ABSTRACT
The DRI values for vitamin A were last reviewed and defined in 2001. At the time, there was very sparse data that could be used to set the DRI values for pregnancy, lactation, and infancy. In the subsequent 20 y since the last formal review, a number of findings relevant to the adequacy indicator of visual dark adaptation in pregnancy, the usual vitamin A content of breast milk across lactation stages, and vitamin A metabolism in women and children have been published. Furthermore, identification of genetic variables affecting the bioconversion of provitamin A carotenoids to vitamin A have provided an improved explanation for interindividual variability in responses to provitamin A carotenoids. The purpose of this collection of articles, introduced herein, is to review and apply recent findings about vitamin A status, address current gaps in knowledge, and suggest avenues for future research needed to refine the DRI values for pregnancy, lactation, and early life.
Keywords: vitamin A, infants, Dietary Reference Intakes, pregnancy, carotenoids, Recommended Dietary Allowance, lactation
Since the vitamin A Dietary Reference Intakes were set 20 years ago, numerous advances have added to what is known about vitamin A status and health in pregnancy, lactation, and early life.
Introduction
The DRI values for vitamin A are now nearly 20 y old (1). A new body of research since the time of the last review suggests that there may be sufficient new data to justify a new review and, possibly, a revision of these values. In June 2019 at the Carotenoids and Vitamin A Research Interactive Group (CARIG) Symposium of the American Society for Nutrition, several presentations addressed new human studies (2–6), as well as data from experimental research (7–12), that appear germane to an evaluation of the DRI values for vitamin A, especially for pregnant women, infants, and children. Our purpose herein is, firstly, to set the stage for the papers that follow in this collection, and secondly, to provide our own review of the DRI values for vitamin A—the purposes they serve; the evidence on which our current DRI values for vitamin A are based; newer research that helps in understanding the relation between dietary provitamin A carotenoids and vitamin A; and, finally, our thoughts on why it may be time to revisit the DRI values for vitamin A for infants, children, and pregnant and lactating women.
Why DRI values? The DRI values are important to the USA and Canada, and are considered internationally when other nations develop their own nutrient reference values. The development of the DRI values represent a collaborative, exhaustive review process conducted between the USA and Canada (13) and overseen by the Institute of Medicine, IOM (which became the National Academy of Medicine in 2015). The fundamental framework is described under “Introduction to Dietary Reference Intakes,” chapter 1 of the report on “Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc” (1), to which we will refer extensively in this article. The development of the DRI values follows a systematic process, starting with a thorough review of published literature that is available at the time of the DRI committee's review. Further information about the current process to establish DRI values can be found in chapter 2 of (14).
As the purpose is to establish dietary reference values, studies that have examined the relation of diet to health outcomes, known as indicators of nutrient adequacy and indicators of nutrient excess, are the most valuable in setting DRI values. Randomized controlled trials are taken as the “highest” level of evidence, whereas other types of data are also considered. Each nutrient or group of nutrients is reviewed by a group of experts (study committee) that deliberates in complete independence from the US and Canadian governments and other study sponsors. The DRI values are meant to address the nutrient requirements and recommended dietary intakes of the “general population,” (i.e. they do not address special medical needs). The quantitative values established in the DRI review and recommendation process become reference values for a wide range of purposes, and must be considered in formulating other recommendations of public health importance in the USA. As some examples, the DRI values are used as measures of adequacy for USDA programs including School Lunch; for military feeding programs which, of relevance, increasingly involve females in the military; for Women, Infants, and Children (WIC) programs, and others [Table 2-2 in reference (14) provides a comprehensive listing of uses]. DRI values are also used by public health researchers and workers to evaluate the adequacy of nutrient intakes by a population. Further, health professionals use the values for planning appropriate diets. For these reasons, it is truly important to have meaningful and accurate criteria for the assessment of nutrient adequacy. Furthermore, the US Dietary Guidelines are based on DRI values as fundamental underpinnings of nutrient requirements (15). Moreover, and especially relevant to the new 2020 US Dietary Guidelines, greater attention is being focused on the life span from birth to 24 mo, and to women of reproductive age (13). Thus, accurate nutrient adequacy information specific to these age groups is especially timely. The purpose of this collection is to present some recent research on vitamin A and carotenoids that may be important in informing the DRI process going forward. We wish to provide a critical analysis of what we know, what is missing and still needs to be determined, and where there are promising new directions for research, with the overarching goal of being able in the future to better define or even redefine the requirement for vitamin A and carotenoids during the critical life stages of pregnancy, infancy, and childhood.
The State of Science in 2001
Vitamin A plays many roles in the body. However, the DRI process relies on identification of 1 or more indicators that can be used to define nutrient adequacy or nutrient excess. Often, when one looks for such indicators, it is difficult to identify markers or processes that are sufficiently specific to a single nutrient to be useful for DRI development. This is not unusual for most micronutrients, as multiple vitamins and minerals often participate together in biochemical pathways, and thus their deficiency or excess states may exhibit similar characteristics. As an example, vitamin A is required for cell differentiation and growth, but impaired growth is not specific to vitamin A. Thus, identifying a useful indicator, especially a clinical one, for vitamin A for setting DRI values is in itself a difficult process. Fortunately, there is a physiological process that is nearly specific to vitamin A, namely, the formation of rhodopsin in the rods of the retina, which is responsible for vision in dim light. Thus, impaired visual dark adaptation, commonly called “night blindness,” is a physiological indicator that can be used to answer a DRI-relevant question, “How much vitamin A is necessary to prevent impaired dark adaptation?”
