Abstract
Introduction
This study aimed to predict brain amyloid beta (Aβ) status in older adults using collected information from an online registry focused on cognitive aging.
Methods
Aβ positron emission tomography (PET) was obtained from multiple in‐clinic studies. Using logistic regression, we predicted Aβ using self‐report variables collected in the Brain Health Registry in 634 participants, as well as a subsample (N = 533) identified as either cognitively unimpaired (CU) or mild cognitive impairment (MCI). Cross‐validated area under the curve (cAUC) evaluated the predictive performance.
Results
The best prediction model included age, sex, education, subjective memory concern, family history of Alzheimer's disease, Geriatric Depression Scale Short‐Form, self‐reported Everyday Cognition, and self‐reported cognitive impairment. The cross‐validated AUCs ranged from 0.62 to 0.66. This online model could help reduce between 15.2% and 23.7% of unnecessary Aβ PET scans in CU and MCI populations.
Disucssion
The findings suggest that a novel, online approach could aid in Aβ prediction.
Keywords: amyloid, Brain Health Registry, cognitively unimpaired, dementia, IDEAS study, Internet, mild cognitive impairment, prediction, research registry, self‐report
1. BACKGROUND
The current National Institutes on Aging and Alzheimer's Association research framework biological definition of Alzheimer’ disease (AD) emphasizes the importance of the presence of the following three biomarkers: amyloid beta (Aβ) plaques, tau pathology, and neurodegeneration (A/T/N criteria). 1 Aβ plaques constitute a key hallmark of the pathophysiology of AD. 2 , 3 There is growing recognition of AD as a continuum which includes a lengthy preclinical phase during which changes in brain Aβ are believed to precede the development of clinical symptoms. 2 , 3 , 4 Recent studies have confirmed the association of high brain Aβ levels with greater cognitive decline 5 as well as greater clinical progression in cognitively unimpaired (CU) older adults with high versus low levels of Aβ. 6 Aβ may be considered the earliest biomarker of AD and could therefore be a crucial indicator of increased risk for subsequent cognitive decline and dementia. 5 At‐risk non‐demented older adults may be most likely to benefit from disease‐modifying treatments.
The ability to determine brain Aβ pathology has clinical benefits (eg, increase the confidence in diagnosis and care and treatment decisions) 7 , 8 and can facilitate AD prevention trial recruitment (eg, reduced screening fails). 9 , 10 It is possible to assess amyloid burden in vivo by Aβ positron emission tomography (PET) 11 or cerebral spinal fluid (CSF). 12 , 13 Despite the robustness of both methods, they are not suitable for screening of large populations because of the invasiveness, high cost, and low technological availability in primary care clinical and research settings. 14 , 15 Thus, there is a need to identify non‐invasive, easily accessible, and cost‐effective markers and/or models that can accurately predict brain Aβ burden.
Many recent studies have investigated the development of practical and low‐cost markers for predicting Aβ status. A variety of modalities, including demographics, apolipoprotein E (APOE), cognition, 9 , 16 , 17 , 18 polygenic risk scores, 19 , 20 magnetic resonance imaging (MRI), 21 , 22 , 23 plasma Aβ, 24 , 25 , 26 and other blood biomarkers approaches 27 , 28 were found to aid the prediction of Aβ burden. However, these studies focused on markers collected in‐clinic. With technology adoption increasing among older adults, 29 the Internet offers a low‐cost, scalable, and remote approach to collecting similar markers. Thus, the overall goal of this study was to leverage the inexpensive and easily available data collected in an online research and recruitment registry for cognitive aging, the Brain Health Registry (BHR), 30 , 31 to assess the predictive value of this remotely collected information in identifying older adults with elevated levels of Aβ. More specifically, the aims were to develop the strongest predictive models for Aβ status using online self‐reported data and to determine the number of PET scans that could be reduced when applying the model to facilitate the clinical trial screening.
2. METHODS
2.1. Study sample
Data were obtained from the BHR database. BHR is a public online registry for research recruitment, assessment, and longitudinal monitoring with a focus on cognitive aging. 30 Participants register online, complete an online informed consent form, and complete a variety of self‐report questionnaires and self‐administered cognitive assessments. This study focused specifically on data obtained from self‐report questionnaires. At the time of the analysis, 70,992 were enrolled. The mean age of the overall BHR sample was 60 (standard deviation [SD] = 14.1), participants had an average of 15.9 years of education (SD = 2.5), 69.8% were female, and 71.7% identified as White.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using electronic data bases (eg, PubMed) and search engines (Google Scholar). Previous studies have found that various in‐clinic measures (eg, cognition, apolipoprotein E [APOE], polygenic risk factors, plasma amyloid, plasma analytes, magnetic resonance imaging) can aid the prediction of positron emission tomography (PET) and cerebrospinal fluid (CSF) amyloid status. However, the utility of online collected self‐report measures is not yet well understood.
Interpretation: Results highlight the utility of online self‐reported measures in the prediction of amyloid beta status. The inexpensive, non‐invasive, easily accessible, and scalable approach of online self‐reported information makes this an attractive and useful alternative to determining amyloid using PET imaging or CSF.
Future directions: Future studies will need to extend these findings by evaluating the predictive utility of these online measures in external and more heterogenous samples. Apart from the self‐reported sociodemographic variables and selected health measures used in this study, future research could test other online measures.
