Abstract
Background
The present study was designed to study the ability of preoperative serum concentrations of the tumor-associated biomarkers carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9) and adjusted CA19-9 to assess the resectability of advanced gallbladder cancer (GBC).
Material/Methods
This retrospective study included patients with potentially resectable stage II–IV (AJCC 8th) GBC examined at our institution between January 2012 and December 2016. Receiver operating characteristic (ROC) curve analysis was used to determine the predictive value and optimal cut-off point of tumor-associated biomarkers for curative resection.
Results
Pathological examination of the 309 patients included in this study found that 169 (54.7%) underwent R0 (curative) resection, whereas 121 (39.2%) underwent R1/2 (non-curative) resection, and 19 (6.1%) were unresectable. The mean serum concentrations of CEA, CA19-9 and adjusted CA19-9 were significantly lower in patients who underwent R0 resection than in the other groups. ROC curve analysis showed that adjusted CA19-9 concentration was better able to predict resectability (area under the curve, 0.774; 95% confidence interval, 0.722–0.826; P<0.001) than total bilirubin, CEA, and CA19-9 concentrations. The optimal cut-off for adjusted CA19-9 concentration was 47.63 U/mL, which had a sensitivity of 69.82%, a specificity of 75%, a positive predictive value of 77.12% and a negative predictive value of 67.31%.
Conclusions
Adjusted CA19-9 concentration is an easily calculated parameter superior to CA19-9 and CEA concentrations in predicting the resectability of advanced gallbladder cancer.
MeSH Keywords: Biological Markers, CA-19-9 Antigen, Carcinoembryonic Antigen, Gallbladder Neoplasms
Background
Gallbladder cancer (GBC) is a relatively rare but highly lethal tumor of the biliary tract system, accounting for about 165000 cancer-related deaths per year worldwide [1,2]. The overall prognosis of patients with GBC is poor, with a 5-year mean survival rate of 5% to 10% [3]. Despite most patients being diagnosed at an advanced stage, because of the non-obvious symptoms and highly invasive nature of GBC, patients with advanced stage may benefit from aggressive radical resection, which may be the only potentially curative treatment [4,5]. Eligibility for radical resection is dependent on tumor stage and tumor biology [2,6], making preoperative assessment of the feasibility of curative resection for patients with advanced stage GBC essential. Moreover, accurate evaluation can avoid unnecessary surgery in patients ineligible for resection and reduce mortality rates.
Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA19-9) are cancer-associated antigens, measurements of which have been widely used to determine the diagnosis and prognosis of patients with tumors of the digestive system, including pancreatic [7], bile duct [8], colorectal [9], and gastric [10] cancer. Moreover, measurements of CEA and CA19-9 concentrations can be used to evaluate the resectability of various cancers [11,12], including GBCs [13]. Radical resection is possible in most patients with early stage GBC (stage 0–I, AJCC 8th), with a satisfactory prognosis [14,15]. However, the radical resection rate of advanced GBC (stages II–IV) is much lower and variable. The present study therefore evaluated the ability of serum concentrations of CEA, CA19-9 and CA19-9 relative to serum total bilirubin concentration (adjusted CA19-9) to determine the resectability of advanced (stages II–IV) GBC.
Material and Methods
This retrospective, single-institution study analyzed patients who underwent surgery for potentially resectable stage II–IV GBC between January 2012 and December 2016 at the Third Affiliated Hospital of Second Military Medical University. The study protocol was approved by the Ethics Committee of our institution. The diagnosis of GBC was confirmed by pathological examination of a resected specimen or intraoperative biopsy. Tumor stage was intraoperatively classified based on the eighth edition of the American Joint Committee on Cancer (AJCC/TNM) staging system. All tumor resections were performed by senior surgeons. R0 resection was defined as resection with microscopically determined negative resection margins, R1 as microscopically positive resection margins and R2 as macroscopically minimal positive resection margins. Tumors were determined to be unresectable by intraoperative exploration. Patients with incomplete clinical data, those who received preoperative radiotherapy or chemotherapy, and patients with other tumors were excluded.
