Abstract
Aim
To describe the epidemiological and clinical characteristics along with outcomes of hospitalized Coronavirus Disease 2019 (COVID-19) patients with and without diabetes.
Methods
This retrospective, single-center study included 595 consecutive hospitalized patients with confirmed COVID-19 at Baqiyatallah Hospital in Tehran, Iran, from February 26, 2020 to March 26, 2020. Demographic data, clinical, laboratory, and radiological findings were collected and compared between patients based on diabetes status. Complications and clinical outcomes were followed up until April 4, 2020.
Results
From among the 595 hospitalized patients with COVID-19, the median age was 55 years and 401 (67.4%) were male. The most common symptoms included fever (419 [70.4%]), dry cough (368 [61.8%]) and dyspnea (363 [61%]). A total of 148 patients (24.9%) had diabetes, and compared with patients without diabetes, these patients had more comorbidities (eg, hypertension [48.6% vs. 22.3%; P < 0.001]); had higher levels of white blood cell count, neutrophil count, C-reactive protein, erythrocyte sedimentation rate and blood urea nitrogen, and had a higher proportion of patchy ground-glass opacity in chest computed tomography findings (52.7% vs. 25.7%; P < 0.001). Significantly, patients with diabetes had more complications and needed more respiratory support than those without diabetes (P < 0.001). At the end of the follow-up, treatment failure and death was significantly higher in patients with diabetes compared to those without diabetes (17.8% vs. 8.7%; P = 0.003).
Conclusion
COVID-19 patients with diabetes are at a higher risk of complications and a higher in-hospital mortality during hospitalization. Diabetes status of COVID-19 patients and frequent monitoring of glycemia would be helpful to prevent deteriorating clinical conditions.
Keywords: COVID-19, Diabetes, Hospitalized, Clinical characteristics
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by a novel coronavirus named the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was initially reported in Wuhan, China and has rapidly become a global pandemic [1], [2]. The first patient with confirmed COVID-19 in Iran was announced from Qom province on February 19, 2020 [3]. Shortly afterwards, the infection spread rapidly throughout the country, and Iran became one of the epicenters of the COVID-19 pandemic [4]. As of July 13, 2020, a total of 257.303 laboratory-confirmed cases and over 12.000 deaths in Iran have been documented [5].
The COVID-19 infection causes an intricate situation for people with underlying diseases including cardiac disease, diabetes, hypertension and respiratory diseases, which result in rising rates of hospitalization and mortality [6], [7]. Notably, the highest number of comorbidities has been seen in infected patients admitted to the intensive care unit (ICU), suggesting that the chronic diseases are likely to be risk factors for adverse clinical outcomes [8]. In this context, the largest COVID-19 study in America found that diabetes was one of the most frequent comorbidities (33.8%) among 5700 hospitalized patients with COVID-19 [9]. Moreover, it has been shown that expression of angiotensin-converting enzyme II (ACE2) as a cell entry receptor for SARS-CoV-2 is significantly increased in diabetes patients treated with angiotensin-converting enzymes (ACE) inhibitors and angiotensin II receptor blockers (ARBs) [10]. Consequently, the ACE2 overexpression make them highly vulnerable to COVID-19 infection and may have an unfavorable prognosis.
Currently, there is limited data on the characteristics and outcomes of diabetes patients hospitalized with COVID-19 in Iran. Further awareness of the baseline characteristics and risk factors for COVID-19 in different clinical settings is needed for better patient management and mitigation of disease complications. Hence, in this study it was aimed to evaluate and compare demographic and clinical characteristics, laboratory findings, treatment and outcomes of hospitalized COVID-19 patients with and without diabetes from a single medical center in Iran.
2. Methods
2.1. Study design and participants
This research was a retrospective study of 595 consecutive patients with confirmed COVID-19 who were admitted to Baqiyatallah Hospital from February 26, 2020 to March 26, 2020. Baqiyatallah Hospital, affiliated to Baqiyatallah University of Medical Sciences, located in Tehran province, Iran, is one of the major assigned hospitals for the treatment of COVID-19 patients by the government. All COVID-19 patients included in this study were hospitalized and were diagnosed according to the World Health Organization (WHO) interim guidance [11]. The cases infected with SARS-CoV-2 were confirmed by reverse transcription-polymerase chain reaction (RT-PCR) assay on throat and nose swab samples. The clinical outcomes of these patients were monitored to April 4, 2020, the date of the last follow-up.
This study was reviewed and approved by the ethical committee of the Baqiyatallah University of Medical Sciences, Iran (IR.BMSU.REC.1399.183). The need for written informed consent was waived by the ethics committee due to the retrospective nature of this study and the patient data anonymity. However, a verbal consent was obtained from either each patient or their next of kin before their data were included in this study.
2.2. Data collection
The demographic data, exposure history, clinical symptoms and signs, laboratory findings, chest X-ray or computed tomography (CT) scans, underlying comorbidities, treatment measures and outcomes data of each patient were obtained using standardized data collection forms from electronic medical records. All data collected were reviewed by an experienced team of physicians and entered into the computer database. Patients with missing data or medical recorded unknown on characteristics studied were excluded. Diabetes cases were identified based on patient’s self-report or medical records confirmed by endocrinologists. Diagnosed diabetes was defined according to the WHO diagnostic criteria of fasting plasma glucose ≥ 126 mg/dL (≥7.0 mmol/L). The date of disease onset was defined as the day when the first sign or symptom was appeared. Acute respiratory distress syndrome (ARDS) was diagnosed according to the Berlin definition [12]. All clinical outcomes of patients were presented after completing the hospital period at the end of the study. In this study, patients were clinically stratified into three groups of moderate, severe and critical disease according to the criteria defined as follows:
Diagnostic criteria for moderate cases were: fever, respiratory symptoms and CT manifestation of pneumonia. At least one of the diagnostic criteria for severe cases was: dyspnea with a respiratory rate ≥ 30 breaths/min, blood oxygen saturation ≤ 93% at rest or partial pressure of oxygen in arterial blood (PaO2)/ fraction of inspired oxygen (FiO2) ≤ 300 mmHg as hypoxemia and chest imaging with progression in lesion of more than 50% within 24–48 h. The diagnostic criteria for critical cases was: respiratory failure with mechanical ventilation need, shock and dysfunction of other organ requiring ICU care.