This analysis focused on BHR participants with available Aβ status by June 2019 (N = 930). Figure S1 in the supporting information shows a flow diagram of the numbers of participants excluded from the entire BHR sample down to the two included samples. BHR allows co‐enrollments, meaning that participants can be enrolled simultaneously in the BHR and another study, with data linkage between the two studies. 30 Co‐enrolling studies in which participants have Aβ data included the Imaging Dementia‐Evidence for Amyloid Scanning (IDEAS) study 32 , 33 and clinical studies at the San Francisco Veterans Affairs Medical Center (SFVAMC). Apart from having a known Aβ status, the key inclusion criterion was that participants had completed the BHR self‐report measures of interest for the analysis; 634 participants met these criteria and were included in the modelling. This final sample included participants regardless of their impairment level. The models were also tested in a subsample of participants (N = 533) who were classified as CU or mild cognitive impairment (MCI). The impairment level for this subgroup was clinician‐rated for participants coming from the IDEAS study and self‐reported for participants from SFVAMC studies. Clinician‐rated impairment level in IDEAS was determined as described in Nosheny et al. 32 Briefly, the participant's impairment level of MCI or dementia was reported by IDEAS dementia specialists prior to the participant's PET scan and required objective evidence of cognitive impairment. All IDEAS participants were required to have an impairment level of MCI or dementias as an inclusion criterion of the IDEAS study. The supporting information (Figures S2 and S3) includes more details regarding the eligibility criteria for IDEAS and the SFVAMC studies.
2.2. Self‐reported online measures
The BHR self‐reported online predictors used in this study included three sociodemographic measures and five health‐related measures. We estimate that it takes BHR participants ≈25 minutes to complete these measures. Table 1 provides the measures and individual time estimates.
TABLE 1.
Time estimates for each self‐reported Brain Health Registry (BHR) measures included in this analysis
| Self‐report measure | Time estimate |
|---|---|
| Age | 30 seconds |
| Sex | 30 seconds |
| Education | 30 seconds |
| Memory concern | 30 seconds |
| Family history of Alzheimer's disease | 30 seconds |
| Diagnosis of mild cognitive impairment | 30 seconds |
| Everyday Cognition Scale (39 items) | 16 minutes |
| Geriatric Depression Scale‐Short Form (15 items) | 6 minutes |
| Total | 25 minutes |
2.2.1. Sociodemographic measures
BHR participants complete a self‐report questionnaire during registration which asks about sociodemographic information. For this analysis, we focused on the following sociodemographic variables: age, sex (male, female), and education, which are known to be associated with Aβ and often included as predictors in similar Aβ prediction models using in‐clinic data. 34 , 35 , 36 The categorical variable education was converted to years of education, which ranged from 6 to 20 years.
2.2.2. Self‐reported health‐related measures
BHR participants are invited to complete further online self‐report questionnaires including an assessment of detailed medical history and overall health. This analysis used subjective memory concern (“Are you concerned that you have a memory problem?”), family history of AD (“Have you, your sibling[s], or parent[s] ever been diagnosed with Alzheimer's disease?”), self‐reported Everyday Cognition Scale (ECog) score, and Geriatric Depression Scale‐Short Form (GDS‐SF) score, which have previously been shown to be associated with Aβ. 35 , 37 , 38 , 39 We also used self‐reported cognitive impairment (“Please indicate whether you currently have or have had any of the following conditions in the past: MCI or dementia/AD”).
Everyday Cognition Scale
BHR participants complete an online adaptation of the ECog. 31 The ECog is a 39‐item measure of functional change and assesses the participant's self‐ or study partner‐reported capability to perform everyday tasks in comparison to activity levels 10 years prior including activities that map to cognitive abilities across six domains. 40 This analysis focused on the self‐reported score.
Geriatric Depression Scale‐Short Form
The GDS‐SF is a 15‐item screening tool to rate severity of depressive symptoms in older adults. 41 , 42 In the BHR the GDS‐SF is completed in an online survey form; the item text and response options are identical to the paper version and higher scores represent greater symptoms of depression.
2.3. Aβ
Aβ PET scan results were directly provided by IDEAS (N = 520) or studies conducted at the SFVAMC (N = 114). Determination of IDEAS participants’ Aβ status has previously been described in detail. 32 Briefly, Aβ PET images were interpreted by IDEAS study imaging specialists using approved reading methodologies for each tracer (fluorine 18 [18F]‐labeled florbetapir, 18F‐labeled flutemetamol, and 18F‐labeled florbetaben). Scan interpretation was “negative” when the retention of the Aβ tracer was in cerebral white matter only and “positive” when Aβ tracer retention was also found in cortical gray matter. The IDEAS PET scan preceded the BHR data collection. All studies done at the SFVAMC followed the same protocol. The PET scans with 18F florbetapir were performed to establish Aβ status and scans were interpreted using visual read. For this group, the BHR online data collection was not restricted to time of PET scan.
2.4. Statistical analysis
The participants’ characteristics were summarized and compared by Aβ status using Mann‐Whitney and chi‐square tests. We used a complete‐case logistic regression to construct the models that predict Aβ status based on online self‐report measures. Here, prediction refers to estimating the probability that someone is Aβ positive (diagnostic prediction model). 43 For the logistic regression analysis, the online self‐reported predictors of interest were divided into two groups: first group: demographic variables (age, sex, years of education); second group: self‐reported health variables (family history of AD, subjective memory concern [SMC], self‐ECog score, GDS‐SF score, self‐reported cognitive impairment). The predictive performance of all logistic models was assessed by calculating the area under the receiver operating characteristic (AUC) curve. We corrected for optimism by calculating 10‐fold cross‐validated estimates of the AUCs. Estimates of sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) were also calculated using a cut‐off point of 0.5. We calibrated the logistic regression prediction models by computing Hosmer‐Lemeshow goodness‐of‐fit tests for overall calibration, by plotting the observed and model‐based predictions used in the Hosmer‐Lemeshow tests, and fitting a linear regression model to the data in the plots. We assessed calibration separately for the full sample and the sample that included only CU and MCI participants. These analyses were performed in two samples: (1) including all impairment levels and (2) CU and MCI only. Statistical analyses were performed using SAS version 9.4, STATA, and R and a P value of .05 was taken as statistically significant.