Demographic characteristics, including age and sex, were recorded, as were concentrations of tumor-associated biomarkers and total bilirubin measured within one week before surgery. Because serum CA19-9 concentrations are affected by tumor-associated elevations in total bilirubin concentrations, CA19-9 may not reflect the actual tumor burden [16,17]. Therefore, CA19-9 concentrations were adjusted for total bilirubin concentrations. The upper limits of normal were 39 U/ml for CA19-9, 10 μg/L for CEA and 23 μmol/L for total bilirubin. If both CA19-9 and total bilirubin concentrations were above the upper limits of normal, adjusted CA19-9 concentration was calculated as the serum CA19-9 concentration divided by the fold elevation in total bilirubin concentration. If total bilirubin concentrations were within the normal range, the actual CA19-9 concentration was used.
Statistical analysis
Continuous data were reported as the mean±standard deviation (SD) and compared by one-way ANOVA or the Kruskal-Wallis test. Categorical data were compared by Pearson’s chi-squared test or Fisher’s exact test. The optimal cut-offs for tumor-associated biomarkers were determined by receiver operating characteristic (ROC) curve analysis and determination of the area under the ROC curve (AUC). The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) for resectability were calculated for each cutoff value of each biomarker. Survival was analyzed by the Kaplan-Meier method and compared by log-rank tests. All statistical analyses were performed using SPSS 22.0 software, with P<0.05 considered statistically significant.
Results
During the study period from January 2012 to December 2016, 309 patients at our institution underwent surgery for potentially resectable of stage II–IV GBC, Of these 169 (54.7%) underwent R0 resection, as determined by postoperative pathological examination, 121 (39.2%) underwent R1/2 resection and 19 (6.1%) were unresectable. The demographic and clinical characteristics of these patients are summarized in Table 1. Patients were of mean age 60.0 years (range, 24 to 84 years), and 178 (57.6%) were women. Tumors were located in the body and fundus of the gallbladder in 169 (54.7%) patients, and in the neck and duct of the gallbladder in 140 (45.3%). Tumors in 238 (77.0%) patients were histologically classified as well and moderately differentiated, whereas tumors in 71 (23.0%) patients were poorly differentiated. Sex distribution, tumor site, and tumor differentiation did not differ significantly in the three groups of patients with R0, R1/2, and unresectable tumors. In contrast, AJCC stage correlated significantly with tumor resectability (P<0.001). Patients with unresectable tumors were younger than those who underwent R0 and R1/2 resection.
Table 1.
Baseline characteristics of patients with gallbladder cancer.
| Total | R0 | R1/R2 | Unresected | P value | |
|---|---|---|---|---|---|
| No. patients | 309 | 169 | 121 | 19 | |
| Age. Yr, mean±SD | 59.99±8.66 | 59.95±7.61 | 60.97±9.86 | 54.12±7.24 | 0.006 |
| Gender (%) | 0.855 | ||||
| Male | 131 (42.4) | 74 (43.8) | 49 (40.5) | 8 (42.1) | |
| Female | 178 (57.6) | 95 (56.2) | 72 (59.5) | 11 (57.9) | |
| Tumor location (%) | 0.526 | ||||
| Body and fundus | 169 (54.7) | 88 (52.1) | 69 (57.0) | 12 (63.2) | |
| Neck and duct | 140 (45.3) | 81 (47.9) | 52 (43.0) | 7 (36.8) | |
| Tumor differentiation (%) | 0.439 | ||||
| Well and moderate | 238 (77.0) | 133 (78.7) | 89 (73.6) | 16 (84.2) | |
| Poor | 71 (23.0) | 36 (21.3) | 32 (26.4) | 3 (15.8) | |
| AJCC staging, n | 0.000 | ||||
| II | 25 | 25 | 0 | 0 | |
| IIIA | 60 | 56 | 4 | 0 | |
| IIIB | 56 | 46 | 10 | 0 | |
| IVA | 70 | 42 | 28 | 0 | |
| IVB | 98 | 0 | 79 | 19 |
AJCC – American Joint Committee on Cancer; SD – standard deviation.
