Abstract
Background:
Initial biopsy of basal cell carcinoma (BCC) may fail to show aggressive histologic subtypes. Additionality, the clinical evaluation of BCC prior to surgery can miss subclinical extension. Reflectance confocal microscopy (RCM) and optical coherence tomography (OCT) are emerging tools that can help in the presurgical evaluation of BCCs.
Objective:
To assess the feasibility of a combined RCM-OCT imaging modality for presurgical evaluation of biopsy-proven BCCs for residual tumor, margins-status, and depth.
Methods:
Thirty-eight BCCs in 35 patients referred to a tertiary cancer center for Mohs micrographic surgery (MMS) were imaged using combined RCM-OCT. Images were correlated to MMS-frozen-sections.
Results:
Thirty-eight BCCs were analyzed; mean age 67.34 (range 36–84 years); 57.14% females; 63.16% located on head and neck. Mean size was 8.58 mm (range 3–30 mm). RCM-OCT showed an overall agreement of 91.1% with MMS-frozen sections. A sensitivity of 82.6 % [95% CI 069–92%], specificity of 93.8 % [95% CI 88–97%], and receiver operating characteristic curve of 0.88 [95% CI 0.82–0.94] was found. OCT depth was highly correlated with MMS depth (r2 = 0.9).
Limitations:
Small sample size and difficulty evaluating certain challenging anatomical sites.
Conclusion:
Combined RCM-OCT may emerge as a useful tool for presurgical evaluation of BCCs.
Keywords: basal cell carcinoma, margins, residual, reflectance confocal microscopy, optical coherence tomography, biopsy, surgery, Mohs
Introduction:
Basal cell carcinoma (BCC) is the most common keratinocyte carcinoma in the United States with an annual incidence greater than 2 million cases per year, and an estimated cost of $4.8 billion.1–3 The National Comprehensive Cancer Network (NCCN) guidelines recommend performing a biopsy for the diagnosis of BCC and define ‘high-risk’ and ‘low-risk’ based on clinic-pathological variables.4 Despite the increasing burden of disease to both the patient and health care system, presurgical evaluation has remained unchanged. Clinical and dermoscopic assessments have been shown to be poor predictors of BCC extent after a biopsy.5 Moreover, up to 15% of BCCs display a more aggressive histologic subtype at the time of surgery than at the initial biopsy.
Multiple non-invasive imaging modalities, including reflectance confocal microscopy (RCM) and optical coherence tomography (OCT), have emerged as helpful tools in the diagnosis and management of BCCs. RCM is a non-invasive diagnostic technique that allows for in vivo visualization of the skin with quasi-histological resolution.6 RCM has shown good sensitivity and specificity for the diagnosis of BCC.7–9 It has also been used to monitor BCC non-invasive treatments10–12 and to evaluate lesions for residual BCC prior to Mohs micrographic surgery (MMS).13 RCM’s main limitation is inability to image beyond 200–250μm in depth.6 This limitation can potentially lead to missing deeper tumors or more aggressive subtypes.13 In contrast, OCT is a non-invasive imaging modality that generates cross-sectional images. While it lacks cellular resolution, it can image at depths of 1000–2000 μm. OCT has also been independently used to assess BCC margins and estimate tumor depth.14, 15
A recent study has validated a combined RCM-OCT imaging modality for the diagnosis of BCC, and highlighted the synergistic benefits of using a simultaneous, real-time, multimodal imaging approach.16–18 In this study, we sought to assess the feasibility of a combined RCM-OCT imaging modality in the presurgical evaluation of residual disease and surgical margins after biopsy and prior to MMS. We also sought to correlate the depth assessed by OCT and final depth on histologic frozen sections.
Patients and methods:
This was a prospective study performed at a tertiary cancer center, between October 2018 and April 2019. The study was IRB approved (#99–099) and patients signed informed consent before imaging. All patients met MMS criteria as per NCCN guidelines4 and were screened by 2 investigators (S.A and C.N-D) for inclusion/exclusion criteria. All patients underwent MMS after RCM-OCT imaging as per standard of care. Mohs surgeons were blinded to the imaging results and imaging findings did not change surgical planning.
Inclusion and exclusion criteria:
Adult patients (≥ 18 years old) with a biopsy-proven BCC, regardless of the subtype, were included. The tumor site needed to be healed with no ulcers or crusts present at the time of RCM-OCT examination. Patients were excluded if they declined to participate in the study and if a complete RCM-OCT evaluation was not possible due to anatomic location (e.g. nasal crease).
Clinical features:
Patients’ demographic characteristics (age, gender, and skin type) were recorded. Tumor characteristics from the pathology report were also recorded: BCC subtype, location, and size. All biopsies were evaluated by an expert dermatopathologist who confirmed the diagnosis and subtype of BCC prior to MMS.
