Abstract
Both acute and chronic stress can cause allostatic overload, or long-term imbalance in mediators of homeostasis, that results in disruptions in the maternal-placental-fetal endocrine and immune system responses. During pregnancy, disruptions in homeostasis may increase the likelihood of preterm birth and preeclampsia. Expectant mothers traditionally have high rates of anxiety and depressive disorders, and many are susceptible to a variety of stressors during pregnancy. These common life stressors include financial concerns and relationship challenges and may be exacerbated by the biological, social, and psychological changes occurring during pregnancy. In addition, external stressors such as major weather events (eg, hurricanes, tornados, floods) and other global phenomena (eg, the coronavirus disease 2019 pandemic) may contribute to stress during pregnancy.
This review investigates recent literature published about the use of nonpharmacologic modalities for stress relief in pregnancy and examines the interplay between psychiatric diagnoses and stressors, with the purpose of evaluating the feasibility of implementing nonpharmacologic interventions as sole therapies or in conjunction with psychotherapy or psychiatric medication therapy. Further, the effectiveness of each nonpharmacologic therapy in reducing symptoms of maternal stress is reviewed. Mindfulness meditation and biofeedback have shown effectiveness in improving one’s mental health, such as depressive symptoms and anxiety. Exercise, including yoga, may improve both depressive symptoms and birth outcomes. Expressive writing has successfully been applied postpartum and in response to pregnancy challenges. Although some of these nonpharmacologic interventions can be convenient and low cost, there is a trend toward inconsistent implementation of these modalities. Future investigations should focus on methods to increase ease of uptake, ensure each option is available at home, and provide a standardized way to evaluate whether combinations of different interventions may provide added benefit.
Key words: allostatic load, anxiety symptoms, depressive symptoms, exercise, mindfulness, perinatal mental health, stress relief, therapeutic writing
Introduction
Stress is a frequently used, ambiguous term.1 The allostatic model has been developed to help clarify these ambiguities and explain the effects of acute vs chronic stress responses.2 Initial conceptions of stress response were centered around homeostasis, a concept referring to self-regulating processes that maintain the stability of an individual’s essential systems. Cohen et al3 provide a working definition of stress as “when environmental demands tax or exceed the adaptive capacity of an organism, resulting in psychological and biological changes that may place persons at risk for disease.” These environmental demands may be internal, relating to an individual’s disposition, or external, relating to an individual’s life circumstances. Acute stress is an intense but relatively short-lived response to stressors, whereas chronic stress is the result of unresolved stressors that are experienced for a longer period of time.
Repeated or chronic stress contributes to the cumulative allostatic load—the wear and tear on the bod or a sum of the lifetime stress exposure.4 , 5 Allostatic load increases over time and represents physiological consequences of heightened neural or neuroendocrine responses.6 Hypothalamic-pituitary-adrenal axis hormones, including cortisol, catecholamines such as epinephrine, and cytokines, are all primary mediators impacting allostasis.2 When these primary mediators go beyond the limits of homeostatic mechanisms and become unbalanced, the body is only able to sustain this state without negative effects for a limited time.2 A prolonged imbalance of these primary homeostatic mediators results in allostatic overload. In addition, chronic stressors or repeated acute stressors may result in changes related to glucocorticoid genes by alterations of the epigenome and/or transcriptome and hasten disease. However, although the initial stressful insult cannot always be prevented, epigenetic and transcriptomic effects are dynamic and potentially reversible through treatment.7, 8, 9, 10, 11, 12
Stress and Pregnancy
Stress is common among pregnant women. From 2009 to 2010, data from the US-wide Centers for Disease Control Pregnancy Risk Assessment Monitoring System found that nearly 75% of postpartum mothers reported at least 1 major stressful event in the year leading up to delivery of their baby.13 The most commonly cited stressors experienced during pregnancy included moving to a new address, arguing with a partner more than usual, serious illness and hospitalization of a family member, and inability to pay bills. In addition, external stressors such as extreme weather events (eg, hurricanes, tornados, floods) and other global adverse events (eg, the coronavirus disease 2019 pandemic) may contribute to significant acute and chronic stress during pregnancy. Reports of perceived stress varied widely by race and ethnicity, with non-Hispanic American Indian and Alaska Native women reporting highest levels of stress and non-Hispanic Asian women reporting the lowest.13
Possible mechanisms linking stress and adverse pregnancy outcomes
Chronic stress may be associated with adverse pregnancy outcomes through a positive feedback loop. Maternal-placental-fetal neuroendocrine interaction and immune responses are stress-sensitive and thus may affect birth outcomes. Maternal stress is associated with cortisol release.14 , 15 High cortisol levels reduce lymphocyte sensitivity to glucocorticoids by binding to glucocorticoid receptors; subsequently, as steroid resistance is developed, there is an increased release of proinflammatory cytokines.15 Furthermore, maternal stress influences circulating levels of inflammatory markers by increasing proinflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor α, and decreasing antiinflammatory cytokine IL-10.14 These inflammatory markers dampen the immune system response, increasing the susceptibility to adverse pregnancy outcomes such as preterm birth (PTB).14 Women who develop adverse pregnancy complications requiring early delivery or resulting in other maternal or neonatal morbidities may then experience additional stress, furthering the loop.
