, in the absence of the esterification catalyst, to favor the acyl migration reaction. After this time, there was observed to be a tendency toward an increase in x1,2L, the maintenance of x1,2,3D, and the x1T/x2T and x1,3L/x1,2L ratios equal to 10 and 2, respectively (Table S7). The x1,3L/x1,2L ratio around 2 has also been observed for poly(glycerol suberate) and poly(oleic diacid-co-glycerol) prepared via CALB catalysis and for poly(oleic diacid-co-glycerol) prepared via metal catalysis.22,29 The ratios x1T/x2T and x1,3L/x1,2L were very close for the polymers synthesized in different solvents at a given temperature (Figure S9), indicating that the acyl migration rate was not affected by the solvent. This tendency agrees with that observed by Duan et al.37 and Li et al.73 for the CALB-catalyzed esterification of glycerol and oleic acid in solvents with log P values that ranged from −0.24 to 4.7. In their study, it was reported that the reactions performed in polar solvents with log P < 1.0 have similar lower acyl migration constant rates and higher CALB selectivity toward glycerol primary hydroxyl compared to the use of apolar solvents with log P > 1.0.
Based on these results and the works of Rao et al.22 and Lortie et al.,42 we propose a mechanism of the chain growth behavior and branching of glycerol-based polyesters prepared via CALB catalysis, Scheme 1.
Scheme 1. Representation of the Mechanism of Glycerol-Based Polyester Chain Growth and Branching during the Simultaneous Occurrence of the CALB-Catalyzed Esterification and Acyl Migration (A.M.).
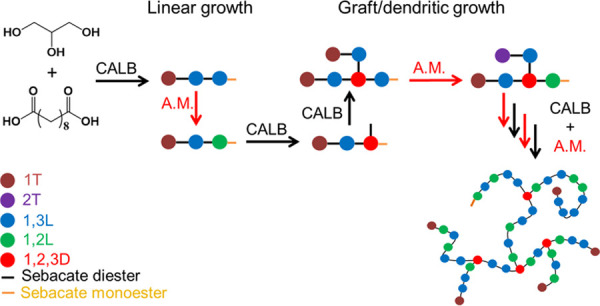
The enzyme is mainly responsible for the esterification of glycerol primary hydroxyl groups to produce 1T and 1,3L units. The 2T and 1,2L units result mainly from acyl migration, and further esterification of 1,2L units by enzyme catalysis produces 1,2,3D units. The esterification of the primary hydroxyl of a 1,2L unit should be faster than the esterification of the secondary hydroxyl of a 1,3L unit, and the consumption of 1,2L units by esterification is compensated by acyl migration equilibrium, which restores 1,2L units from 1,3L units. Both reactions will proceed until the polymerization and acyl migration reactions achieve thermodynamic equilibrium and the molar fractions of each glyceridic unit reach a constant value with x1T/x2T = 10 and x1,3L/x1,2L = 2. The 2T unit molar fraction remains low and constant throughout the reaction, indicating a steady-state (Figure 5). Thus, the 2T unit formation rate and consuming rate in 1T and 1,2L due to acyl migration and CALB-catalyzed esterification, respectively, should be equivalent. Moreover, based on this mechanism, the CALB-catalyzed esterification is mainly responsible for the increase of the polymer degree of polymerization, whereas the acyl migration only changes the ester position from a primary to a secondary hydroxyl. Therefore, the kapp values describe mainly the CALB-catalyzed esterification reaction.
This set of results indicates that branching suppression is not possible for a polycondensation catalyzed by CALB under the reaction conditions investigated due to the occurrence of the acyl migration reaction. However, the PGS architecture, molecular weight, and degree of branching can be tailored by changing the reaction parameters such as the solvent, temperature, CALB amount, and sebacic acid/glycerol feed ratio, which are important strategies to tune the PGS elastomer properties.