2.3. Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median and interquartile range (IQR). Categorical variables were expressed as frequencies and percentages (%). The Fisher exact test or χ2 test was applied to compare categorical variables and independent t-test or Mann-Whitney U test was applied to compare continuous variables, as appropriate. The data were analyzed using SPSS software (version 22.0; IBM). For all the statistical analyses, p-value < 0.05 was considered statistically significant.
3. Results
A total of 595 hospitalized patients with COVID-19 confirmed by RT-PCR detection of SARS-CoV-2 were included in this retrospective study. Among these patients, 148 (24.9%) and 447 (75.1%) were identified as diabetic and non-diabetic COVID-19 patients, respectively. The demographic and clinical characteristics of the studied patients according to diabetes status are shown in Table 1 . The patients had a very wide age span (IQR, 45–63; range, 22–94 years), with the median age of 55 years, and most patients (401 [67.4%]) were male. The median time from symptom onset to hospital admission was seven days (IQR, 3.7–9). The median duration of hospitalization was 11 days (IQR, 7.5–15). Most patients (365 [61.3%]) had a history of referral to medical centers in the last two weeks, and 40 patients (6.7%) were current smokers. The most common symptoms were fever (419 [70.4%]), dry cough (368 [61.8%]), dyspnea (363 [61%]), fatigue (332 [55.8%]), Myalgia/arthralgia (320 [54%]) and chill (317 [53%]) at the illness onset. Less common symptoms included headache, nausea or vomiting, chest pain, diarrhea, taste loss, sputum production and smell loss. Hemoptysis (43 [7.2%]) and earache (40 [7%]) were relatively rare. From among the 595 patients, 332 (55.8%) presented at least one coexisting condition; the most common of which were obesity (176 [29.6%]), hypertension (172 [28.9%]) and diabetes (148 [24.9%]). The disease severity on admission was moderate in 487 (81.8%) of the patients, severe in 85 (14.3%), and critical in 23 (%3.9).
Table 1.
Baseline demographics and clinical characteristics of hospitalized patients with COVID-19.
| Characteristic |
Patients, No. (%) |
P valuea | ||
|---|---|---|---|---|
| Total (n = 595) | Diabetes (n = 148) | Non-diabetes (n = 447) | ||
| Age, median (IQR), years | 55 (45–63) | 54 (43–63) | 56 (47–69) | 0.323 |
| Sex | ||||
| Male | 401 (67.4) | 99 (66.9) | 302 (67.6) 145 (32.4) |
0.478 |
| Female | 194 (32.6) | 49 (33.1) | ||
| Exposure history | ||||
| Referral to medical centers in the last 2 weeks | 365 (61.3) | 93 (62.8) | 272 (60.8) | 0.343 |
| Contact with suspected or confirmed cases in the last 2 weeks | 264 (44.3) | 70 (47.2) | 194 (43.4) | 0.166 |
| Travel to epidemic area in the last 2 weeks | 140 (23.5) | 42 (28.3) | 98 (22) | 0.074 |
| Current smoker | 40 (6.7) | 9 (6) | 31 (6.9) | 0.436 |
| Signs and symptoms at admission | ||||
| Fever (temperature ≥37.3 °C) | 419 (70.4) | 103 (69.6) | 316 (70.7) | 0.438 |
| Dry cough | 368 (61.8) | 97 (65.5) | 271 (60.6) | 0.172 |
| Dyspnea | 363 (61) | 89 (60) | 274 (61.3) | 0.391 |
| Fatigue | 332 (55.8) | 79 (53.4) | 253 (56.6) | 0.505 |
| Myalgia/arthralgia | 320 (54) | 80 (54) | 240 (53.6) | 0.537 |
| Chill | 317 (53) | 77 (52) | 240 (53.7) | 0.532 |
| Headache | 207 (34.7) | 49 (33.1) | 158 (35.3) | 0.341 |
| Nausea or vomiting | 188 (31.5) | 52 (35.1) | 136 (36.4) | 0.179 |
| Chest pain | 145 (24.3) | 44 (30) | 101 (22.6) | 0.059 |
| Diarrhea | 116 (19.5) | 32 (21.6) | 84 (18.8) | 0.280 |
| Taste loss | 109 (18.3) | 25 (16.8) | 84 (18.8) | 0.250 |
| Sputum production | 107 (18) | 35 (23.8) | 72 (16.1) | 0.031 |
| Smell loss | 102 (17.1) | 18 (12.1) | 84 (18.8) | 0.020 |
| Sore throat | 81 (13.6) | 19 (12.9) | 62 (13.9) | 0.410 |
| Dizziness | 75 (12.6) | 22 (14.8) | 53 (11.8) | 0.270 |
| Rhinorrhea | 61 (10.2) | 26 (17.6) | 35 (7.8) | 0.001 |
| Hemoptysis | 43 (7.2) | 12 (8.1) | 31 (6.9) | 0.375 |
| Earache | 40 (7) | 7 (4.8) | 33 (7.3) | 0.141 |
| Comorbidities | ||||
| Obesity (BMI ≥30) | 176 (29.6) | 48 (32.4) | 128 (28.6) | 0.205 |
| Hypertension | 172 (28.9) | 72 (48.6) | 100 (22.3) | <.001 |
| Cardiovascular disease | 112 (18.8) | 40 (27) | 72 (16.1) | 0.003 |
| Asthma and allergic diseases | 95 (16) | 22 (14.9) | 73 (16.3) | 0.390 |
| Pulmonary disease | 87 (14.6) | 24 (16.2) | 63 (14.09) | 0.308 |
| Chronic kidney disease | 58 (9.7) | 24 (16.2) | 34 (7.6) | 0.030 |
| Chronic liver disease | 32 (5.3) | 14 (9.4) | 18 (4) | 0.013 |
| Thyroid disease | 21 (3.5) | 6 (4) | 15 (3.3) | 0.541 |
| Rheumatic disease | 16 (2.6) | 3 (2) | 13 (2.9) | 0.407 |
| Cerebrovascular disease | 13 (2.2) | 8 (5.4) | 5 (1.1) | 0.005 |
| Cancer | 12 (2) | 5 (3.4) | 7 (1.6) | 0.184 |
| Disease onset to admission, median (IQR), days | 7 (3.7–9) | 7 (4.5–8) | 8 (3–9.5) | 0.407 |
| Hospitalization duration, median (IQR), days | 11 (7.5–15) | 12 (9–16) | 10.5 (7–14) | 0.103 |
IQR, interquartile range; COVID-19, coronavirus disease 2019; BMI, body mass index; No., number.