Previous studies have found that minority and low-income pregnant women may have higher baseline levels of cortisol than women of other situations, supporting the association between chronic stress, increased allostatic load, and higher rates of adverse pregnancy outcomes among these high-risk obstetrical populations.16 One stressor of particular concern to pregnant women of color in the United States is racism. Racism (and discrimination) is typically defined as differential treatment based on one’s skin color or racial identity. Racism affects over 68% non-Hispanic black women in the United States. It is hypothesized that racism may be a significant contributor to the disparities in adverse birth outcomes (eg, PTB and preeclampsia) among non-Hispanic black women compared with non-Hispanic white women.15 The chronic stress associated with racism contributes to an increased allostatic load and a more rapid decline in health during an individual’s lifetime; it is a major contributing factor to the weathering hypothesis. Weathering is the premature aging of the body because of endurance of adverse events; this can be both physical and psychological. This hypothesis is supported by studies suggesting that age-related increases in PTB are higher among non-Hispanic black women than non-Hispanic white women17 and that racial and ethnic disparities in PTB and other adverse pregnancy outcomes persist among women of high socioeconomic status.18
Effects of stress on pregnancy outcomes
Acute stress, chronic stress, and allostatic overload have all been associated with a variety of adverse pregnancy outcomes, including spontaneous PTB, preeclampsia, neonatal morbidity, and low birthweight (Table ).14 , 15 , 19 In turn, PTB (regardless of indication) is associated with a higher risk of short-term neonatal morbidities (including neurologic, pulmonary, cardiovascular, gastrointestinal, immune, and metabolic complications) and long-term complications among survivors (eg, cerebral palsy, neurodevelopmental delay, visions problems, and hearing loss).20, 21, 22, 23, 24, 25, 26 As the total allostatic load increases, the likelihood of adverse pregnancy outcomes may also increase (Figure 1 ).
Table.
Selected studies evaluating effects of stress and related exposures on birth outcomes
| Study and year | N | Population | Key findings |
|---|---|---|---|
| Barrett et al,19 2018 | 836 | Assessment of Multiple Intrauterine Gestations from Ovarian Stimulation Study | Each 1-unit increase in allostatic load associated with preeclampsia (aOR, 1.62; 95% CI, 1.14–2.38) and preterm birth (aOR, 1.44; 95% CI, 1.02–2.08) |
| Hux et al,20 2015 | 113 | University of Pittsburgh, Prenatal Exposures and Preeclampsia Prevention Study |
Allostatic load <15 weeks associated with preeclampsia (OR, 2.91; 95% CI, 1.50–5.65) |
| Harville et al,88 2010 | 4865 | Great Britain, National Child Development Study | Exposure to ≥4 hardships in childhood (across financial, family, structural) associated with preterm birth (OR, 1.44; 95% CI, 1.08–1.92) |
| Mendez-Figueroa et al,89 2019 | 29,179 | Ben Taub Hospital and Texas Children’s Pavilion for Women | Delivery after Hurricane Harvey associated with 50% increase in neonatal morbidity (7.8% vs 11.9%; aOR, 1.27; 95% CI, 1.34–1.71) |
| Oyarzo et al,90 2012 | 2553 | Chile, Herminda Martin Clinic Hospital | Earthquake during first trimester associated with smaller newborns (3340±712 vs 3426±576 g; P=.007) |
aOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
Traylor. Nonpharmacologic interventions to reduce stress in pregnancy. AJOG MFM 2020.