The strategy to achieve linear polymers should be kinetic control using reaction conditions for which the esterification of primary hydroxyl and formation of 1T and 1,3L units are much faster than the rate of acyl migration. For example, this condition is probably achieved by the use of diarylborinic acid catalysts in which a catalyst–substrate adduct is formed, preventing the acyl migration reaction and the esterification of glycerol secondary hydroxyls, and the PGS formed is mostly composed of 1,3L units with less than 1% of the 1,2,3D unit.19 The use of activated esters or bulk reactions for the CALB-catalyzed synthesis of glycerol-based polyester is other strategies that seem to delay acyl migration and allow the preparation of linear polymers with the higher molecular weight than achieved in the conditions studied here. However, after some time, acyl migration also seems to occur in these conditions and some degree of branching is observed as the polymer molecular weight increases.29,31
Conclusions
The use of CALB as a catalyst for the polycondensation of glycerol and sebacic acid in low-hazard polar solvents and mild reaction conditions allows the preparation of PGS. These polyesters have pendant hydroxyl groups, low to relatively high molecular weight (Mw ranging from 3 to 16 kDa), and a degree of branching ranging from 24 to 42%. The influence of temperature on the polymerization is governed by good enzyme performance at temperatures up to 50 °C and enzyme denaturation at above 50 °C. The solvents seem to affect both polymer coil solvation and the enzyme structure and, consequently, the extent of the polymerization. However, the solvent does not seem to affect the acyl migration rate or CALB selectivity. The enzyme amount of 13.6 wt % leads to a higher PGS molecular weight for a 24 h reaction time, and by controlling the sebacic acid/glycerol feed ratio, it is possible to control the PGS architecture, molecular weight, degree of branching, and end-group functionality. Using acetone as a solvent at 40 °C, a higher rate constant (kapp = 1.5 h–1), a higher molecular weight (Mn = 9.4 kDa and Mw = 16 kDa), and a higher degree of branching (42%) were achieved for PGS. Therefore, the CALB-catalyzed synthesis of PGS is a suitable alternative to the conventional preparation of PGS. The kinetic study allowed us to understand polymer growth and branching. During the polycondensation reaction, CALB-catalyzed esterification and acyl migration occur simultaneously, and the latter is mainly responsible for the esterification of secondary hydroxyls and to give rise to branching.
Acknowledgments
The authors would like to thank FAPESP (Process nos. 2018/01562-6 and 2015/25406–5), CNPq (Process no. 444392/2014-9), and Capes (Finance Code “001”) for the financial funding.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.macromol.0c01709.
Summarized polycondensation conditions and characteristics of glycerol-based polyesters prepared via CALB catalysis, IR spectrum, 13C NMR spectrum, 1H–13C HSQC contour map, 1H–1H COSY contour map, temporal evolution of 1H NMR spectra signals, 1H NMR curve-fitted signals of methylene protons adjacent to the sebacic acid/ester carbonyl, structural parameters calculations, comparison of results from 1H NMR and quantitative 13C NMR, general data from the 1H NMR and GPC analyses, DLS analyses and CAC estimation, x1T/x2T and x1,3L/x1,2L molar fraction ratio as a function of time, and acyl migration experiment (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Rai R.; Tallawi M.; Grigore A.; Boccaccini A. R. Synthesis, properties and biomedical applications of poly(glycerol sebacate) (PGS): A review. Prog. Polym. Sci. 2012, 37, 1051–1078. 10.1016/j.progpolymsci.2012.02.001. [DOI] [Google Scholar]