Reflectance confocal microscopy and optical coherence tomography imaging technique and analysis:
Clinical images were taken with a digital camera (VEOS DS3, Canfield INC, NJ, USA). All biopsy sites were imaged with a combined RCM-OCT handheld probe with the aid of paper rings, as previously described.19–21 Briefly, the RCM imaging provided an en face field of view (FOV) of 750μm×750μm, with a resolution of approximately 1μm and an optical sectioning of approximately 3μm. The OCT imaging provided vertical FOV of 1000μm deep×2000μm wide with a resolution of approximately 5μm and an axial resolution of 10μm.21 Both images were co-registered and viewed simultaneously in real-time on a single monitor.
RCM-OCT evaluations were performed in a systematic fashion. First, the center of the lesion was evaluated (providing 1 data point) to assess for residual status with the intent to scout a 100% of the area. Margins were then assessed; each quadrant was circumferentially imaged from 12–3, 3–6, 6–9 and 9–12 o’clock, respectively (providing 4 independent data points). (Figure 1). RCM images of the lesion were taken at each data point. OCT images recorded simultaneously, and OCT estimated-depth was measured. A total of 5 independent RCM-OCT data points were analyzed per lesion.
Figure 1.
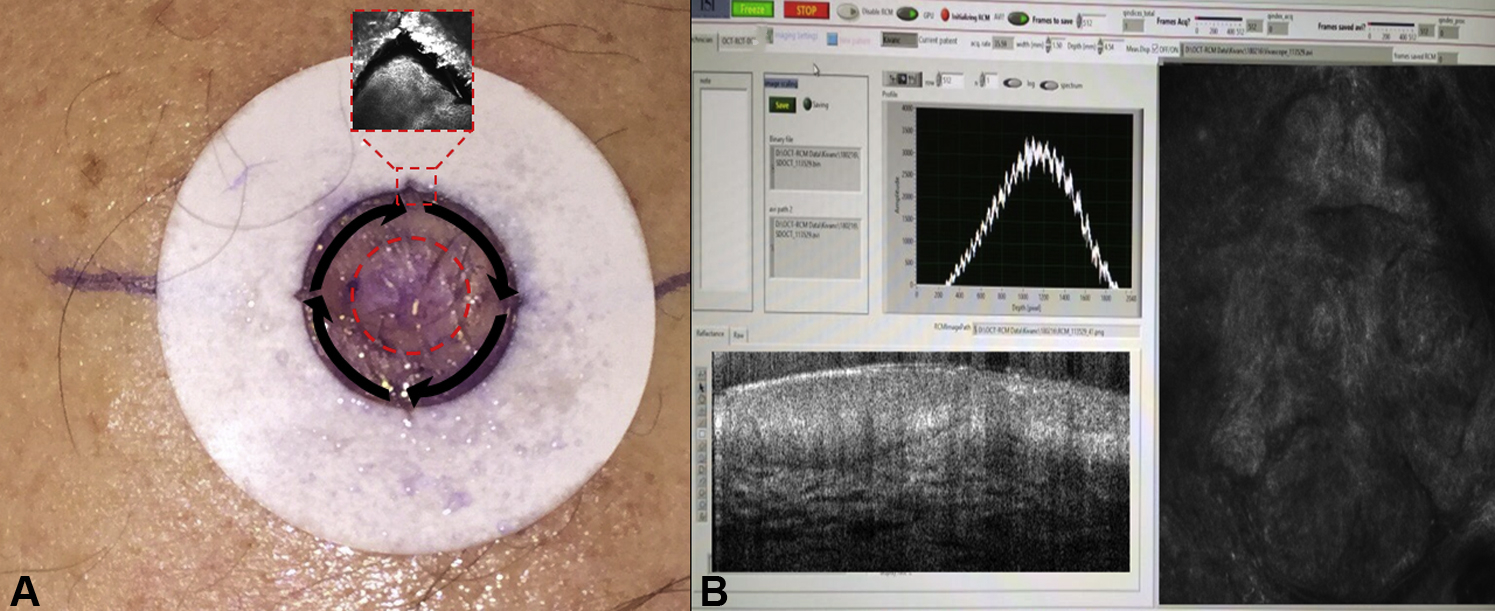
A. Biopsy-proven basal cell carcinoma. A paper ring is placed around the surgical margin as determined by the Mohs surgeon. There are notches at each quadrant of the paper ring. The dashed red circle in the center represents the 1st data point that is imaged to assess for the presence of residual BCC. The dashed red square shows an RCM image of the notch at 12 o’clock. Black arrows represent the quadrants to be imaged: 12–3, 3–6, 6–9, 9–12 o’clock respectively. Each quadrant represents an individual data point that is compared to the corresponding Mohs frozen section. B. Simultaneous co-registration of both RCM and OCT in real-time
The combined (co-registered) RCM-OCT images were evaluated and recorded bedside in a database in ‘real-time’ for consensus by two expert investigators (SA and C.N-D). If BCC criteria were seen at any point under the RCM-OCT evaluation of the BCCs, the case was labeled as ‘RMC-OCT positive’ and depth was recorded. If no BCC criteria were found under RCM-OCT evaluation, the case was labeled as ‘RCM-OCT negative’. The RCM criteria used for the diagnosis of BCC were those previously described.22, 23 OCT criteria used were those described by Sahu et al.24
Mohs tissue processing:
Mohs surgeons were blinded to the imaging results. Surgery was performed as per standard of care and was not modified by the imaging findings. The first Mohs layer was taken and sent for Mohs sectioning (frozen section, H&E staining). If residual BCC was found on a Mohs frozen section margin, the case was labeled as ‘frozen-section positive’ for BCC and the margin was correlated to the corresponding quadrant on RCM-OCT imaging. Additionally, 15μm-serial vertical sectioning of the excised tissue was performed until tissue was exhausted, in all patients. If residual BCC was observed in these sections, the case was also labeled as ‘frozen-section positive’ for residual BCC. If the case was negative on the evaluation of the Mohs margins and negative after serial sectioning, the case was labeled as ‘frozen-section negative’ for residual BCC.