The mean concentrations of CA19-9, CEA, and adjusted CA19-9 were significantly lower in patients who underwent R0 resection than in those who underwent R1/2 resection and those with unresectable tumors (Table 2). These three concentrations were higher in patients with elevated (>23 μmol/L) than with lower (≤23 μmol/L) serum total bilirubin concentrations and correlated significantly with AJCC stages. Taken together these findings indicate that the serum concentrations of these three parameters correlated with the severity of GBC.
Table 2.
Relationships of preoperative CEA, CA19-9, and adjusted CA19-9 concentrations with resectability, total bilirubin concentration and AJCC staging.
| Variable | No. | CEA | CA19-9 | Adjusted CA19-9 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Mean±SD | P value | Median | Mean±SD | P value | Median | Mean±SD | P value | ||
| Resectability | ||||||||||
| R0 | 169 | 2.8 | 11.55±33.29 | 0.000 | 41 | 175.18±294.60 | 0.000 | 26.97 | 43.93±58.34 | 0.000 |
| R1/R2 | 121 | 5.0 | 27.17±71.24 | 264.2 | 417.81±402.80 | 78.90 | 115.11±156.76 | |||
| Unresected | 19 | 5.3 | 57.51±95.76 | 149 | 412.73±436.10 | 97.50 | 158.53±219.00 | |||
| Total bilirubin | ||||||||||
| ≤23 μmol/L | 165 | 2.5 | 17.85±59.44 | 0.000 | 30 | 72.51±159.39 | 0.000 | 30 | 72.51±159.39 | 0.000 |
| >23 μmol/L | 144 | 5.1 | 23.52±54.41 | 403.1 | 528.04±390.75 | 68.16 | 86.12±69.41 | |||
| AJCC staging | ||||||||||
| II | 25 | 1.9 | 2.56±1.87 | 0.000 | 17 | 53.21±117.68 | 0.000 | 17 | 25.10±25.91 | 0.000 |
| IIIA | 60 | 2.75 | 8.17±24.49 | 46.3 | 195.10±321.34 | 27.40 | 42.72±42.55 | |||
| IIIB | 56 | 4 | 13.63±29.87 | 71.5 | 264.86±361.82 | 41.68 | 57.38±80.93 | |||
| IVA | 70 | 4.2 | 17.39±44.19 | 120.35 | 288.79±345.74 | 53.49 | 76.01±66.50 | |||
| IVB | 98 | 5.1 | 38.75±88.92 | 159.50 | 407.32±415.73 | 77.26 | 128.98±194.19 | |||
AJCC – American Joint Committee on Cancer; SD – standard deviation; CA19-9 – carbohydrate antigen 19-9; CEA – carcinoembryonic antigen.
ROC curves were constructed and AUC analysis performed to evaluate the predictive value of each parameter. Adjusted CA19-9 had greater predictive value (AUC, 0.774; 95% confidence interval [CI], 0.722–0.826; P<0.001) than total bilirubin (AUC, 0.572; 95% CI, 0.507–0.636; P=0.03), CEA (AUC, 0.648; 95%CI, 0.586–0.71; P <0.001), and CA19-9 (AUC, 0.728; 95%CI, 0.672–0.784; P<0.001) concentrations (Figure 1). The optimal cut-off points to assess resectability were 4.05 ng/mL (sensitivity, 66.9%; specificity, 60.0%) for CEA, 78.5 U/mL (sensitivity, 64.5%; specificity, 70.7%) for CA19-9 and 47.63 U/mL (sensitivity, 69.82%; specificity, 75%) for adjusted CA19-9 (Table 3). At a threshold of 78.5 U/mL, serum CA19-9 had a 72.67% positive predictive value (PPV) and a 62.26% negative predictive value (NPV) for predicting respectability, whereas an adjusted CA19-9 concentration <47.63 U/mL had a PPV of 77.12% and an NPV of 67.31%. These results indicated that adjusted CA19-9 was better than CA19-9 at predicting the resectability of GBC.