- Zamboulis A.; Nakiou E. A.; Christodoulou E.; Bikiaris D. N. Polyglycerol Hyperbranched Polyesters: Synthesis, Properties and Pharmaceutical and Biomedical Applications. Int. J. Mol. Sci. 2019, 20, 6210 10.3390/ijms20246210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh X. J.; Karim A. A.; Owh C. Poly(glycerol sebacate) biomaterial: synthesis and biomedical applications. J. Mater. Chem. B 2015, 3, 7641–7652. 10.1039/C5TB01048A. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Ameer G. A.; Sheppard B. J.; Langer R. A tough biodegradable elastomer. Nat. Biotechnol. 2002, 20, 602–606. 10.1038/nbt0602-602. [DOI] [PubMed] [Google Scholar]
- Li Y.; Cook W. D.; Moorhoff C.; Huang W.; Chen Q. Synthesis, characterization and properties of biocompatible poly(glycerol sebacate) pre-polymer and gel. Polym. Int. 2013, 62, 534–547. 10.1002/pi.4419. [DOI] [Google Scholar]
- Chen Q.; Bismarck A.; Hansen U.; Junaid S.; Tran M. Q.; Harding S. E.; Ali N. N.; Boccaccini A. R. Characterisation of a soft elastomer poly(glycerol sebacate) designed to match the mechanical properties of myocardial tissue. Biomaterials 2008, 29, 47–57. 10.1016/j.biomaterials.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Li X.; Hong A. T.; Naskar N.; Chung H. Criteria for Quick and Consistent Synthesis of Poly (glycerol sebacate) for Tailored Mechanical Properties. Biomacromolecules 2015, 16, 1525–1533. 10.1021/acs.biomac.5b00018. [DOI] [PubMed] [Google Scholar]
- Barrett D. G.; Yousaf M. N. Design and applications of biodegradable polyester tissue scaffolds based on endogenous monomers found in human metabolism. Molecules 2009, 14, 4022–4050. 10.3390/molecules14104022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Kim Y. M.; Langer R. In vivo degradation characteristics of poly(glycerol sebacate). J. Biomed. Mater. Res., Part A 2003, 66A, 192–197. 10.1002/jbm.a.10534. [DOI] [PubMed] [Google Scholar]
- Cai W.; Liu L. Shape-memory effect of poly(glycerol – sebacate) elastomer. Mater. Lett. 2008, 62, 2171–2173. 10.1016/j.matlet.2007.11.042. [DOI] [Google Scholar]
- Mortensen P. B. C6-C10-dicarboxylic aciduria in starved, fat-fed and diabetic rats receiving decanoic acid or medium-chain triacylglycerol. An in vivo measure of the rate of beta-oxidation of fatty acids. Biochim. Biophys. Acta, Lipids Lipid Metab. 1981, 664, 349–355. 10.1016/0005-2760(81)90057-6. [DOI] [PubMed] [Google Scholar]
- Liu G.; Hinch B.; Beavis A. D. Mechanisms for the Transport of α,ω-Dicarboxylates through the Mitochondrial Inner Membrane. J. Biol. Chem. 1996, 271, 25338–25344. 10.1074/jbc.271.41.25338. [DOI] [PubMed] [Google Scholar]
- Sundback C. A.; Shyu J. Y.; Wang Y.; Faquin W.; Langer R. S.; Vacanti J. P.; Hadlock T. A. Biocompatibility analysis of poly(glycerol sebacate) as a nerve guide material. Biomaterials 2005, 26, 5454–5464. 10.1016/j.biomaterials.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Lau C. C.; Bayazit M. K.; Knowles J. C.; Tang J. Tailoring degree of esterification and branching of poly(glycerol sebacate) by energy efficient microwave irradiation. Polym. Chem. 2017, 8, 3937–3947. 10.1039/C7PY00862G. [DOI] [Google Scholar]
- Conejero-garcía Á.; Gimeno H. R.; Sáez Y. M.; Vilariño-feltrer G.; Ortuño-lizarán I.; Vallés-lluch A. Correlating synthesis parameters with physicochemical properties of poly(glycerol sebacate). Eur. Polym. J. 2017, 87, 406–419. 10.1016/j.eurpolymj.2017.01.001. [DOI] [Google Scholar]