However, although a reasonably good indicator of vitamin A sufficiency was available at the time of the 2001 DRI report, the number and scope of studies that had used this indicator was very limited. It may not be widely appreciated how little information was available to the DRI committee at that time, on which to make recommendations for DRI values (Table 1). As noted, fortunately, vitamin A studies had been conducted that had examined a relatively specific indicator of vitamin A deficiency, but these studies had included only a very small number of adult men and women. Published reports were available of studies that had been conducted in the USA or UK in the 1940s and 1950s, which had used a paradigm of depletion-repletion in which subjects were first fed controlled diets lacking in vitamin A until they reached a point at which they exhibited ocular manifestations of vitamin A deficiency and thus were considered vitamin A-depleted. These manifestations, which are due to a relative lack of 11-cis-retinal required for rhodopsin formation, included either impaired dark adaptation (changes in visual threshold after exposure to bright light) or an altered dark adaptometry (electroretinogram) response. Both of these responses can be measured quantitatively and are considered specific enough to vitamin A to be useful as indicators of low vitamin A status. As was tabulated in the 2001 report [Table 4.4 in reference (1)], data were available from 4 studies, conducted from ∼1940 to 1974, each having just 1 to 6 subjects and comprising a grand total of 13 subjects. Of these subjects, the descriptions of the sex and age were complete for only 8 of them. Two subjects, interestingly, were simply described as “medical students,” with neither sex nor age given! Each person exhibited abnormal dark adaptation at the beginning of the repletion period, and in just a few days after the beginning of repletion with escalating doses of vitamin A, the abnormal dark adaptation was corrected. By identifying the lowest doses of vitamin A that resulted in correction, it was possible for the DRI committee to estimate values for the Estimated Average Requirement (EAR) for the population. In the DRI framework (17), the EAR represents the intake at which the biological requirement of 50% of the healthy population (in a specific age-sex group) is attained. Citing the 2001 report, “The data by Sauberlich et al. (1974) were given greater consideration because 1) the actual amount (μg) of vitamin A and β-carotene consumed was cited, 2) varied amounts of vitamin A or β-carotene were consumed by each individual, and 3) a greater sample size was employed (6 versus 2 subjects).” In the 2001 report, 2 studies of dark adaptometry in children were also cited, but as neither of these studies had included dietary data they could not be used to determine the EAR. Another approach used in the 2001 report was referred to as the factorial (computational) model. It utilized estimates of vitamin A intake that would be just sufficient to attain liver vitamin A stores at a concentration considered sufficient to maintain a steady concentration of plasma retinol. Data were available from a small number of studies conducted in the 1970s and 1 study from 1997 that provided limited but relevant data on retinol absorption and indicators of vitamin A storage in the liver. From these 2 approaches, which are explained in the chapter on vitamin A in the 2001 publication, EAR values for vitamin A were developed for adults. Then, using these values, the corresponding RDA was determined by adding to the EAR twice the CV, estimated at 15%, in order to raise the value to an RDA that is expected to cover the requirement for vitamin A for 97–98% of the population.
TABLE 1.
Current RDA values for vitamin A expressed in Retinol Activity Equivalents for infants, children, adults, and women during pregnancy and lactation
| Age | Male | Female | Pregnancy | Lactation |
|---|---|---|---|---|
| 0–6 mo1 | 400 | 400 | ||
| 7–12 mo1 | 500 | 500 | ||
| 1–3 y | 300 | 300 | ||
| 4–8 y | 400 | 400 | ||
| 9–13 y | 600 | 600 | ||
| 14–18 y | 900 | 700 | 750 | 1200 |
| 19–50 y | 900 | 700 | 770 | 1300 |
| 51 + y | 900 | 700 |
Adequate Intake, equivalent to the mean intake of vitamin A in healthy, breastfed infants. RDAs for vitamin A are given as retinol activity equivalents to account for the different bioactivities of retinol and provitamin A carotenoids, all of which are converted by the body into retinol. One RAE is equivalent to 1 µg retinol, 2 µg supplemental β-carotene, 12 µg dietary β-carotene, or 24 µg dietary α-carotene or β-cryptoxanthin. Based upon (16). RAE, retinol activity equivalents.
Regarding Women, Pregnant Women, Children, and Infants; What was Known in 2001?
Women
Citing the report, “Using this method, there was insufficient evidence to support setting a different EAR for men and for women, as there were too few women studied.” Indeed, there were 2 (unless the 2 medical students were female).
Pregnancy and lactation
The situation at the time of the last DRI values for vitamin A was similar, “Direct studies of the requirement for vitamin A during pregnancy are lacking” (1). However, estimates were included of the amount of vitamin A needed for transfer to the fetus. For pregnant women, considered for 3 different age groups, estimates of the EAR and specification of the RDA were based on values for nonpregnant women of the same age group to which were added estimates of fetal accumulation of vitamin A and the efficiency of vitamin A absorption by the woman. Fetal vitamin A accumulation was estimated from the liver vitamin A measurement of 10 stillborn Thai infants carried to term by mothers who were reported to be generally healthy (18). The result yielded an estimate of how much additional vitamin A per day would be necessary for maintenance.
For lactation, estimates were available for the amount of vitamin A transferred daily to the infant in breast milk. The latter estimates might be the most reliable in that actual determinations of milk volume transferred per day were available, as were measurements of milk vitamin A concentration, although the range of values observed for the 9 subjects were relatively wide. Nevertheless, some direct analytical data were available to inform this DRI decision. In this manner, 400 µg/d was added to the EAR value that was determined for nonlactating females.