aP values indicate differences between diabetes and non-diabetes patients. P < 0.05 was considered statistically significant.
There was no significant difference between the patients with and without diabetes in terms age, sex and exposure history. Compared with non-diabetic patients, patients with diabetes had more sputum production (35 [23.8%] vs. 72 [16.1%]; P = 0.031) and rhinorrhea (26 [17.6%] vs. 35 [7.8%]; P = 0.001) and less smell loss (18 [12.1%] vs. 84 [18.8%]; P = 0.020). Moreover, underlying comorbidities, including hypertension (72 [48.6%] vs. 100 [22.3%]; P < 0.001), cardiovascular disease (40 [27%] vs. 72 [16.1%]; P = 0.003), chronic kidney disease (24 [16.2%] vs. 34 [7.6%]; P = 0.030), chronic liver disease (14 [9.4%] vs. 18 [4%]; P = 0.013), cerebrovascular disease (8 [5.4%] vs. 5 [1.1%]; P = 0.005).
The laboratory and radiographic findings at admission are shown in Table 2 . In all the patients, the counts of lymphocytes (0.87 [IQR, 0.65–1.3] × 109/L) were below the normal range and erythrocyte sedimentation rate (ESR; 27 [IQR, 20–39] mm/h), C-reactive protein (CRP; 22 [IQR, 12–48] mg/L) and lactate dehydrogenase (LDH; 625 [IQR, 517–789] U/L) were above the normal range, while the values of other laboratory indices showed a normal change. According to chest radiography or CT findings, 527 patients (88.6%) showed bilateral pneumonia, and 193 patients (32.4%) showed patchy ground-glass opacity.
Table 2.
Laboratory and radiographic findings among hospitalized COVID-19 patients with diabetes and without diabetes.
| Characteristic | Normal range |
Median (IQR) |
P valuea | ||
|---|---|---|---|---|---|
| Total (n = 595) | Diabetes (n = 148) | Non-diabetes (n = 447) | |||
| Laboratory findings at admission | |||||
| Red blood cells count, ×1012/L | 4.3–5.8 | 4.8 (4.4–5.1) | 4.6 (4.2–5) | 4.8 (4.5–5.2) | 0.001 |
| White blood cell count, ×109/L | 3.5–9.5 | 5.8 (4.4–7.2) | 5.9 (4.7–7.4) | 5.3 (4.2–6.9) | 0.028 |
| Lymphocyte count, ×109/L | 1.1–3.2 | 0.87 (0.65–1.3) | 0.83 (0.59–1.3) | 0.94 (0.71–1.3) | 0.045 |
| Neutrophil count, ×109/L | 1.8–6.3 | 3.7 (2.4–5.6) | 4.2 (2.9–6.2) | 3.3 (2.3–4.8) | 0.001 |
| Platelet count, ×109/L | 125–350 | 180 (138–229) | 178 (138–246) | 181 (138–226) | 0.975 |
| Hemoglobin, g/L | 115–150 | 144 (134–154) | 142 (132–150) | 145 (134–156) | 0.012 |
| ESR, mm/h | 0–20 | 27 (20–39) | 35 (25–46) | 26 (17–36) | 0.001 |
| PT, s | 9–13 | 12.6 (11.8–13) | 12.7 (11.9–13) | 12.6 (11.8–12.9) | 0.109 |
| APTT, s | 29–42 | 31 (28.4–35.7) | 31 (28.8–36.4) | 30.4 (28.1–34.8) | 0.486 |
| CRP, mg/L | 0–10 | 22 (12–48) | 27 (15–59) | 21 (11–45) | 0.010 |
| Pao2, mmHg | 80–100 | 91 (87–94) | 89 (82–94) | 91 (88–94) | 0.006 |
| LDH, U/L | 207–414 | 625 (517–789) | 628 (543–748) | 624 (502–791) | 0.850 |
| ALT, U/L | ≤45 | 31 (19–47) | 31 (19–54) | 31 (19–46) | 0.650 |
| AST, U/L | ≤35 | 35 (26–47) | 36 (26–51) | 35 (25–45) | 0.292 |
| BUN, mmol/L | 2.8–7.6 | 5 (4.4–6.7) | 5.3 (4.3–7.7) | 4.6 (3.5–5.7) | 0.001 |
| Creatinine, mg/dL | 0.5–1.2 | 1.1 (0.9–1.3) | 1.2 (0.9–1.3) | 1.1 (0.8–1.2) | 0.503 |
| Creatine kinase, U/L | ≤170 | 133 (89–225) | 133 (87–232) | 130 (97–213) | 0.777 |
| Sodium, mEq/L | 136–145 | 136 (134–138) | 136 (134–138) | 136 (133–138) | 0.585 |
| Potassium, mEq/L | 3.5–5 | 4.1 (3.9–4.4) | 4.1 (3.7–4.4) | 4.1 (3.9–4.4) | 0.175 |
| Chest CT findings, No.% | |||||
| Unilateral pneumonia | NA | 68 (11.4) | 15 (10.1) | 53 (11.9)394 (88.1) | 0.343 |
| Bilateral pneumonia | NA | 527 (88.6) | 133 (89.9) | ||
| Patchy ground-glass opacity | NA | 193 (32.4) | 78 (52.7) | 115 (25.7) | <.001 |
IQR, interquartile range; COVID-19, coronavirus disease 2019; ESR, erythrocyte sedimentation rate; PT, prothrombin time; APTT, activated partial thromboplastin time; CRP, C-reactive protein; Pao2, partial pressure of oxygen; LDH, lactate dehydrogenase; ALT, alanine aminotransferase; AST, aspartate aminotransferase, BUN, blood urea nitrogen; NA, not available; No., number.
aP values indicate differences between diabetes and non-diabetes patients. P < 0.05 was considered statistically significant.
