Abstract
Silicon (Si) plays an important role in the sustainable agriculture industry. The increasing demand for crop production with a significant reduction of synthetic chemical fertilizers and pesticide use is a big challenge nowadays. The use of Si has been proven to be an environmentally sound way of enhancing crop productivity by facilitating plant growth and development through either a direct or indirect mechanism, especially in tropical and subtropical regions. In particular, it has been investigated for its role in water stress management. The aim of the current experiment was to examine the protective role of Si in the photosynthetic capacity of different leaf segments and the ultrastructure of sugarcane (Saccharum officinarm) plants under water stress. Sugarcane cv. GT 42 plants were supplied with 0, 100, 300, and 500 mg L–1 Si and exposed for 60 days under each stress condition such as 100–95, 55–50, and 35–30% of field capacity. For the photosynthetic responses, each leaf was observed and separated into three equal parts (base, middle, and tip). We used intact leaves and were able to assess leaf photosynthetic responses. Under moderate and severe stress conditions, applied Si increased the photosynthesis (base, ∼16–143%; middle, 20–66%; and tip leaf part, 41–71%), transpiration rate (base, 15–97%; middle, 26–68%; and tip leaf part, 6–61%), and stomatal conductance (base, 26–137%; middle, 12–70%; and tip leaf part, 7–75%) in sugarcane plants. Ultrastructural examination of sugarcane leaves using scanning electron microscopy showed the remarkable effects on stomata ultrastructure. Silicon increased plant growth development, photosynthetic efficiency, and biomass/yield, and promoted better adaptation of stomata to drought. This study suggests that the application of Si may be used to increase the stress tolerance of sugarcane plants.
1. Introduction
Drought is one of the major environmental stresses that hinders plant growth, development, and productivity worldwide.1,2 It causes a broad range of growth, photosynthetic, metabolic, and ultrastructural variations in plants.1 It also escalates leaf senescence, chlorosis, and necrosis, and degrades photosynthetic pigments, which in turn reduces photosynthetic efficiency and canopy size, resulting in reduced crop productivity and sometimes in total failure of the crop plants.2−4
Plant stomata are important channels among plants and the environment may play an important role in plant responses to atmospheric variables.5,6 In addition, various studies show that stomatal density responds to various environmental variables like extreme temperature,7 elevated CO2 concentration,8 salinity,9 insufficient water,10,11 precipitation change,12 and plant density.13
Photosynthesis is an important physiological process for plant carbon uptake, development, and productivity.14,15 It is commonly considered that stomatal limitation, which affects the substomatal CO2 content, is the major source of the loss of photosynthetic efficiency under stress conditions.15−17 The photosynthetic apparatus of plants appears to be more sensitive to drought.18,19 Improving photosynthetic traits is the basis for the enhancement of plant biomass and crop productivity. Water stress triggers closure of stomata, affects the electron transport rate (ETR), aggravates photoinhibition induced by excessive light intensity, and lowers rates of photosynthesis as well as reduction of photosynthetic pigments. All of these variations could further lead to a loss in crop production and affect plant development.20−22 Under water stress conditions, Si can enhance soil water-use efficiency, root growth zone, and uptake of nutrients and consequently enhance the crop yield.15,23
Silicon has been commonly recognized as a fertilizer, biostimulating plant protection under atmospheric environmental variables.15,24−26 Silicon is absorbed by root hairs through an active uptake by a transpiration stream, being later transported as monosilic acid to the plant tissues, where it is polymerized as solid amorphous silica bodies (SiO2·nH2O) called phytoliths.27,28 In particular, the connection of silicon with tolerance to environmental stresses has been studied extensively in various crops.15,24,28−31 Silicon might be associated with physiologic and molecular mechanisms in plants32,33 and potentially alleviates the detrimental impact of water stress, a severe abiotic stress.
Sugarcane (Saccharum officinarum L.) is a major cash crop and is cultivated in the arid and semiarid areas of the world. In relation to geoponics, water scarcity is one of the major factors that limits sugarcane productivity, while the utilization of fertilizers exerts a significant impact on photosynthetic performance and yield.6,15,20,21 The synergy of water and fertilizers or the combined use of both is an important measure to save water in agricultural crops.34−36
However, knowledge about how Si modulates the photosynthetic capacity in S. officinarum “GT 42” during drought remains elusive. Although the importance of this element to crop plants is still debated, there have been beneficial impacts on our understanding of the uptake of Si in plants. The purpose of this study was to assess the responses of stomata morphology to different water irrigation levels with Si amendment and to evaluate the importance of stomatal conductance with photosynthesis and transpiration rate in sugarcane based on a greenhouse experiment.
2. Results
2.1. Leaf Gas Exchange Measurement
To characterize sugarcane leaf positions (base, middle, and tip parts), photosynthetic traits were measured. The silicon fertilization influenced photosynthesis (PN) (Figure 1A–C). The highest PN was observed in the middle part of the leaf (Figure 1B) as compared to the base and tip leaf parts under control, moderate, and severe water stress with Si. Maximum photosynthetic rates were obtained at 40.7, 27.6, and 43.7% of control, 92.4, 53.4, and 82.8% of moderate, and 143.2, 66.4, and 70.9% of severe water stress conditions with 500 mg L–1 Si supplied as compared to 0–300 mg L–1 Si with different irrigation levels (Figure 1A–C).
Figure 1.
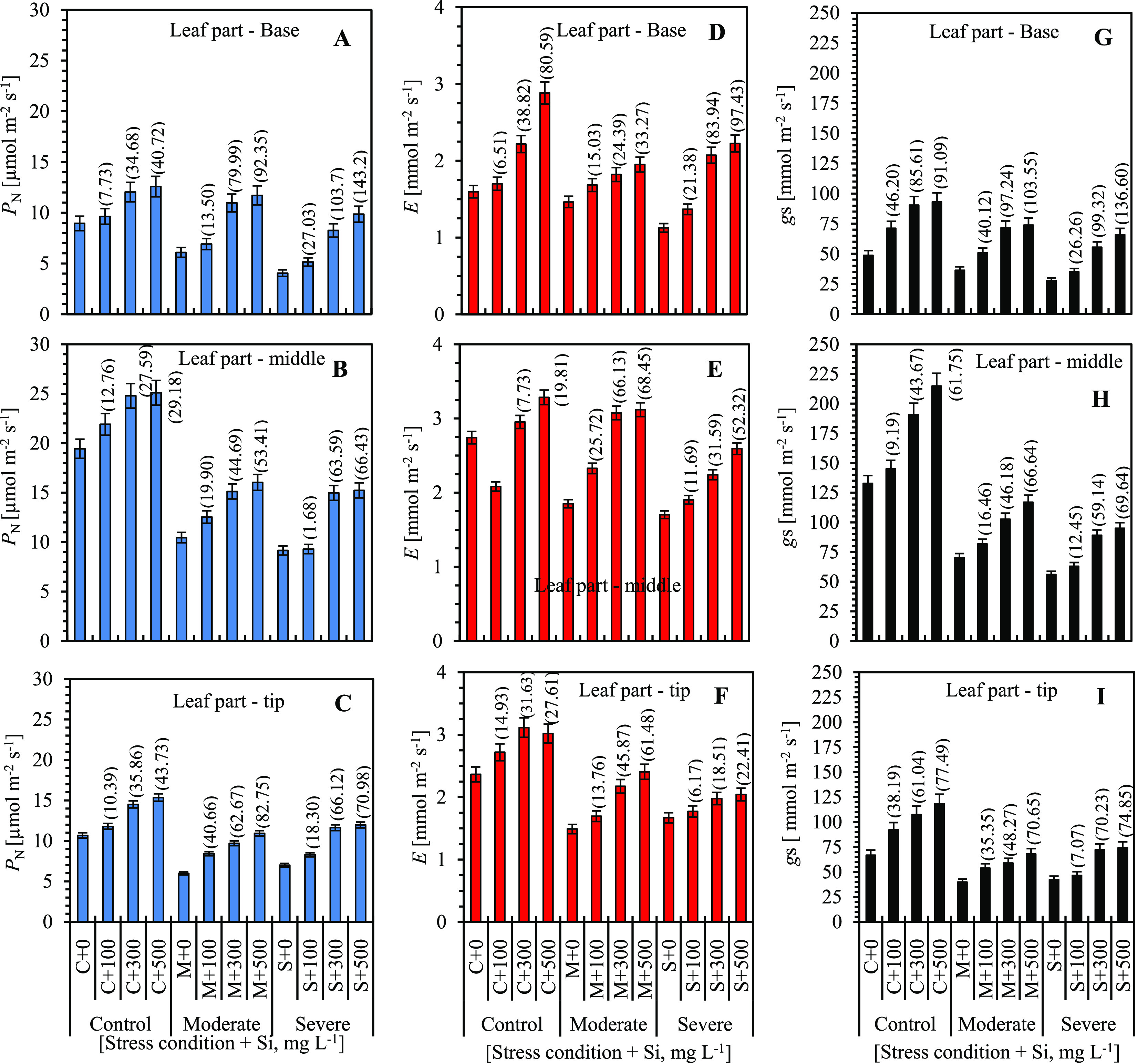
Changes in photosynthesis (PN), transpiration (E), and stomatal conductance (gs) in the base (A, D, G), middle (B, E, H), and tip (C, F, I) parts of sugarcane leaves under well-watered and limited water supplies with different silicon concentrations at 60 days after the stress condition. Five independent biological replicates are shown for each leaf part. Parenthesis values indicate percentage gain in Si-amended soil of various irrigation levels. Vertical bars indicate the standard error (SE) (n = 5).