Infants
The approach to micronutrient DRI values for infants has been based on a standard paradigm, which is to estimate an Adequate Intake (AI) based on a standard volume of milk consumed per day as consumed by infants aged 0–6 and 7–12 mo, and on milk vitamin A concentration. A review of literature on milk vitamin A in healthy women showed that there is considerable variation, but nevertheless it could be estimated that: “…human milk-fed infants consume on average 400 μg/d of vitamin A in the first 6 mo of life.”
Additionally, as milk is known to contain carotenoids, they too were considered. Interestingly, the 2001 report refers to an earlier DRI report: “The carotenoid content of human milk has been summarized in Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (19). Because the bioconversion of carotenoids in milk and in infants is not known, the contribution of carotenoids in human milk to meeting the vitamin A requirement of infants was not considered.” Therefore, the AI values for vitamin A for infants in the 2001 report on micronutrients are based only on preformed retinol. It seems likely they may underestimate what infants actually consume, if provitamin A carotenoids were to be included.
Children
At the time of the preparation of the 2001 DRI report on vitamin A, no direct studies had been conducted in the age groups from 1 to 18 y. Indeed, the 2001 report states: “No data are available to estimate an average requirement for children and adolescents. A computational method is used that includes an allowance for adequate liver vitamin A stores to set the Estimated Average Requirement (EAR)… The EAR for children and adolescents is extrapolated from adults by using metabolic body weight” (i.e. body weight to the 2/3 power). Thus, it can be seen that in the absence of direct dose-response data to establish an EAR, the approach used was simply to scale the values that were determined from the study of adult men, downwards, by a scaling factor based on body weight.
A further comment about carotenoids may be of interest. The 2001 report on DRIs for vitamin A included consideration of carotenoids with regard to their conversion to retinol, taking into account types and physical forms of provitamin A carotenoids present in the diet. This report published new “conversion” or “equivalency factors” (1), retinol activity equivalency ratios (RAEs) (Figure 1) based on newer data on the bioavailability of, e.g. ß-carotene in oily solution compared with ß-carotene contained within food matrices, and of the provitamin A carotenoids α-carotene and ß-cryptoxanthin in foods, as compared with ß-carotene also in foods. Thus, a significant advance was made at this time regarding the bioequivalency of various food-based carotenoids. These factors could then be used along with tables of carotenoid contents of foods to compute RAEs for the carotenoids of various forms in the diet. However, it is also interesting to note that carotenoids were considered independently of vitamin A in another DRI report, being included as a chapter in the 2000 report on nutrients with antioxidant properties: Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids (19). In this context, carotenoids were considered for their antioxidant potential and no DRI values specific for carotenoids were set then, or have been set since then. Neither were data on, for example, the carotenoid content of human milk considered in the context of this report, and there was essentially no focus in the 2000 publication on carotenoid nutrition in the context of pregnancy and lactation, or for infants and children.
FIGURE 1.
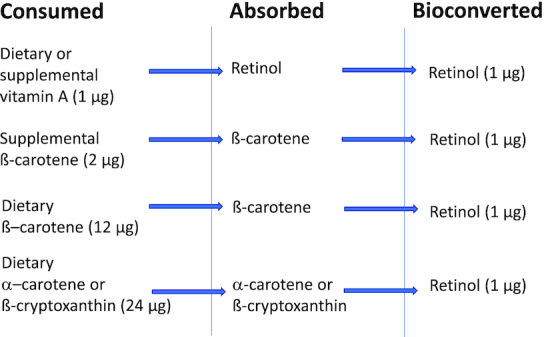
Schematic of the bioavailability of carotenoids to vitamin A as published in (1).
In sum, this historical briefing is meant to provide some context regarding the limitations of information on which the DRI values currently in use are based, and thereby lead the way to considering what had been learned since that time that may be relevant for the development of DRI values for vitamin A in the future.
Looking Forward: What has Changed, and Why Might it be Time for New Vitamin A DRI Values?
Since the 2001 report, a number of advances have been made to address the effects of dietary vitamin A on the indicator of dark adaptation, usual vitamin A and carotenoid intake by infants from breast milk, and the accretion and maintenance of vitamin A stores in pregnancy, lactation, and childhood. Of direct relevance to vitamin A requirements in pregnancy, several studies have been published on the relation between dietary intake of vitamin A and provitamin A carotenoids with visual dark adaptation in pregnant Nepali as well as Ethiopian women (20–24). In children, a cluster-randomized trial on the effect of provitamin A carotenoid-containing maize on night blindness was published (25), since the 2001 report (1), a significant addition to the literature where there previously were no publications. There is also now a much greater body of literature on the vitamin A and carotenoid content of human milk, across different populations and across different lactation stages [e.g. (2, 3, 26)], since the 2001 report (1), which may have the potential to expand upon the previous sample size of 9 subjects. Several more advances are described and applied in the following 3 articles in this special collection of articles published by Current Developments in Nutrition (CDN), and thus they will be mentioned just briefly here.