Figure 1.
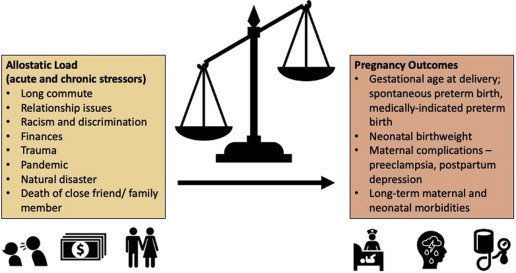
As the burden of chronic and acute stress increases, adverse pregnancy outcomes may also increase
Traylor. Nonpharmacologic interventions to reduce stress in pregnancy. AJOG MFM 2020.
Natural disasters and adverse national and international events (eg, pandemics) provide a unique opportunity to study the effects of a universally stressful exposure on pregnancy outcomes. Although it can be difficult to navigate ethical considerations of human research involving an imposed stress variable, populations and individuals intrinsically experience varying levels of impact, and thus stress exposure, in the event of a population-wide stressor. These stressful experiences, including destructive weather events (eg, hurricanes, earthquakes) and more chronic population-wide stressors (eg, local political unrest, war, pandemics) may also activate both the acute and chronic stress response feedback loops in pregnant individuals.
Stress and psychiatric comorbidities
Depression and anxiety during pregnancy are very common. Of note, 1 in 5 women will have an anxiety disorder in pregnancy,27 and 10% to 14% of women in the general obstetrical population meet criteria for major depression during pregnancy.28 However, anxiety and depressive symptoms are as high as 25% to 50% in pregnancy, when symptoms are present but insufficient to meet full diagnostic criteria for a specific anxiety or depressive disorder.29, 30, 31 In a study of pregnant low-income black women engaging in home-visiting programs in an urban environment, over 20% met full criteria for major depressive disorder in pregnancy,31 further supporting that non-Hispanic black women who face higher stress also have higher rates of depression.
Women with a previous diagnosis of depression or anxiety before pregnancy, past pregnancy or delivery complications including pregnancy loss and stillbirth, history of adverse life events (eg, abuse), and particularly those with multiple traumatic events have a higher allostatic load and higher rates of antenatal depression and anxiety.32 Similar to biologic findings seen in those with chronic stress, women with major depressive disorder during pregnancy have increased proinflammatory cytokines and a blunted cortisol awakening response.33 Further, prenatal anxiety is associated with increased cortisol levels and proinflammatory cytokines.34 Women with both severe depression and severe anxiety during the third trimester had higher levels of IL-6, IL-2, IL-9, and IL-17A.35
Potential benefits of reducing stress during pregnancy
Although many pregnant women are exposed to both acute and chronic stressors, not all women who are exposed have adverse pregnancy outcomes. This may explain, in part, why pregnancy outcomes remain variable in the setting of more widespread adverse events. The sum of an individual’s prior social experiences (both positive and negative) and their reaction to these experiences influence whether exposure to new acute or chronic stressors disrupts homeostasis and results in disease or adverse outcomes. Hogue et al36 positioned racial discrimination and spontaneous PTB within a stress and coping framework, whereby effective coping may reduce the negative impact of the stress of discrimination but ineffective coping may allow the stress to cause a disruption in homeostasis and contribute to adverse birth outcomes. Others report that although women have an elevated risk of spontaneous PTB when reporting lifetime racism (odds ratio [OR], 1.5; 95% confidence interval [CI], 0.9–2.8) and racism in the previous year (OR, 2.5; 95% CI, 1.2–5.2), this risk can be abrogated by active coping.37 Finally, although 1 study of 3021 women in Canada found that stress was a significant risk factor for PTB (OR, 1.73; 95% CI, 1.07–2.81), the risk of prematurity was highest among those with low levels of social support or optimism.38
On the basis of these data, the successful reduction on the biological effects of stress during pregnancy has the potential for profound impacts on maternal health and pregnancy outcomes for certain populations. Although pharmacologic therapy, including selective serotonin reuptake inhibitors (SSRIs) and benzodiazepines, has a role in treating depression or anxiety in pregnancy, pharmacologic treatments for the different alterations in stress responses that have built up over time are more elusive, and the effect on pregnancy outcomes remains underinvestigated. Of concern, benzodiazepine use in combination with SSRI therapy has been associated with worse adverse behavioral effects in the infants.39 Furthermore, although rates of depression are high, only 8.6% receive adequate treatment in pregnancy, and data regarding remission rates of depression with medication during pregnancy are limited.40 However, in the Sequenced Treatment Alternatives to Relieve Depression trial, which included nonpregnant men and women, 67% of participants achieved remission of depression after completing 1 to 4 medication treatment steps.41 In a large randomized multicenter trial on men and women with major depressive disorder, those with anxious depression and lower heart rate variability (HRV) had poorer response to antidepressant medication; conversely, participants with nonanxious depression had poorer response with higher HRV.42 HRV is a measure of parasympathic activity and is emerging as an increasingly important tool for assessing the autonomic nervous system; measurement of HRV is comparable to composite allostatic measures.43 , 44 Pregnant women with higher trait anxiety had significantly lower HRV at 30 and 36 weeks of gestation than pregnant women with lower trait anxiety,45 indicating that anxiety in pregnancy decreased HRV. Further, these data suggest that response to antidepressant medication and other interventions (such as the nonpharmacologic interventions reviewed here) may vary depending on HRV and type of anxiety and depression.
