Abstract
Adolescence is characterized by changes in behavior, such as increases in sensation seeking and risk taking, and increased vulnerability to developing a range of psychiatric disorders, including substance abuse disorders (SUD) and mood disorders. The mesolimbic dopamine system plays an essential role in mediating these behaviors and disorders. Therefore, it is imperative to understand how the dopamine system and its regulation are changing during this period of development. Here, we used ex vivo fast scan cyclic voltammetry to compare stimulated dopamine release and its local circuitry regulation between early adolescent and adult male and female Sprague-Dawley rats. We found that, compared to adults, adolescent males have decreased stimulated dopamine release in the NAc core, while adolescent females have increased dopamine release in the NAc shell, NAc core, and DMS. We also found sex- and region-specific differences in other dopamine dynamics, including maximal dopamine uptake (Vmax), release across a range of stimulation frequencies, and autoreceptor regulation of dopamine release. Better understanding how the dopamine system develops during adolescence will be imperative for understanding what mediates adolescent vulnerability to developing psychiatric disorders and how disruptions during this period of reorganization could alter behaviors and vulnerability into adulthood.
Keywords: Adolescence, Dopamine, Striatum, Voltammetry
1. Introduction
Adolescence is a developmental time-period characterized by increases in reward sensitivity, sensation seeking, and peer-focused social behavior (Spear, 2000, 2011, 2013; Nelson et al., 2005). These changes in behavior are proposed to be biologically advantageous, supporting the development of independence necessary for the movement from childhood to adulthood (Spear, 2000). However, this is also a time of increased vulnerability to both the consequences of increased risk-taking and the development of a range of psychiatric disorders, including depression, schizophrenia, and substance use disorders (SUDs) (Casey et al., 2008; Fareri et al., 2008; Steinberg, 2008).
Developmental reorganization of the mesolimbic dopamine system is hypothesized to drive altered reward-related decision making, including increasing risk-taking behaviors, and to play a role in the increased vulnerability to a variety of psychiatric disorders that is seen during adolescence (Spear, 2000, 2013; Nelson et al., 2005; Wahlstrom et al., 2010a,b). A number of changes occur to the mesolimbic dopamine system during adolescence (see Galvan, 2010; Wahlstrom et al., 2010a,b; Padmanabhan & Luna, 2014). For example, D1 and D2 receptor density peaks in the striatum during adolescence (Teicher et al., 1995; Tarazi et al., 1999; Philpot et al., 2009), as does dopamine neuron firing rates (McCutcheon et al., 2012).
Despite the importance of changes to the dopamine system on adolescent behaviors and vulnerabilities, changes in striatal dopamine levels and their regulation are not well understood. Previous studies have used microdialysis to compare extracellular dopamine levels in the ventral striatum of adolescents and adults, but these studies have found conflicting results showing both increased and decreased levels of dopamine in adolescent animals (Badanich et al., 2006; Cao et al., 2007; Philpot et al., 2009). Additionally, one study examined rapid dopamine release and uptake in the striatum using in vivo fast scan cyclic voltammetry (FSCV) in anesthetized rats and found decreased dopamine release in the striatum of adolescents (Stamford, 1989). However, no one has yet compared rapid dopamine release and its modulation in adolescent and adult rats throughout the striatum. In addition, little research has examined whether the dopamine system develops differently in male and females. Ex vivo FSCV provides unique advantages for studying local circuitry modulation of dopamine release. It has high spatial and temporal resolution and can be used to examine uptake kinetics and the role of transporters and autoreceptors in mediating dopamine release (Ferris et al., 2013). Additionally, electrical stimulation can model both tonic (~4–5 Hz) and phasic (2–5 spikes at 20–100 Hz) neuronal firing, which may be particularly important for the salience of rewarding information in adolescence (Luciana et al., 2012).
Here, we used ex vivo FSCV to compare sub-second dopamine release and uptake in adult and early adolescent male and female rats across several regions of the striatum that may play a role in mediating behaviors and vulnerability unique to adolescence. In particular, we focused on the nucleus accumbens shell (NAc shell) and core (NAc core), which are important in incentive motivated behavior and reward-related learning (see Di Chiara, 2002; Saddoris et al., 2013). Additionally, the NAc core plays a role in goal-directed decision making (Saddoris et al., 2013). We also examined the dorsomedial striatum (DMS) and the dorsolateral striatum (DLS), which are necessary for flexible, goal-directed actions and habit formation, respectively (see Balleine & O’Doherty, 2010). Various stimulation parameters were used to model a range of dopamine neuron firing patterns. We then used selective D2/D3 agonists and antagonists to examine the potential role of autoreceptors in mediating developmental changes in dopamine release.
2. Materials and Methods
2.1. Animals
Adolescent (P30-37) and adult (P70-90) male and female Sprague-Dawley rats (Envigo, Dublin, VA) were pair housed and maintained on a 12:12 h reverse light/dark cycle (4:00 a.m. lights off; 4:00 p.m. lights on) with food and water available ad libitum. All animals were maintained according to the National Institutes of Health guidelines in Association for Assessment and Accreditation of Laboratory Animal Care accredited facilities. All experimental protocols were approved by the Institutional Animal Care and Use Committee at Wake Forest School of Medicine.