Figure 1.
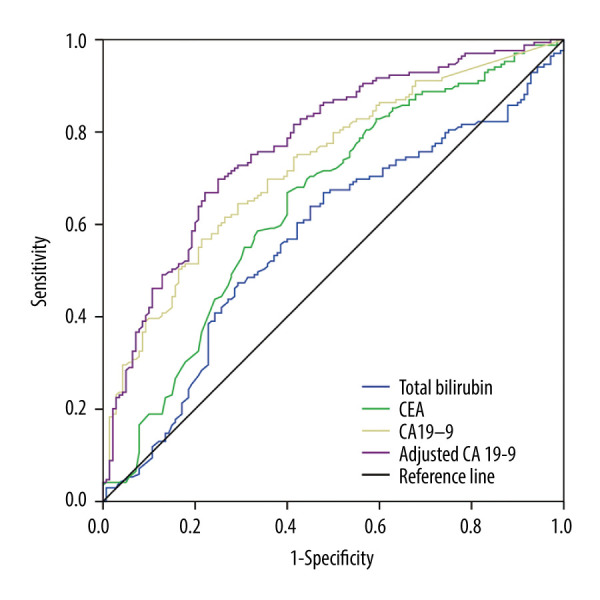
ROC curves for preoperative serum CEA, total bilirubin, CA19-9, and adjusted CA19-9 levels in the determination of the resectability of gallbladder cancer.
Table 3.
The predictive accuracy of preoperative serum CEA, CA19-9, and adjusted CA19-9 concentrations for gallbladder cancer respectability.
| Resectability (R0 resection) | |||
|---|---|---|---|
| CEA <4.05 ng/mL | CA19-9 <78.5 U/mL | Adjusted CA19-9 <47.63 U/mL | |
| No. patients | 169 | 150 | 153 |
| Sensitivity (%) | 66.90 | 64.50 | 69.82 |
| Specificity (%) | 60.00 | 70.70 | 75.00 |
| PPV (%) | 66.90 | 72.67 | 77.12 |
| NPV (%) | 60.00 | 62.26 | 67.31 |
CA19-9 – carbohydrate antigen 19-9; CEA – carcinoembryonic antigen; PPV – positive predictive value; NPV – negative predictive value.
The 309 patients had a median survival time of 12 months and a 5-year survival rate of 5.83% (Figure 2). The 1-, 2-, and 5-year cumulative survival rates in patients who underwent R0 resection for GBC were 73.96%, 31.36%, and 9.47%, respectively, significantly better than for patients who underwent R1/2 resection. None of the patients with unresectable GBC survived for more than one year.
Figure 2.

Kaplan-Meier survival curves for patients who underwent R0 and R1/2 resection and those with unresectable tumors.
Discussion
GBC is the most common cancer of the biliary tract and the sixth most common gastrointestinal cancer [18]. Due to their atypical symptoms, frequent local invasion, early and frequent metastases, most tumors are diagnosed at advanced stage, with only about 1 in 5 GBCs diagnosed in early stages [19]. Despite improvements in surgical methods, only 25% of patients are eligible for potentially curative surgery [20]. The radical resection rate in patients with for advanced GBC, remains low and the proportion of fatal postoperative complications remains high. Moreover, unnecessary surgery for advanced GBC may delay appropriate systemic chemotherapy and increase health-care costs. An reliable and accurate preoperative assessment of GBC resectability is therefore of significant clinical importance.