Statistical analysis:
Descriptive statistics including means, medians, range, standard deviation, and relative frequencies were used to describe the study participants, the characteristics of the procedures, and the RCM and OCT characteristics. The primary outcome variable was presence or absence of residual BCC and margin assessment observed on RCM and or OCT evaluation coded as a dichotomous variable. The distributions of study variables were assessed by residual BCC using relative frequencies. Percent agreement and kappa were used to assess level of agreement between RCM and OCT readings. Prevalence adjusted bias adjusted kappa (PABAK) was also estimated with the prevalence of tumor was less than 10% in the sample. Measures of diagnostic accuracy along with exact 95% binomial confidence intervals for overall RCM classification and individual RCM features were estimated. Scatterplots and measures of concordance were used to assess the relationship between histologic depth and OCT depth. Analyses were performed using Stata v.14.2, Stata Corporation, College Station, TX.
Results:
Thirty-eight biopsy-proven BCCs on 35 patients met the inclusion criteria; mean age was 67.3 years (SD 11.8, range 36–84 years); 57.1% were females. Most cases were located on the head and neck (63.2%). All biopsies were performed by shave technique with the intent of diagnosis. Mean clinical size was 8.6 mm (SD 1.8; range 3–30mm). Table 1 presents demographic and clinical characteristics.
Table 1.
Patient demographics, lesion location, subtype and size (n=35 patients/38 BCCs).
| Variable | n. | % |
|---|---|---|
| Gender | ||
| Male | 15 | 42.86 |
| Female | 20 | 57.14 |
| Age: Mean (SD) | 67.34 (11.84) | |
| Age: Median (IQR) | 47(23) | |
| Phototype | ||
| I | 1 | 2.86 |
| II | 17 | 48.57 |
| III | 17 | 48.57 |
| Personal History of NMSC | ||
| No | 7 | 20 |
| Yes | 24 | 68.57 |
| Not recorded | 4 | 11.43 |
| Lesion contributed per participant | ||
| 1 | 32 | 91.42 |
| 2 | 3 | 8.57 |
| Location (specific) | ||
| Back | 6 | 15.79 |
| Cheek | 8 | 21.05 |
| Chest | 5 | 13.16 |
| Arm | 2 | 5.26 |
| Forehead | 8 | 21.05 |
| Lip | 2 | 5.26 |
| Neck | 1 | 2.63 |
| Nose | 2 | 5.26 |
| Scalp | 2 | 5.26 |
| Temple | 1 | 2.63 |
| Thigh | 1 | 2.63 |
| Laterality | ||
| Right | 17 | 44.74 |
| Left | 16 | 42.11 |
| Center | 5 | 13.16 |
| Histology | ||
| Superficial | 6 | 15.79 |
| Nodular | 21 | 55.26 |
| Infiltrative | 2 | 5.26 |
| Basosquamous | 1 | 2.63 |
| Mixed | 4 | 10.53 |
| NOS* | 4 | 10.53 |
| Final Histology Status | ||
| Negative | 11 | 28.95 |
| Positive | 27 | 71.05 |
| Lesion largest diameter (mm), mean (SD) | 8.58 (5.03) | |
Combined reflectance confocal microscopy-optical coherence tomography findings:
After analyzing 190 data points (5 data points per lesion), RCM-OCT showed an overall agreement with MMS frozen-sections of 91.1% with an expected agreement (due to random allocation) of 63.1%, kappa of 0.76, and prevalence-adjusted and bias-adjusted kappa (PABAK) of 0.81. There were 38 true positive data points, 9 false negative, 8 false positive and 135 true negative; this led to a sensitivity of 82.6 % [95% CI 069–92%], with a specificity of 93.8% [95% CI 88–97%], positive predictive value of 80.9% [95% CI 0.69–0.89] and negative predictive value of 94.4% [95% CI 0.89–0.97]. The receiver operating characteristic (ROC) curve was 0.88 [95% CI 0.82–0.94]. When analyzing the data at a lesional level (n=38), the sensitivity of the combined RCM-OCT was 100% [95% CI 0.87–1.0], specificity 81.8% [95% CI 0.48–0.97], positive predictive value 93.1% [95% CI 0.79–0.97] and negative predictive value was 100 % [95% CI NA]
Diagnostic accuracy measures were consistent across subsets of the study sample with no differences observed by patient sex or age. A sub-analysis based on tumor histopathological “risk” (‘high-risk’ defined as ‘infiltrative, basosquamous and mixed’ subtypes [n=7] and ‘low-risk’ defined as ‘superficial and nodular’ [n=31]) showed no difference between the groups (92.7% vs 90.4%; p=0.81).