3. RESULTS
3.1. Sample characteristics
Six hundred thirty‐four BHR participants with complete data for all predictors of interest and Aβ status (Aβ+ n = 345, 54%) were included in the analyses. Participant characteristics by Aβ status are presented and compared in Table 2. From the total sample 92 (14.5%) were CU, 441 (69.6%) MCI, and 101 (15.9%) dementia. The mean age was 73.5 (SD = 5.4) with a mean of 16.3 (SD = 2.7) years of education. Females represented 44.6% of the sample and Whites 91.5%. No significant differences in sex, years of education, ethnicity, or self‐reported ECog were found between Aβ+ and Aβ– individuals. There were significant differences in age (P = .018), race (P = .018), family history of AD (P = .0023), self‐reported memory concern (P = .0020), GDS‐SF scores (P = .014), and self‐reported cognitive impairment (P < .0001).
TABLE 2.
Demographic and key sample characteristics by Aβ status
| Aβ+ (N = 345; 54.4%) | Aβ+ (N = 289; 45.9%) | P | Total (N = 634) | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years M (SD) | 74 (5.5) | 73 (5.3) | .018 a | 73.5 (5.4) |
| Female, n (%) | 142 (41.2%) | 141 (48.8%) | .054 b | 283 (44.6%) |
| Education, years M (SD) | 16.1 (2.8) | 16.4 (2.5) | .23 a | 16.3 (2.7) |
| White, n (%) | 324 (93.9%) | 256 (88.6%) | .017 b | 580 (91.5%) |
| Latino, n (%) | 7 (2.0%) | 6 (2.1%) | .079 b | 13 (2.1%) |
| Self‐reported data | ||||
| Family history of AD, n (%) | 130 (37.7%) | 213 (73.7%) | .0023 b | 428 (67.5%) |
| Self‐reported memory concern, n (%) | 320 (92.8%) | 246 (85.1%) | .0020 b | 566 (89.3%) |
| Self‐reported ECog, M (SD) | 1.79 (0.6) | 1.77 (0.6) | .69 a | 1.78 (0.6) |
| GDS‐SF, M (SD) | 2.21 (2.5) | 2.75 (2.9) | .014 a | 2.45 (2.7) |
| Self‐reported cognitive impairment n (%) | 284 (82.3%) | 177 (61.2%) | <.0001 b | 461 (72.7%) |
| Impairment level | <.0001 b | |||
| Cognitively unimpaired n (%) | 36 (10.4%) | 56 (19.4%) | 92 (14.5%) | |
| Mild cognitive impairment n (%) | 229 (66.4%) | 212 (73.4%) | 441 (69.6%) | |
| Dementia n (%) | 80 (23.2%) | 21 (7.3%) | 101 (15.9%) | |
| Study origin | .0009 b | |||
| IDEAS, n (%) | 299 (86.7%) | 221 (76.5%) | 520 (82.0%) | |
| SFVAMC studies, n (%) | 46 (13.3%) | 68 (23.5%) | 114 (18.0%) | |
Abbreviations: Aβ, amyloid beta; AD, Alzheimer's disease; ECog, Everyday Cognition Scale; GDS‐SF, Geriatric Depression Scale‐Short Form; IDEAS, Imaging Dementia‐Evidence for Amyloid Scanning; SD, standard deviation; SFVAMC, San Francisco Veterans Affairs Medical Center.
Based on Mann‐Whitney test.
based on Chi‐square test.
3.2. Amyloid prediction models including participants from all impairment levels
Table 3 contains a summary of multivariate logistic regression models tested in the sample including all impairment levels. Model accuracy ranged from AUC 0.57 to 0.68. The most accurate predictive model (AUC = 0.68) combined demographic and self‐reported health information with a sensitivity of 76.23%, specificity of 48.79%, PPV of 63.99%, and NPV of 63.23%. The ROC curve for this model is shown in Figure 1. The cross‐validated estimated AUC was 0.66. In this model, family history of AD, GDS‐SF score, and self‐report of cognitive impairment were all significantly associated with Aβ status. Self‐reporting a family history of AD (odds ratio [OR] = 1.73, confidence interval [CI] = 1.21, 2.48, P = .003), lower GDS‐SF scores (OR = 0.90, CI = 0.84, 0.97, P = .003) indicating fewer symptoms of depression, and self‐reported cognitive impairment (OR = 2.85, CI = 1.88, 4.32, P < .001) were associated with a greater probability of Aβ positivity. Removing the self‐reported cognitive impairment predictor lowered the predictive accuracy to an AUC of 0.65.
TABLE 3.
Summary of multivariable logistic regression models predicting amyloid status (positive vs negative) in a sample including all impairment levels
| Model 1 (demographics) (n = 634) | Model 2 (self‐reported health information) (n = 634) | Model 3 (demographics + self‐reported health information) (n = 634) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Characteristics | |||
| Age | 1.03 (1.00, 1.06) | 1.03 (0.99, 1.06) | |
| Female sex | 0.72 (0.52, 0.99) | 0.75 (0.53, 1.05) | |
| Education in years (6–20) | 0.95 (0.89, 1.01) | 0.94 (0.88, 1.00) | |
| Family history of AD | 1.67 (1.17, 2.38) a | 1.73 (1.21, 2.48) a | |
| Self‐reported memory concern | 1.39 (0.77, 2.52) | 1.44 (0.79, 2.64) | |
| Geriatric Depression Scale‐Short Form score | 0.90 (0.85 0.97) a | 0.90 (0.84, 0.97) a | |
| Self‐reported Everyday Cognition Scale score | 0.89 (0.66, 1.22) | 0.89 (0.66, 1.22) | |
| Self‐reported cognitive impairment | 2.99 (1.98, 4.53) b | 2.84 (1.88, 4.32) b | |
| AUC (cAUC) | 0.57 | 0.67 | 0.68 (0.66) |
| Sensitivity | 76.23% | 78.55% | 76.23% |
| Specificity | 30.10% | 45.33% | 48.79% |
| PPV | 56.56% | 63.17% | 63.99% |
| NPV | 51.48% | 63.90% | 63.23% |
Abbreviations: AD, Alzheimer's disease; AUC, area under the curve; cAUC, cross‐validated area under the curve; CI, confidence interval; MCI, mild cognitive impairment; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value.
= P‐values < .05.
= P‐values < .001.
FIGURE 1.