With reference to the transpiration rate (E, Figure 1D–F), an increase in Si induced the highest E in the base, middle, and tip parts of the sugarcane leaves at all of the irrigation levels. The highest enhancement of E was measured in the 500 mg L–1 Si treatment in the base part (80.6, 33.3, 97.4%), middle part (19.8, 68.5, 52.3%), and tip part (27.6, 61.5, 22.4%) of the leaves in the control and limited water, while in the 100 and 300 mg L–1 Si applications, only a slight increase was observed (nearly 7–39, 15–24, and 21–84% in the base part; 8–20, 26–66, and 12–32% in the middle part; and 15–32, 14–46, and 6–19% in the tip part) as shown in Figure 1.
Silicon supply also positively influenced the stomatal conductance in the base, middle, and tip parts of leaves in the limited water irrigation treatments (Figure 1G–I). In terms of water stress tolerance, as compared with the control without Si, the Si treatments applied led to a great increase in the maximum gs in the moderate (∼103.6, 66.6, and 70.7%) and severely stressed plants (136.6, 69.6, and 74.9%) at 500 mg L–1, while only a slight increase was found in the 100 mg L–1 Si-treated plants in different leaf segments of the normal and stressed plants.
2.2. Correlation of Photosynthesis and Transpiration with Stomatal Conductance
Leaf stomatal conductance to water vapor (gs) was positively correlated with photosynthesis (PN, RL2 0.896–1.000, RP2 0.963–1.000) and transpiration rate (E, RL2 0.594–0.984, RP2 0.727–0.996) in different leaf segments, i.e., base, middle, and tip parts (Figure 2A–I). Leaf transpiration rate was enhanced with increasing stomata aperture. Responses of photosynthesis and transpiration rate to limited water supply (Figure 2B,C,E,F,H,I) showed a significant correlation with stomatal conductance, which indicated that upregulated leaf gs was closely associated with PN and E in the base, middle, and tip segments of sugarcane plant leaves. However, the correlation of leaf area expansion with gs was also significant, suggesting that the leaf area expansion may be closely associated with the occurrence of stomatal guard cells during various water irrigation levels.
Figure 2.
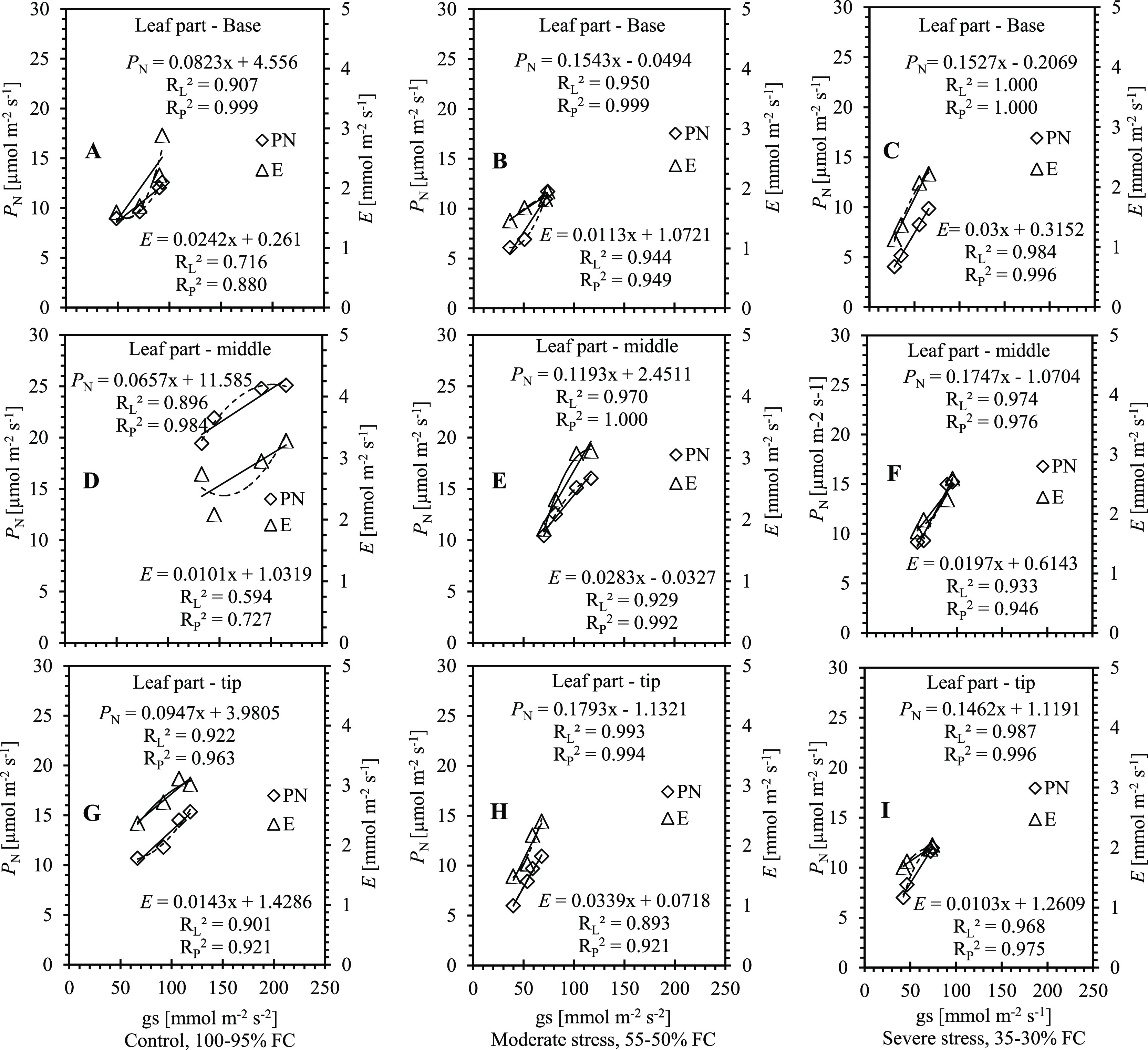
Correlations of stomatal conductance (gs) with photosynthesis (PN, A–I) and transpiration rate (E, A–I) in different leaf parts (base, A–C; middle, D–F; and tip, G–I) of sugarcane plants under control (A, D, G; 100–95% FC), moderate water stress (B, E, H; 55–50% FC), and severe water stress (C, F, I; 35–30% FC), respectively. RL2 and RP2 are R square values on the chart of linear and polynomial regression types, respectively (n = 5).