Finally, women may be taking appropriate doses of pharmacotherapy but need or desire an additional adjunct therapy. Psychotherapy also has a role in the treatment of stress during pregnancy through cognitive behavioral therapy and other approaches but is excluded from this review because access to psychotherapy may be limited because of provider availability or financial concerns. Therefore, the objective here is to review the available use of low cost, logistically feasible, nonpharmacologic therapies to reduce stress during pregnancy (Figure 2 ) and to discuss future avenues for research and clinical care in this area.
Figure 2.
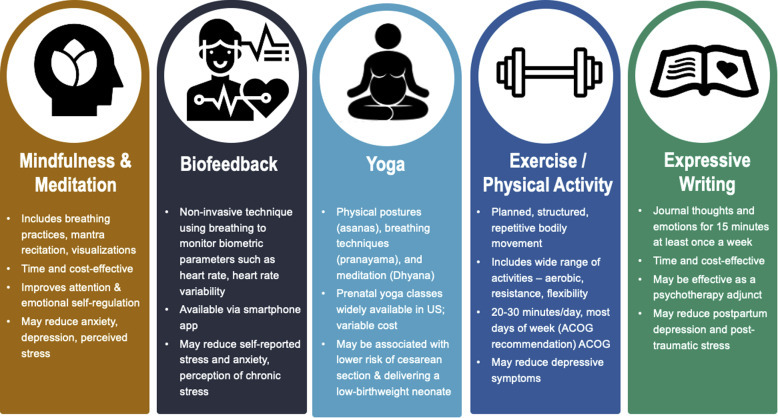
Overview of nonpharmacologic methods for stress reduction in pregnancy
ACOG, American College of Obstetricians and Gynecologists.
Traylor. Nonpharmacologic interventions to reduce stress in pregnancy. AJOG MFM 2020.
Interventions to Reduce Stress During Pregnancy
Meditation and mindfulness and biofeedback
Meditation is a mental exercise that improves attention and emotional self-regulation. Some types of meditation include mindfulness, breathing, mantra recitation, and visualizations.46 Grounded in Buddhist origins, mindfulness is described as attention to and awareness of present perceptions.47 It is a form of experiential processing in which instances are observed from a wider perspective that recognizes influential judgments and associations.45 In contrast, during conceptual processing, an individual evaluates situations within the context of self-concern. In recent literature, mindfulness has been of prominent interest as a potential therapeutic tool because it is low cost and only requires a relatively short investment of time each day. An integrative review of mindfulness in the workplace reported improvements in attention, cognitive capacity, emotional reactivity, self-regulation, and stress response.48 As a targeted intervention for stress, mindfulness has shown effectiveness in reducing negative outcomes such as anxiety, depression, and chronic pain.46
In pregnancy, mindfulness has potential as a therapy to reduce stress and improve birth outcomes. A metaanalysis of 17 studies in 2016 reported mindfulness interventions resulting in significant improvements in depression, anxiety, and stress with small to medium effect sizes (g=0.36–0.51).49 However, these findings included uncontrolled studies and cited limitations in terms of potentially confounding variables, such as lack of tracking other simultaneous interventions.49 Another systematic review and metaanalysis of 14 studies in 2017 also found that this intervention may help with anxiety, depression, and perceived stress; however, there is a lack of evidence from adequately powered randomized controlled trials (RCTs).50 The article called for future research into the potential of mindfulness in pregnancy beyond pilot and nonrandomized studies.50
In 2019, an RCT in Taiwan was published that assessed the effectiveness of a mindfulness program involving a series of 8 weekly 3-hour classes and 1 7-hour silent meditation.51 A total of 74 pregnant women were randomized to either the intervention group or treatment-as-usual (control), and psychological state was assessed though the Perceived Stress Scale, Edinburgh Postnatal Depression Scale, and Five Facet Mindfulness Questionnaire at midpregnancy (baseline) and 3 months postpartum.49 The intervention group showed a significant decline in self-reported stress (perceived stress score decreased by mean of 3.77, from 15.41 to 11.64, vs a change of +0.49 in the control group pre- and postintervention), and more intervention group participants (69% vs 46%) experienced a significant decrease in stress (P=.009).51 This recent study provides promising results for the effectiveness of mindfulness in reducing stress during pregnancy.