One very significant advance has been the use of stable isotope methodologies applied to human studies, including in women and children, as well as the use of isotopic tracer studies in animal models, which can provide information relevant to the DRI process. Such methods allow for estimation of tissue stores that cannot be assessed directly in humans, or only under very limited circumstances. These tracer methods allow the determination of actual micronutrient turnover rates, so that it is now possible to estimate a vitamin A requirement based on how much of the nutrient is required for balance, as opposed to depending on a physical indicator such as dark adaptation to assess nutrient adequacy. Secondly, advances have also been made in assessing vitamin A intake such that country-specific datasets have improved, and more information is available on average intakes by infants, children, adolescents, women, and others. Additionally, new experimental studies in animal models have resulted in a deeper understanding of retinol metabolism in the neonate, for example by showing that a much larger portion of chylomicron vitamin A is taken up by extrahepatic tissues in the neonate compared with the adult, and that supplementation with vitamin A alters turnover rates significantly (11). Such studies have also shown that storage tissues such as the liver and lung do not hold on to the vitamin A provided in a high-dose supplement for more than a few days, whereas in contrast, vitamin A provided to the mother for transfer in milk results in a more gradual but longer-sustained increase in tissue vitamin A, and thus accumulation over a longer period of time (12). The results also suggest that neonates grow well and are able to maintain a normal concentration of plasma retinol in the face of much lower hepatic reserves of vitamin A, as compared with adult animals (11). These data suggest that strategies delivering vitamin A directly to the nursing mother may be most effective in producing a sustained response to supplemental vitamin A in the neonate. Such data also imply that one cannot accurately calculate the vitamin A EAR for children using adult data, and thus data directly from studies in children are essential.
Advances in Understanding the Metabolism of Carotenoids in the Intestine
Another major step forward since the time of the 2 DRI reports cited above has been a new understanding of how carotenoids, especially ß-carotene, are processed in the intestine. Advances have been made at the genetic-molecular level in understanding some of the root causes for variation in carotenoid utilization, specifically the identification, cloning, and molecular analysis of 2 carotenoid cleavage genes, β-carotene-15, 15’-oxygenase 1 (BCO1) and carotene 9’,10’ oxygenase 2 (BCO2) (27, 28). We now have glimpses of how BCO1 gene variants within the coding and noncoding regions may affect either the efficiency or catalytic conversion (cleavage) or the expression, or both, of these enzymes (29, 30). Population differences have been suggested (29) in terms of allelic frequency of BCO1 and other genes related to carotenoid and vitamin A metabolism. Thus, these methods can be applied more widely in human studies, as illustrated in (31–33). In the case of ß-carotene, it has long been known that cleavage in the small intestine is a major source of vitamin A, certainly for all herbivores and for many omnivores whose diets are largely plant-based. For humans, it is well known that the majority of persons living in middle- and low-income countries obtain a larger proportion of their vitamin A intake in the form of provitamin A carotenoid precursors, as compared with preformed retinol. Prior to the 2001 DRI report on vitamin A, studies in humans had already revealed wide person-to-person variability in the bioconversion of ß-carotene to vitamin A (34, 35), which had been inferred from monitoring the appearance of retinol or retinyl esters in plasma a few hours after subjects in various studies had been given rather large oral doses of ß-carotene. From these studies the concept of “responders” (those persons who showed a bump-up in plasma retinol after ß-carotene dosing) and nonresponders (those for whom no increase was apparent) was already present in the literature. A few years after the 2001 report, additional information was obtained from stable isotope kinetic studies conducted in a group of older subjects in Boston which traced the conversion of deuterated ß-carotene into deuterated retinol (36); the results of this study not only confirmed and quantified wide subject-to-subject differences in the efficiency of conversion of ß-carotene to retinol, but also suggested that there can be a slow postintestinal conversion of a small fraction of the ß-carotene that was absorbed intact from the intestine. Thus, the notion of variability was already on the minds of investigators in the early 2000s, but a detailed understanding of “why” was lacking.
It is now appreciated that genetic polymorphisms may be important factors in the metabolism of ß-carotene. From studies in the last decade, it is well understood that the major cleavage enzyme (BCO1) is actually a dioxygenase (37), and that BCO1 cleaves ß-carotene at its central 15,15’ double bond, thus forming retinal that subsequently either is reduced to retinol and then esterified for transport in chylomicrons as retinyl esters, or, in a minor pathway, oxidized to generate retinoic acid (RA) in the enterocyte. It has also been shown that the BCO1 has a number of common, single nucleotide polymorphisms (SNPs), some of which lie in the protein-coding region of the gene and thus directly affect the amino acid sequence of the enzyme (29), whereas other SNPs lie outside the coding region but may still play a regulatory role (30, 38). Moreover, the prevalence of different SNP genotypes differs across human populations, which may potentially explain why some populations may respond to dietary interventions differently than other populations. In a study testing in vitro expressed versions of the BCO1 protein that differed in 2 specific amino acid residues, the SNPs affected the BCO1 in vitro enzyme kinetics, suggesting that these polymorphisms in the BCO1 gene could influence the efficiency of ß-carotene cleavage in vivo (17). However, at present there has been no systematic examination of the implications of various BCO1 SNPs across human populations. Thus, there is fertile ground for future studies to understand population- and individual-level differences in carotenoid metabolism. Furthermore, newer studies have identified a second BCO gene, BCO2, that is capable of cleaving carotenoids at double bond positions other than the central 15,15’ double bond (39), and therefore can form an array of carotenoid metabolites that were not recognized at all in 2001. Although less is known about BCO2 as compared with BCO1, it too should be ready for investigation in more detail at the population level, in combination with metabolic studies on carotenoid bioavailability from different food sources and at different life stages.