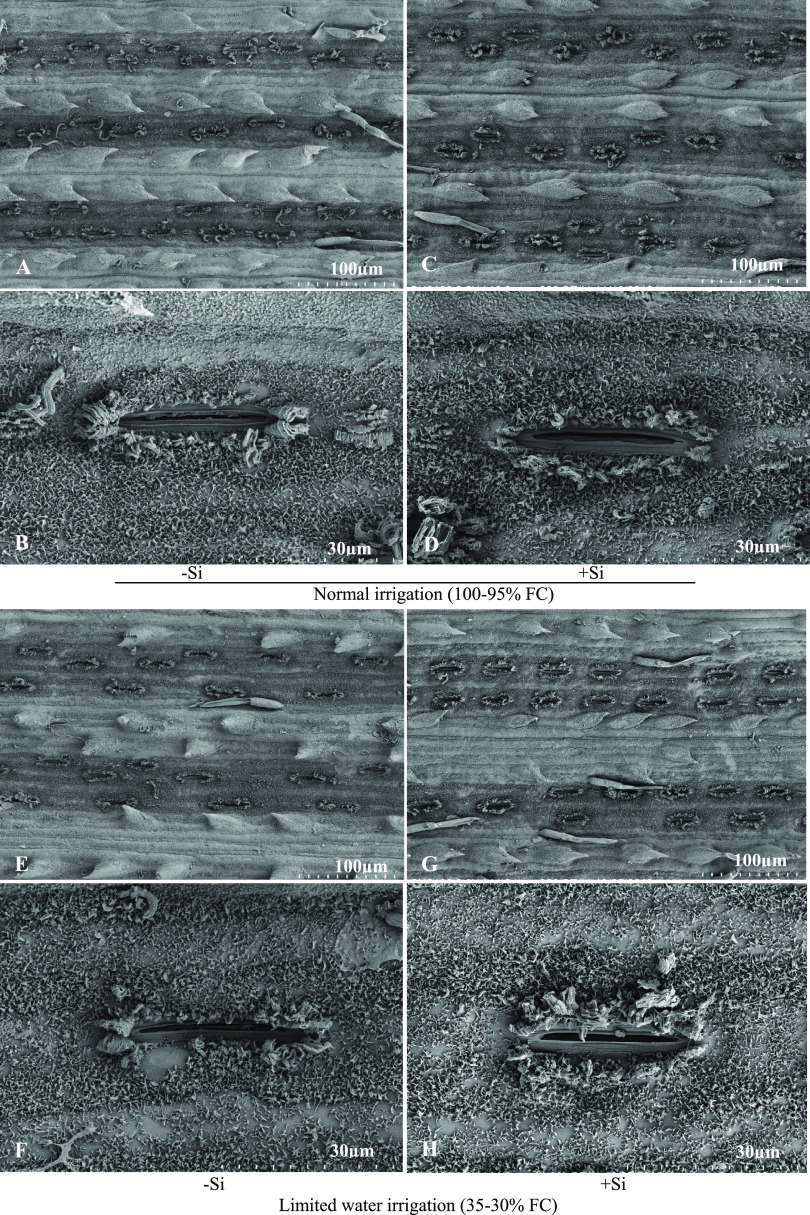
2.3. Effect of Limited Water Irrigation and Si on Stomata
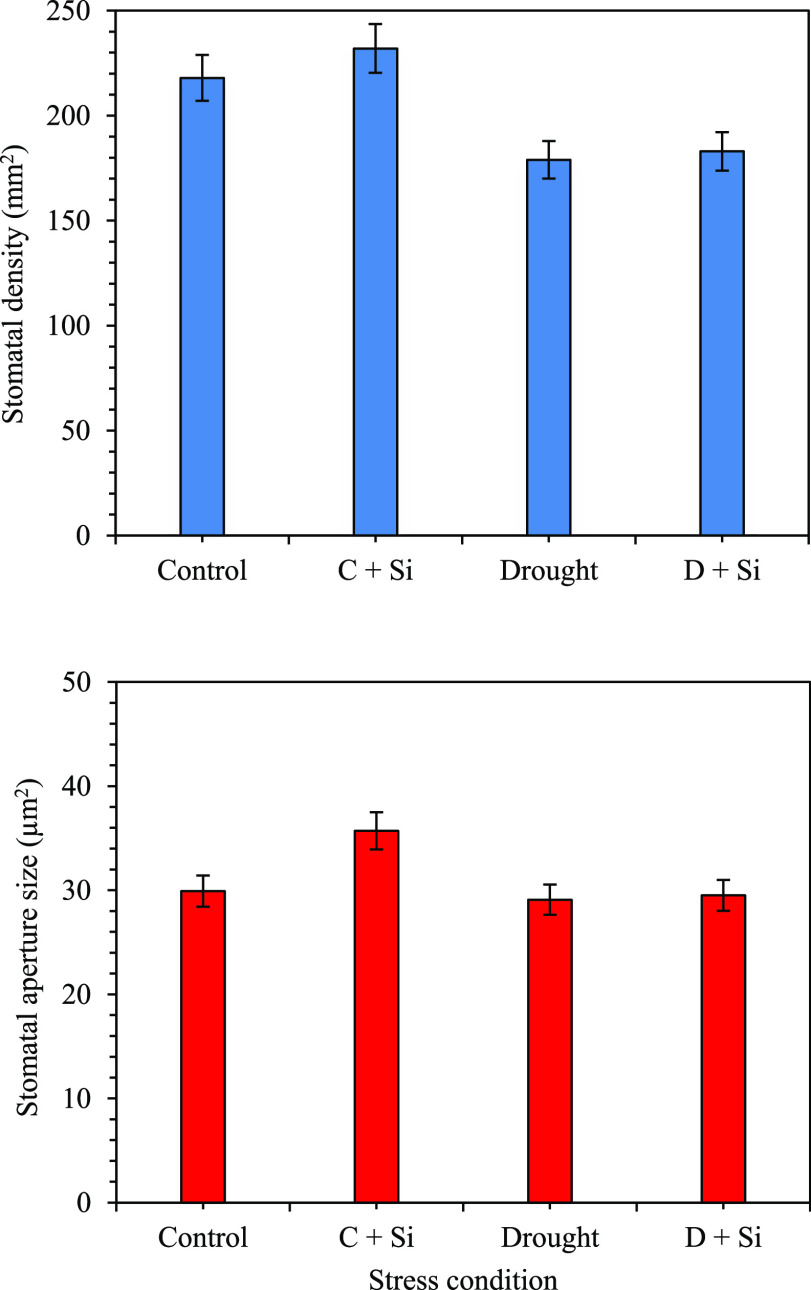
Scanning electron microscopic observations found that stomata morphology in different irrigation levels with Si was amended as shown in Figure 3. Under severe water stress, stomata aperture size was decreased as compared with normal irrigation (100–95% FC) (Figure 3A,B,E,F). The addition of Si enhanced stomatal aperture size, regardless of normal and limited water supply (Figure 3C,D,G,H). Stomatal alterations in response to water stress were observed to cause reductions in the stomatal density and stomatal aperture size in sugarcane plant leaves. The enhanced stomatal density and aperture size were monitored in control and stressed plants with Si application. The average stomatal density and stomatal aperture size of each treatment were found to be 218, 232, 179, and 183 mm2 and 29.92, 35.70, 29.09, and 29.51 μm2 for control, control with Si, stress, and stress with Si application, respectively (Figure 4).