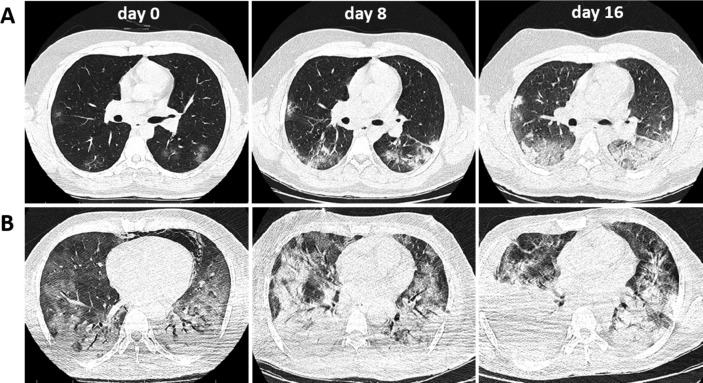
In terms of laboratory test results, diabetes patients compared with patients without diabetes showed higher white blood cell count (5.9 [IQR, 4.7–7.4] vs. 5.3 [IQR, 4.2–6.9] × 109/L; P = 0.028), neutrophil count (4.2 [IQR, 2.9–6.2] vs. 3.3 [IQR, 2.3–4.8] × 109/L; P = 0.001) and levels of CRP (27 [IQR, 15–59] vs. 21 [IQR, 11–45] mg/L; P = 0.010), ESR (35 [IQR, 25–46] vs. 26 [IQR, 17–36] mm/h; P = 0.001) and blood urea nitrogen (BUN; 5.3 [IQR, 4.3–7.7] vs. 4.6 [IQR, 3.5–5.7] mmol/L; P = 0.001), and lower red blood cells count (RBC; 4.6 [IQR, 4.2–5] vs. 4.8 [IQR, 4.5–5.2] × 1012/L; P = 0.001), lymphocyte count (0.83 [IQR, 0.59–1.3] vs. 0.94 [IQR, 0.71–1.3] × 109/L; P = 0.045), and level of hemoglobin (142 [IQR, 132–150] vs. 145 [IQR, 134–156] g/L; P = 0.012) and Pao2 (89 [IQR, 82–94] vs. 91 [IQR, 88–94] mmHg; P = 0.006) (Table 2). These laboratory data indicated that the COVID-19 patients with diabetes were more involved in severe inflammatory response, which may lead to poorer prognosis compared to patients without diabetes. Moreover, diabetes patients had higher prevalence of patchy ground-glass opacity (78 [52.7%] vs. 115 [25.7%]; P < 0.001) (Table 2). Fig. 1 shows the chest CT results of the COVID-19 patients with and without diabetes.
Fig. 1.
Chest CT images of the representative patients infected with covid-19 during the hospitalization. A: chest CT of a 29-year-old male patient without diabetes; B: chest CT from a 49-year-old male patient with diabetes. CT images of both patients were obtained on February 29th (day 0), March 8th (day 8) and March 16th (day 16), showing the patchy ground-glass opacity in both lungs, with pleural effusion at the late stage.
During hospitalization, a total of 511 patients (85.9%) received oxygen therapy, and the oxygen inhalation, noninvasive ventilation, and invasive mechanical ventilation were used in 402 (67.6%), 81 (13.6%), and 28 (4.7%) patients, respectively. Most patients were given antiviral therapy (581 [97.6%]), followed by antibiotic therapy (503 [84.5%]), glucocorticoid therapy (190 [31.9%]) and intravenous immunoglobulin therapy (94 [15.8%]). Fourteen (2.3%) patients received antifungal therapy. Antiviral therapy has been empirically performed by prescribing at least one antiviral agent such as oseltamivir, ribavirin, favipiravir, remdesivir and lopinavir/ritonavir without clinical trials. Antibiotics, including quinolones, cephalosporins, carbapenems and macrolides, were administered for secondary bacterial infection according to the antibiogram results. Moreover, 14 (2.3%) patients received plasma treatment, and 10 (1.7%) patients were treated with interferon administration. Overall, common complications included ARDS (91 [15.3%]), shock (83 [13.9%]) and secondary infection (75 [12.6%]). During the follow-up, a total of 65 patients (10.9%) died, 156 patients (26.2%) were discharged, and 374 patients (62.9%) stayed hospitalized.
Compared with those without diabetes, patients with diabetes required more oxygen (119 [80.4%] vs. 283 [63.3%]; P < 0.001), noninvasive ventilation (53 [35.8%] vs. 28 [6.3%]; P < 0.001), invasive mechanical ventilation (16 [10.8%] vs. 12 [2.7%]; P < 0.001) and antifungal therapy (8 [5.4%] vs. 6 [1.3%]; P = 0.009) (Table 3 ). In addition to, ARDS (36 [24.3%] vs. 55 [12.3%]; P = 0.001), shock (31 [20.9%] vs. 52 [11.6%]; P = 0.004) and secondary infection (29 [19.6%] vs. 46 [10.3%]; P = 0.003) were more prevalent in patients with diabetes than in those without diabetes.
Table 3.