2.2. Slice preparation
Rats were anesthetized with isoflurane and euthanized (9:00 a.m.) by decapitation in a ventilated area. Brains were rapidly removed and transferred into pre-oxygenated (95% O2 / 5% CO2) artificial cerebral spinal fluid (aCSF) containing (in mM): NaCl (126), KCl (2.5), monobasic NaH2PO4 (1.2), CaCl2 (2.4), MgCl2 (1.2), NaHCO3 (25), dextrose (D-glucose) (11), and L-ascorbic acid (0.4). Tissue was sectioned into 400 μm-thick coronal slices on a compresstome® VF-300 vibrating microtome (Precisionary Instruments Inc., San Jose, CA). Brain slices were transferred to testing chambers containing oxygenated aCSF (32 °C) flowing at 1 mL/min.
2.3. Ex vivo voltammetry
Ex vivo FSCV was used to characterize presynaptic dopamine release in the NAc shell (AP +1, ML ±1.4, DV −8.2), NAc core (AP +1.2, ML ±2.2, DV −7), DMS (AP +1, ML ±1.3, DV −5), and DLS (AP +1, ML ±3.9, DV −4)(region coordinates are approximate averages of electrode placement; AP of electrode placement varies by ±0.2 mm based on slice location)(Figure 1A,2A). A carbon-fiber recording microelectrode (100–200 μM length, 7 μMdiameter) was placed 100–150 μM from a bipolar stimulating electrode. Extracellular dopamine was recorded by applying a triangular waveform from −0.4 to 1.2 V and back to −0.4 (Ag vs AgCl) at a scan rate of 400 V/s.
Figure 1. Male adolescents have decreased dopamine release in the nucleus accumbens core.
(A) Ex vivo fast scan cyclic voltammetry was used to examine dopamine release throughout the striatum in adult and adolescent male rats. Representative traces of stimulated single-pulse dopamine release in the nucleus accumbens shell (NAc shell), nucleus accumbens core (NAc core), dorsomedial striatum (DMS), and dorsolateral striatum (DLS) are shown. (B) Dopamine release in the NAc shell was not different in adult and adolescent animals (adults: n=6; adolescents: n=8). (C) However, dopamine release was significantly lower in adolescent than adult rats in the NAc core across a range of stimulation parameters that model tonic and phasic firing of dopamine neurons (adults: n=6; adolescents: n=7). (D) Developmental stage did not impact levels of dopamine release in the DMS (adults: n=8; adolescents: n=5) or (E) DLS (adults: n=9; adolescents: n=9). Insets: Individual data points for single-pulse dopamine release. In line graphs, symbols represent means ± SEMs. In bar graphs, bars represent means and symbols represent individual data points. *p < 0.05.
Figure 2. Female adolescents have increased dopamine release in the nucleus accumbens shell and core and in the dorsomedial striatum a.
(A) Stimulated dopamine release in female adult and adolescent rats was compared throughout the striatum using ex vivo fast scan cyclic voltammetry. Representative traces of stimulated single-pulse dopamine release in the nucleus accumbens shell (NAc shell), nucleus accumbens core (NAc core), dorsomedial striatum (DMS), and dorsolateral striatum (DLS) are shown. (B) Dopamine release in the NAc shell (adults: n=8; adolescents: n=6), (C) NAc core (adults: n=6; adolescents: n=6), and (D) DMS (adults: n=7; adolescents: n=5) was significantly higher in adolescent female rats across a range of stimulation parameters that model tonic and phasic firing of dopamine neurons. (E) Developmental stage did not impact levels of dopamine release in the DLS (adults: n=5; adolescents: n=5). Insets: Individual data points for single-pulse dopamine release. In line graphs, symbols represent means ± SEMs. In bar graphs, bars represent means and symbols represent individual data points. *p < 0.05.
Dopamine release was initially evoked by a single electrical pulse (750 μA, 2 msec, monophasic) applied to the tissue every 3 minutes. Once the extracellular dopamine response was stable (3 collections within <10% variability), five-pulse stimulations were applied at varying burst frequencies (5, 10, 20, or 100 Hz) to model the physiological range of dopamine neuron firing. After assessing the dopamine response to single- and multi-pulse stimulations, sulpiride (1 μM), a dopamine D2/D3 receptor antagonist, was bath applied and dopamine response was equilibrated to single-pulse stimulation and five-pulse stimulations were reassessed. On separate slices, single electrical pulse stimulations were applied until dopamine response was stable (as above). Once stable, quinpirole, a selective dopamine D2/D3 receptor agonist, was bath applied in increasing concentrations (3 nM − 1 μM), stabilizing dopamine response between each ascending dose.
2.4. Drugs
(-)-Quinpirole hydrochloride ((4aR-trans)-4,4a,5,6,7,8,8a,9-Octahydro-5-propyl-1H-pyrazolo[3,4-g]quinoline hydrochloride; Tocris Bioscience, Bristol, UK) was dissolved in distilled water. (RS)-(±)-Sulpiride((RS)-(±)-5-Aminosulfonyl-N-[(1-ethyl-2-pyrrolidinyl)methyl]-2-methoxybenzamide; Tocris Bioscience, Bristol, UK) was dissolved in DMSO. 1 mM concentration aliquots were stored at −20°C and diluted with oxygenated aCSF to final concentration before bath application on slices.