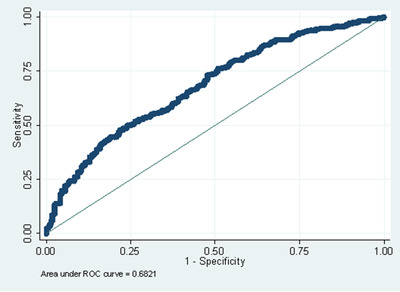
Receiver operating characteristic (ROC) curve for the model combining demographic and self‐reported health in participants from all impairment levels
3.3. Amyloid prediction models in CU and MCI participants
Because a crucial need in the field is the ability to identify Aβ+ in older adults with mild or no symptoms, we then performed similar analysis, but limited our sample to CU and MCI participants. Table 4 shows a summary of multivariable logistic regression models tested in this sample. Model accuracy ranged from AUC 0.57 to 0.65. Similar to the predictions in the sample including all impairment levels, the most accurate predictive model (AUC = 0.65) also combined demographic and self‐reported health information with a sensitivity of 65.28%, specificity of 57.46%, PPV of 60.28%, and NPV of 62.60%. The ROC curve for this model is shown in Figure 2. Here, the cross‐validated estimated AUC was 0.62. Self‐reported family history of AD (OR = 1.72, CI = 1.17,2.52, P = .005) and self‐reporting cognitive impairment (OR = 2.21, CI = 1.42, 3.42, P < .001) was associated with being Aβ positive. Removing the self‐reported cognitive impairment predictor from the model resulted in an AUC of 0.63.
TABLE 4.
Summary of multivariable logistic regression models predicting amyloid status (positive vs negative) in CU and MCI
| Model 1 (demographics) (n = 533) | Model 2 (self‐reported health information) (n = 533) | Model 3 (demographics + self‐reported health information) (n = 533) | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Characteristics | |||
| Age | 1.03 (0.99, 1.06) | 1.03 (0.99,1.06) | |
| Female sex | 0.69 (0.48, 0.98) b | 0.71 (0.49, 1.02) | |
| Education in years (6–20) | 0.96 (0.89, 1.02) | 0.96 (0.89, 1.03) | |
| Family history of AD | 1.64 (1.13, 240) b | 1.72 (1.17,2.52) b | |
| Self‐reported memory concern | 1.40 (0.75, 2.61) | 1.41 (0.76, 2.65) | |
| Geriatric Depression Scale‐Short Form score | 0.93 (0.87, 0.99) b | 0.93 (0.87, 1.00) | |
| Self‐reported Everyday Cognition Scale score | 0.885 (0. 63, 1.25) | 0.88 (0.62,1.25) | |
| Self‐reported cognitive impairment | 2.33 (1.51, 3.60) b | 2.21 (1.42, 3.42) b | |
| AUC (cAUC) | 0.57 | 0.64 | 0.65 (0.62) |
| Sensitivity | 55.09% | 65.66% | 65.28% |
| Specificity | 57.46% | 51.12% | 57.46% |
| PPV | 56.15% | 57.05% | 60.28% |
| NPV | 56.41% | 60.09% | 62.60% |
Abbreviations: AD, Alzheimer's disease; AUC, area under the curve; cAUC, cross‐validated area under the curve; CI, confidence interval; MCI, mild cognitive impairment; NPV, negative predictive value; OR, odds ratio; PPV, positive predictive value
= P‐values = < .05.
= P‐values < .001.
FIGURE 2.
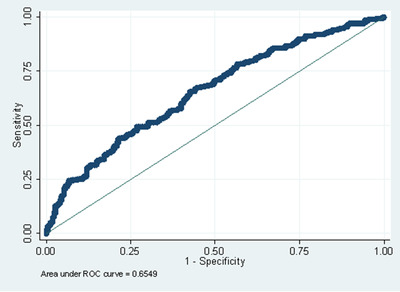
Receiver operating characteristic (ROC) curve for the model combining demographic and self‐reported health in CU and MCI participants
3.4. Calibration of the logistic regression prediction models
The Hosmer‐Lemeshow goodness‐of‐fit statistics for the final models were 10.29, P = 0.25, and 2.63, P = 0.96, for the full and cognitively impaired/MCI samples, respectively, indicating that we did not detect statistically significant lack of fit of the logistic prediction models. Figures S4 and S5 in the supporting information present the calibration plots, and the comparison of observed and model‐based predictions, for the two samples. The points in both plots correspond reasonably well with the 45‐degree line indicating reasonable calibration. The estimated linear regression coefficients that assess the association of the predicted probabilities with the observed in Figures S4 and S5 were 1.01, 95% CI = (0.97, 1.04) and 0.97, 95% CI = (0.95, 0.99) for the full and cognitively impaired/MCI samples, respectively, again indicating reasonable calibration. We note that for a perfectly calibrated model the regression coefficient would be 1.0.
3.5. Trial enrichment estimation in CU and MCI participants
One of the uses of Aβ prediction models is to facilitate prodromal AD clinical trial screening. We used PPV to assess the ability of the model to enrich clinical trial screening by calculating the percent reduction of participants that would need to undergo an Aβ PET scan. The PPV depends on the prevalence of Aβ+ in the sampled population. To address this issue, we calculated PPVs for several possible values of Aβ prevalence. This calculation focused on the subsample that includes MCI and CU as these impairment levels are often candidates for such trials. Based on previous estimates of prevalence of MCI and dementia in the general older population 44 and prevalence of Aβ in MCI and CU individuals, 45 we estimate that Aβ+ prevalence in this population ranges between 30% and 55%. The calculation used the sensitivity (65%) and specificity (57%) of the combined online BHR prediction model for the CU and MCI sample and results are presented in Table 5. For the prevalence ranging from 30% to 55%, PPVs ranged from 39.3% to 64.9%. The number of saved PET scans ranged from 55 to 158 with reductions of unnecessary Aβ PET scans ranging from 15.2% to 23.7%.
TABLE 5.