Optical coherence tomography assessment of basal cell carcinoma depth:
OCT showed high concordance with final MMS frozen section depth with a Pearson’s correlation coefficient of 0.9. The average difference between histologic depth and OCT depth was −21.11 μm (Figure 2).
Figure 2.
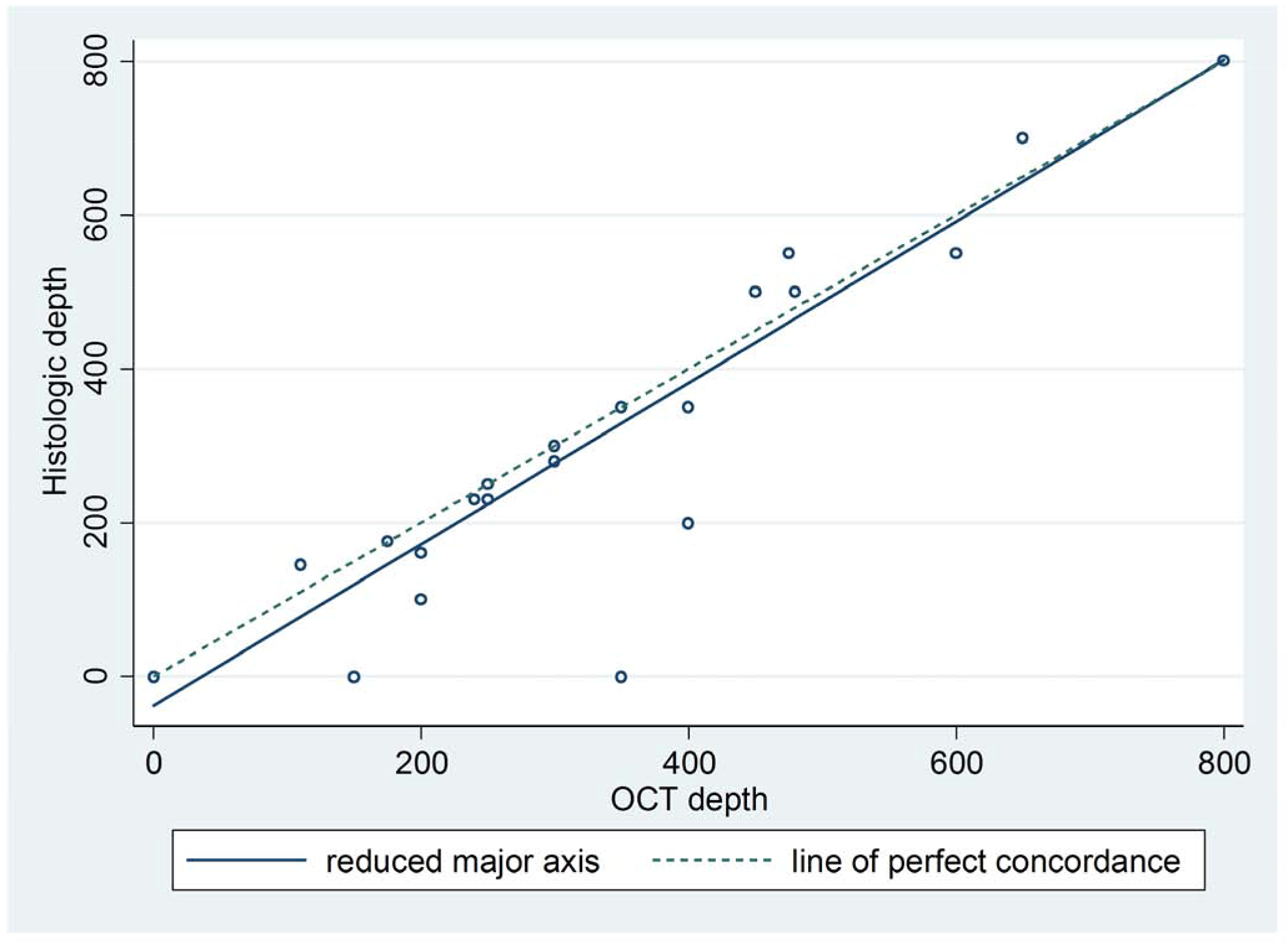
Concordance between histologic depth and OCT depth.