Currently, the resectability of GBC is evaluated primarily by assessing clinical manifestations and performing radiological tests. Endoscopic ultrasonography (EUS) was regarded as the most sensitive diagnostic method for GB polypoid lesions, with a diagnostic sensitivity of 86% [21]. Multi-slice CT (MSCT) and MRI can provide more information about depth of tumor invasion, local extension and lymph node metastasis, but these methods frequently miss small occult hepatic and peritoneal metastases, resulting in unnecessary surgical exploration [22–24]. Although PET can be used for early detection of GBCs, as well as small (<1 cm) metastatic lymph nodes and lesions [25,26], its application is limited by the need for experienced radiologists and high costs. Staging laparoscopy has been recommended before laparotomy, as up to 37% of GBCs were determined to be unresectable by simple laparoscopy [27]. However, a study of 46 patients with potentially resectable GBC found that tumors were unresectable in 10 patients, two found by laparoscopy and eight at the time of laparotomy [28]. Simple and cost-effective measurements of tumor markers in serum may supplement radiological tests and staging laparoscopy to predict the resectability of GBCs.
CEA and CA19-9 are tumor-associated biomarkers in serum widely used to diagnose gastrointestinal cancers, including GBCs and to predict patient prognosis [29,30], indicating their importance in evaluating GBC resectability GBC. Serum CEA and CA19-9 concentrations may also predict the resectability of pancreatic cancers [31–33], whereas preoperative measurements of serum CEA, CA125, and CA19-9 concentrations can assist in evluating the resectability of cholangiocarcinomas, a type of biliary tract cancer [12]. Moreover, a study of 292 patients with stage I–IV GBC reported that preoperative CA19-9 could predict tumor resectability [13], although that study did not consider the effect of tumor-associated elevation of total bilirubin concentration on serum levels of CEA and CA19-9.. The radical resection rate of stage 0-I GBC is high, with these patients having a 5-year survival rate over 60% [15,19].
The present study assessed the ability of CEA, CA19-9 and adjusted CA19-9 concentrations to predict the resectability of advanced stage GBC. An AUC between 0.5 and 0.7 is regarded as having low diagnostic value, whereas an AUC between 0.7 and 0.9 has moderate diagnostic value. In assessing the ability of these potential biomarkers to predict the resectability of GBC, we found that the AUCs were 0.648 for CEA, 0.728 for CA19-9 and 0.774 for adjusted CA19-9, making the latter parameter more diagnostic of GBC respectability than CEA and C19-9 concentrations.
Because of the high mortality rate and limited role of palliative resection in patients with advanced GBC, resectability in the present study was defined as R0 resection. Kaplan-Meier survival analysis showed that survival rates were significantly higher in patients who underwent R0 resection than in those who underwent R1/2 resection and patients with unresectable tumors [13].
In general, it is not accurate and reasonable to assess the resectability of GBC only by measuring serum biomarkers. These markers, however, could supplement the clinical features, imaging findings, and other correlated factors, with comprehensive analysis providing better guidelines for treatment.
Conclusions
Adjusted CA19-9 concentration, which is easily calculated, is better than CA19-9 and CEA concentrations in predicting the resectability of advanced gallbladder cancer. Aberrantly high levels of adjusted CA19-9 and CA19-9 may indicate unresectable GBC. Prospective multicenter studies with larger patient cohorts are needed to assess this issue.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Puhalla H, Wild T, Bareck E, et al. Long-term follow-up of surgically treated gallbladder cancer patients. Eur J Surg Oncol. 2002;28:857–63. doi: 10.1053/ejso.2002.1301. [DOI] [PubMed] [Google Scholar]