2.5. Data Analysis
Demon Voltammetry and Analysis software was used to acquire and model FSCV data (Yorgason et al., 2011). Recording electrodes were calibrated by recording electrical current responses (in nA) to a known concentration of dopamine (3 μM) using a flow-injection system. This was used to convert electrical current to dopamine concentration. Michaelis-Menten kinetics were used to determine maximal rate of dopamine uptake (Vmax) (Ferris et al., 2013).
2.6. Statistics
Baseline dopamine release to single- and multi-pulse stimulations and percent changes in dopamine release following sulpiride and quinpirole application were compared bytwo-way mixed-factor ANOVA. In the case of significant interactions, Bonferroni or Tukey post-hoc comparisons were used. Dopamine release following quinpirole application was transformed into log scale and a non-linear regression was used to generate IC50 and area under the curve (AUC) for each curve. Mean IC50 were compared by extra sum-of-squares F test. Mean AUC and single-pulse dopamine release were compared by students t-test. Graph Pad Prism (version 8, La Jolla, CA) or SPSS (version 24, International Business Machine Corporation, Armonk, NY) were used to statistically analyze data sets and compose graphs.Values >2 standard deviations above or below the mean were considered outliers and excluded. Data are presented as mean ± SEM for group data across multiple variables or mean with individual data points for bar graphs.
3. Results
3.1. Adolescent male rats have decreased NAc core dopamine release and female adolescent rats have increased dopamine release in the NAc shell, NAc core, and DMS
We first compared stimulated dopamine release in the striatum of adolescent (P30-37) and adult (>P70) rats. To examine dopamine release at frequencies that model tonic- and phasic-like firing patterns, we stimulated single pulse and five pulses across the range of physiological dopamine firing rates. Figure 1A and 2A show representative traces of single pulse stimulated dopamine release across four regions of the striatum in males and females, respectively. Developmental phase did not impact dopamine release in the NAc shell, DMS, or DLS (Figure 1B, D, and E) of male rats. However, male adolescents had significantly less dopamine release in the NAc core in response to the full range of stimulation frequencies (age × frequency interaction: F4, 40=3.473, p=0.0158) and to single-pulse stimulations (t10=4.3, p=0.0016)(Figure 1C).In contrast, female adolescent rats had significantly higher dopamine release across the range of frequencies and to single-pulse stimulations in the NAc shell (main effect of age: F1, 12=5.975, p=0.0309; t12=3.351, p=0.0058)(Figure 2B), NAc core (main effect of age: F1, 10=10.28, p=0.0094; t10=3.116, p=0.011)(Figure 2C), and DMS (main effect of age: F1, 10=22.39, p=0.0008; t10=3.857, p=0.0032)(Figure 2D). There was no age-related difference in stimulated dopamine release in the DLS of female rats (Figure 2E).
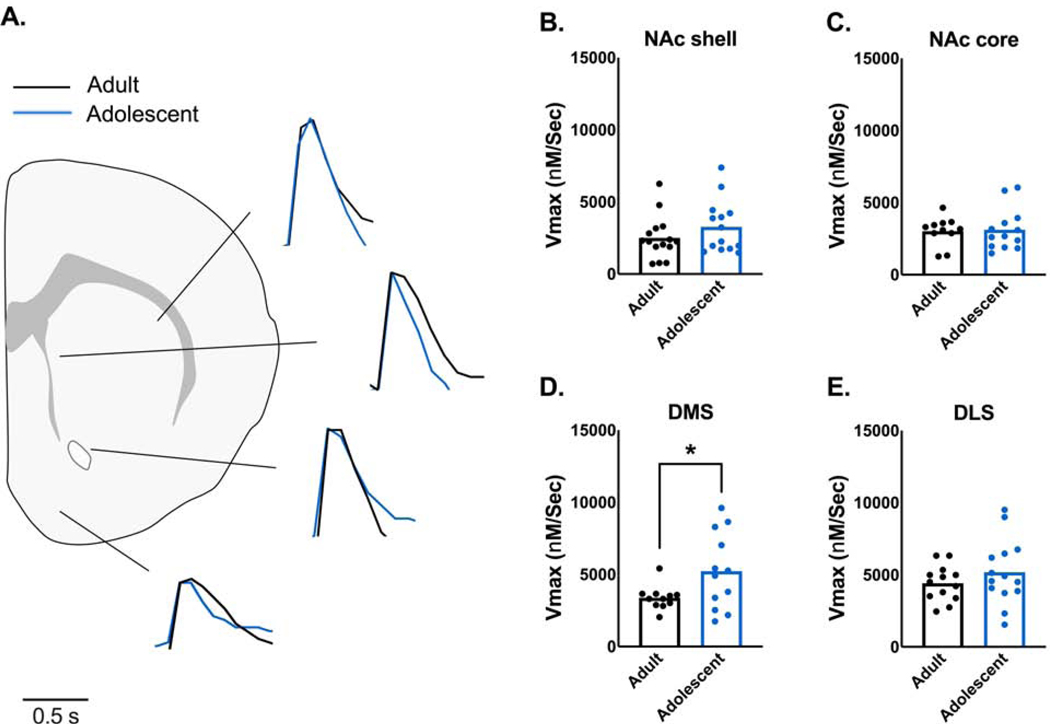
Activity of the dopamine transporter (DAT) can impact evoked dopamine release (see Ferris et al., 2013). To examine whether DAT uptake was mediating differences in dopamine release, we examined the maximal uptake rate of dopamine (Vmax) across the striatum (Figure 3A, 4A; representative traces with peak height normalized for uptake comparison). There was no significant difference in Vmax between adolescent and adult males in the NAc shell, NAc core, or DLS (Figure 3B, C, and E). However, Vmax was significantly greater (i.e. faster maximal dopamine uptake) in male adolescents than adults in the DMS (t21=2.212, p=0.0382)(Figure 3D). In female rats, there were no significant age-related differences in Vmax in any examined region of the striatum (Figure 4A–D).