In an RCT of nonpregnant students with chronic pain and anxiety, mindful breathing facilitated by a 12-minute smartphone-based task is proposed to decrease HRV.52 Similarly, HRV biofeedback is a noninvasive technique that utilizes metronomic breathing while monitoring one’s parasympathetic activity to improve HRV measures.43 HRV biofeedback has been shown to improve control in response to negative situations43 and has been associated with reductions in self-reported perceived stress and anxiety.53 In addition, 2 studies of perinatal women, 1 including women with threatened preterm labor and the other including women in the early postpartum period, utilized HRV biofeedback vs control groups.54, 55, 56 In the study of antenatal women with threatened preterm labor, 48 women were randomized to HRV biofeedback vs standard care at an average of 29 weeks' gestation; those randomized to HRV biofeedback had a decrease in their perception of chronic stress during the study period, and the rate of PTB was lower than in the control group (13% vs 33%; not significant P value, exact value not provided in the article).55 In the early postpartum study, use of HRV biofeedback was associated with significant improvements in HRV measures and in scores on the Edinburgh Postnatal Depression Scale compared with those who did not use HRV feedback.54 An additional RCT of HRV biofeedback training for pregnant women demonstrated a reduction in anxiety symptoms and improvement in psychological well-being.57 Taken together, these data suggest that biofeedback, particularly when linked to HRV monitoring, may be a method of determining who is responding physiologically to mindfulness and deep breathing practices. Studies have already shown efficacy in reducing perceived stress and anxiety during pregnancy, including among pregnant women with threatened preterm labor.
Yoga
According to the National Center for Health Statistics, more than 35 million American adults actively practiced yoga in 2017, and this number is still growing.58 Yoga is the most commonly used complementary health approach in the United States and consists of the following 3 aspects: physical postures (asanas), breathing techniques (pranayama), and meditation (dhyana).58 Originating from India as a spiritual practice, yoga has grown and evolved into many different styles such as Hatha, Iyengar, Bikram, and integrated approaches.59 Although Hatha yoga is the most popular form, these different styles do not significantly differ in the probability of reaching positive conclusions in recent research (P=.191).59
Yoga is a popular nonpharmacologic intervention available to pregnant women that may improve both birth outcomes and mental health. Prenatal yoga classes are commonly available across the United States. In addition, yoga instruction specifically tailored for pregnant women is available for free online through smartphone apps and on publicly available websites. In a study investigating the effects of prenatal yoga on birth outcomes, 84 women with depressive symptoms were randomized to yoga, massage therapy, or standard prenatal care from 20 to 32 weeks’ gestation. A greater improvement in depression scores, decreased anxiety scores, decreased anger scores, decreased back and leg pain scores, and increased relationship scores were seen for those in both the yoga group and the massage therapy group but not the control group. In addition, those in the yoga and massage group delivered later (mean, 38.6 and 38.4 weeks, respectively) than those in the control group (mean, 36.7 weeks gestation).60 A Taiwanese study evaluating the effects of prenatal yoga on stress and immune function randomized 94 healthy pregnant women to a 20-week long intervention of either twice weekly 70-minute yoga sessions or routine prenatal care, beginning at 16 weeks’ gestation.61 Both clinical outcomes and biological measures of stress were evaluated. Participants’ salivary cortisol and immunoglobulin A levels were collected between and after yoga every 4 weeks. Women randomized to the yoga group had lower salivary cortisol (P<.001) and higher immunoglobulin A (P<.001) levels immediately after yoga, higher long-term immunoglobulin A levels (P=.018), and their babies had an increased birthweight (P<.001) compared with the control group.61 These results suggest that prenatal yoga can reduce stress and improve immune function in pregnant women and potentially affect neonatal outcomes.62