- 3.Hueman MT, Vollmer CM, Jr, Pawlik TM. Evolving treatment strategies for gallbladder cancer. Ann Surg Oncol. 2009;16:2101–15. doi: 10.1245/s10434-009-0538-x. [DOI] [PubMed] [Google Scholar]
- 4.Kohya N, Miyazaki K. Hepatectomy of segment 4a and 5 combined with extra-hepatic bile duct resection for T2 and T3 gallbladder carcinoma. J Surg Oncol. 2008;97:498–502. doi: 10.1002/jso.20982. [DOI] [PubMed] [Google Scholar]
- 5.Shirai Y, Wakai T, Sakata J, Hatakeyama K. Regional lymphadenectomy for gallbladder cancer: Rational extent, technical details, and patient outcomes. World J Gastroenterol. 2012;18:2775–83. doi: 10.3748/wjg.v18.i22.2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ito H, Matros E, Brooks DC, et al. Treatment outcomes associated with surgery for gallbladder cancer: A 20-year experience. J Gastrointest Surg. 2004;8:183–90. doi: 10.1016/j.gassur.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Del Favero G, Fabris C, Plebani M, et al. CA 19-9 and carcinoembryonic antigen in pancreatic cancer diagnosis. Cancer. 1986;57:1576–79. doi: 10.1002/1097-0142(19860415)57:8<1576::aid-cncr2820570823>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 8.Grunnet M, Mau-Sorensen M. Serum tumor markers in bile duct cancer – a review. Biomarkers. 2014;19:437–43. doi: 10.3109/1354750X.2014.923048. [DOI] [PubMed] [Google Scholar]
- 9.Gao Y, Wang J, Zhou Y, et al. Evaluation of serum CEA, CA19-9, CA72-4, CA125 and ferritin as diagnostic markers and factors of clinical parameters for colorectal cancer. Sci Rep. 2018;8:2732. doi: 10.1038/s41598-018-21048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ning S, Wei W, Li J, et al. Clinical significance and diagnostic capacity of serum TK1, CEA, CA 19-9 and CA 72-4 levels in gastric and colorectal cancer patients. J Cancer. 2018;9:494–501. doi: 10.7150/jca.21562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo G, Xiao Z, Long J, et al. CA125 is superior to CA19-9 in predicting the resectability of pancreatic cancer. J Gastrointest Surg. 2013;17:2092–98. doi: 10.1007/s11605-013-2389-9. [DOI] [PubMed] [Google Scholar]
- 12.Fang T, Wang H, Wang Y, et al. Clinical significance of preoperative serum CEA, CA125, and CA19-9 levels in predicting the resectability of cholangiocarcinoma. Dis Markers. 2019;2019 doi: 10.1155/2019/6016931. 6016931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu F, Wang JK, Ma WJ, et al. Clinical value of preoperative CA19-9 levels in evaluating resectability of gallbladder carcinoma. ANZ J Surg. 2019;89:E76–80. doi: 10.1111/ans.14893. [DOI] [PubMed] [Google Scholar]
- 14.Lee SE, Jang JY, Lim CS, et al. Systematic review on the surgical treatment for T1 gallbladder cancer. World J Gastroenterol. 2011;17:174–80. doi: 10.3748/wjg.v17.i2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hickman L, Contreras C. Gallbladder cancer: Diagnosis, surgical management, and adjuvant therapies. Surg Clin North Am. 2019;99:337–55. doi: 10.1016/j.suc.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Marrelli D, Caruso S, Pedrazzani C, et al. CA19-9 serum levels in obstructive jaundice: Clinical value in benign and malignant conditions. Am J Surg. 2009;198:333–39. doi: 10.1016/j.amjsurg.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 17.Mann DV, Edwards R, Ho S, et al. Elevated tumour marker CA19-9: Clinical interpretation and influence of obstructive jaundice. Eur J Surg Oncol. 2000;26:474–79. doi: 10.1053/ejso.1999.0925. [DOI] [PubMed] [Google Scholar]