Figure 3. Maximal rate of dopamine uptake is faster in the dorsomedial striatum of male adolescent rats.
(A) Dopamine uptake rates can impact stimulated dopamine release levels (Ferris et al., 2013), so maximal rate of dopamine uptake (Vmax) was examined using ex vivo fast scan cyclic voltammetry in adult and adolescent male rats. Representative traces of dopamine release and uptake in the nucleus accumbens shell (NAc shell), nucleus accumbens core (NAc core), dorsomedial striatum (DMS), and dorsolateral striatum (DLS) are shown. Peaks of dopamine release were aligned to best compare rate of uptake. (B) Vmax was not different between adult and adolescent males in the NAc shell (adults: n=15; adolescents: n=14) or (C) the NAc core (adults: n=11; adolescents: n=13). (D) Vmax was significantly higher in the DMS of adolescent rats, indicating faster uptake rates (adults: n=11; adolescents: n=12). (E) However, Vmax did not differ by age in the DLS (adults: n=13; adolescents: n=14). Bars represent means and symbols represent individual data points. *p < 0.05.
Figure 4. Maximal rate of dopamine uptake did not differ between adolescent and adult female rats.
(A) We also comparedmaximal rate of dopamine uptake (Vmax) using ex vivo fast scan cyclic voltammetry in adult and adolescent female rats. Representative traces of dopamine uptake in the nucleus accumbens shell (NAc shell), nucleus accumbens core (NAc core), dorsomedial striatum (DMS), and dorsolateral striatum (DLS) are shown. Peaks of dopamine release were aligned to best compare rate of uptake. (B) Vmax was not significantly different between adolescent and adult females in the NAc shell (adults: n=5; adolescents: n=6). (C) NAc core (adults: n=6; adolescents: n=6), (D) DMS (adults: n=6; adolescents: n=5), or (E) DLS (adults: n=5; adolescents: n=6). Bars represent means and symbols represent individual data points. *p < 0.05.
3.2. Increased D2/D3 receptor sensitivity in male, but not female, adolescent rats
Next, we hypothesized that autoreceptors may be mediating the difference between adolescent and adult rats in stimulated dopamine, since D2 autoreceptors play a large role in mediating dopamine release (Zhang & Sulzer, 2012). Importantly, ex vivo FSCV is an excellent technique for isolating the contribution of autoreceptor on their respective neurotransmitter signals (Maina & Mathews, 2010; Bello et al., 2011). To examine this, we bath applied increasing doses of quinpirole, a selective D2/D3 receptor agonist, and calculated an inhibition dose effect curve for each striatal region (Males: Figure 5A–D; Females: Figure 6A–D). The IC50 of the inhibition dose effect curve was not impacted by age for any region of the striatum in males or females (data not shown). However, the dose response curve was significantly lower and the area under the curve (AUC) was significantly smaller in the NAc core (main effect of age: F1, 10=17.83, p=0.0018; t10=4.152, p=0.002)(Figure 5B) and DLS (main effect of age: F1, 12=5.551, p=0.0363; t12=2.42, p=0.0323)(Figure 5D) of male adolescent rats. In contrast, the dose response curve was significantly higher and the AUC significantly greater in the NAc shell of female adolescents (main effect of age: F1, 10=12.72, p=0.0051; t10=2.935, p=0.0149)(Figure 6A). There were no age-related differences in quinpirole dose-response curves in other striatal regions of female rats (Figure 6B–D).
Figure 5. Dopamine autoreceptors in the nucleus accumbens core and dorsolateral striatum are hypersensitive in male adolescent rats.
(A) Dopamine autoreceptors also play an important role in regulating terminal dopamine release (Ferris et al., 2013). To examine whether autoreceptors differentially regulate dopamine release in adult and adolescent males, we bath applied increasing doses of quinpirole, a dopamine D2/D3 receptor agonist, and generated inhibition curves. Quinpirole decreased dopamine release in the nucleus accumbens shell (NAc shell), but the inhibition curve was not differentially impacted by age (adults: n=6; adolescents: n=6). (B) In contrast, adolescents have a down-shifted quinpirole inhibition curve in the nucleus accumbens core (NAc core) and a significantly decreased area under of the curve (inset)(adults: n=6; adolescents: n=6). This indicates adolescents have more sensitive dopamine autoreceptors than adults in the NAc core. Interestingly, the slope of the curve is not different between adolescents and adults, indicating there is not an efficacy shift in quinpirole’s effects. (C) Quinpirole inhibition curves were similar in adults and adolescents in the dorsomedial striatum (DMS)(adults: n=7; adolescents: n=8) and (D) dorsolateral striatum (DLS)(adults: n=7; adolescents: n=7). Insets: Area under the curve values for each rat’s inhibition curve. In line graphs, symbols represent means ± SEMs. In bar graphs, bars represent means and symbols represent individual data points. *p < 0.05.