Treatments, complications and clinical outcomes of hospitalized COVID-19 patients with diabetes and without diabetes.
| Characteristic |
Patients, No. (%) |
P valuea | ||
|---|---|---|---|---|
| Total (n = 595) | Diabetes (n = 148) | Non-diabetes (n = 447) | ||
| Treatment | ||||
| Oxygen inhalation | 402 (67.6) | 119 (80.4) | 283 (63.3) | <.001 |
| Noninvasive ventilation | 81 (13.6) | 53 (35.8) | 28 (6.3) | <.001 |
| Invasive mechanical ventilation | 28 (4.7) | 16 (10.8) | 12 (2.7) | <.001 |
| Antiviral therapy | 581 (97.6) | 146 (98.6) | 435 (97.3) | 0.535 |
| Antibiotic therapy | 503 (84.5) | 129 (87.1) | 374 (83.7) | 0.188 |
| Antifungal therapy | 14 (2.3) | 8 (5.4) | 6 (1.3) | 0.009 |
| Glucocorticoid therapy | 190 (31.9) | 57 (38.5) | 133 (29.7) | 0.310 |
| Intravenous immunoglobulin therapy | 94 (15.8) | 22 (14.9) | 72 (16.1) | 0.415 |
| Plasma therapy | 14 (2.3) | 5 (3.4) | 9 (2) | 0.353 |
| Interferon administration | 10 (1.7) | 4 (2.7) | 6 (1.3) | 0.276 |
| Complications | ||||
| ARDS | 91 (15.3) | 36 (24.3) | 55 (12.3) | 0.001 |
| Shock | 83 (13.9) | 31 (20.9) | 52 (11.6) | 0.004 |
| Secondary infection | 75 (12.6) | 29 (19.6) | 46 (10.3) | 0.003 |
| Clinical outcome | ||||
| Hospital Discharge | 156 (26.2) | 33 (22.3) | 123 (27.5) | 0.295 |
| Hospital stay | 374 (62.9) | 89 (60.1) | 285 (63.7) | |
| Death | 65 (10.9) | 26 (17.8) | 39 (8.7) | 0.003 |
COVID-19, coronavirus disease 2019; ARDS, acute respiratory distress syndrome; No., number.
aP values indicate differences between diabetes and non-diabetes patients. P < 0.05 was considered statistically significant.
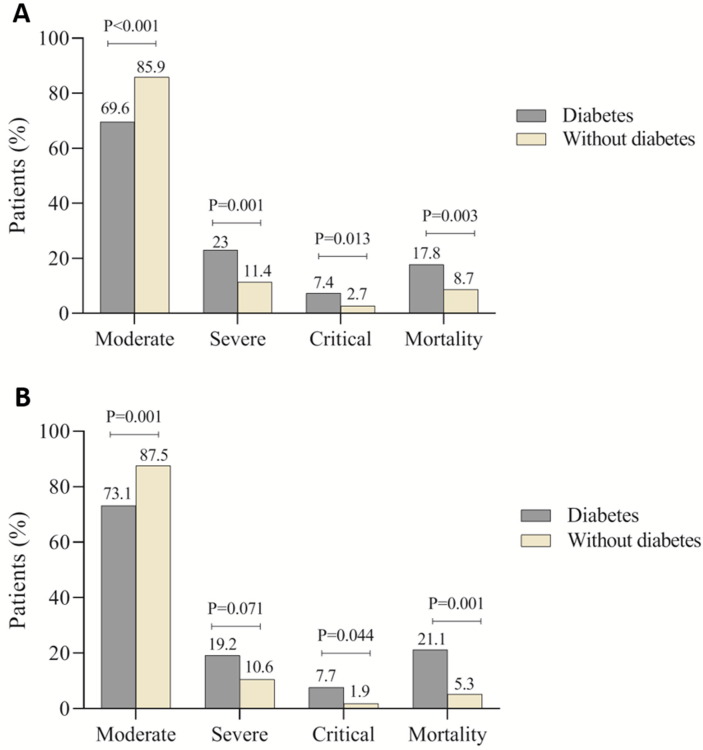
Next, a similar analysis was performed in the absence of other comorbidities to investigate whether diabetes increases the disease severity and death per se. We found that white blood cell count (5.8 [IQR, 4.5–7.2] vs. 5.4 [IQR, 4.3–6.5] × 109/L; P = 0.066), neutrophil count (4 [IQR, 2.4–6.1] vs. 3.1 [IQR, 2.6–4.3] × 109/L; P = 0.021) and levels of CRP (43.4 [IQR, 16–64] vs. 18.7 [IQR, 10–48] mg/L; P = 0.003), ESR (40 [IQR, 27–50] vs. 25 [IQR, 15–33] mm/h; P = 0.001), LDH (497 [IQR, 346–630] vs. 380 [IQR, 281–545] U/L; P = 0.026) and blood urea nitrogen (BUN; 4.8 [IQR, 3.5–6.5] vs. 4.3 [IQR, 3.2–5] mmol/L; P = 0.020) were significantly higher in diabetes patients compared to patients without diabetes. The levels of CRP, ESR and LDH were above the normal range in patients with diabetes, indicating a more severe inflammatory response and tissue damage in these patients. Patients with diabetes were more likely to have suffered ARDS (10 [19.2%] vs. 23 [8.7%]; P = 0.001), shock (9 [17.6%] vs. 17 [6.5%]; P = 0.004) and secondary infection (10 [19.2%] vs. 21 [8%]; P = 0.003) compared with patients without diabetes (Table 4 ). Comparisons of the disease severity and mortality between COVID-19 patients with diabetes and those without diabetes in the presence and absence of other comorbidities showed statistically significant differences (Fig. 2 ).
Table 4.