Several studies since the last vitamin A DRI report have further revealed that ß-carotene metabolism in the intestine is subject to an elegant molecular feedback mechanism that is capable of “sensing” RA, a minor yet crucial product of ß-carotene and retinol metabolism. In this mechanism, RA binds to its nuclear receptor, the retinoic acid receptor (RAR), in the small intestine and thereby functions as a transcriptional inducer of the expression of the intestine-specific homeobox gene, ISX, which in turn is a transcriptional repressor for BCO1 (38, 40). Thus, as more RA is produced, more ISX protein is expressed and consequently the expression of BCO1 is reduced. The ISX-mediated feedback mechanism with RA as a crucial signal provides a way to control the efficiency of ß-carotene utilization, such as could be important when dietary ß-carotene is highly abundant over long periods of time. In fact, it may help to explain the well-known lack of toxicity of ß-carotene. Moreover, ISX has been shown to not only repress the BCO1 gene, but also the gene encoding the SCARB1, scavenger receptor class B type 1, an apical plasma membrane-associated transporter through which ß-carotene enters the enterocyte (41–43). This suggests that the uptake of carotenoids into the absorptive cells as well as their intracellular conversion within are both sensitive to RA as the downstream product of the carotenoid cleavage reaction (Figure 2). The molecular details elucidated to date have come only from studies in genetically modified mice tested with extremely high intakes of ß-carotene (38, 40). However, future human studies using moderate carotenoid doses could possibly reveal results concerning regulation that would be important for future DRI development. As the homology of these genes (BCO1, ISX, SCARB1) between mice and humans is high, the results from animal studies suggest that there is still much to be learned about the utilization of the various precursor forms of vitamin A in humans. Furthermore, the existence of this feedback loop suggests the potential for dose-response interactions between preformed vitamin A, due to its conversion to RA, and the bioavailability of dietary carotenoids.
FIGURE 2.
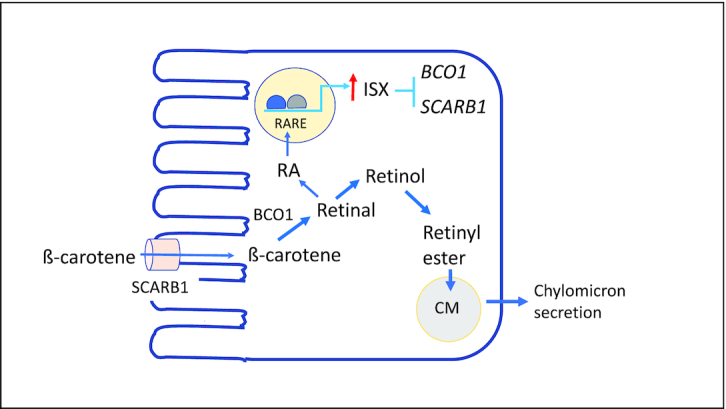
Schematic of intestinal β-carotene metabolism and its feedback regulation by retinoic acid. An enterocyte is illustrated. Newer information since the time of the 2001 report on vitamin A has identified brush border-associated transporters involved in carotene uptake, SCARB1; a mechanism for the enzymatic cleavage of β-carotene by BCO1; and a feedback mechanism involving the intestine-specific transcription factor, ISX, which is activated by RA, an active downstream product of vitamin A and β-carotene metabolism. When the RA concentration rises and the expression of the ISX gene is induced, then ISX protein serves to repress the transcription of the SCARB1 and BCO1 genes, thereby reducing the efficiency of cleavage of β-carotene. See refs (38–43) and text for discussion. Abbreviations: BCO1, ß-carotene oxygenase 1; CM, chylomicron; ISX, intestine-specific transcription factor; RA, retinoic acid; RARE, retinoic acid receptor response element; SCARB1, scavenger receptor class B type 1.
Towards Reevaluation of the Vitamin A DRI Values for Infants, Children, and Pregnant Women
In the 3 articles that follow in this collection, firstly, Gannon, Jones, and Mehta (44) will address vitamin A requirements in pregnancy and lactation, by reviewing a variety of methods and experimental approaches that can be used to estimate the requirements for vitamin A that are applicable during these life stages. Some of the assessment measures discussed are of the kind that can be performed in field studies, and others are more limited but likely to provide more precise information on vitamin A stores, absorption, and transfer from mother to infant in breast milk. These authors will consider placental transfer, transfer from mother to infant during lactation, and studies of vitamin A supplementation in the pregnancy and the neonatal period, and include a discussion of public health implications of using dietary approaches as compared with large-dose supplementation to improve vitamin A status.
Secondly, Ford and Lopez-Teros (45) will address advances in defining vitamin A requirements in early life and childhood. In their article, they will not only provide a review but also present new analyses that are now possible from data obtained by using newer methods for determining vitamin A storage in young children, using mostly data from studies that have been conducted in low- and middle-income countries. These authors have taken a novel experimental approach that makes use of country-specific data on the intake of vitamin A as well as new estimates of vitamin A (retinol) turnover derived from recent stable isotope tracer studies in children, and other relevant data including from studies of vitamin A supplementation, to estimate how vitamin A stores accumulate in infants and children. These up-to-date approaches provide direct estimates of whether children in these countries are likely to be receiving sufficient vitamin A in their diet and/or supplements to build body reserves, and what amounts of vitamin A intake may provide more than enough for this purpose; they also provide indirect evidence of country-to-country variability that might be related to country-specific programs for vitamin A distribution.
Thirdly, Tan et al. (46) address the question, using an experimental approach, of how the macronutrient content of the maternal diet can affect vitamin A status in the neonate. Specifically, they probe whether the offspring of mother rats fed a high-fat diet differ from those of control-fed dams. Such information is more important now than ever, as rates of maternal and child obesity have increased significantly since the last DRI report on vitamin A in 2001.
We may also ask the question, what is still lacking? What more is necessary or would be especially helpful to establish new DRI values for vitamin A in the future? Each of the articles in this collection will have some comments and suggestions for ways forward to improve upon the current estimates used to set the DRI values for vitamin A. One type of data that is clearly needed for DRI development is dose-response data, since the determination of the EAR rests on having a clear understanding of the distribution of requirements within a population, thus data on outcome as a function of intake are required. In this regard, the old studies mentioned from the 1950s had the right idea—to gradually increase intake and observe an outcome response—however, newer studies have not always had sufficient dose variation to allow for construction of the dose-response relations needed to estimate the EAR.