Percent reduction of Aβ PET scans for varying prevalence rates based on the BHR online prediction model in CU and MCI participants
| Prevalence | PPV | N of Aβ+ predicted by online BHR model | Aβ PET scans needed without prescreen | N of Aβ PET scans saved | % reduction of unnecessary PET scans |
|---|---|---|---|---|---|
| 30.0% | 39.3% | 509 | 667 | 158 | 23.7% |
| 35.0% | 44.9% | 446 | 571 | 126 | 22.0% |
| 40.0% | 50.2% | 398 | 500 | 102 | 20.3% |
| 45.0% | 55.3% | 362 | 444 | 83 | 18.6% |
| 50.0% | 60.2% | 332 | 400 | 68 | 16.9% |
| 55.0% | 64.9% | 308 | 364 | 55 | 15.2% |
Abbreviations: Aβ, amyloid beta; BHR, Brain Health Registry; CU, cognitively unimpaired; MCI, mild cognitive impairment; PET, positron emission tomography; PPV, positive predictive value.
Notes: The calculations are based on a hypothetical trial which aims to identify 200 Aβ+ older adults. For calculating the PPV by prevalence, the sensitivity = 65% and specificity = 57% from the combined online BHR model in the CU and MCI group were used (Section 3.3).
4. DISCUSSION
The two main findings were that self‐reported online data collected through BHR aided the classification of Aβ status in older adults, and that this model could lead to estimated reductions of unnecessary Aβ PET scans ranging between 15.2% and 23.7% when used as a pre‐screener for clinical trial recruitment in a population of CU and MCI older adults. This suggests that online self‐reported demographic and health information has the potential to facilitate prodromal AD clinical trial recruitment.
In both tested samples (including all impairment levels and restricted to CU and MCI), the models combining self‐reported demographics (age, sex, ethnicity) and health information (self‐reported memory concern, family history of AD, GDS‐SF score, self‐ECog score, self‐reported cognitive impairment) to predict Aβ status yielded the highest AUCs. The discrimination was modest, but this nonetheless indicates that the online collection of these eight self‐reported variables, which can take as little as ≈25 minutes, could aid the prediction. Other groups have explored the predictive abilities of self‐reported data; however, those studies used information collected in‐clinic. The discriminative abilities of our online models was comparable to previous in‐clinic prediction models, with AUCs from in‐clinic predictive models ranging from 0.51 to 0.70. 34 , 35 , 46 , 47 Comparisons to previous in‐clinic models are limited by the fact that these studies varied in: (1) the diagnostic groups included (CU, subjective memory concern, MCI, dementia, or combined diagnostic groups), (2) how the Aβ status was determined (PET or CSF), (3) the degree of validation (no validation, internal validation, and external validation), and (4) inclusion of specific self‐reported predictors. In addition, in contrast to our study, none of the reviewed studies specifically aimed at just using demographic and health information to predict Aβ status. Other models evaluated in these studies, for example, included cognitive performance, APOE, and plasma Aβ levels. 34 , 35 , 46 The study with the highest AUC (0.70) used age to predict Aβ status in CU individuals with subjective memory concern. However, the results were not cross‐validated. Only one study used external validation and found in a sample of MCI and AD that age, sex, education, and hypertension together predicted Aβ status with an AUC of 0.54. 34 Another study used CU adults from the population‐based Mayo Clinic Study of Aging and received an AUC of 0.64 with age, sex, family history of dementia/AD, and SMC as predictors. 35 However, the results from this study were not cross‐validated and focused on CU adults. Taken together, our results demonstrate that a brief online assessment of self‐report variables, which are all easily obtainable with little participant burden, can aid the prediction of Aβ status, and the discrimination ability is comparable to similar in‐clinic data prediction models.
A popular inclusion criterion for many AD trials is Aβ biomarker positivity. Thus, one of the uses of Aβ prediction models is to enrich clinical trial screening. For clinical trial enrichment purposes, a prediction model with a large value of PPV is desired as it helps reduce the cost of identifying individuals who are Aβ positive. In this study, the PPVs for the final models were 64% in the full sample and 60% in the CU and MCI sample. The PPV in previous in‐clinic data prediction studies ranged from 50% to 73%, with lower PPVs in CU samples 9 , 35 and larger PPVs in AD dementia samples. 21 One study used a similar sample to our study (CU and early MCI) and found a slightly higher PPV of 67% for a model including age, sex, and education. 48 Based on the PPVs we estimated using a range of possible Aβ+ prevalence, we found that using this online self‐report measure model as clinical trial recruitment pre‐screener could lead to reductions of unnecessary Aβ PET scans ranging between 15.2% and 23.7%. While screening on these variables will help reduce the costs of identifying individuals who are Aβ positive, the NPV of 63% is modest, meaning a number of potential participants who are Aβ positive will screen negative and therefore be excluded. Furthermore, because we used family history of AD as a predictor, our sample might overrepresent APOE ε4 positive participants, which could affect the generalizability of trial results to an APOE ε4 negative population. However, because the cost and burden of prescreening and excluding false negatives using our online self‐reported measures is relatively small compared to PET, using this online pre‐screener could still greatly reduce the cost of identifying candidates who are Aβ positive for AD trials.
In the final models, self‐reported family history of AD and self‐reported cognitive impairment were significant predictors of Aβ status. As expected, self‐reporting a family history of AD or cognitive impairment was associated with a greater probability of Aβ positivity. Consistent with these findings, Aβ+ participants have been found to be more likely to have a family history of dementia. 49 To our knowledge, no other study has used self‐reported cognitive impairment as a predictor; however, it was a strong predictor in our models indicating that it could be a useful in the prediction of Aβ positivity. However, it is unclear whether individuals can accurately report whether they have MCI or dementia.