Clinical characteristics, laboratory findings and outcomes of hospitalized COVID-19 patients with and without diabetes in the absence of other comorbidities.
| Characteristic |
Patients, No. (%) |
P valuea | |||
|---|---|---|---|---|---|
| Total (n = 315) | Diabetes (n = 52) | Non-diabetes (n = 263) | |||
| Age, median (IQR), years | 54 (44–63) | 54 (47–63) | 49 (44–58) | 0.236 | |
| Sex | |||||
| Male | 206 (65.4) | 33 (63.5) | 173 (65.8) 90 (34.2) |
0.432 | |
| Female | 109 (34.6) | 19 (36.5) | |||
| Signs and symptoms at admission | |||||
| Fever (temperature ≥37.3 °C) | 229 (72.7) | 37 (71.2) | 192 (73) | 0.452 | |
| Dry cough | 212 (67.3) | 36 (69.2) | 176 (66.9) | 0.440 | |
| Dyspnea | 164 (52.1) | 30 (57.7) | 134 (51) | 0.231 | |
| Fatigue | 173 (54.9) | 29 (55.8) | 144 (54.8) | 0.508 | |
| Myalgia/arthralgia | 153 (48.6) | 26 (50) | 127 (48.3) | 0.470 | |
| Chill | 178 (56.5) | 31 (59.6) | 147 (55.9) | 0.368 | |
| Headache | 118 (37.5) | 17 (32.7) | 101 (38.4) | 0.269 | |
| Nausea or vomiting | 97 (30.8) | 19 (36.5) | 78 (29.7) | 0.205 | |
| Chest pain | 59 (18.7) | 11 (21.2) | 48 (18.3) | 0.374 | |
| Diarrhea | 65 (20.6) | 14 (26.9) | 51 (19.4) | 0.150 | |
| Taste loss | 47 (14.9) | 6 (11.5) | 41 (15.6) | 0.305 | |
| Sputum production | 39 (12.4) | 8 (15.4) | 31 (11.8) | 0.302 | |
| Smell loss | 32 (10.2) | 3 (5.8) | 29 (11) | 0.188 | |
| Sore throat | 29 (9.2) | 5 (9.6) | 24 (9.1) | 0.541 | |
| Dizziness | 28 (8.9) | 7 (13.5) | 21 (8) | 0.157 | |
| Rhinorrhea | 10 (3.2) | 4 (7.7) | 6 (2.3) | 0.065 | |
| Hemoptysis | 8 (2.5) | 3 (5.8) | 5 (1.9) | 0.129 | |
| Earache | 7 (2.2) | 0 | 7 (2.7) | 0.279 | |
| Laboratory findings, median (IQR) | Normal Range | ||||
| Red blood cells count, ×1012/L | 4.3–5.8 | 4.9 (4.5–5.2) | 4.5 (4.3–4.9) | 4.9 (4.7–5.4) | 0.016 |
| White blood cell count, ×109/L | 3.5–9.5 | 5.7 (4.3–6.9) | 5.8 (4.5–7.2) | 5.4 (4.3–6.5) | 0.066 |
| Lymphocyte count, ×109/L | 1.1–3.2 | 0.94 (0.61–1.3) | 0.64 (0.5–0.88) | 1.3 (1.1–1.6) | 0.001 |
| Neutrophil count, ×109/L | 1.8–6.3 | 3.8 (2.6–5.3) | 4 (2.4–6.1) | 3.1 (2.6–4.3) | 0.021 |
| Platelet count, ×109/L | 125–350 | 184 (141–241) | 184 (141–254) | 197 (147–239) | 0.125 |
| Hemoglobin, g/L | 115–150 | 144 (135–154) | 140 (130–149) | 146 (136–156) | 0.045 |
| ESR, mm/h | 0–20 | 28 (20–40) | 40 (27–50) | 25 (15–33) | 0.001 |
| PT, s | 9–13 | 12.3 (11.5–13) | 12.4 (11.6–13) | 12.3 (11.5–12.8) | 0.574 |
| APTT, s | 29–42 | 29 (26.5–32) | 29 (27–34.5) | 28.8 (26.2–32) | 0.230 |
| CRP, mg/L | 0–10 | 20.8 (14–48) | 43.4 (16–64) | 18.7 (10–48) | 0.003 |
| Pao2, mmHg | 80–100 | 90 (86–94) | 85 (78–90) | 91 (87–96) | 0.001 |
| LDH, U/L | 207–414 | 420 (315–580) | 497 (346–630) | 380 (281–545) | 0.026 |
| ALT, U/L | ≤45 | 26 (18–41) | 29 (18–55) | 25 (17–41) | 0.192 |
| AST, U/L | ≤35 | 32 (23–44) | 33 (24–49) | 31 (22–44) | 0.490 |
| BUN, mmol/L | 2.8–7.6 | 4.3 (3.2–6) | 4.8 (3.5–6.5) | 4.3 (3.2–5) | 0.020 |
| Creatinine, mg/dL | 0.5–1.2 | 1 (0.8–1.1) | 1 (0.8–1.2) | 0.9 (0.7–1.1) | 0.103 |
| Creatine kinase, U/L | ≤170 | 119 (67–209) | 124 (70–223) | 118 (67–209) | 0.557 |
| Sodium, mEq/L | 136–145 | 138 (134–140) | 137 (134–140) | 138 (135–142) | 0.263 |
| Potassium, mEq/L | 3.5–5 | 4.3 (3.9–4.7) | 4.1 (3.8–4.5) | 4.3 (3.9–4.8) | 0.023 |
| Complications | |||||
| ARDS | 33 (10.5) | 10 (19.2) | 23 (8.7) | 0.028 | |
| Shock | 26 (8.3) | 9 (17.6) | 17 (6.5) | 0.015 | |
| Secondary infection | 31 (9.8) | 10 (19.2) | 21 (8) | 0.018 | |
| Mortality | 25 (7.9) | 11 (21.2) | 14 (5.3) | 0.001 | |
IQR, interquartile range; COVID-19, coronavirus disease 2019; ESR, erythrocyte sedimentation rate; PT, prothrombin time; APTT, activated partial thromboplastin time; CRP, C-reactive protein; Pao2, partial pressure of oxygen; LDH, lactate dehydrogenase; ALT, alanine aminotransferase; AST, aspartate aminotransferase, BUN, blood urea nitrogen; ARDS, acute respiratory distress syndrome; No., number.
aP values indicate differences between diabetes and non-diabetes patients. P < 0.05 was considered statistically significant.