Lin’s concordance correlation coefficient = 0.9.
Pearson’s correlation coefficient = 0.9
Average difference between histologic depth and OCT depth, −21.11
Figure 3 and 4 show case examples of RCM-OCT imaging of BCCs.
Figure 3:
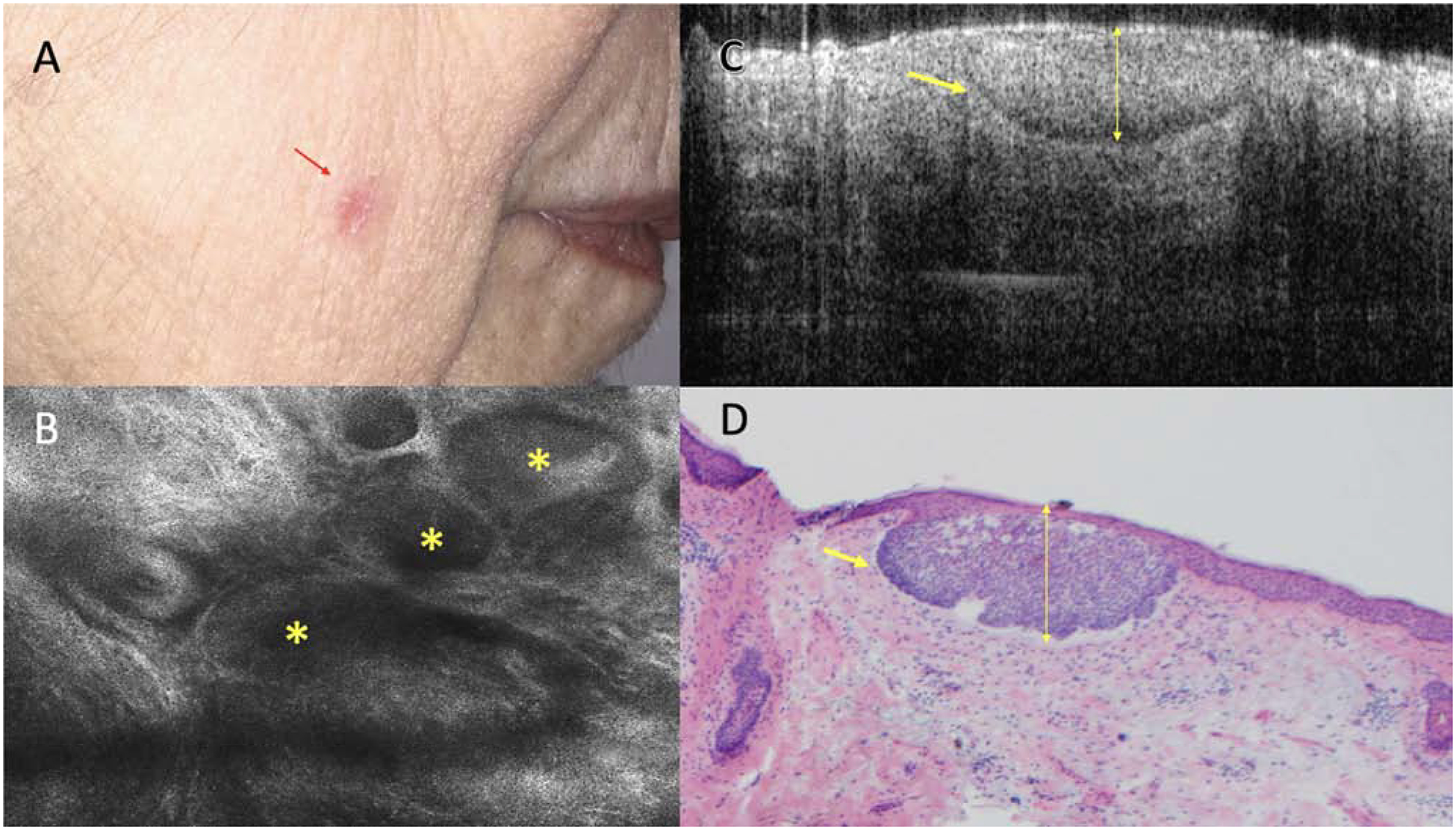
Superficial basal cell carcinoma (BCC) on the cheek with no clinical evidence of residual tumor. A. Clinical appearance of the biopsy site (red arrow). B. Reflectance confocal microscopy showing tumor islands (yellow asterisk) with palisading and clefting (750 × 750 μm). C. Optical coherence tomography image showing hyporeflective tumor island with retraction space (yellow arrow) measuring 200 μm in depth (1000 × 2000 μm) D. Mohs frozen section showing corresponding superficial BCC (yellow arrow), 200 μm depth. (H&E, 4X magnification).