- 18.Wistuba II, Gazdar AF. Gallbladder cancer: Lessons from a rare tumour. Nat Rev Cancer. 2004;4:695–706. doi: 10.1038/nrc1429. [DOI] [PubMed] [Google Scholar]
- 19.Rawla P, Sunkara T, Thandra KC, Barsouk A. Epidemiology of gallbladder cancer. Clin Exp Hepatol. 2019;5:93–102. doi: 10.5114/ceh.2019.85166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Aloia TA, Jarufe N, Javle M, et al. Gallbladder cancer: Expert consensus statement. HPB (Oxford) 2015;17:681–90. doi: 10.1111/hpb.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang JY, Kim SW, Lee SE, et al. Differential diagnostic and staging accuracies of high resolution ultrasonography, endoscopic ultrasonography, and multidetector computed tomography for gallbladder polypoid lesions and gallbladder cancer. Ann Surg. 2009;250:943–49. doi: 10.1097/SLA.0b013e3181b5d5fc. [DOI] [PubMed] [Google Scholar]
- 22.Kim SJ, Lee JM, Lee JY, et al. Accuracy of preoperative T-staging of gallbladder carcinoma using MDCT. Am J Roentgenol. 2008;190:74–80. doi: 10.2214/AJR.07.2348. [DOI] [PubMed] [Google Scholar]
- 23.Oikarinen H. Diagnostic imaging of carcinomas of the gallbladder and the bile ducts. Acta Radiol. 2006;47:345–58. doi: 10.1080/02841850600580317. [DOI] [PubMed] [Google Scholar]
- 24.Kim SJ, Lee JM, Lee ES, et al. Preoperative staging of gallbladder carcinoma using biliary MR imaging. J Magn Reson Imaging. 2015;41:314–21. doi: 10.1002/jmri.24537. [DOI] [PubMed] [Google Scholar]
- 25.Ramos-Font C, Gomez-Rio M, Rodriguez-Fernandez A, et al. Ability of FDG-PET/CT in the detection of gallbladder cancer. J Surg Oncol. 2014;109:218–24. doi: 10.1002/jso.23476. [DOI] [PubMed] [Google Scholar]
- 26.Chun YJ, Jeung HC, Park HS, et al. Significance of metabolic tumor volume and total lesion glycolysis measured using 18F-FDG PET/CT in locally advanced and metastatic gallbladder carcinoma. Yonsei Med J. 2019;60:604–10. doi: 10.3349/ymj.2019.60.7.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goere D, Wagholikar GD, Pessaux P, et al. Utility of staging laparoscopy in subsets of biliary cancers: laparoscopy is a powerful diagnostic tool in patients with intrahepatic and gallbladder carcinoma. Surg Endosc. 2006;20:721–25. doi: 10.1007/s00464-005-0583-x. [DOI] [PubMed] [Google Scholar]
- 28.Butte JM, Gonen M, Allen PJ, et al. The role of laparoscopic staging in patients with incidental gallbladder cancer. HPB (Oxford) 2011;13:463–72. doi: 10.1111/j.1477-2574.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang YF, Feng FL, Zhao XH, et al. Combined detection tumor markers for diagnosis and prognosis of gallbladder cancer. World J Gastroenterol. 2014;20:4085–92. doi: 10.3748/wjg.v20.i14.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wen Z, Si A, Yang J, et al. Elevation of CA19-9 and CEA is associated with a poor prognosis in patients with resectable gallbladder carcinoma. HPB (Oxford) 2017;19:951–56. doi: 10.1016/j.hpb.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 31.Kim YC, Kim HJ, Park JH, et al. Can preoperative CA19-9 and CEA levels predict the resectability of patients with pancreatic adenocarcinoma? J Gastroenterol Hepatol. 2009;24:1869–75. doi: 10.1111/j.1440-1746.2009.05935.x. [DOI] [PubMed] [Google Scholar]
- 32.Fujioka S, Misawa T, Okamoto T, et al. Preoperative serum carcinoembryonic antigen and carbohydrate antigen 19-9 levels for the evaluation of curability and resectability in patients with pancreatic adenocarcinoma. J Hepatobiliary Pancreat Surg. 2007;14:539–44. doi: 10.1007/s00534-006-1184-3. [DOI] [PubMed] [Google Scholar]
- 33.Schlieman MG, Ho HS, Bold RJ. Utility of tumor markers in determining resectability of pancreatic cancer. Arch Surg. 2003;138:951–55. doi: 10.1001/archsurg.138.9.951. discussion 955–56. [DOI] [PubMed] [Google Scholar]