Figure 6. Dopamine autoreceptors in the nucleus accumbens shell are hyposensitive in female adolescent rats.
(A) To compare the regulation of dopamine release by dopamine autoreceptors in adult and adolescent female rats, we bath applied increasing doses of quinpirole, a dopamine D2/D3 receptor agonist, and generated inhibition curves. Quinpirole decreased dopamine release in the nucleus accumbens shell (NAc shell), but the inhibition curve was up-shifted in adolescent females (adults: n=6; adolescents: n=6). (B) In contrast, the slope of the curve is not different between adolescents and adult females in the NAc core (adults: n=7; adolescents: n=6), (C) dorsomedial striatum (DMS)(adults: n=6; adolescents: n=5), or (D) dorsolateral striatum (DLS)(adults: n=5; adolescents: n=6), indicating there is not a efficacy shift in quinpirole’s effects. Insets: Area under the curve values for each rat’s inhibition curve. In line graphs, symbols represent means ± SEMs. In bar graphs, bars represent means and symbols represent individual data points. *p < 0.05.
To further characterize striatal dopamine autoreceptors, we examined dopamine release to single- and multi-pulse stimulations following bath application of sulpiride, a D2/D3receptor antagonist. We performed this experiment exclusively in males since there were more robust differences in quinpirole-induced changes in dopamine release in this group. We did not see any differences between male adolescents and adults in changes in dopamine release following sulpiride application in any examined striatal region (Figure 7A–D).
Figure 7. Sulpiride, a dopamine D2/D3 receptor antagonist, does not differentially impact dopamine release in adult and adolescent rats.
(A) Sulpiride (1 μM) was bath applied to slices and the percent baseline dopamine release was compared across adult and adolescent animals for multi-pulse stimulations that model both tonic (5 Hz) and phasic (20 Hz) firing of dopamine neurons. Age did not impact the effect of sulpiride on dopamine release in the nucleus accumbens shell (NAc shell)(adults: n=5; adolescents: n=5), (B) nucleus accumbens core (NAc core)(adults: n=5; adolescents: n=6), (C) dorsomedial striatum (DMS)(adults: n=5; adolescents: n=6), or (D) dorsolateral striatum (DLS)(adults: n=5; adolescents: n=5). Bars represent means and symbols represent individual data points.
4. Discussion
Here, we utilized ex vivo FSCV to compare stimulated dopamine release and uptake across the striatum in adolescent and adult male and female rats. There is significant reorganization of the mesolimbic dopamine system between adolescence and adulthood, including changes in dopamine and dopamine receptor levels, firing rates of dopamine neurons, and dopamine neuron innervation patterns (see Galvan, 2010; Wahlstrom et al., 2010a,b; Padmanabhan & Luna, 2014). The reorganization of the dopamine system during adolescence has been implicated in the unique behavioral changes and vulnerability of this developmental stage (Spear, 2013; Nelson et al., 2005; Wahlstrom et al., 2010a,b). Thus, our study aimed to characterize adolescent differences in striatal dopamine dynamics and its local circuitry regulation.
Some evidence indicates that dopamine activity is lower in the striatum of adolescents. For example, studies have found decreased basal levels of extracellular dopamine in the striatum of adolescent male rats (Andersen & Gazzara, 1993; Laviola et al., 2001; Badanich et al., 2006; Cao et al., 2007), although not all studies find this relationship (Philpot et al., 2009). Additionally, adolescent male rats have lower DA synthesis and turnover rates in the NAc (Andersen et al., 1997a) and decreased stimulated dopamine release in the striatum (Stamford, 1989; Palm & Nydler, 2014). We found decreased stimulated dopamine release in the NAc core of adolescent male rats, supporting decreased dopamine activity in adolescents. However, we did not find differences in dopamine release in other examined regions of the striatum.
In contrast, female adolescent rats have significantly higher dopamine release than adults in the NAc shell, NAc core, and DMS, but similar dopamine release in the DLS. There is extensive evidence that sex differences exist in the dopamine system and its regulation in adult rodents (see Kuhn, 2015; Becker, 2016), yet few studies have compared the dopamine system in adolescent and adult female rats. Our results indicate there may be striking sex differences in how dopamine release in the striatum, and possibly other aspects of the dopamine system, changes between adolescence and adulthood. Further studies are needed to understand how development of the dopamine system throughout adolescence differs between males and females, and what behavioral differences these disparate developments may underlie.