Figure 4:

Basal cell carcinoma (BCC) on the forehead, read as ‘superficial BCC’ on biopsy report with no clinical evidence of residual tumor. A. Clinical appearance of the biopsy site (red arrow). B. Reflectance confocal microscopy (RCM) showing no evidence of residual tumor (750 × 750 μm) C. Optical coherence tomography (OCT) showing Hypo-reflective tumor cords surrounded by hyper-reflective collagen strands (yellow arrows). D. Mohs frozen section showing infiltrative BCC (H&E, 4X magnification). In this case, RCM did not show any evidence of residual BCC, but OCT suggested an infiltrative component. This case highlights the synergist value of the combined RCM-OCT device.
Discussion:
In this prospective study, the combined RCM-OCT probe obtained a sensitivity of 82.6%, specificity of 93.8% for the diagnosis of residual BCC and margin evaluation. There was a 91% agreement, on all 190 data points, between the RCM-OCT imaging and final histopathological status on frozen sections. Moreover, there was a high correlation between the tumor depth on RCM-OCT imaging and the final depth on MMS frozen sections (r2=0.9). These results illustrate the potential diagnostic accuracy of the combined RCM-OCT probe and are similar to the pilot study using the same probe for the diagnosis of non-biopsied BCCs.24
Prior studies have demonstrated that biopsies can miss features of aggressive BCC subtypes in 11–26% of cases and the rate of tumor upgrading at the time of MMS ranges from 10–33%.25–29 Furthermore, in a recent study showed RCM alone can be helpful in the presurgical evaluation of BCC. However, due to the depth limitation of RCM (200–250 μm) deeper tumors or more aggressive sub-types were not detected.13 In this study, 3 patients (8.6%) who presented with a ‘superficial BCC’ per biopsy report were correctly identified as deeper tumors using RCM-OCT. These studies highlight how the two imaging modalities can complement each other and take advantage of the higher cellular resolution of RCM and the OCT increased depth assessment.17
Previous studies using RCM have also shown to be helpful in evaluating for residual BCC prior to MMS.13 In the present study 29% of the biopsied BCCs showed no evidence of residual tumor on MMS frozen sections and serial vertical sectioning, similar to previous reports.13, 30 RCM-OCT may potentially help better triage these patients and possibly discuss non-invasive treatment options (PDT, chemotherapeutic agents), or even observation if no deep tumor is found or no residual tumor is detected. Larger randomized studies are needed to confirm our findings.
The discrepancy between the pathology report and more aggressive subtypes on the final MMS frozen sections that Kyllo et al. demonstrated in their study highlights the possible need and relevance of a presurgical evaluation tool, as ‘upgraded’ BCCs required more stages, a larger postoperative defect size, and more complicated repairs.31 The present study showed a 100% NPV and no difference between low-and high-risk histologic BCC subtypes.
The information provided by RCM-OCT can potentially help address patients’ and physicians’ concerns such as presence of residual BCC and surgical necessity, margin extent, depth, and possible type of surgical repair needed. If all these questions could be addressed preoperatively and non-invasively, it could potentially lead to downstream benefits with a potential impact on the patient’s surgical experience, satisfaction, and improved shared decision-making process. This in turn can decrease patient anxiety, reduce cost by better assessing surgical margins and potentially reducing the number of Mohs stages, and improve pre-operative surgical planning by discussing appropriate reconstruction options and potential non-invasive treatment options.
Limitations:
The main limitation was the relatively small sample size. Larger clinical studies are needed to confirm these finding. Furthermore, previously biopsied lesions can be challenging to assess on both RCM and OCT due to fibrosis and inflammation. In addition, certain anatomical sites are difficult to evaluate due to the size of the probe tip (e.g. conchal ear and peri-ocular). Hopefully future technological advances can improve miniaturizing the RCM-OCT probe, and increasing its application towards a wider dermatologic use. The combined RCM-OCT device is not currently commercially available, but we anticipate more interest in non-invasive imaging given the recent category I CPT code granted to RCM and the category III to OCT.
Conclusion:
The combined RCM-OCT with simultaneous co-registration of both imaging modalities, as described in this study, may emerge as a useful tool for the presurgical evaluation of BCCs and usher in a new area of presurgical assessment. RCM-OCT takes advantage of the higher cellular resolution of RCM and the ability to measure up to 1000μm with OCT. While there is associated cost, training, and additional patient time required, these can potentially be offset by increasing surgical efficiency and planning, enhancing patient experience, and may prove helpful in triaging selected low-risk patients to less invasive treatment options or observation.
Capsule summary:
Basal cell carcinoma (BCC) is the most common skin cancer yet presurgical evaluation has remained largely unchanged.
Combined reflectance confocal microscopy-optical coherence tomography may be helpful in presurgical evaluation of BCCs by detecting residual tumor after a biopsy, aiding in margin assessment, and tumor depth prior to Mohs micrographic surgery.