- Maliger R.; Halley P. J.; Cooper-White J. J. Poly(glycerol-sebacate) bioelastomers-kinetics of step-growth reactions using Fourier Transform (FT)-Raman spectroscopy. J. Appl. Polym. Sci. 2013, 127, 3980–3986. 10.1002/app.37719. [DOI] [Google Scholar]
- You Z.; Cao H.; Gao J.; Shin P. H.; Day B. W.; Wang Y. A functionalizable polyester with free hydroxyl groups and tunable physiochemical and biological properties. Biomaterials 2010, 31, 3129–3138. 10.1016/j.biomaterials.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You Z.; Bi X.; Wang Y. Fine Control of Polyester Properties via Epoxide ROP Using Monomers Carrying Diverse Functional Groups. Macromol. Biosci. 2012, 12, 822–829. 10.1002/mabi.201200035. [DOI] [PubMed] [Google Scholar]
- Slavko E.; Taylor M. S. Catalyst-controlled polycondensation of glycerol with diacyl chlorides: Linear polyesters from a trifunctional monomer. Chem. Sci. 2017, 8, 7106–7111. 10.1039/C7SC01886J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline B. J.; Beckman E. J.; Russell A. J. One-step biocatalytic synthesis of linear polyesters with pendant hydroxyl groups. J. Am. Chem. Soc. 1998, 120, 9475–9480. 10.1021/ja9808907. [DOI] [Google Scholar]
- Iglesias L. E.; Fukuyama Y.; Nonami H.; Erra-Balsells R.; Baldessari A. A simple enzymatic procedure for the synthesis of a hydroxylated polyester from glycerol and adipic acid. Biotechnol. Tech. 1999, 13, 923–926. 10.1023/A:1008958212814. [DOI] [Google Scholar]
- Rao Z. K.; Ni H. L.; Li Y.; Zhu H. Y.; Liu Y.; Hao J. Y. Macroscopic Scaffold Control for Lipase-Catalyzed Dendritic Polyol-Polyesters. Macromol. Chem. Phys. 2019, 220, 1900048 10.1002/macp.201900048. [DOI] [Google Scholar]
- Zeng F.; Yang X.; Li D.; Dai L.; Zhang X.; Lv Y.; Wei Z. Functionalized polyesters derived from glycerol: Selective polycondensation methods toward glycerol-based polyesters by different catalysts. J. Appl. Polym. Sci. 2019, 48574 10.1002/app.48574. [DOI] [Google Scholar]
- Uyama H.; Inada K.; Kobayashi S. Regioselective polymerization of divinyl sebacate and triols using lipase catalyst. Macromol. Rapid. Commun. 1999, 20, 171–174. . [DOI] [Google Scholar]
- Uyama H.; Inada K.; Kobayashi S. Regioselectivity Control in Lipase-Catalyzed Polymerization of Divinyl Sebacate and Triols. Macromol. Biosci. 2001, 1, 40–44. . [DOI] [Google Scholar]
- Kumar A.; Kulshrestha A. S.; Gao W.; Gross R. A. Versatile Route to Polyol Polyesters by Lipase Catalysis. Macromolecules 2003, 36, 8219–8221. 10.1021/ma0351827. [DOI] [Google Scholar]
- Kulshrestha A. S.; Gao W.; Gross R. A. Glycerol copolyesters: Control of branching and molecular weight using a lipase catalyst. Macromolecules 2005, 38, 3193–3204. 10.1021/ma0480190. [DOI] [Google Scholar]
- Kallinteri P.; Higgins S.; Hutcheon G. A.; St. Pourçain C. B.; Garnett M. C. Novel functionalized biodegradable polymers for nanoparticle drug delivery systems. Biomacromolecules 2005, 6, 1885–1894. 10.1021/bm049200j. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Lu W.; Cai J.; Hou Y.; Ouyang S.; Xie W.; Gross R. A. Poly(oleic diacid-co-glycerol): Comparison of polymer structure resulting from chemical and lipase catalysis. Macromolecules 2011, 44, 1977–1985. 10.1021/ma102939k. [DOI] [Google Scholar]
- Naolou T.; Weiss V. M.; Conrad D.; Busse K.; Mäder K.; Kressler J.. Fatty Acid Modified Poly(glycerol adipate) - Polymeric Analogues of Glycerides. In Tailored Polymer Architectures for Pharmaceutical and Biomedical Applications; Scholz C.; Kressler J., Eds.; ACS Symposium Series; American Chemical Society: Washington, 2013; Chapter 4, pp 39–52. [Google Scholar]