It is noteworthy that DRI values by nature depend on an understanding of the underlying structure of the populations of interest, including the variability inherent in estimates of effect (indicators). Studies must include a sufficient number of individuals to provide the estimate of variability needed to convert an EAR to an RDA. Previously in 2002, a 20% CV was “guesstimated,” but the number of samples was too small to be able to consider this a very solid estimate. For more precise DRI values, better information is needed that is age- and sex-specific, and, for the future, that tests whether other factors like baseline micronutrient status, race, obesity, or even habitual dietary patterns need to be considered as factors in DRI development. Furthermore, the DRI process itself has evolved to now consider how DRI values can be useful for the prevention of chronic diseases (14). Although more attention has been focused on the role of nutrition in gestation and early life on long-term health outcomes (47), this remains a relatively understudied aspect of vitamin A nutriture. Further, there is also a need for more information related to at-risk populations, such as the obese, prediabetic, and others that constitute parts of the generally healthy population to which the DRI values apply (14).
We hope that the articles in this CDN collection will raise awareness of the important advances that have taken place since the last publication of DRI values for vitamin A in 2001 and carotenoids in 2000, highlight gaps that still remain, and suggest ways that new information could be used to create a much more solid, evidence-based foundation for future new DRI values, especially for women, infants, and children.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ACR and NEM: wrote the paper; ACR: had primary responsibility for final content; and all authors have read and approved the final manuscript.
Notes
This work was supported by the NIH National Center for Complementary and Integrative Health (NCCIH) and the Office of Dietary Supplements (ODS) (R00 AT008576; NEM), the National Institute of Child Health and Human Development (NICHD; R01 HD006982; ACR), and the Agricultural Research Service of the USDA (CRIS 3092-51000-059; NEM). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the USDA/ARS, or NIH, NCCIH, ODS, or NICHD.
Author disclosures: ACR and NEM report no conflicts of interest.
Abbreviations used: AI, Adequate Intake; BCO1, β-carotene-15, 15’-oxygenase 1; BCO2, carotene 9’,10’ oxygenase 2; EAR, Estimated Average Requirement; ISX, intestine-specific homeobox; RA, retinoic acid; RAE, retinol activity equivalents; SCARB1, scavenger receptor class B type 1; SNP, single nucleotide polymorphism.
Contributor Information
A Catharine Ross, Email: acr6@psu.edu, Department of Nutritional Sciences, Pennsylvania State University, University Park, PA, USA.
Nancy E Moran, USDA/Agricultural Research Service Children's Nutrition Research Center/Department of Pediatrics, Baylor College of Medicine, Houston, TX, USA.
References
- 1. Institute of Medicine (U.S.), Panel on Micronutrients. Dietary Reference Intakes for vitamin A, vitamin K, arsenic, boron, chromium, copper, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. [Internet] Washington (DC): National Academies Press; 2001. [Accessed 2020 Jun 14]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK222310/. [PubMed] [Google Scholar]
- 2. Lipkie TE, Morrow AL, Jouni ZE, McMahon RJ, Ferruzzi MG. Longitudinal survey of carotenoids in human milk from urban cohorts in China, Mexico, and the USA. PLoS One. 2015;10:e0127729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Song BJ, Jouni ZE, Ferruzzi MG. Assessment of phytochemical content in human milk during different stages of lactation. Nutrition. 2013;29:195–202. [DOI] [PubMed] [Google Scholar]
- 4. Cheatham CL, Sheppard KW.. Synergistic effects of human milk nutrients in the support of infant recognition memory: an observational study. Nutrients. 2015;7:9079–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lopez-Teros V, Limon-Miro AT, Astiazaran-Garcia H, Tanumihardjo SA, Tortoledo-Ortiz O, Valencia ME. ‘Dose-to-mother’ deuterium oxide dilution technique: an accurate strategy to measure vitamin A intake in breastfed infants. Nutrients. 2017;9:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lopez-Teros V, Ford JL, Green MH, Tang G, Grusak MA, Quihui-Cota L, Muzhingi T, Paz-Cassini M, Astiazaran-Garcia H. Use of a “super-child” approach to assess the vitamin A equivalence of Moringaoleifera leaves, develop a compartmental model for vitamin A kinetics, and estimate vitamin A total body stores in young Mexican children. J Nutr. 2017;147:2356–63. [DOI] [PubMed] [Google Scholar]
- 7. Lipkie TE, Banavara D, Shah B, Morrow AL, McMahon RJ, Jouni ZE, Ferruzzi MG. Caco-2 accumulation of lutein is greater from human milk than from infant formula despite similar bioaccessibility. Mol Nutr Food Res. 2014;58:2014–22. [DOI] [PubMed] [Google Scholar]