Next, we wanted to examine dopamine dynamics that could be mediating the decrease in stimulated dopamine release in the NAc core of male adolescent rats and the increase in dopamine release across the striatum, except in the DLS, in female adolescent rats. The activity of the dopamine transporter (DAT) can influence terminal dopamine release (see Ferris et al., 2013). However, we did not observe differences in Vmax (i.e. the rate of maximal dopamine uptake) in the NAc core of male rats or any region of the striatum in female rats, indicating that the DAT functions similarly in adolescents and adults even in areas where dopamine release differs. The only striatal region in which we found age-related differences in Vmax was the male DMS, where we saw increased rate of dopamine uptake, likely driven by an increase in dopamine transporter levels (Salahpour et al., 2008; Ferris et al., 2013). This is consistent with other literature that finds increased numbers of dopamine transporters in the striatum of adolescent rats (Moll et al., 2000) and greater dopamine release in the dorsal striatum of adolescents following administration of monoamine transporter blockers, but not monoamine releasers (Walker et al., 2010).
We then hypothesized that stimulated dopamine release may be mediated by differences between adolescents and adults in the sensitivity of D2/D3 autoreceptors. Autoreceptors can impact terminal dopamine release and ex vivo FSCV is an ideal technique for examining their role in mediating dopamine release (Maina & Mathews, 2010; Bello et al., 2011). We observed no developmental shift in potency (as indicated by IC50) of the effects of quinpirole, a selective D2/D3 dopamine receptor agonist, across the striatum in either male or female rats. However, there was a downward curve shift and a significantly reduced AUC of the inhibitory curve in the NAc core and DLS of male adolescents, indicating increased sensitivity of D2/D3 receptors. Conversely, female adolescent rats had an upward shift of the inhibitory curve and significantly greater AUC in the NAc shell, indicating adolescent female rats have hyposensitive D2/D3 receptors. There was no age-related shift in the potency or efficacy of quinpirole in the other examined regions of the striatum of female rats. One possible mediator of the sex differences in D2/D3 receptor sensitivity changes is the sexual dimorphism in the developmental proliferation and pruning of dopamine receptors. The density of D1 and D2 receptors peaks in the striatum of males during adolescence (Teicher et al., 1995; Tarazi et al., 1999; Philpot et al., 2009), but the developmental changes in dopamine receptor density are much less pronounced in female rats (Andersen et al., 1997b). Future studies are needed to understand what is driving sex differences in response to D2/D3 receptor activation and how post-synaptic response to dopamine release is altered in adolescents compared to adults.
The hypersensitive D2/D3 receptors in the NAc core of male adolescent rats and the hyposensitive D2/D3 receptors in the NAc shell of female adolescent rats may play a role in decreasing NAc core and increasing NAc shell stimulated dopamine release, respectively. This hypothesis as an overarching explanation for age-related differences in stimulated dopamine release is weakened, however, by our findings that the increased sensitivity of D2/D3 receptors in the DLS of adolescent male rats did not translate to decreased dopamine release and by the lack of age-related changes in sensitivity of D2/D3 receptors in the NAc core and DMS of female rats, despite increased dopamine release in these regions in adolescents. Additionally, the D2/D3 antagonist sulpiride did not differentially impact dopamine release in in the NAc core or DLS of adolescent and adult male rats, which seemingly counters the increased sensitivity we observed in the NAc core and DLS of male adolescents following administration of a D2 agonist. One possible explanation for this difference between agonist and antagonist is that our dopamine stimulation parameters were chosen to model endogenous firing patterns of dopamine neurons. These stimulation parameters may not isolate differences in D2 modulation of dopamine release, particularly because there is no dopamine tone that can constitutively activate D2 receptors in a slice preparation (Phillips et al., 2002). Moreover, our stimulation patterns (2 ms duration) likely did not evoke dopamine for enough time to activate D2 receptors, as previous work examining the impact of sulpiride on dopamine release found that sulpiride does not impact dopamine release unless the duration of stimulation exceeds 500 ms (Wieczorek & Kruk, 1995). Another possible interpretation is that the differential effects of quinpirole are being driven by quinpirole-induced inhibition of acetylcholine (Stoof & Kebabian, 1982) via D2 receptors on cholinergic interneurons. This circuit is known to regulate dopamine terminal release (Rice & Cragg, 2004).
Acetylcholine is tonically released in the striatum, and pauses in acetylcholine signaling drive changes in striatal dopamine release (Cragg, 2006). Since striatal dopamine release is modulated through decreases in acetylcholine levels, and acetylcholine is degraded rapidly at the synapse, the inhibition in acetylcholine release caused by quinpirole may alter dopamine release more than any increases caused by sulpiride application. This interpretation would also indicate that acetylcholine signaling differentially regulates dopamine release in the NAc core and DLS of male adolescent rats. In addition, differences in acetylcholine signaling could also play a role in the differences we see between adolescents and adults in stimulated dopamine release. Further studies are needed to examine these possibilities.
This study compared dopamine release and its regulations across the striatum at two time points: early adolescence (P30-37) and adulthood (>P70). However, there is obviously a continuum in how the dopamine system is developing throughout adolescence and more studies are needed to understand the full developmental progression of sub-second striatal dopamine release and its regulation. Additionally, while these findings are consistent with other studies examining dopamine release in male Wistar rats (Stamford, 1989; Palm & Nylander, 2014), they were performed exclusively in Sprague-Dawley rats, so more studies are needed to understand the generalizability of these findings.