- Taresco V.; Creasey R. G.; Kennon J.; Mantovani G.; Alexander C.; Burley J. C.; Garnett M. C. Variation in structure and properties of poly(glycerol adipate) via control of chain branching during enzymatic synthesis. Polymer 2016, 89, 41–49. 10.1016/j.polymer.2016.02.036. [DOI] [Google Scholar]
- Valerio O.; Misra M.; Mohanty A. K. Poly(glycerol-co-diacids) Polyesters: From Glycerol Biorefinery to Sustainable Engineering Applications, A Review. ACS Sustainable Chem. Eng. 2018, 6, 5681–5693. 10.1021/acssuschemeng.7b04837. [DOI] [Google Scholar]
- Ortiz C.; Ferreira M. L.; Barbosa O.; dos Santos J. C. S.; Rodrigues R. C.; Berenguer-Murcia Á.; Briand L. E.; Fernandez-Lafuente R. Novozym 435: The ‘perfect’ lipase immobilized biocatalyst?. Catal. Sci. Technol. 2019, 9, 2380–2420. 10.1039/C9CY00415G. [DOI] [Google Scholar]
- Yoon K. R.; Hong S. P.; Kong B.; Choi I. S. Polycondensation of sebacic acid with primary and secondary hydroxyl groups containing diols catalyzed by Candida antarctica lipase B. Synth. Commun. 2012, 42, 3504–3512. 10.1080/00397911.2011.585267. [DOI] [Google Scholar]
- Cha H. J.; Park J. B.; Park S. Esterification of Secondary Alcohols and Multi-hydroxyl Compounds by Candida antarctica Lipase B and Subtilisin. Biotechnol. Bioprocess. Eng. 2019, 24, 41–47. 10.1007/s12257-018-0379-1. [DOI] [Google Scholar]
- Schmid R. D.; Verger R. Lipases: Interfacial Enzymes with Attractive Applications. Angew. Chem., Int. Ed. 1998, 37, 1608–1633. . [DOI] [PubMed] [Google Scholar]
- Duan Z. Q.; Du W.; Liu D. H. The solvent influence on the positional selectivity of Novozym 435 during 1,3-diolein synthesis by esterication. Bioresour. Technol. 2010, 101, 2568–2571. 10.1016/j.biortech.2009.11.087. [DOI] [PubMed] [Google Scholar]
- Duan Z. Q.; Du W.; Liu D. H. The mechanism of solvent effect on the positional selectivity of Candida antarctica lipase B during 1,3-diolein synthesis by esterification. Bioresour. Technol. 2011, 102, 11048–11050. 10.1016/j.biortech.2011.09.003. [DOI] [PubMed] [Google Scholar]
- Uyama H.; Klegraf E.; Wada S.; Kobayashi S. Regioselective Polymerization of Sorbitol and Divinyl Sebacate Using Lipase Catalyst. Chem. Lett. 2000, 29, 800–801. 10.1246/cl.2000.800. [DOI] [Google Scholar]
- Lang K.; Bhattacharya S.; Ning Z.; Sanchez-Leija R. J.; Bramson M. T. K.; Centore R.; Corr D. T.; Linhardt R. J.; Gross R. A. Enzymatic polymerization of poly(glycerol-1,8-octanediol-sebacate): versatile PGS analogs that form mono-component biodegradable fiber scaffolds. Biomacromolecules 2020, 21, 3197–3206. 10.1021/acs.biomac.0c00641. [DOI] [PubMed] [Google Scholar]
- d’Almeida Gameiro M.; Goddard A.; Taresco V.; Howdle S. M. Enzymatic one-pot synthesis of renewable and biodegradable surfactants in supercritical carbon dioxide (scCO2). Green Chem. 2020, 22, 1308–1318. 10.1039/C9GC04011K. [DOI] [Google Scholar]
- Lortie R.; Trani M.; Ergan F. Kinetic study of the lipase-catalyzed synthesis of triolein. Biotechnol. Bioeng. 1993, 41, 1021–1026. 10.1002/bit.260411104. [DOI] [PubMed] [Google Scholar]
- Kapoor M.; Gupta M. N. Lipase promiscuity and its biochemical applications. Process Biochem. 2012, 47, 555–569. 10.1016/j.procbio.2012.01.011. [DOI] [Google Scholar]
- Nguyen H. D.; Löf D.; Hvilsted S.; Daugaard A. E. Highly branched bio-based unsaturated polyesters by enzymatic polymerization. Polymers 2016, 8, 363 10.3390/polym8100363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt V. T.; Strahan G. D. Degree of branching in hyperbranched poly(glycerol-co-diacid)s synthesized in toluene. Polymers 2012, 4, 396–407. 10.3390/polym4010396. [DOI] [Google Scholar]
- Holter D.; Burgath A.; Frey H. Degree of branching in hyperbranched polymers. Acta Polym. 1999, 50, 67–76. 10.1002/actp.1997.010480105. [DOI] [Google Scholar]