- 8. Spiegler E, Kim Y-K, Wassef L, Shete V, Quadro L. Maternal–fetal transfer and metabolism of vitamin A and its precursor β-carotene in the developing tissues. Biochim Biophys Acta. 2012;1821:(1):88–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Spiegler E, Kim Y-K, Hoyos B, Narayanasamy S, Jiang H, Savio N, Curley RW, Harrison EH, Hammerling U, Quadro L. β-apo-10′-carotenoids support normal embryonic development during vitamin A deficiency. Sci Rep. 2018;8:8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Costabile BK, Kim Y-K, Iqbal J, Zuccaro MV, Wassef L, Narayanasamy S, Curley RW, Harrison EH, Hussain MM, Quadro L. β-Apo-10′-carotenoids modulate placental microsomal triglyceride transfer protein expression and function to optimize transport of intact β-carotene to the embryo. J Biol Chem. 2016;291:18525–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan L, Green MH, Ross AC. Vitamin A kinetics in neonatal rats vs. adult rats: comparisons from model-based compartmental analysis. J Nutr. 2015;145:403–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tan L, Babbs AE, Green MH, Ross AC. Direct and indirect vitamin A supplementation strategies result in different plasma and tissue retinol kinetics in neonatal rats. J Lipid Res. 2016;57:1423–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raiten DJ, Raghavan R, Porter A, Obbagy JE, Spahn JM. Executive summary: evaluating the evidence base to support the inclusion of infants and children from birth to 24 mo of age in the Dietary Guidelines for Americans – “the B-24 Project”. Am J Clin Nutr. 2014;99:663S–91S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Committee on the Development of Guiding Principles for the Inclusion of Chronic Disease Endpoints in Future Dietary Reference Intakes, Food and Nutrition Board, Health and Medicine Division, National Academies of Sciences, Engineering, and Medicine. Guiding principles for developing Dietary Reference Intakes based on chronic disease. [Internet] Kumanyika S, Oria MP editors. Washington (DC): National Academies Press; 2017. [Accessed 2020 Jun 14]. Available from: https://www.nap.edu/catalog/24828/guiding-principles-for-developing-dietary-reference-intakes-based-on-chronic-disease. [PubMed] [Google Scholar]
- 15. Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. Washington (DC): U.S. Department of Agriculture, Agricultural Research Service; 2015. [Google Scholar]
- 16. National Institutes of Health: Office of Dietary Supplements. Vitamin A—fact sheet for health professionals. [Internet] 2020; [cited 2020 May 4]. Available from: https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/. [Google Scholar]
- 17. Otten JJ, Hellwig JP, Meyers LD editors. DRI, Dietary Reference Intakes: the essential guide to nutrient requirements. Washington (DC): National Academies Press; 2006. p. 543. [Google Scholar]
- 18. Montreewasuwat N, Olson JA. Serum and liver concentrations of vitamin A in Thai fetuses as a function of gestational age. Am J Clin Nutr. 1979;32:601–6. [DOI] [PubMed] [Google Scholar]
- 19. Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for vitamin C, vitamin E, selenium, and carotenoids. [Internet] Washington (DC): National Academies Press; 2000; [cited 2017 Feb 1]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK225483/. [PubMed] [Google Scholar]
- 20. Congdon NG, Dreyfuss ML, Christian P, Navitsky RC, Sanchez AM, Wu LS, Khatry SK, Thapa MD, Humphrey J, Hazelwood D et al. Responsiveness of dark-adaptation threshold to vitamin A and β-carotene supplementation in pregnant and lactating women in Nepal. Am J Clin Nutr. 2000;72:1004–9. [DOI] [PubMed] [Google Scholar]
- 21. Christian P, Khatry SK, Yamini S, Stallings R, LeClerq SC, Shrestha SR, Pradhan EK, West KP. Zinc supplementation might potentiate the effect of vitamin A in restoring night vision in pregnant Nepalese women. Am J Clin Nutr. 2001;73:1045–51. [DOI] [PubMed] [Google Scholar]
- 22. Haskell MJ, Pandey P, Graham JM, Peerson JM, Shrestha RK, Brown KH. Recovery from impaired dark adaptation in nightblind pregnant Nepali women who receive small daily doses of vitamin A as amaranth leaves, carrots, goat liver, vitamin A-fortified rice, or retinyl palmitate. Am J Clin Nutr. 2005;81:461–71. [DOI] [PubMed] [Google Scholar]
- 23. Graham JM, Haskell MJ, Pandey P, Shrestha RK, Brown KH, Allen LH. Supplementation with iron and riboflavin enhances dark adaptation response to vitamin A-fortified rice in iron-deficient, pregnant, nightblind Nepali women. Am J Clin Nutr. 2007;85:1375–84. [DOI] [PubMed] [Google Scholar]
- 24. Abebe H, Abebe Y, Loha E, Stoecker BJ. Consumption of vitamin A rich foods and dark adaptation threshold of pregnant women at Damot Sore District, Wolayita, Southern Ethiopia. Ethiop J Health Sci. 2014;24:219–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Palmer AC, Healy K, Barffour MA, Siamusantu W, Chileshe J, Schulze KJ, West KP, Labrique AB. Provitamin A carotenoid-biofortified maize consumption increases pupillary responsiveness among Zambian children in a randomized controlled trial. J Nutr. 2016;146:2551–8. [DOI] [PubMed] [Google Scholar]
- 26. Dror DK, Allen LH.. Retinol-to-fat ratio and retinol concentration in human milk show similar time trends and associations with maternal factors at the population level: a systematic review and meta-analysis. Adv Nutr. 2018;9:332S–46S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Von Lintig J, Vogt K. Filling the gap in vitamin A research. Molecular identification of an enzyme cleaving β-carotene to retinal. J Biol Chem. 2000;275:11915–20. [DOI] [PubMed] [Google Scholar]