Funding source:
This research is funded in part by a grant from the National Cancer Institute / National Institutes of Health (P30-CA008748) made to the Memorial Sloan Kettering Cancer Center.
Conflicts of interest disclosures:
Anthony Rossi: Dr. Rossi has no relevant conflicts of interest related to this manuscript but has received grant funding from The Skin Cancer Foundation and the Award Ford Memorial Grant for research related to this work. He also served on advisory board, as a consultant, or given educational presentations: for Allergan, Inc; Galderma Inc; Evolus Inc; Elekta; Biofrontera, Quantia; Merz Inc; Dynamed; Skinuvia, Perf-Action, and LAM therapeutics.
Kishwer S. Nehal: Dr. Nehal received royalties from publishing companies for books and book chapters.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Consent for publication: The authors consent the publication of this submission (manuscript and figures).
Prior presentation: Preliminary data on this manuscript was presented at the American College of Mohs Surgery Annual Meeting held in Baltimore MD, May 3rd, 2019.
References:
- 1.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012;166:1069–80. [DOI] [PubMed] [Google Scholar]
- 2.Rogers HW, Weinstock MA, Feldman SR, Coldiron BM. Incidence Estimate of Nonmelanoma Skin Cancer (Keratinocyte Carcinomas) in the U.S. Population, 2012. JAMA Dermatol 2015;151:1081–6. [DOI] [PubMed] [Google Scholar]
- 3.Drucker AM, Adam GP, Rofeberg V, Gazula A, Smith B, Moustafa F et al. Treatments of Primary Basal Cell Carcinoma of the Skin: A Systematic Review and Network Meta-analysis. Ann Intern Med 2018;169:456–66. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. Melanoma (Version 2.2018).
- 5.Sasor SE, Nosrati NN, Katona T, Wooden WA, Cohen A, Munshi IA et al. Predicting the Presence of Nonmelanoma Skin Cancers After Biopsy: A Method to Reduce Unnecessary Surgical Procedures. JAMA Surg 2016;151:289–90. [DOI] [PubMed] [Google Scholar]
- 6.Rajadhyaksha M, Marghoob A, Rossi A, Halpern AC, Nehal KS. Reflectance confocal microscopy of skin in vivo: From bench to bedside. Lasers Surg Med 2017;49:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castro RP, Stephens A, Fraga-Braghiroli NA, Oliviero MC, Rezze GG, Rabinovitz H et al. Accuracy of in vivo confocal microscopy for diagnosis of basal cell carcinoma: a comparative study between handheld and wide-probe confocal imaging. J Eur Acad Dermatol Venereol 2015;29:1164–9. [DOI] [PubMed] [Google Scholar]
- 8.Kadouch DJ, Schram ME, Leeflang MM, Limpens J, Spuls PI, de Rie MA. In vivo confocal microscopy of basal cell carcinoma: a systematic review of diagnostic accuracy. J Eur Acad Dermatol Venereol 2015;29:1890–7. [DOI] [PubMed] [Google Scholar]
- 9.Liopyris K, Navarrete-Dechent C, Yelamos O, Marchetti MA, Rabinovitz H, Marghoob AA. Clinical, dermoscopic and reflectance confocal microscopy characterization of facial basal cell carcinomas presenting as small white lesions on sun-damaged skin. Br J Dermatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Navarrete-Dechent C, Cordova M, Liopyris K, Yelamos O, Aleissa S, Hibler B et al. Reflectance confocal microscopy-guided carbon dioxide laser ablation of low-risk basal cell carcinomas: A prospective study. J Am Acad Dermatol 2019;In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Torres A, Niemeyer A, Berkes B, Marra D, Schanbacher C, Gonzalez S et al. 5% imiquimod cream and reflectance-mode confocal microscopy as adjunct modalities to Mohs micrographic surgery for treatment of basal cell carcinoma. Dermatol Surg 2004;30:1462–9. [DOI] [PubMed] [Google Scholar]
- 12.Pasquali P, Segurado-Miravalles G, Freites-Martinez A, Gonzalez-Rodriguez S. Cryosurgical management of basal cell carcinoma: in vivo follow-up using reflectance confocal microscopy. Int J Dermatol 2018. [DOI] [PubMed] [Google Scholar]
- 13.Navarrete-Dechent C, Cordova M, Aleissa S, Liopyris K, Dusza SW, Phillips W et al. Reflectance confocal microscopy confirms residual basal cell carcinoma on clinically negative biopsy sites before Mohs micrographic surgery: A prospective study. J Am Acad Dermatol 2019;81:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng HM, Lo S, Scolyer R, Meekings A, Carlos G, Guitera P. Accuracy of optical coherence tomography for the diagnosis of superficial basal cell carcinoma: a prospective, consecutive, cohort study of 168 cases. Br J Dermatol 2016;175:1290–300. [DOI] [PubMed] [Google Scholar]