Figure 3.
Scanning electron micrographs of stomata ultrastructure in the epidermis of sugarcane leaves in normal (A, B) and limited water supply treatments without Si (E, F) or with Si supplementation for normal (C, D) and limited water (G, H) at 500 mg L–1 Si. The scale bars for the micrographs were 30 μm (B, D, F, and H) and 100 μm (A, C, E, and G), respectively.
Figure 4.
Effect of stomatal density [SD, number of stomata per unit area, (A)] and stomatal aperture size [guard cell area, based on guard cell pair length and width, (B)] in sugarcane plants subjected to drought and silicon application. Average values ± SE of 10 biological replicates. C = control, D = drought, and Si = silicon level (500 mg L–1).
Plant growth and biomass traits were significantly inhibited under limited water supply as compared with the normal irrigation. Insufficient water supply reduced noticeably leaf area expansion, shoot, root, and plant dry masses of sugarcane plants (data not shown). However, the inhibition of plant development by water stress was mitigated by silicon application. Leaf area expansion was significantly different in the treatments with different Si concentrations. Actually, it was augmented significantly when Si application was increased from 0 to 500 mg L–1. The fresh and dry biomasses of the sugarcane plants subjected to the combined treatments with Si (100–500 mg L–1) and limited water (55–50 and 35–30% FC) were clearly enhanced compared to the control plants without Si application (data not shown). The application of Si (500 mg L–1) with limited water led to better plant performance than application with 100–300 mg L–1 Si.
3. Discussion
Plant leaves are the main organs for photosynthetic CO2 assimilation, and leaf area determines light harvesting, affects photosynthetic performance, and the accumulation of photosynthetic products.37,38 This study found that for all of the irrigation levels, leaf area expansion was larger in the treatments with Si than those without Si supply. In this experiment, Si fertilizers can guarantee a relatively higher photosynthetic performance under the limited water irrigation. Under water stress conditions, the significant impact of silicon on plant water status has been broadly investigated in many agricultural crop plants such as Sorghum bicolor,39,40Triticum aestivum,41Zea mays,42Oryza sativa,43Cucumis sativus,29Kentucky bluegrass,44Brassica napus,45Helianthus annuus,46Cicer arietinum,47Glycine max,48Medicago sativa,49 and Solanum lycopersicum.50 Under water stress, the plant’s initial response is to exclude the minimum water potential by adjusting its water maintenance between root water uptake and water loss in leaves.51,52 The plants can decrease leaf water loss by controlling the exhalation of water vapor through the stomata and also by reducing their leaf area expansion.15 When plants initially begin to experience water deficit, they reduce the leaf water loss mainly by reducing the transpiration rate through stomatal closure.15,53 Ming et al.43 and Chen et al.54 reported that the leaf transpiration rate was increased by silicon when the plants were facing water shortage. Numerous scientific reports published on insufficient water for plants have been shown to be compatible with upregulated leaf transpiration rates by Si amendment.15,28,41,54−57
In this study, stomata ultrastructure was destroyed in the severely water-stressed plants but stabilized considerably with the addition of silicon. Therefore, silicon may upgrade or enhance the rate of stomatal conductance by protecting the stomatal aperture from destruction. The number of stomata was similar in different leaf segments when considering both surfaces, i.e., adaxial and abaxial. This indicates that stomata density does not contribute to the changes observed at leaf gas exchange. Although the stomatal aperture has a significant link with PN, E, and gs of crop plants,6,586,58 in this study, the leaf stomatal appearance was reduced with limited water irrigation, which is similar to the results reported by Xu and Zhou,6 Yang et al.,59 and Meng et al.60 Stomatal density was also negatively associated with the length of stomata on various watering levels in Platanus acerifolia(61) and Leymus chinensis.8 However, the stomatal length was increased in the water-stressed plants, whereas its width decreased. Nevertheless, various impacts of environmental variables on stomata morphology may depend on plant genotypes.62,63
Dynamic adjustments to the opening degree of stomatal pores are linked to regulation of gs in the short term, allowing plants to immediately reduce water loss according to atmospheric conditions.64 Over a longer duration, anatomical adaptations, i.e., variations to SS and SD, can modify the range of gs by altering the higher gs.65,66 Changes in the size and density of stomata may arise due to genetic factors and/or plant growth and development against various environmental variables. Loss in gs due to a smaller SS has been linked to higher water conservation, as demonstrated for plants subjected to water deficit.67 Growth during insufficient soil moisture capacity has been shown to cause a reduction in SS in various plant species/cultivars,6,67−69 but the effect on SD is less consistent.6,67−70 In response to variations in water availability, leaf morphology and ultrastructure can vary considerably.71 Specifically, with respect to stomatal numbers, variations in atmospheric variables that influence mature leaf gs will have lasting effects on the stomatal differentiation of newly developing leaves.72 Our results regarding improvement in stomatal ultrastructure due to exogenous Si application against water stress are shown in Figure 4. The optimum level of Si application maintained/upgraded the stomatal functions by enabling plants to reopen their stomata against stressed conditions, suggesting a significant role of Si in stomatal regulation.1,73,74
Water deficit may initially inhibit plant development, significantly decreasing leaf area expansion,75,76 although stomatal density is closely linked to leaf growth and development.6,59 Casson and Hentherington77 reported that stomata morphology or size directly affected the photosynthetic CO2 assimilation and transpiration rate of plant leaves and that the key factors linked to plant environmental adaptation were two irrelevant indications: adjustment of stomatal motion and optimization of stomatal density and appearance.
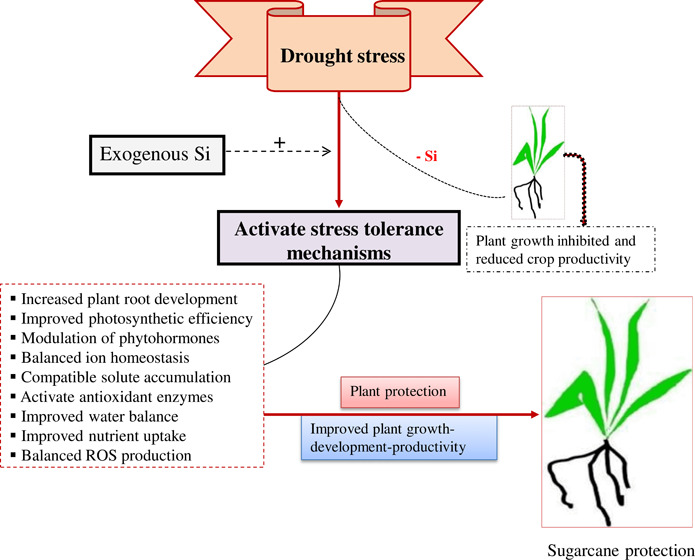
Application of irrigation water combined with silicon fertilizers can induce water stress tolerance and sustain sugarcane biomass production under water stress conditions, which is of great importance for sustainable development of sugarcane plants (Figure 5). Thus, appropriate Si fertilization is recommended for agricultural crops to improve photosynthetic performance inhibited by limited water and to facilitate plant establishment under limited water irrigation management.