- 28. Kiefer C, Hessel S, Lampert JM, Vogt K, Lederer MO, Breithaupt DE, von Lintig J. Identification and characterization of a mammalian enzyme catalyzing the asymmetric oxidative cleavage of provitamin A. J Biol Chem. 2001;276:14110–6. [DOI] [PubMed] [Google Scholar]
- 29. Leung WC, Hessel S, Meplan C, Flint J, Oberhauser V, Tourniaire F, Hesketh JE, von Lintig J, Lietz G. Two common single nucleotide polymorphisms in the gene encoding beta-carotene 15,15’-monoxygenase alter beta-carotene metabolism in female volunteers. FASEB J. 2009;23:1041–53. [DOI] [PubMed] [Google Scholar]
- 30. Lietz G, Oxley A, Leung W, Hesketh J. Single nucleotide polymorphisms upstream from the beta-carotene 15,15’-monooxygenase gene influence provitamin A conversion efficiency in female volunteers. J Nutr. 2012;142:161S–5S. [DOI] [PubMed] [Google Scholar]
- 31. Ferrucci L, Perry JRB, Matteini A, Perola M, Tanaka T, Silander K, Rice N, Melzer D, Murray A, Cluett C et al. Common variation in the β-carotene 15,15′-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Borel P, Desmarchelier C, Nowicki M, Bott R. A combination of single-nucleotide polymorphisms is associated with interindividual variability in dietary β-carotene bioavailability in healthy men. J Nutr. 2015;145:1740–7. [DOI] [PubMed] [Google Scholar]
- 33. Moran NE, Thomas-Ahner JM, Fleming JL, McElroy JP, Mehl R, Grainger EM, Riedl KM, Toland AE, Schwartz SJ, Clinton SK. Single nucleotide polymorphisms in β-carotene oxygenase 1 are associated with plasma lycopene responses to a tomato-soy juice intervention in men with prostate cancer. J Nutr. 2019;149:381–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. van den Berg H, van Vliet T. Effect of simultaneous, single oral doses of beta-carotene with lutein or lycopene on the beta-carotene and retinyl ester responses in the triacylglycerol-rich lipoprotein fraction of men. Am J Clin Nutr. 1998;68:82–9. [DOI] [PubMed] [Google Scholar]
- 35. Borel P, Grolier P, Mekki N, Boirie Y, Rochette Y, Roy BL, Alexandre-Gouabau MC, Lairon D, Azais-Braesco V. Low and high responders to pharmacological doses of β-carotene: proportion in the population, mechanisms involved and consequences on β-carotene metabolism. J Lipid Res. 1998;39:2250–60. [PubMed] [Google Scholar]
- 36. Tang G, Qin J, Dolnikowski GG, Russell RM. Short-term (intestinal) and long-term (postintestinal) conversion of β-carotene to retinol in adults as assessed by a stable-isotope reference method. Am J Clin Nutr. 2003;78:259–66. [DOI] [PubMed] [Google Scholar]
- 37. Seña C dela, Riedl KM, Narayanasamy S, Curley RW, Schwartz SJ, Harrison EH. The human enzyme that converts dietary provitamin A carotenoids to vitamin A is a dioxygenase. J Biol Chem. 2014;289:13661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lobo GP, Amengual J, Baus D, Shivdasani RA, Taylor D, von Lintig J. Genetics and diet regulate vitamin A production via the homeobox transcription factor ISX. J Biol Chem. 2013;288:9017–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seña C dela, J Sun, Narayanasamy S, Riedl KM, Yuan Y, Curley RW, Schwartz SJ, Harrison EH. Substrate specificity of purified recombinant chicken β-carotene 9′,10′-oxygenase (BCO2). J Biol Chem. 2016;291:14609–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lobo GP, Hessel S, Eichinger A, Noy N, Moise AR, Wyss A, Palczewski K, von Lintig J. ISX is a retinoic acid-sensitive gatekeeper that controls intestinal, beta-carotene absorption and vitamin A production. FASEB J. 2010;24:1656–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Borel P, Lietz G, Goncalves A, de Edelenyi FS, Lecompte S, Curtis P, Goumidi L, Caslake MJ, Miles EA, Packard C et al. CD36 and SR-BI are involved in cellular uptake of provitamin A carotenoids by Caco-2 and HEK cells, and some of their genetic variants are associated with plasma concentrations of these micronutrients in humans. J Nutr. 2013;143:448–56. [DOI] [PubMed] [Google Scholar]
- 42. During A, Dawson HD, Harrison EH. Carotenoid transport is decreased and expression of the lipid transporters SR-BI, NPC1L1, and ABCA1 is downregulated in Caco-2 cells treated with ezetimibe. J Nutr. 2005;135:2305–12. [DOI] [PubMed] [Google Scholar]
- 43. van Bennekum A, Werder M, Thuahnai ST, Han C-H, Duong P, Williams DL, Wettstein P, Schulthess G, Phillips MC, Hauser H. Class B scavenger receptor-mediated intestinal absorption of dietary β-carotene and cholesterol. Biochemistry. 2005;44:4517–25. [DOI] [PubMed] [Google Scholar]
- 44. Gannon BM, Jones C, Mehta S. Vitamin A requirements in pregnancy and lactation. Curr Dev Nutr. 2020;4:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ford JL, Lopez-Teros V. Prediction of vitamin A stores in young children provides insights into the adequacy of current dietary reference intakes. Curr Dev Nutr. 2020;4:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tan L, Zhang Y, Crowe-White KM, Senkus KE, Erwin ME, Wang H. Vitamin A supplementation during suckling and postweaning periods attenuates the adverse metabolic effects of maternal high-fat diet consumption in Sprague-Dawley rats. Curr Dev Nutr. 2020;4:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Singhal A. The role of infant nutrition in the global epidemic of non-communicable disease. Proc Nutr Soc. 2016;75:162–8. [DOI] [PubMed] [Google Scholar]