- 15.Olmedo JM, Warschaw KE, Schmitt JM, Swanson DL. Correlation of thickness of basal cell carcinoma by optical coherence tomography in vivo and routine histologic findings: a pilot study. Dermatol Surg 2007;33:421–5; discussion 5–6. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan A, Xu T, Hutfless S, Park A, Stasko T, Vidimos AT et al. Outlier Practice Patterns in Mohs Micrographic Surgery: Defining the Problem and a Proposed Solution. JAMA Dermatol 2017;153:565–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Navarrete-Dechent C, Rajadhyaksha M, Nehal KS. Can optical coherence tomography improve the management of basal cell carcinoma? Br J Dermatol 2018;Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 18.Rossi AM, Navarrete-Dechent C, Nehal KS. Beyond skin deep: taking bedside dermatology to the next level with noninvasive technologies. Br J Dermatol 2018;178:994–6. [DOI] [PubMed] [Google Scholar]
- 19.Marino ML, Rogers T, Sierra Gil H, Rajadhyaksha M, Cordova MA, Marghoob AA. Improving lesion localization when imaging with handheld reflectance confocal microscope. Skin Res Technol 2016;22:519–20. [DOI] [PubMed] [Google Scholar]
- 20.Yelamos O, Cordova M, Blank N, Kose K, Dusza SW, Lee E et al. Correlation of Handheld Reflectance Confocal Microscopy With Radial Video Mosaicing for Margin Mapping of Lentigo Maligna and Lentigo Maligna Melanoma. JAMA Dermatol 2017;153:1278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iftimia N, Yelamos O, Chen CJ, Maguluri G, Cordova MA, Sahu A et al. Handheld optical coherence tomography-reflectance confocal microscopy probe for detection of basal cell carcinoma and delineation of margins. J Biomed Opt 2017;22:76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Longo C, Lallas A, Kyrgidis A, Rabinovitz H, Moscarella E, Ciardo S et al. Classifying distinct basal cell carcinoma subtype by means of dermatoscopy and reflectance confocal microscopy. J Am Acad Dermatol 2014;71:716–24 e1. [DOI] [PubMed] [Google Scholar]
- 23.Peppelman M, Wolberink EA, Blokx WA, van de Kerkhof PC, van Erp PE, Gerritsen MJ. In vivo diagnosis of basal cell carcinoma subtype by reflectance confocal microscopy. Dermatology 2013;227:255–62. [DOI] [PubMed] [Google Scholar]
- 24.Sahu A, Yelamos O, Iftimia N, Cordova M, Alessi-Fox C, Gill M et al. Evaluation of a Combined Reflectance Confocal Microscopy-Optical Coherence Tomography Device for Detection and Depth Assessment of Basal Cell Carcinoma. JAMA Dermatol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mosterd K, Thissen MR, van Marion AM, Nelemans PJ, Lohman BG, Steijlen PM et al. Correlation between histologic findings on punch biopsy specimens and subsequent excision specimens in recurrent basal cell carcinoma. J Am Acad Dermatol 2011;64:323–7. [DOI] [PubMed] [Google Scholar]
- 26.Kamyab-Hesari K, Seirafi H, Naraghi ZS, Shahshahani MM, Rahbar Z, Damavandi MR et al. Diagnostic accuracy of punch biopsy in subtyping basal cell carcinoma. J Eur Acad Dermatol Venereol 2014;28:250–3. [DOI] [PubMed] [Google Scholar]
- 27.Wolberink EA, Pasch MC, Zeiler M, van Erp PE, Gerritsen MJ. High discordance between punch biopsy and excision in establishing basal cell carcinoma subtype: analysis of 500 cases. J Eur Acad Dermatol Venereol 2013;27:985–9. [DOI] [PubMed] [Google Scholar]
- 28.Izikson L, Seyler M, Zeitouni NC. Prevalence of underdiagnosed aggressive non-melanoma skin cancers treated with Mohs micrographic surgery: analysis of 513 cases. Dermatol Surg 2010;36:1769–72. [DOI] [PubMed] [Google Scholar]
- 29.Stiegel E, Lam C, Schowalter M, Somani AK, Lucas J, Poblete-Lopez C. Correlation Between Original Biopsy Pathology and Mohs Intraoperative Pathology. Dermatol Surg 2018;44:193–7. [DOI] [PubMed] [Google Scholar]
- 30.Holmkvist KA, Rogers GS, Dahl PR. Incidence of residual basal cell carcinoma in patients who appear tumor free after biopsy. J Am Acad Dermatol 1999;41:600–5. [PubMed] [Google Scholar]
- 31.Kyllo RL, Staser KW, Rosman I, Council ML, Hurst EA. Histopathologic upgrading of nonmelanoma skin cancer at the time of Mohs micrographic surgery: A prospective review. J Am Acad Dermatol 2019. [DOI] [PubMed] [Google Scholar]