- Flory P. J.Principles of Polymer Chemistry; Cornell University Press: New York, 1953. [Google Scholar]
- Prat D.; Wells A.; Hayler J.; Sneddon H.; McElroy C. R.; Abou-Shehada S.; Dunn P. J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2016, 18, 288–296. 10.1039/C5GC01008J. [DOI] [Google Scholar]
- Nijst C. L. E.; Bruggeman J. P.; Karp J. M.; Ferreira L.; Zumbuehl A.; Bettinger C. J.; Langer R. Synthesis and Characterization of Photocurable Elastomers from Poly(glycerol-co-sebacate). Biomacromolecules 2007, 8, 3067–3073. 10.1021/bm070423u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louage B.; Tack L.; Wang Y.; De Geest B. G. Poly(glycerol sebacate) nanoparticles for encapsulation of hydrophobic anti-cancer drugs. Polym. Chem. 2017, 8, 5033–5038. 10.1039/C6PY02192A. [DOI] [Google Scholar]
- Laane C.; Boeren S.; Vos K.; Veeger C. Rules for optimization of biocatalysis in organic solvents. Biotechnol. Bioeng. 1987, 30, 81–87. 10.1002/bit.260300112. [DOI] [PubMed] [Google Scholar]
- Yao D.; Li G.; Kuila T.; Li P.; Kim N. H.; Kim S.-I.; Lee J. H. Lipase-Catalyzed Synthesis and Characterization of Biodegradable Polyester Containing L-Malic Acid Unit in Solvent System. J. Appl. Polym. Sci. 2011, 120, 1114–1120. 10.1002/app.33257. [DOI] [Google Scholar]
- Dutta Banik S.; Nordblad M.; Woodley J. M.; Peters G. H. A Correlation between the Activity of Candida antarctica Lipase B and Differences in Binding Free Energies of Organic Solvent and Substrate. ACS Catal. 2016, 6, 6350–6361. 10.1021/acscatal.6b02073. [DOI] [Google Scholar]
- Douka A.; Vouyiouka S.; Papaspyridi L. M.; Papaspyrides C. D. A review on enzymatic polymerization to produce polycondensation polymers: The case of aliphatic polyesters, polyamides and polyesteramides. Prog. Polym. Sci. 2018, 79, 1–25. 10.1016/j.progpolymsci.2017.10.001. [DOI] [Google Scholar]
- Distel K. A.; Zhu G.; Wang P. Biocatalysis using an organic-soluble enzyme for the preparation of poly(lactic acid) in organic solvents. Bioresour. Technol. 2005, 96, 617–623. 10.1016/j.biortech.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Azim H.; Dekhterman A.; Jiang Z.; Gross R. A. Candida antarctica lipase B-catalyzed synthesis of poly(butylene succinate): Shorter chain building blocks also work. Biomacromolecules 2006, 7, 3093–3097. 10.1021/bm060574h. [DOI] [PubMed] [Google Scholar]
- Mahapatro A.; Kumar A.; Kalra B.; Gross R. A. Solvent-free adipic acid/1,8-octanediol condensation polymerizations catalyzed by Candida antartica lipase B. Macromolecules 2004, 37, 35–40. 10.1021/ma025796w. [DOI] [Google Scholar]
- Jiang Y.; Woortman A. J. J.; Alberda Van Ekenstein G. O. R.; Loos K. Environmentally benign synthesis of saturated and unsaturated aliphatic polyesters via enzymatic polymerization of biobased monomers derived from renewable resources. Polym. Chem. 2015, 6, 5451–5463. 10.1039/C5PY00660K. [DOI] [Google Scholar]
- Kuperkar V. V.; Lade V. G.; Prakash A.; Rathod V. K. Synthesis of isobutyl propionate using immobilized lipase in a solvent free system: Optimization and kinetic studies. J. Mol. Catal. B: Enzym. 2014, 99, 143–149. 10.1016/j.molcatb.2013.10.024. [DOI] [Google Scholar]
- Lopresto C. G.; Calabrò V.; Woodley J. M.; Tufvesson P. Kinetic study on the enzymatic esterification of octanoic acid and hexanol by immobilized Candida antarctica lipase B. J. Mol. Catal. B: Enzym. 2014, 110, 64–71. 10.1016/j.molcatb.2014.09.011. [DOI] [Google Scholar]
- Voit B. I.; Lederer A. Hyperbranched and Highly Branched Polymer Architectures-Synthetic Strategies and Major Characterization Aspects. Chem. Rev. 2009, 109, 5924–5973. 10.1021/cr900068q. [DOI] [PubMed] [Google Scholar]