To summarize, we found decreased dopamine release in the NAc core of male adolescent rats and increased dopamine release in the NAc shell, NAc core, and DMS of female adolescent rats. We also see increased maximal rate of dopamine uptake in the DMS and increased sensitivity of D2/D3 autoreceptor regulation of dopamine in the NAc core and DLS of male adolescent rats. We did not see any additional age-related differences in dopamine dynamics in female rats. Given the importance of dopamine systems in regulating motivated behavior and flexible decision making in healthy and diseased states, and the changes in these behaviors and vulnerability to psychiatric disorders in adolescence, it is imperative to understand how these systems develop in healthy individuals. This study gives us initial insight into sex-, region-, and age-related differences in dopamine release and its regulation.
Adolescent male rats have decreased dopamine release in the nucleus accumbens core
Female adolescents have greater dopamine release in several striatal regions
Autoreceptor regulation of dopamine release differs between adolescents and adults
Acknowledgments
Funding: This work was supported by the National Institutes of Health grants R00 DA031791 (MJF), P50 DA006634 (MJF), P50 AA026117 (MJF), K12 GM102773 (EGP), F32 AA028162 (EGP), and the Peter McManus Charitable Trust
Footnotes
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
5. References
- Andersen SL, Dumont NL, Teicher MH, 1997a. Developmental differences in dopamine synthesis inhibition by (+/−)-7-OH-DPAT. Naunyn Schmiedebergs Arch Pharmacol 356, 173–181. 10.1007/pl00005038 [DOI] [PubMed] [Google Scholar]
- Andersen SL, Gazzara RA, 1993. The ontogeny of apomorphine-induced alterations of neostriatal dopamine release:effects on spontaneous release. J Neurochem 61, 2247–2255. 10.1111/j.1471-4159.1993.tb07466.x [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH, 1997bSex differences in dopamine receptor overproduction and elimination.Neuroreport 8, 1495–1498. 10.1097/00001756-199704140-00034 [DOI] [PubMed] [Google Scholar]
- Badanich KA, Adler KJ, Kirstein CL, 2006. Adolescents differ from adults in cocaine conditioned place preference and cocaine-induced dopamine in the nucleus accumbens septi. Eur J Pharmacol 550, 95–106. 10.1016/j.ejphar.2006.08.034 [DOI] [PubMed] [Google Scholar]
- Balleine BW, O’Doherty JP, 2010. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology 35, 48–69. 10.1038/npp.2009.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, 2016. Sex differences in addiction. Dialogues ClinNeurosci 18, 395–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello EP, Mateo Y, Gelman DM, Noain D, Shin JH, Low MJ, Alvarez VA, Lovinger DM, Rubinstein M, 2011Cocaine supersensitivity and enhanced motivation for reward in mice lacking dopamine D2 autoreceptors. Nat Neurosci 14, 1033–1038. 10.1038/nn.2862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Lotfipour S, Loughlin SE, Leslie FM, 2007. Adolescent maturation of cocaine-sensitive neural mechanisms. Neuropsychopharmacology 32, 2279–2289. 10.1038/sj.npp.1301349 [DOI] [PubMed] [Google Scholar]
- Casey BJ, Getz S, Galvan A, 2008. The adolescent brain. Dev Rev 28, 62–77. 10.1016/j.dr.2007.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cragg SJ, 2006. Meaningful silences: how dopamine listens to the ACh pause. Trends Neurosci 29, 125–131. 10.1016/j.tins.2006.01.003 [DOI] [PubMed] [Google Scholar]
- Di Chiara G, 2002. Nucleus accumbens shell and core dopamine: differential role in behavior and addiction. Behav Brain Res 137, 75–114. 10.1016/s0166-4328(02)00286-3 [DOI] [PubMed] [Google Scholar]
- Fareri DS, Martin LN, Delgado MR, 2008. Reward-related processing in the human brain: developmental considerations. Dev Psychopathol 20, 1191–1211. 10.1017/S0954579408000576 [DOI] [PubMed] [Google Scholar]
- Ferris MJ, Calipari ES, Yorgason JT, Jones SR, 2013. Examining the complex regulation and drug-induced plasticity of dopamine releaseand uptake using voltammetry in brain slices. ACS Chem Neurosci 4, 693–703. 10.1021/cn400026v [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, 2010. Adolescent development of the reward system. Front Hum Neurosci 4, 6 10.3389/neuro.09.006.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn C, 2015. Emergence of sex differences in the development of substance use and abuse during adolescence.Pharmacol Ther 153, 55–78. 10.1016/j.pharmthera.2015.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviola G, Pascucci T, Pieretti S, 2001Striatal dopamine sensitization to D-amphetamine in periadolescent but not in adult rats. Pharmacol Biochem Behav 68, 115–124. 10.1016/s0091-3057(00)00430-5 [DOI] [PubMed] [Google Scholar]