Figure 5.
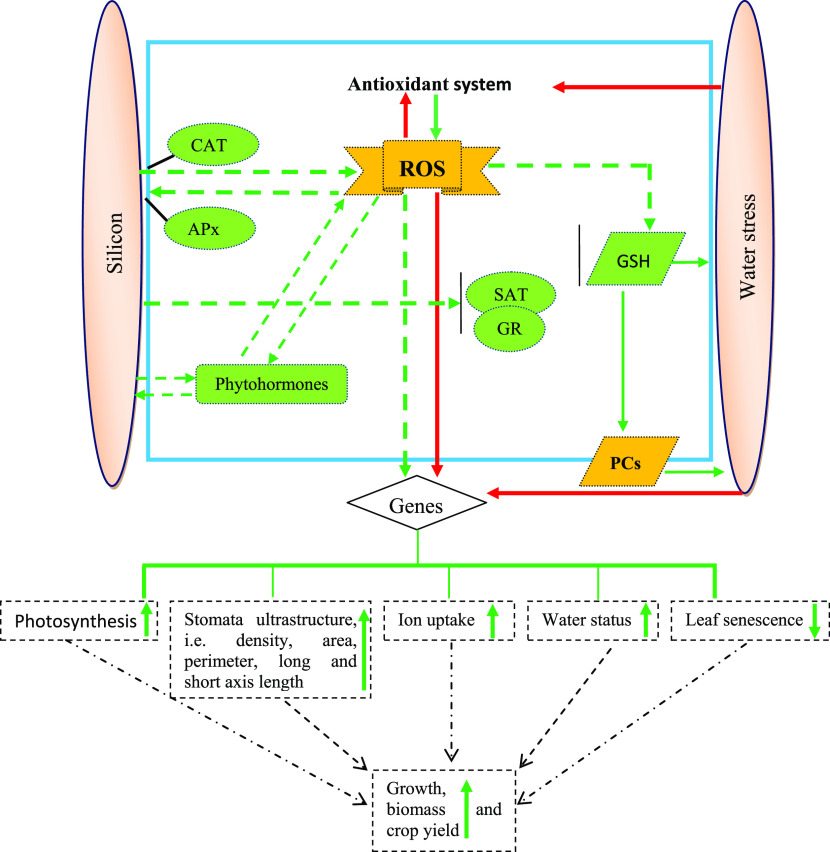
Schematic diagram representing the effects of water stress and defensive system induced by silicon and limited water, underlying sugarcane water stress tolerance. Water stress causes negative impacts by repressing photosynthetic performance, disrupting osmoprotectant status, and negatively influencing water status and ionic balance. Severe water stress can promote the production of reactive oxygen species (ROS). ROS contribute to lipid peroxidation, resulting in oxidative stress that causes growth reduction, biomass loss, and subsequently extreme loss of yield. Conversely, Si shows a protective mechanism against limited water by maintaining or improving water balance, leaf gas exchange, and maintaining ionic balance. As a result, Si improves water stress tolerance by retaining better plant growth and the productivity of stressed plants. CAT—catalase, APx—ascorbate peroxidase, GR—glutathione reductase, GSH—glutathione, PCs—phytochelatins, and SAT—serine acetyltransferase. The dotted arrows indicate possible signaling pathways. The red arrow indicates damage and the green arrow indicates positive effects, respectively.
4. Experimental Section
4.1. Site Description and Sugarcane Growing Conditions
Sugarcane seedcane stalks of cultivar GT 42 were kindly provided by the Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China. Sugarcane stalks were cut in one bud per segment and budded in trays containing vermiculite. The 60-day old plants were transplanted in 3.5 L plastic pots filled with 70% of fertile topsoil and 30% of organic manure and regularly watered with the same water volume. The soil at the experimental site was silty-clay soil and it was top-dressed with N, P, and K fertilizers, following the farmer’s standard practices. Fungicides were applied according to standard practice. The pots were randomly distributed into three irrigation groups such as normal (100–95% of FC), moderate stress (55–50% of FC), and severe stress (35–30% of FC) conditions, with 10 biological replicates per treatment. Subsequently, the fertilization of sugarcane plants was done with 200 mL of silicon at the concentrations of 0, 100, 300, and 500 mg L–1. Calcium metasilicate (CaO·SiO2) was used as a source of Si. The solution was applied directly to the soil. The watering of the sugarcane plants was done manually in each pot. The substrate elements were quantified before treatment, pH 5.95, organic carbon 0.74%, P 9.20 mg kg–1, K 2.74, Ca 4.2, Mg 1.5, and Na 0.085 cmol (+) kg–1, respectively. The availabilities of Cu, Fe, Zn, and Mn were 0.86, 12.3, 1.32, and 18.7 mg kg–1. Soil water percentage was noted using the Soil Moisture Meter (Top Instrument Co. Ltd. Zhejiang, China) from 12 to 15 cm of the soil layer. The average ambient air temperature (°C), relative air humidity (%), precipitation (mm), and light (h) data were monitored inside the greenhouse from March 2019 to September 2019 (Table 1).
Table 1. Atmospheric Data during the Experiments.
| average air temperature (°C) |
light (h) |
||||||
|---|---|---|---|---|---|---|---|
| month | min. | max. | average air humidity (%) | average precipitation (mm) | average rainy days | daylight | sunshine |
| March | 15 | 22 | 82 | 50 | 8 | 12.0 | 2.0 |
| April | 20 | 26 | 81 | 107 | 10 | 12.7 | 3.0 |
| May | 23 | 31 | 80 | 188 | 12 | 13.2 | 4.6 |
| June | 25 | 32 | 81 | 216 | 12 | 13.5 | 5.4 |
| July | 25 | 33 | 82 | 201 | 12 | 13.4 | 6.3 |
| August | 25 | 33 | 82 | 214 | 13 | 12.9 | 6.0 |
| September | 24 | 32 | 78 | 125 | 8 | 12.3 | 6.2 |
4.2. Photosynthetic Responses
A portable photosynthesis system (Li-6800, Li-COR Biosciences, Lincoln, NE) was used to observe the net photosynthetic rate (PN), transpiration rate (E), and stomatal conductance (gs) in the functional top visible dewlap leaf (leaf + 1) of the main stem. In line with the natural light intensity, temperature, and humidity between 9:00 and 11:00 in Nanning, Guangxi, the light intensity provided by the red and blue LED light source was adjusted to 1000 μmol (photon) m–2 s–1, the leaf chamber (6 cm2) temperature was fixed at 25 °C, and the CO2 concentration was set to 400 μmol mol–1 by a CO2 cylinder simulating the current atmospheric CO2 level.
4.3. Scanning Electron Microscopic Examination
Fresh leaves (leaf + 1, middle segment) were obtained and prepared for scanning electron microscopy (SEM) to observe stomata ultrastructure. Photosynthetically fully matured leaves were used for SEM at 60 days after the application of Si and limited water. The middle segment of the leaves was sectioned into small pieces (near 1 mm). To inhibit entry of air bubbles, the samples were fixed in glutaraldehyde (2.5%, 24 h) and further fixed in a sodium sulfide solution (0.5%, pH 7.2, 30 min) and subsequently rinsed with phosphate buffer (0.1 M, pH 7.2) thrice (15 min intervals). The samples were then fixed in OsO4 (1%) in phosphate buffer (0.1 M, pH 7.2) for 12 h (4 °C) and then dehydrated with increasing concentrations of ethanol series (30, 50, 70, 96, and 100%). After dehydration, the samples were embedded in LR white and polymerized (60 °C, 24 h). The leaf samples were seared under CO2 using a critical-point drying instrument. Then, the samples were gold sputter-coated with a JFC-1600 metal sputtering equipment and imaged with a JMS-6490 (Japanese Electronics Companies). The leaf stomata characteristics, i.e., stomatal density (SD) and stomatal aperture size (SS), were determined. These factors are known to be affected by the maturity of the leaf, leaf position, leaf surface (abaxial or adaxial), and various stresses. The stomatal characteristics were analyzed by NIS Element 7.0 software.