Our results indicate that lower levels of depressive symptoms were associated with Aβ positivity. While the majority of existing studies have suggested that increased depression is associated with increased amyloid (eg, Chung et al. 38 ), these studies have not been consistent and have often been conducted in small samples and did not routinely look at APOE (eg, Madsen et al. 50 ). It has also been shown that MCI and AD participants tend to underreport symptoms of depression. 51 Further analysis, including testing this prediction model in other populations, is important to clarify these relationships. Of the demographic variables, only age was positively associated with higher probability for being amyloid positive in the sample including all impairment levels. It is well established that the prevalence of Aβ burden increases with age. 52 Overall, age, self‐reported memory concern, MCI, and GDS‐SF were the most useful in aiding the prediction in our sample. These variables can all easily be collected online, as well as in‐clinic. Based on Table 1, it could take as little as 7.5 minutes to collect this information online. This study focused on self‐report predictors and future work will investigate how self‐administered online cognitive tests might affect the predictive ability.
A few limitations should be mentioned regarding this analysis. The current analysis only focused on the “A” of the A/T/N criteria; however, for a diagnosis of AD, elevated Aβ levels are considered necessary but not sufficient. 1 , 53 Further, our sample was composed of a very limited amount of the overall BHR cohort that had a Aβ PET status, which introduces a selection bias. There was variability in the methods of interpretation of Aβ positivity. Visual reads of amyloid PET scans were not standardized, which poses a risk of bias. For the IDEAS study, visual reads were performed locally by multiple imaging specialists. Further, we analyzed a subset of the total included sample of CU and MCI participants. However, we only had a clinically confirmed MCI diagnosis for IDEAS participants; for the remaining participants self‐reported MCI was used. The generalizability of the study findings may be impacted by the characteristics of the participants, including a lack of racial, ethnic, and educational diversity. The majority of the included participants came from the IDEAS study. Most IDEAS participants were informed about their Aβ status and likely also received recent information about the cause of their cognitive impairments, which could have affected all of the self‐reported cognitive and functional measures, including their self‐reported impairment level used in our models. 54 Further, the predictors used in this study were collected online and self‐administered. Therefore, the sample might be subject to selection biases, for example, for those with Internet and computer access and competencies. This and other biases are likely to result in data not missing at random. Although we internally validated the model using 10‐fold cross‐validation, further external validation is still needed in different samples and larger populations. It needs to be determined how greater sample heterogeneity (eg, more CU participants) would impact the predictive value of these online measures. Another drawback to the current study is the selection criteria. The study did not include participants with available Aβ who did not complete BHR online measures. As such, the model might overestimate the ability of online variables to predict Aβ.
In conclusion, results from this study highlight the potential utility of online self‐reported measures in the prediction of Aβ status. The inexpensive, non‐invasive, easily accessible, and scalable approach of online self‐reported information makes a useful method for prescreening older adults for clinical trials prior to determining Aβ using PET imaging. Although the online self‐reported predictors do not approach perfect discrimination between Aβ+ and Aβ– subjects, they could still be useful in facilitating prodromal AD trial recruitment. Further research is needed to determine the validity of online measures in predicting amyloid status in other samples.
CONFLICTS OF INTEREST
Miriam T. Ashford, John Neuhaus, Chengshi Jin, Monica R. Camacho, Juliet Fockler, Diana Truran, and R. Scott Mackin have no interests to declare.
Supporting information
Supporting Information.
ACKNOWLEDGMENTS
This work was funded by the National Institutes of Health [K01AG055692], Larry L. Hillblom Foundation [2015‐A‐011‐NET], and the California Department of Public Health [16‐10323]. The authors gratefully acknowledge the following funding sources for the Brain Health Registry: National Institute on Aging, the Alzheimer's Association, Patient Centered Research Outcomes Institute, Alzheimer's Drug Discovery Foundation, the Rosenberg Alzheimer's Project, the Ray and Dagmar Dolby Family Fund, Connie and Kevin Shanahan, General Electric, and The Drew Foundation. The authors further appreciate the IDEAS study team, the UCSF‐NVP study team, the entire Brain Health Registry staff, and all BHR participants. R. Scott Mackin has received research support from The National Institute of Mental Health and Janssen Research and Development LLC over the last 2 years. Gil D. Rabinovici receives research support from NIH, Alzheimer's Association, American College of Radiology, Rainwater Charitable Foundation, Avid Radiopharmaceuticals, Eli Lilly, GE Healthcare, and Life Molecular Imaging; has been a paid consultant for Axon Neurosciences, Eisai, GE Healthcare, Johnson & Johnson; and is an Associate Editor for JAMA Neurology. Michael W. Weine receives support for his work from the following funding sources: NIH: 5U19AG024904‐14; 1R01AG053798‐01A1; R01 MH098062; U24 AG057437‐01; 1U2CA060426‐01; 1R01AG058676‐01A1; 1RF1AG059009‐01, 1R33AG062867‐01A1, DOD: W81XWH‐15‐2‐0070; 0W81XWH‐12‐2‐0012; W81XWH‐14‐1‐0462; W81XWH‐13‐1‐0259, PCORI: PPRN‐1501‐26817, California Dept. of Public Health: 16‐10054, U. Michigan: 18‐PAF01312, Siemens: 444951‐54249, Biogen: 174552, Hillblom Foundation: 2015‐A‐011‐NET, Alzheimer's Association: BHR‐16‐459161; The State of California: 19‐10616. He also receives support from Johnson & Johnson, Kevin and Connie Shanahan, GE, VUmc, Australian Catholic University (HBI‐BHR), The Stroke Foundation, and the Veterans Administration. He has served on Advisory Boards for Eli Lilly, Cerecin/Accera, Roche, Alzheon, Inc., Merck Sharp & Dohme Corp., Nestle/Nestec, PCORI/PPRN, Dolby Family Ventures, National Institute on Aging (NIA), Brain Health Registry, and ADNI. He serves on the Editorial Boards for Alzheimer's & Dementia, TMRI, and MRI. He has provided consulting and/or acted as a speaker/lecturer to Cerecin/Accera, Inc., Alzheimer's Drug Discovery Foundation (ADDF), Merck, BioClinica, Eli Lilly, Indiana University, Howard University, Nestle/Nestec, Roche, Genentech, NIH, Health & Wellness Partners, Bionest Partners, American Academy of Neurology (AAN), NYU, Japanese Government Alliance, National Center for Geriatrics and Gerontology (Japan), US Against Alzheimer's, Society for Nuclear Medicine and Molecular Imaging (SNMMI), The Buck Institute for Research on Aging, FUJIFILM‐Toyama Chemical (Japan), Garfield Weston, Baird Equity Capital, JOMDD, Cytox, and T3D Therapeutics. He holds stock options with Alzheon, Inc., Alzeca, and Anven.