- Luciana M, Wahlstrom D, Porter JN, Collins PF, 2012. Dopaminergic modulation of incentive motivation in adolescence: age-related changes in signaling, individual differences, and implications for the development of self-regulation. Dev Psychol 48, 844–861. 10.1037/a0027432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maina FK, Mathews TA, 2010. A functional fast scan cyclic voltammetry assay to characterize dopamine D2 and D3 autoreceptors in the mouse striatum. ACS Chem Neurosci 1, 450–462. 10.1021/cn100003u [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Conrad KL, Carr SB, Ford KA, McGehee DS, Marinelli M, 2012. Dopamine neurons in the ventral tegmental area fire faster in adolescent rats than in adults. J Neurophysiol 108, 1620–1630. 10.1152/jn.00077.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll GH, Mehnert C, Wicker M, Bock N, Rothenberger A, Ruther E, Huether G, 2000. Age-associated changes in the densities of presynaptic monoamine transporters indifferent regions of the rat brain from early juvenile life to late adulthood. Brain Res Dev Brain Res 119, 251–257. 10.1016/s0165-3806(99)00182-0 [DOI] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS, 2005. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med 35, 163–174. 10.1017/s0033291704003915 [DOI] [PubMed] [Google Scholar]
- Padmanabhan A, Luna B, 2014. Developmental imaging genetics: linking dopamine function to adolescent behavior. Brain Cogn 89, 27–38. 10.1016/j.bandc.2013.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm S, Nylander I, 2014. Dopamine release dynamics change during adolescence and after voluntary alcohol intake. PLoS One 9, e96337. 10.1371/journal.pone.0096337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips PEM, Hancock PJ, Stamford JA, 2002. Time window of autoreceptor-mediated inhibition of limbic and striatal dopamine release. Synapse 44, 15–22. 10.1002/syn.10049 [DOI] [PubMed] [Google Scholar]
- Philpot RM, Wecker L, Kirstein CL, 2009. Repeated ethanol exposure during adolescence alters the developmental trajectoryof dopaminergic output from the nucleus accumbenssepti. Int J Dev Neurosci 27, 805–815. 10.1016/j.ijdevneu.2009.08.009 [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ, 2004. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci 7, 583–584. 10.1038/nn1244 [DOI] [PubMed] [Google Scholar]
- Saddoris MP, Sugam JA, Cacciapaglia F, Carelli RM, 2013. Rapid dopamine dynamics in the accumbens core and shell: learning and action. Front Biosci (Elite Ed) 5, 273–288. 10.2741/e615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahpour A, Ramsey AJ, Medvedev IO, Kile B, Sotnikova TD, Holmstrand E, Ghisi V, Nicholls PJ, Wong L, Murphy K, Sesack SR, Wightman RM, Gainetdinov RR, Caron MG, 2008. Increased amphetamine-induced hyperactivity and reward in mice overexpressing the dopamine transporter. Proc Natl Acad Sci U S A 105, 4405–4410. 10.1073/pnas.0707646105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2013. Adolescent neurodevelopment. J Adolesc Health 52, S7–13. 10.1016/j.jadohealth.2012.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2011. Rewards, aversions and affect in adolescence: emerging convergences across laboratory animal and human data. Dev Cogn Neurosci 1, 392–400. 10.1016/j.dcn.2011.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2000The adolescent brain and age-related behavioral manifestations.Neurosci Biobehav Rev 24, 417–463. 10.1016/s0149-7634(00)00014-2 [DOI] [PubMed] [Google Scholar]
- Stamford JA, 1989. Development and ageing of the rat nigrostriatal dopamine system studied with fast cyclic voltammetry. J Neurochem 52, 1582–1589. 10.1111/j.1471-4159.1989.tb09212.x [DOI] [PubMed] [Google Scholar]
- Steinberg L, 2008. A Social Neuroscience Perspective on Adolescent Risk-Taking . Dev Rev 28, 78–106. 10.1016/j.dr.2007.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoof JC, Kebabian JW, 1982. Independent in vitro regulation by the D-2 dopamine receptor of dopamine-stimulated efflux of cyclic AMP and K+-stimulated release of acetylcholine from rat neostriatum. Brain Res 250, 263–270. 10.1016/0006-8993(82)90420-6 [DOI] [PubMed] [Google Scholar]
- Tarazi FI, Tomasini EC, Baldessarini RJ, 1999. Postnatal development of dopamine D1-like receptors in rat cortical and striatolimbic brain regions: An autoradiographic study. Dev Neurosci 21, 43–49. 10.1159/000017365 [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Hostetter JCJ, 1995. Evidence for dopamine receptor pruning between adolescence and adulthood in striatum but not nucleus accumbens. Brain Res Dev Brain Res 89, 167–172. 10.1016/0165-3806(95)00109-q [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M, 2010a. Developmental changes in dopamine neurotransmission in adolescence: behavioral implications and issues in assessment. Brain Cogn 72, 146–159. 10.1016/j.bandc.2009.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlstrom D, White T, Luciana M, 2010b. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev 34, 631–648. 10.1016/j.neubiorev.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM, 2000. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience 95, 1061–1070. 10.1016/s0306-4522(99)00500-x [DOI] [PubMed] [Google Scholar]
- Wieczorek W, Kruk ZL, 1995Influences of neuronal uptake and D2 autoreceptors on regulation of extracellular dopamine in the core, shell and rostral pole of the rat nucleus accumbens. Brain Res 699, 171–182. 10.1016/0006-8993(95)00894-v [DOI] [PubMed] [Google Scholar]
- Yorgason JT, Espana RA, Jones SR, 2011. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. J Neurosci Methods 202, 158–164. 10.1016/j.jneumeth.2011.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Sulzer D, 2012. Regulation of striatal dopamine release by presynaptic auto- and heteroreceptors. Basal Ganglia 2, 5–13. 10.1016/j.baga.2011.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]