Fig. 2.
Comparison of COVID-19 disease severity and mortality between patients with and without diabetes. A: in the presence of other comorbidities; B: in the absence of other comorbidities.
Table 5 showed the characteristics of hospitalized COVID-19 patients with diabetes according to disease severity at the time of admission. Compared with patients with moderate disease, patients with severe/critical COVID-19 were older (59 [IQR, 45–67] vs. 51 [IQR, 38–60] years; P = 0.005), had longer diabetes duration (15 [IQR, 9–20] vs. 11 [IQR, 6–14] years; P = 0.002), had higher HbA1c levels (8 [IQR, 7–9.5] vs. 7.6 [IQR, 6.8–9] %; P = 0.036), had a greater proportion of diabetic ketoacidosis (5 [11.1%] vs. 4 [3.9%]; P = 0.044) and had higher mortality (14 [31.1%] vs. 12 [11.7%]; P = 0.005).
Table 5.
Clinical characteristics of hospitalized COVID-19 patients with diabetes stratified according to disease severity.
| Characteristic |
Patients, No. (%) |
P valuea | |
|---|---|---|---|
| Moderate (n = 103) | Severe/critical (n = 45) | ||
| Age, median (IQR), years | 51 (38–60) | 59 (45–67) | 0.005 |
| Sex | |||
| Male Female |
62 (60.2) 41 (39.8) |
37 (82.2) 8 (17.8) |
0.006 |
| Diabetes duration, median (IQR), years | 11 (6–14) | 15 (9–20) | 0.002 |
| HbA1c, median (IQR), % | 7.6 (6.8–9) | 8 (7–9.5) | 0.036 |
| Diabetes control | |||
| Controlled Uncontrolled |
42 (40.8) 61 (59.2) |
16 (35.6) 29 (64.4) |
0.341 |
| Diabetic complications | |||
| Ketoacidosis | 4 (3.9) | 5 (11.1) | 0.044 |
| Infectious shock | 2 (1.9) | 3 (6.7) | 0.165 |
| Treatment | |||
| Oral medication | 34 (33) | 10 (22.2) | 0.130 |
| Insulin | 48 (46.6) | 22 (48.8) | 0.469 |
| Both | 21 (20.4) | 13 (28.9) | 0.179 |
| Mortality | 12 (11.7) | 14 (31.1) | 0.005 |
IQR, interquartile range; COVID-19, coronavirus disease 2019; HbA1c, Glycated hemoglobin; No., number.
aP values indicate differences between moderate and severe/critical disease among patients with diabetes. P < 0.05 was considered statistically significant.
4. Discussion
The present research was a retrospective study of 595 hospitalized patients with COVID-19 which were analyzed in the cases of baseline demographic, clinical characteristics, and outcomes. The rapid global expansion of COVID-19 has shown that SARS-CoV-2 has a high transmission potential in humans, especially in elderly people and those with underlying diseases.
Greater proportions of hospitalized patients in this study were men (67.4%), indicating that men are at higher risk from COVID-19 infection, which in some studies the cause has been partly attributed to the high prevalence of smoking among men [13], [14]. However, in this study, current smokers accounted for a low percentage of COVID-19 patients and no significant relationship was found between smoking and COVID-19, which was consistent with a recent meta-analysis study [15]. One of the important factors in increasing the transmission of the virus is the long duration of the disease onset to hospital admission. In the present study, the time from symptom onset to hospital admission was relatively long (7 days, IQR 3.7–9 days), which indicates the need for raising awareness and public education to mitigate the spread of this infection.
Recent studies have reported that diabetes is one of the most important underlying comorbidities in patients with COVID-19 and is associated with severity and mortality in these patients [16], [17]. Consistently, the data of this study showed a high prevalence of diabetes in patients with COVID-19 (24.9%) and significant statistical associations between hospitalized COVID-19 patients with diabetes and those without diabetes. According to finding, fever, dry cough, and dyspnea were most common symptoms in patients with diabetes, which were consistent with previous studies [17], [18].
Laboratory findings at admission indicated the white blood cell count increased, neutrophil count increased and lymphocyte count decreased significantly in the COVID-19 patients with diabetes compared to those without diabetes, which are consistent with earlier studies [18], [19], [20]. These findings may indicate the fact that patients with diabetes had suffered a more severe viral infection and had propensity to bacterial infections. In addition, inflammatory markers levels including C-reactive protein and erythrocyte sedimentation rate were significantly higher in patient with diabetes than in patients without diabetes. Previous studies have shown the effect of Th17 cells and Treg cells on immune system imbalance and induction of inflammatory factors secretion in patients with diabetes and obesity [21], [22]. Diabetes-associated inflammatory process can result in a reduction in the immune response and more severe infection along with worse outcomes in COVID-19 patients with diabetes [23]. On the other hand, the level of blood urea nitrogen, kidney damage indicator, significantly increased in patients with diabetes compared to patients without diabetes, suggesting that kidney damage may have occurred. It is worth noting that the high level of LDH in the blood of COVID-19 patients with diabetes in the absence of other comorbidities can partly justify the more severe tissue damage compared with those without diabetes without comorbidities.
Since the CT imaging results were different between patients with diabetes and without diabetes, it can be considered as an indicator to determine the severity of the COVID-19 pneumonia. Radiographic findings demonstrated that the patchy ground-glass opacity obtained from chest CT was significantly more prevalent in patients with diabetes, indicating that pneumonia was more severe in these patients.