Ashford MT, Neuhaus J, Jin C, et al. Predicting amyloid status using self‐report information from an online research and recruitment registry: The Brain Health Registry. Alzheimer's Dement. 2020;12:e12102 10.1002/dad2.12102
REFERENCES
- 1. Jack CR, Jr , Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Villemagne VL, , Burnham S, Bourgeat P, et al. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol. 2013;12(4):357‐367. [DOI] [PubMed] [Google Scholar]
- 3. Jack CR, Jr , Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jack CR, Jr , Knopman DS, Weigand SD, et al. An operational approach to National Institute on Aging–Alzheimer's Association criteria for preclinical Alzheimer disease. Ann Neurol. 2012;71(6):765‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Baker JE, Lim YY, Pietrzak RH, et al. Cognitive impairment and decline in cognitively normal older adults with high amyloid‐β: a meta‐analysis. Alzheimers Dement (Amst). 2017;6:108‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dang C, Harrington KD, Lim YY, et al. Relationship between amyloid‐β positivity and progression to mild cognitive impairment or dementia over 8 years in cognitively normal older adults. J Alzheimers Dis. 2018;65(4):1313‐1325. [DOI] [PubMed] [Google Scholar]
- 7. Sánchez‐Juan P, Ghosh PM, Hagen J, et al. Practical utility of amyloid and FDG‐PET in an academic dementia center. Neurology. 2014;82(3):230‐238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rabinovici GD, et al. Impact of amyloid PET on patient management: early results from the IDEAS study. Alzheimers Dement. 2017;13(7):P1474. [Google Scholar]
- 9. Insel PS, Palmqvist S, Mackin S, et al. Assessing risk for preclinical β‐amyloid pathology with APOE, cognitive, and demographic information. Alzheimers Dement (Amst). 2016;4:76‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ansart M, Epelbaum S, Gagliardi G, et al. Reduction of recruitment costs in preclinical AD trials: validation of automatic pre‐screening algorithm for brain amyloidosis. Stat Methods Med Res. 2019;29(1):151‐164. [DOI] [PubMed] [Google Scholar]
- 11. Doraiswamy PM, Sperling RA, Coleman RE, et al. Amyloid‐β assessed by florbetapir F 18 PET and 18‐month cognitive decline: a multicenter study. Neurology. 2012;79(16):1636‐1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tapiola T, Alafuzoff I, Herukka S‐K, et al. Cerebrospinal fluid β‐amyloid 42 and tau proteins as biomarkers of Alzheimer‐type pathologic changes in the brain. Arch Neurol. 2009;66(3):382‐389. [DOI] [PubMed] [Google Scholar]
- 13. Palmqvist S, Mattsson N, Hansson O. Cerebrospinal fluid analysis detects cerebral amyloid‐β accumulation earlier than positron emission tomography. Brain. 2016;139(4):1226‐1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Johnson KA, Minoshima S, Bohnen NI, et al. Update on appropriate use criteria for amyloid PET imaging: dementia experts, mild cognitive impairment, and education. J Nucl Med. 2013;54(7):1011‐1013. [DOI] [PubMed] [Google Scholar]
- 15. Mitka M. PET imaging for Alzheimer disease: are its benefits worth the cost?. JAMA. 2013;309(11):1099‐1100. [DOI] [PubMed] [Google Scholar]
- 16. Maserejian N, Bian S, Wang W, et al. Practical algorithms for amyloid β probability in subjective or mild cognitive impairment. Alzheimers Dement (Amst). 2019;11:180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Baluchnejadmojarad T, Mohamadi‐Zarch S‐M, Roghani M. Safranal, an active ingredient of saffron, attenuates cognitive deficits in amyloid β‐induced rat model of Alzheimer's disease: underlying mechanisms. Metab Brain Dis. 2019;34(6):1747‐1759. [DOI] [PubMed] [Google Scholar]
- 18. Ko H, Ihm JJ, Kim HG. Cognitive profiling related to cerebral amyloid beta burden using machine learning approaches. Front Aging Neurosci. 2019;11:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Voyle N, Patel H, Folarin A, et al. Genetic risk as a marker of amyloid‐β and tau burden in cerebrospinal fluid. J Alzheimers Dis. 2017;55(4):1417‐1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leonenko G, Shoai M, Bellou E, et al. Genetic risk for alzheimer disease is distinct from genetic risk for amyloid deposition. Ann Neurol. 2019;86(3):427‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tosun D, Chen Y‐F, Yu P, et al. Amyloid status imputed from a multimodal classifier including structural MRI distinguishes progressors from nonprogressors in a mild Alzheimer's disease clinical trial cohort. Alzheimers Dement. 2016;12(9):977‐986. [DOI] [PubMed] [Google Scholar]
- 22. Yasuno F, Kazui H, Morita N, et al. Use of T1‐weighted/T2‐weighted magnetic resonance ratio to elucidate changes due to amyloid β accumulation in cognitively normal subjects. Neuroimage Clin. 2017;13:209‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Casamitjana A, Petrone P, Tucholka A, et al. MRI‐based screening of preclinical Alzheimer's disease for prevention clinical trials. J Alzheimers Dis. 2018;64(4):1099‐1112. [DOI] [PubMed] [Google Scholar]
- 24. Palmqvist S, Janelidze S, Stomrud E, et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease–related β‐amyloid status. JAMA Neurol. 2019;76(9):1060‐1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verberk IMW, Slot RE, Verfaillie SCJ, et al. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol. 2018;84(5):648‐658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schindler SE, Bollinger JG, Ovod V, et al. High‐precision plasma β‐amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93(17):e1647‐e1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park J‐C, Han S‐H, Lee H, et al. Prognostic plasma protein panel for Aβ deposition in the brain in Alzheimer's disease. Prog Neurobiol. 2019;183:101690. [DOI] [PubMed] [Google Scholar]