4.4. Data Analysis
All of the data were subjected to analysis of variance according to a completely randomized block design using GraphPad Prism 5.00 statistical software for Windows (GraphPad Software, San Diego, California). The Tukey test was used to compare the means. Correlation coefficients among photosynthetic capacity and stomatal conductance were calculated to examine the relationship.
Acknowledgments
We are thankful to the Guangxi Academy of Agricultural Sciences, Nanning, Guangxi, China, for providing the necessary facilities for this study. This study was financially supported by the Guangxi R and D Program Fund (GK17195100), the Fund for Guangxi Innovation Teams of Modern Agriculture Technology (gjnytxgxcxtd-03-01), and the Fund of Guangxi Academy of Agricultural Sciences (2015YT02).
Author Contributions
# K.K.V. and X.-P.S. have contributed equally to this work.
The authors declare no competing financial interest.
References
- Cui G.; Zhao X.; Liu S.; Sun F.; Zhang C.; Xi Y. Beneficial effects of melatonin in overcoming drought stress in wheat seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. 10.1016/j.plaphy.2017.06.014. [DOI] [PubMed] [Google Scholar]
- Verma K. K.; Singh P.; Song X. P.; Malviya M. K.; Singh R. K.; Chen G. L.; Solomon S.; Li Y. R. Mitigating climate change for sugarcane improvement: role of silicon in alleviating abiotic stresses. Sugar Tech 2020, 741–749. 10.1007/s12355-020-00831-0. [DOI] [Google Scholar]
- Jiang C.; Cui Q.; Feng K.; Xu D.; Li C.; Zheng Q. Melatonin improves antioxidant capacity and ion homeostasis and enhances salt tolerance in maize seedlings. Acta Physiol. Plant. 2016, 38, 82. 10.1007/s11738-016-2101-2. [DOI] [Google Scholar]
- Naeem M.; Naeem M. S.; Ahmad R.; Ahmad R.; Ashraf M. Y.; Ihsan M. Z.; Nawaz F.; Athar H. U. R. R.; Ashraf M.; Abbas H. T.; Abdullah M. Improving drought tolerance in maize by foliar application of boron: water status, antioxidative defense and photosynthetic capacity. Arch. Agron. Soil Sci. 2018, 64, 626–639. 10.1080/03650340.2017.1370541. [DOI] [Google Scholar]
- Nilson S. E.; Assmann S. M. The control of transpiration. insights from Arabidopsis. Plant Physiol. 2007, 143, 19–27. 10.1104/pp.106.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z.; Zhou G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. 10.1093/jxb/ern185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beerling D. J.; Chaloner W. G. The impact of atmospheric CO2 and temperature change on stomatal density: observations from Quercus robur Lammad leaves. Ann. Bot. 1993, 71, 231–235. 10.1006/anbo.1993.1029. [DOI] [Google Scholar]
- Woodward F. I. Stomatal numbers are sensitive to increases in CO2 from pre-industrial levels. Nature 1987, 327, 617–618. 10.1038/327617a0. [DOI] [Google Scholar]
- Zhao S.; Chen W.; Ma D.; Zhao F. Influence of different salt level on stomatal character in rice leaves. Reclaiming Rice Cultiv. 2006, 6, 26–29. [Google Scholar]
- Zhao R. X.; Zhang Q. B.; Wu X. Y.; Wang Y. The effects of drought on epidermal cells and stomatal density of wheat leaves. Inner Mongolia Agric. Sci. Technol. 2001, 6, 6–7. [Google Scholar]
- Galmés J.; Flexas J.; Save R.; Medrano H. Water relations and stomatal characteristics of Mediterranean plants with different growth forms and leaf habits: responses to water stress and recovery. Plant Soil 2007, 290, 139–155. 10.1007/s11104-006-9148-6. [DOI] [Google Scholar]
- Yang L.; Han M.; Zhou G.; Li J. The changes of water-use efficiency and stoma density of Leymus chinensis along Northeast China Transect. Acta Ecol. Sin. 2007, 27, 16–24. 10.1016/S1872-2032(07)60006-7. [DOI] [Google Scholar]
- Zhang X. Y.; Yang H. M.; Hou Z. D.; Wang G. X. Stomatal density and distributions of spring wheat leaves under different planting densities and soil moisture levels. Chin. J. Plant Ecol. 2003, 27, 133–136. 10.17521/cjpe.2003.0020. [DOI] [Google Scholar]
- Chaves M. M.; Flexas J.; Pinheiro C. Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. 10.1093/aob/mcn125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma K. K.; Liu X. H.; Wu K. C.; Singh R. K.; Song Q. Q.; Malviya M. K.; Song X. P.; Singh P.; Verma C. L.; Li Y. R. The impact of silicon on photosynthetic and biochemical responses of sugarcane under different soil moisture levels. Silicon 2019, 1–13. 10.1007/s12633-019-00228-z. [DOI] [Google Scholar]
- Cornic G. Drought stress inhibits photosynthesis by decreasing stomatal aperture–not by affecting ATPsynthesis. Trends Plant Sci. 2000, 5, 187–188. 10.1016/S1360-1385(00)01625-3. [DOI] [Google Scholar]
- Song X.; Zhou G.; Xu Z.; Lv X.; Wang Y. Detection of photosynthetic performance of Stipa bungeana seedlings under climatic change using chlorophyll fluorescence imaging. Front. Plant Sci. 2016, 6, 1254. 10.3389/fpls.2015.01254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petsas A.; Grammatikopoulos G. Drought resistance and recovery of photosystem II activity in a Mediterranean semi-deciduous shrub at the seedling stage. Photosynthetica 2009, 47, 284–292. 10.1007/s11099-009-0044-1. [DOI] [Google Scholar]
- Zivcak M.; Kalaji H. M.; Shao H. B.; Olsovska K.; Brestic M. Photosynthetic proton and electron transport in wheat leaves under prolonged moderate drought stress. J. Photochem. Photobiol., B 2014, 137, 107–115. 10.1016/j.jphotobiol.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Chen Z. K.; Niu Y. P.; Ma H.; Hafeez A.; Luo H. H.; Zhang W. F. Photosynthesis and biomass allocation of cotton as affected by deep-layer water and fertilizer application depth. Photosynthetica 2017, 55, 638–647. 10.1007/s11099-016-0675-y. [DOI] [Google Scholar]
- Li J. H.; Wang Y. Y.; Li N. N.; Zhao R. H.; Khan A.; Wang J.; Luo H. H. Cotton leaf photosynthetic characteristics, biomass production, and their correlation analysis under different irrigation and phosphorus application. Photosynthetica 2019, 57, 1066–1075. 10.32615/ps.2019.118. [DOI] [Google Scholar]
- Verma K. K.; Wu K.-C.; Singh P.; Malviya M. K.; Singh R. K.; Song X.-P.; Li Y. R. The protective role of silicon in sugarcane under water stress: photosynthesis and antioxidant enzymes. Biomed. J. Sci. Tech. Res. 2019, 15, 002685. [Google Scholar]
- Sonobe K.; Hattori T.; An P.; Tsuji W.; Eneji E.; Kobayashi S.; Kawamura Y.; Tanaka K.; Inanaga S. Effect of silicon application on sorghum root responses to water stress. J. Plant Nutr. 2010, 34, 71–82. 10.1080/01904167.2011.531360. [DOI] [Google Scholar]