- Zhu X.; Zhou Y.; Yan D. Influence of branching architecture on polymer properties. J. Polym. Sci., Part B: Polym. Phys. 2011, 49, 1277–1286. 10.1002/polb.22320. [DOI] [Google Scholar]
- Murillo E. A.; Vallejo P. P.; López B. L. Characterization of hydroxylated hyperbranched polyesters of fourth and fifth generation. e-Polymers 2010, 10, 1347–1358. 10.1515/epoly.2010.10.1.1347. [DOI] [Google Scholar]
- Xiang F.; Stuart M.; Vorenkamp J.; Roest S.; Timmer-Bosscha H.; Stuart M. C.; Fokkink R.; Loontjens T. One-pot synthesis for biocompatible amphiphilic hyperbranched polyurea micelles. Macromolecules 2013, 46, 4418–4425. 10.1021/ma400552x. [DOI] [Google Scholar]
- Žagar E.; Grdadolnik J. An infrared spectroscopic study of H-bond network in hyperbranched polyester polyol. J. Mol. Struct. 2003, 658, 143–152. 10.1016/S0022-2860(03)00286-2. [DOI] [Google Scholar]
- Godinho B.; Gama N.; Barros-Timmons A.; Ferreira A. Enzymatic synthesis of poly(glycerol sebacate) pre-polymer with crude glycerol, by-product from biodiesel prodution. AIP Conf. Proc. 2018, 1981, 020031 10.1063/1.5045893. [DOI] [Google Scholar]
- Brandner J. D.; Birkmeier R. L. Relative esterifiability of the primary and secondary hydroxyl groups of glycerol. J. Am. Oil Chem. Soc. 1960, 37, 390–396. 10.1007/BF02672644. [DOI] [Google Scholar]
- Serdarevich B. Glyceride isomerizations in lipid chemistry. J. Am. Oil Chem. Soc. 1967, 44, 381–393. 10.1007/BF02666775. [DOI] [Google Scholar]
- Mao J.; Hu Z.; Hu J.; Zhu X.; Xiong H. A Density Functional Theory (DFT) study of the Acyl migration occurring during lipase-catalyzed transesterifications. Int. J. Mol. Sci. 2019, 20, 3438 10.3390/ijms20143438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.; Skands A. R. H.; Høy C. E.; Mu H.; Balchen S.; Adler-Nissen J. Production of specific-structured lipids by enzymatic interesterification: Elucidation of acyl migration by response surface design. J. Am. Oil Chem. Soc. 1998, 75, 1179–1186. 10.1007/s11746-998-0132-6. [DOI] [Google Scholar]
- Laszlo J. A.; Compton D. L.; Vermillion K. E. Acyl migration kinetics of vegetable oil 1,2-diacylglycerols. J. Am. Oil Chem. Soc. 2008, 85, 307–312. 10.1007/s11746-008-1202-5. [DOI] [Google Scholar]
- Compton D. L.; Vermillion K. E.; Laszlo J. A. Acyl migration kinetics of 2-Monoacylglycerols from soybean oil via 1H NMR. J. Am. Oil Chem. Soc. 2007, 84, 343–348. 10.1007/s11746-007-1049-1. [DOI] [Google Scholar]
- Li W.; Du W.; Li Q.; Li R.-w.; Liu D. Dependence on the properties of organic solvent: Study on acyl migration kinetics of partial glycerides. Bioresour. Technol. 2010, 101, 5737–5742. 10.1016/j.biortech.2010.03.018. [DOI] [PubMed] [Google Scholar]
- Li W.; Du W.; Li Q.; Sun T.; Liu D. Study on acyl migration kinetics of partial glycerides: Dependence on temperature and water activity. J. Mol. Catal. B: Enzym. 2010, 63, 17–22. 10.1016/j.molcatb.2009.11.012. [DOI] [Google Scholar]
- Fureby A. M.; Virto C.; Adlercreutz P.; Mattiasson B. Acyl group migrations in 2-monoolein. Biocatal. Biotransform. 1996, 14, 89–111. 10.3109/10242429609106879. [DOI] [Google Scholar]
- Freeman I. P.; Morton D. Acyl Migration in Diglycerides. J. Chem. Soc. C 1966, 1710–1711. 10.1039/J39660001710. [DOI] [Google Scholar]
- Li W.; Li R. W.; Li Q.; Du W.; Liu D. Acyl migration and kinetics study of 1(3)-positional specific lipase of Rhizopus oryzae-catalyzed methanolysis of triglyceride for biodiesel production. Process Biochem. 2010, 45, 1888–1893. 10.1016/j.procbio.2010.03.034. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.