In the present study, patients with diabetes showed a more severe disease and a higher mortality rate than patients without diabetes which could be significantly related to a higher incidence of complications and coexistence with other comorbidities, especially obesity and hypertension. Many recent studies have also noticed an association between obesity and hypertension with more severe COVID-19 illness [9], [16], [24], [25]. Additionally, further analysis showed that COVID-19 patients with diabetes without these comorbidities had also more complication and death compared to those without diabetes without comorbidities. This means that other comorbidities may have only little impact on the prognosis and outcome of COVID-19 patients with diabetes. Thus, more attention should be paid to COVID-19 patients with diabetes during hospitalization.
The strengths of this study were the large sample size of COVID-19 patients, comprehensive clinical records and admission of a wide range of ethnicities. In addition, as the studied hospital is one of the major designated-government hospitals for COVID-19 patients’ treatment in Tehran, so, it would be a good representative of patients in the region. To the best of our knowledge, this is the first study in Iran to investigate the clinical characteristics and outcomes of hospitalized COVID-19 patients with and without diabetes. However, this study also faced some limitations. First, all included patients were only from a single center within the Tehran metropolitan area which means a nationwide multicenter study is needed. Second, the data were collected from the electronic medical records and some indicators have not been tested in all patients, so bias from missing data might exist. Third, given that most patients were still hospitalized at the end of the study and due to short-term outcome follow-up, it was difficult to assess risk factors for a poor prognosis.
5. Conclusion
In conclusion, COVID-19 patients with diabetes presented a more severe infection and a worse prognosis than that of non-diabetes patients. Moreover, diabetes could be considered as a risk factor for disease progression and increase in-hospital death in patients with COVID-19. The study’s findings highlighted the importance of understanding the clinical features of COVID-19 to implement effective control measures and more intensive disease management in patients with diabetes worldwide.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
Thanks to guidance and advice from the “Clinical Research Development Unit of Baqiyatallah Hospital”.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Wang C., Horby P.W., Hayden F.G., Gao G.F. A novel coronavirus outbreak of global health concern. Lancet. 2020;395(10223):470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdi M. Coronavirus disease 2019 (COVID-19) outbreak in Iran: Actions and problems. Infect Control Hosp Epidemiol. 2020;41:754–755. doi: 10.1017/ice.2020.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikpouraghdam M., Farahani A.J., Alishiri G., Heydari S., Ebrahimnia M., Samadinia H., et al. Epidemiological characteristics of coronavirus disease 2019 (COVID-19) patients in IRAN: A single center study. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104378. 104378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization. Coronavirus disease 2019 (COVID-19): Situation Report-174. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200713-covid-19-sitrep-175.pdf?sfvrsn=d6acef25_2. [accessed 13 July 2020].
- 6.Garg S., Kim L., Whitaker M., O’Halloran A., Cummings C., Holstein R., et al. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019—COVID-NET, 14 States, March 1–30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kobayashi T., Jung S., Linton N.M., Kinoshita R., Hayashi K., Miyama T., et al. Communicating the risk of death from novel coronavirus disease (COVID-19) J Clin Med. 2020;9(2):580. doi: 10.3390/jcm9020580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan. China JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Richardson S., Hirsch J.S., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fang L., Karakiulakis G., Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30116-8. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Clinical management of severe acute respiratory infection when novel coronavirus (2019-nCoV) infection is suspected: interim guidance. Available from: https://apps.who.int/iris/bitstream/handle/10665/330893/WHO-nCoV-Clinical-2020.3-eng.pdf?sequence51&isAllowed5y. [accessed 28 January 2020].
- 12.Force A.D.T., Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., et al. Acute respiratory distress syndrome: the berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 13.Cai H. Sex difference and smoking predisposition in patients with COVID-19. Lancet Respir Med. 2020;8(4) doi: 10.1016/S2213-2600(20)30117-X. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung J.M., Yang C.X., Tam A., Shaipanich T., Hackett T.-L., Singhera G.K., et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020;55:2000688. doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farsalinos K., Barbouni A., Poulas K., Polosa R., Caponnetto P., Niaura R. Current smoking, former smoking, and adverse outcome among hospitalized COVID-19 patients: a systematic review and meta-analysis. Ther Adv Chronic Dis. 2020;11:1–14. doi: 10.1177/2040622320935765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guan W., Liang W., Zhao Y., Liang H., Chen Z., Li Y., et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: A nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi Q., Zhang X., Jiang F., Zhang X., Hu N., Bimu C., et al. Clinical characteristics and risk factors for mortality of COVID-19 patients with diabetes in Wuhan, China: a two-center, retrospective study. Diabetes Care. 2020;43(7):1382–1391. doi: 10.2337/dc20-0598. [DOI] [PubMed] [Google Scholar]
- 18.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. e001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C., et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43(7):1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 20.Guo W., Li M., Dong Y., Zhou H., Zhang Z., Tian C., et al. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020:e3319 doi: 10.1002/dmrr.3319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang C., Xiao C., Wang P., Xu W., Zhang A., Li Q., et al. The alteration of Th1/Th2/Th17/Treg paradigm in patients with type 2 diabetes mellitus: relationship with diabetic nephropathy. Hum Immunol. 2014;75:289–296. doi: 10.1016/j.humimm.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Guzmán-Flores J.M., López-Briones S. Cells of innate and adaptive immunity in type 2 diabetes and obesity. Gac Med Mex. 2012;148:381–389. [PubMed] [Google Scholar]
- 23.Hussain A, Bhowmik B, do Vale Moreira NC. COVID-19 and diabetes: Knowledge in progress. Diabetes Res Clin Pract 2020;162:108142. https://doi.org/10.1016/j.diabres.2020.108142 [DOI] [PMC free article] [PubMed]
- 24.Lighter J., Phillips M., Hochman S., Sterling S., Johnson D., Francois F., et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin Infect Dis. 2020:ciaa415. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang S., Wang J., Liu F., Liu J., Cao G., Yang C., et al. COVID-19 patients with hypertension have more severe disease: a multicenter retrospective observational study. Hypertens Res. 2020:1–8. doi: 10.1038/s41440-020-0485-2. [DOI] [PMC free article] [PubMed] [Google Scholar]