- Epstein E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 641–664. 10.1146/annurev.arplant.50.1.641. [DOI] [PubMed] [Google Scholar]
- Savvas D.; Ntatsi G. Biostimulant activity of silicon in horticulture. Sci. Hortic. 2015, 196, 66–81. 10.1016/j.scienta.2015.09.010. [DOI] [Google Scholar]
- Latef A. A. A.; Tran L.-S. P. Impacts of priming with silicon on the growth and tolerance of maize plants to alkaline stress. Front. Plant Sci. 2016, 7, 243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds O. L.; Padula M. P.; Zeng R.; Gurr G. M. Silicon: potential to promote direct and indirect effects on plant defense against arthropod pests in agriculture. Front. Plant Sci. 2016, 7, 744. 10.3389/fpls.2016.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carneiro-Carvalho A.; Anjos R.; Aires A.; Marques T.; Pinto T.; Gomes-Laranjo J. Ecophysiological study of the impact of SiK fertilization on Castanea sativa Mill. Seedling tolerance to high temperature. Photosynthetica 2019, 57, 1165–1175. 10.32615/ps.2019.099. [DOI] [Google Scholar]
- Ma C. C.; Li Q. F.; Gao Y. B.; Xin T. R. Effects of silicon application on drought resistance of cucumber plants. Soil Sci. Plant Nutr. 2004, 50, 623–632. 10.1080/00380768.2004.10408520. [DOI] [Google Scholar]
- Liang Y. C.; Sun W. C.; Zhu Y. G.; Christie P. Mechanisms of silicon mediated alleviation of abiotic stresses in higher plants: a review. Environ. Pollut. 2007, 147, 422–428. 10.1016/j.envpol.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Ju S.; Yin N.; Wang L.; Zhang C.; Wang Y. Effects of silicon on Oryza sativa L. seedling roots under simulated acid rain stress. PLoS One 2017, 12, e0173378 10.1371/journal.pone.0173378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivanesan I.; Jeong B. R. Silicon promotes adventitious shoot regeneration and enhances salinity tolerance of Ajuga multiflora bunge by altering activity of antioxidant enzyme. Sci. World J. 2014, 521703 10.1155/2014/521703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahebi M.; Hanafi M. M.; Akmar A. S. N.; Rafii M. Y.; Azizi P.; Tengoua F. F.; Azwa J. N. M.; Shabanimofrad M. Importance of silicon and mechanisms of biosilica formation in plants. BioMed Res. Int. 2015, 2015, 396010 10.1155/2015/396010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabello M. J.; Castellanos M. T.; Romojaro F.; Martnez-Madrid C.; Ribas F. Yield and quality of melon grown under different irrigation and nitrogen rates. Agric. Water Manage. 2009, 96, 866–874. 10.1016/j.agwat.2008.11.006. [DOI] [Google Scholar]
- Patanè C. Leaf area index, leaf transpiration and stomatal conductance as affected by soil water deficit and VPD in processing tomato in semi arid mediterranean climate. J. Agron. Crop Sci. 2011, 197, 165–176. 10.1111/j.1439-037X.2010.00454.x. [DOI] [Google Scholar]
- Dinh T. H.; Watanabe K.; Takaragawa H.; Nakabaru M.; Kawamitsu Y. Photosynthetic response and nitrogen use efficiency of sugarcane under drought stress conditions with different nitrogen application levels. Plant Prod. Sci. 2017, 20, 412–422. 10.1080/1343943X.2017.1371570. [DOI] [Google Scholar]
- Lawlor D. W. Photosynthesis, productivity and environment. J. Exp. Bot. 1995, 46, 1449–1461. 10.1093/jxb/46.special_issue.1449. [DOI] [Google Scholar]
- Dai J.; Zheng W.; Yu Y. Z.; Yang J. S. The relationship between yield and photosynthetic area after flowering in rapeseed. Chin. J. Oil Crop Sci. 2001, 2, 20–23. [Google Scholar]; [in Chinese]
- Hattori T.; Sonobe K.; Inanaga S.; An P.; Tsuji W.; Araki H.; Eneji A. E.; Morita S. Short term stomatal responses to light intensity changes and osmotic stress in sorghum seedlings raised with and without silicon. Environ. Exp. Bot. 2007, 60, 177–182. 10.1016/j.envexpbot.2006.10.004. [DOI] [Google Scholar]
- Ahmed M.; Asif M.; Hassan F. (2014) Augmenting drought tolerance in sorghum by silicon nutrition. Acta Physiol. Plant. 2014, 36, 473–483. 10.1007/s11738-013-1427-2. [DOI] [Google Scholar]
- Gong H. J.; Chen K. M. The regulatory role of silicon on water relations, photosynthetic gas exchange, and carboxylation activities of wheat leaves in field drought conditions. Acta Physiol. Plant. 2012, 34, 1589–1594. 10.1007/s11738-012-0954-6. [DOI] [Google Scholar]
- Amin M.; Ahmad R.; Basra S. M. A.; Murtaza G. Silicon induced improvement in morpho-physiological traits of maize (Zea mays L.) under water deficit. Pak. J. Agric. Sci. 2014, 51, 187–196. [Google Scholar]
- Ming D. F.; Pei Z. F.; Naeem M. S.; Gong H. J.; Zhou W. J. Silicon alleviates PEG-Induced water-deficit stress in upland rice seedlings by enhancing osmotic adjustment. J. Agron. Crop Sci. 2012, 198, 14–26. 10.1111/j.1439-037X.2011.00486.x. [DOI] [Google Scholar]
- Saud S.; Li X.; Chen Y.; Zhang L.; Fahad S.; Hussain S.; Sadiq A.; Chen Y. Silicon application increases drought tolerance of Kentucky Bluegrass by improving plant water relations and morpho-physiological functions. Sci. World J. 2014, 368694 10.1155/2014/368694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habibi G. Silicon supplementation improves drought tolerance in canola plants. Russ. J. Plant Physiol. 2014, 61, 784–791. 10.1134/S1021443714060077. [DOI] [Google Scholar]
- Gunes A.; Pilbeam D. J.; Inal A.; Coban S. (2008) Influence of silicon on sunflower cultivars under drought stress, I: growth, antioxidant mechanisms, and lipid peroxidation. Commun. Soil Sci. Plant Anal. 2008, 39, 1885–1903. 10.1080/00103620802134651. [DOI] [Google Scholar]
- Gunes A.; Inal A.; Bagci E. G.; Pilbeam D. J. Silicon-mediated changes of some physiological and enzymatic parameters symptomatic for oxidative stress in spinach and tomato grown in sodic-B toxic soil. Plant Soil 2007, 290, 103–114. 10.1007/s11104-006-9137-9. [DOI] [PubMed] [Google Scholar]
- Shen X. H.; Zhou Y. Y.; Duan L. S.; Li Z. H.; Eneji A. E.; Li J. M. Silicon effects on photosynthesis and antioxidant parameters of soybean seedlings under drought and ultraviolet-B radiation. J. Plant Physiol. 2010, 167, 1248–1252. 10.1016/j.jplph.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Liu H. X.; Guo Z. G. Forage yield and water use efficiency of alfalfa applied with silicon under water deficit conditions. Philipp. Agric. Sci. 2013, 96, 370–376. [Google Scholar]