- 28. Ashton NJ, Nevado‐Holgado AJ, Barber IS, et al. A plasma protein classifier for predicting amyloid burden for preclinical Alzheimer's disease. Sci Adv. 2019;5(2):eaau7220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Anderson M, Perrin A. Tech Adoption Climbs Among Older Adults. 2017, Pew Research Center. https://www.pewresearch.org/internet/2017/05/17/tech-adoption-climbs-among-older-adults/
- 30. Weiner MW, Nosheny R, Camacho M, et al. The Brain Health Registry: an internet‐based platform for recruitment, assessment, and longitudinal monitoring of participants for neuroscience studies. Alzheimers Dement. 2018;14(8):1063‐1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Nosheny RL, Camacho MR, Insel PS, et al. Online study partner‐reported cognitive decline in the Brain Health Registry. Alzheimers Dement (N Y). 2018;4:565‐574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rabinovici GD, Gatsonis C, Apgar C, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321(13):1286‐1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nosheny RL, Camacho MR, Jin C, et al. Validation of online functional measures in cognitively impaired older adults. Alzheimer's & Dementia. 2020; 10.1002/alz.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lee JH, Byun MS, Yi D, et al. Prediction of cerebral amyloid with common information obtained from memory clinic practice. Front Aging Neurosci. 2018;10:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mielke MM, Wiste HJ, Weigand SD, et al. Indicators of amyloid burden in a population‐based study of cognitively normal elderly. Neurology. 2012;79(15):1570‐1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arenaza‐Urquijo EM, Bejanin A, Gonneaud J, et al. Association between educational attainment and amyloid deposition across the spectrum from normal cognition to dementia: neuroimaging evidence for protection and compensation. Neurobiol Aging. 2017;59:72‐79. [DOI] [PubMed] [Google Scholar]
- 37. Snitz BE, Weissfeld LA, Cohen AD, et al. Subjective cognitive complaints, personality and brain amyloid‐beta in cognitively normal older adults. Am J Geriatr Psychiatry. 2015;23(9):985‐993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chung JK, Plitman E, Nakajima S, et al. Lifetime history of depression predicts increased amyloid‐β accumulation in patients with mild cognitive impairment. J Alzheimers Dis. 2015;45(3):907‐919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nosheny RL, et al. Increasing the impact of the ideas study using brain health registry online data collection. Alzheimers Dementia. 2019;15(7):P1216‐P1217. [Google Scholar]
- 40. Farias ST, Mungas D, Reed BR, et al. The measurement of everyday cognition (ECog): scale development and psychometric properties. Neuropsychology. 2008;22(4):531‐544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yesavage JA, Brink TL, Rose TL, et al. Development and validation of a geriatric depression screening scale: a preliminary report. J Psychiatr Res. 1982;17(1):37‐49. [DOI] [PubMed] [Google Scholar]
- 42. Sheikh JI, Yesavage JA. Recent evidence and development of a shorter version In: Brink TL, ed. Clinical Gerontology: A Guide to Assessment and Intervention. New York: The Haworth Press; 1986:165‐173. [Google Scholar]
- 43. Moons KG, Wolff RF, Riley RD, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med. 2019;170(1):W1‐W33. [DOI] [PubMed] [Google Scholar]
- 44. Alzheimer's Association . 2019 Alzheimer's disease facts and figures. Alzheimers Dement, 2019;15(3):321‐387. [DOI] [PubMed] [Google Scholar]
- 45. Nosheny RL, Insel PS, Mattsson N, et al. Associations among amyloid status, age, and longitudinal regional brain atrophy in cognitively unimpaired older adults. Neurobiol Aging. 2019;82:110‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. de Rojas I, Romero J, Rodríguez‐Gomez O, et al. Correlations between plasma and PET beta‐amyloid levels in individuals with subjective cognitive decline: the Fundacio ACE healthy brain initiative (FACEHBI). Alzheimers Res Ther. 2018;10(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vermunt L, Muniz‐Terrera G, Ter Meulen L, et al. Prescreening for European Prevention of Alzheimer Dementia (EPAD) trial‐ready cohort: impact of AD risk factors and recruitment settings. Alzheimers Res Ther. 2020;12(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tosun D, Joshi S, Weiner MW, the Alzheimer's Disease Neuroimaging Initiative . Multimodal MRI‐based imputation of the Aβ+ in early mild cognitive impairment. Ann Clin Transl Neurol. 2014;1(3):160‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sperling RA, Donohue MC, Raman R, et al. Association of factors with elevated amyloid burden in clinically normal older individuals. JAMA Neurol. 2020;77(6):735‐745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Madsen K, Hasselbalch BJ, Frederiksen KS, et al. Lack of association between prior depressive episodes and cerebral [11C] PiB binding. Neurobiol Aging. 2012;33(10):2334‐2342. [DOI] [PubMed] [Google Scholar]
- 51. Gold D, Rosowsky E, Piryatinsky I, Sinclair SJ. Comparing patient and informant ratings of depressive symptoms in various stages of Alzheimer's disease. Neuropsychology. 2020;34(5):535‐550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Jansen WJ, Ossenkoppele R, Knol DL, et al. Prevalence of cerebral amyloid pathology in persons without dementia: a meta‐analysis. JAMA. 2015;313(19):1924‐1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Jack CR, Jr , Bennett DA, Blennow K, et al. NIA‐AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stites SD, Karlawish J, Harkins K, Rubright JD, Wolk D. Awareness of mild cognitive impairment and mild Alzheimer's disease dementia diagnoses associated with lower self‐ratings of quality of life in older adults. J Gerontol B Psychol Sci Soc Sci. 2017;72(6):974‐985. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information.