- Shi Y.; Zhang Y.; Han W.; Feng R.; Hu Y.; Guo J.; Gong H. Silicon enhances water stress tolerance by improving root hydraulic conductance in Solanum lycopersicum L. Front. Plant Sci. 2016, 7, 196. 10.3389/fpls.2016.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luu D. T.; Maurel C. Aquaporins in a challenging environment: molecular gears for adjusting plant water status. Plant Cell Environ. 2005, 28, 85–96. 10.1111/j.1365-3040.2004.01295.x. [DOI] [Google Scholar]
- Verslues P. E.; Agarwal M.; Katiyar-Agarwal S.; Zhu J.; Zhu J. K. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006, 45, 523–539. 10.1111/j.1365-313X.2005.02593.x. [DOI] [PubMed] [Google Scholar]
- Chen D.; Wang S.; Yin L.; Deng X. How does silicon mediate plant water uptake and loss under water deficiency?. Front. Plant Sci. 2018, 9, 281. 10.3389/fpls.2018.00281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W.; Yao X.; Cai K.; Chen J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 2011, 142, 67–76. 10.1007/s12011-010-8742-x. [DOI] [PubMed] [Google Scholar]
- Hattori T.; Inanaga S.; Araki H.; An P.; Morita S.; Luxova M.; Lux A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 2005, 123, 459–466. 10.1111/j.1399-3054.2005.00481.x. [DOI] [Google Scholar]
- Sonobe K.; Hattori T.; An P.; Tsuji W.; Eneji E.; Tanaka K.; Inanaga S. Diurnal variations in photosynthesis, stomatal conductance and leaf water relation in Sorghum grown with or without silicon under water stress. J. Plant Nutr. 2009, 32, 433–442. 10.1080/01904160802660743. [DOI] [Google Scholar]
- Kang J.; Zhao W.; Zhu X. Silicon improves photosynthesis and strengthens enzyme activities in the C-3 succulent xerophyte Zygophyllum xanthoxylum under drought stress. J. Plant Physiol. 2016, 199, 76–86. 10.1016/j.jplph.2016.05.009. [DOI] [PubMed] [Google Scholar]
- Mattiello L.; Riano-Pachon D. M.; Martins M. C. M.; de Cruz L. P.; Bassi D.; Marchiori P. E. R.; Ribeiro R. V.; Labate M. T. V.; Labate C. A.; Menossi M. Physiological and transcriptional analysis of developmental stage along sugarcane leaf. BMC Plant Biol. 2015, 15, 300. 10.1186/s12870-015-0694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J.; Jonathan W.; Zhu Q.; Peng Z. Effect of water deficit stress on the stomatal frequency, stomatal conductance and abscisic acid in rice leaves. Acta Agron. Sin. 1995, 21, 533–539. [Google Scholar]
- Meng L.; Li L.; Chen W.; Xu Z.; Liu L. Effect of water stress on stomatal density, length, width and net photosynthetic rate in rice leaves. J. Shenyang Agric. Univ. 1999, 30, 477–480. [Google Scholar]
- Zhang H.; Wang X.; Wang S. A study on stomatal traits of Platanus acerifolia under urban stress. J. Fudan Univ. 2004, 43, 651–656. [Google Scholar]
- Maherali H.; Reid C. D.; Polley H. W.; Johnson H. B.; Jachson R. B. Stomatal acclimation over a subambient to elevated CO2 gradient in a C3/C4 grassland. Plant Cell Environ. 2002, 25, 557–566. 10.1046/j.1365-3040.2002.00832.x. [DOI] [Google Scholar]
- Liu S.; Liu J.; Cao J.; Bai C.; Shi R. Stomatal distribution and character analysis of leaf epidermis of jujube under drought stress. J. Anhui Agric. Sci. 2006, 34, 1315–1318. [Google Scholar]
- Farquhar G. D.; Sharkey T. D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. 10.1146/annurev.pp.33.060182.001533. [DOI] [Google Scholar]
- Franks P. J.; Beerling D. J. Maximum leaf conductance driven by CO2 effects on stomatal size and density over geologic time. Proc. Natl. Acad. Sci. U.S.A. 2009, 106, 10343–10347. 10.1073/pnas.0904209106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dow G. J.; Bergmann D. C.; Berry J. A. An integrated model of stomatal development and leaf physiology. New Phytol. 2014, 201, 1218–1226. 10.1111/nph.12608. [DOI] [PubMed] [Google Scholar]
- Doheny-Adams T.; Hunt L.; Franks P. J.; Beerling D. J.; Gray J. E. Genetic manipulation of stomatal density influences stomatal size, plant growth and tolerance to restricted water supply across a growth carbon dioxide gradient. Philos. Trans. R. Soc., B 2012, 367, 547–555. 10.1098/rstb.2011.0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y.; Yan F.; Cui X.; Liu F. Plasticity in stomatal size and density of potato leaves under different irrigation and phosphorus regimes. J. Plant Physiol. 2014, 171, 1248–1255. 10.1016/j.jplph.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Zhao W.; Sun Y.; Kjelgren R.; Liu X. Response of stomatal density and bound gas exchange in leaves of maize to soil water deficit. Acta Physiol. Plant. 2015, 37, 1704. 10.1007/s11738-014-1704-8. [DOI] [Google Scholar]
- Clifford S. C.; Black C. R.; Roberts J. A.; Stronach I. M.; Singleton-Jones P. R.; Mohamed A. D.; Azam-Ali S. N. The effect of elevated atmospheric CO2 and drought onstomatal frequency in groundnut (Arachis hypogaea L.). J. Exp. Bot. 1995, 46, 847–852. 10.1093/jxb/46.7.847. [DOI] [Google Scholar]
- Peña-Rojas K.; Aranda X.; Joffre R.; Fleck I. Leaf morphology, photochemistry and water status changes in resprouting Quercus ilex during drought. Funct. Plant Biol. 2005, 32, 117–130. 10.1071/FP04137. [DOI] [PubMed] [Google Scholar]
- Miyazawa S.-I.; Livingston N. J.; Turpin D. H. Stomatal development in new leaves is related to the stomatal conductance of mature leaves in poplar (Populus trichocarpa × P. deltoides). J. Exp. Bot. 2006, 57, 373–380. 10.1093/jxb/eri278. [DOI] [PubMed] [Google Scholar]
- Li C.; Tan D. X.; Liang D.; Chang C.; Jia D.; Ma F. Melatonin mediates the regulation of ABA metabolism, free-radical scavenging, and stomatal behaviour in two Malus species under drought stress. J. Exp. Bot. 2015, 66, 669–680. 10.1093/jxb/eru476. [DOI] [PubMed] [Google Scholar]
- Li H.; Chang J.; Chen H.; Wang Z.; Gu X.; Wei C.; Zhang Y.; Ma J.; Yang J.; Zhang X. Exogenous melatonin confers salt stress tolerance to watermelon by improving photosynthesis and redox homeostasis. Front. Plant Sci. 2017, 8, 1–9. 10.3389/fpls.2017.00295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves M. M.; Maroco J. P.; Pereira J. S. Understanding plant responses to drought-from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. 10.1071/FP02076. [DOI] [PubMed] [Google Scholar]
- Gazanchian A.; Hajheidari M.; Sima N. K.; Salekdeh G. H. Proteome response of Elymus elongatum to severe water stress and recovery. J. Exp. Bot. 2006, 58, 291–300. 10.1093/jxb/erl226. [DOI] [PubMed] [Google Scholar]
- Casson S. A.; Hetherington A. M. Environmental regulation of stomatal development. Curr. Opin. Plant Biol. 2010, 13, 90–95. 10.1016/j.pbi.2009.08.005. [DOI] [PubMed] [Google Scholar]