Yoga also has positive findings as a mental health intervention. A metaanalysis evaluating the efficacy of yoga in the treatment of prenatal depression evaluated 6 moderate quality RCTs including a sample of 374 pregnant women.63 Depressive symptoms of both prenatally depressed women (standardized mean difference [SMD], −0.46; 95% CI, −0.90 to −0.03; P=.04) and nondepressed women (SMD, −0.87; 95% CI, −1.22 to −0.52; P<.00001) were significantly lower in the yoga intervention groups than control groups.63 A 2019 metaanalysis of prenatal yoga for maternal depression found similar results. Six RCTs including a total of 405 pregnant women with mild depressive symptoms were analyzed.64 The pooled SMD from baseline depressive score was −0.452 (95% CI, −0.816 to −0.880; P=.015).64 Both of these analyses provide significant support for yoga as a prenatal intervention. However, it is it important to note that the trials evaluated in both analyses were typically preliminary with small sample sizes.
Exercise
As defined by the American College of Sports Medicine, exercise is “a type of physical activity consisting of planned, structured, and repetitive bodily movement” that is produced by skeletal muscle contraction and leads to increased energy expenditure.65 Exercise is known to help prevent and treat metabolic or cardiovascular diseases; it has also been shown to reduce depressive symptoms.65 The American College of Obstetricians and Gynecologists recommends women with uncomplicated pregnancies should complete moderate-intensity physical activity for at least 20 to 30 minutes on most or all days of the week.66 Historically, intense exercise has been assumed to have negative consequences for mother and child.66 In fact, women tend to reduce their physical activity during pregnancy, and fewer than half of pregnant women meet exercise recommendations.61 Although there may be a theoretical concern regarding vigorous exercise in some situations (eg, women with premature cervical dilation or threatened preterm labor), there is minimal evidence to suggest harm, and 1 observational study of women with short cervix suggested a higher risk of PTB among women with activity restrictions than those without such restrictions.67 An analysis of exercise and pregnancy loss referencing 6 cohort studies and reviews including over 120,000 women found that regular exercise for up to 7 hours a week, including low- and high-intensity activity, is not associated with increased rates of miscarriage.68
The benefits of exercise during pregnancy span both physical and mental capacities. A systematic review and metaanalysis of low-impact physical activity in pregnancy evaluated 30 RCTs and 51 cohort studies for maternal-child health outcomes.69 Regular exercise was associated with lower weight gain during pregnancy, a lower likelihood of gestational diabetes mellitus, and a lower risk of preterm delivery. Less is understood about the relationship between stress and exercise in pregnancy. However, there is a direct association between low exercise frequency and higher reports of stress-related symptoms.70 , 71 One RCT of 167 women explored depression reduction and exercise during pregnancy.72 Women randomized to the exercise group completed 3 60-minute sessions of supervised physical activity per week throughout pregnancy. Compared with the control group, women in the exercise intervention group scored significantly lower on the Center for Epidemiologic Studies Depression Scale (7.76±6.30 vs 11.34±9.74, P=.005) at the end of the study.72
Expressive writing
Expressive writing involves a personal and often emotional reflection of thoughts or memories; it focuses on detailing one’s feelings while writing with the purpose of potentially easing emotional trauma. It was developed as a type of therapy by James W. Pennebaker in the late 1980s after his research found that writing for 15 minutes a day for at least 3 consecutive days about previous distressing experiences was associated with significantly fewer visits to a physician in the following months.73 Subsequently, various medical disciplines have started using expressive writing; it is an attractive therapeutic option as it is accessible, customizable, does not require significant time commitment, and is low cost. In addition, it may be more convenient than traditional psychotherapy given the lack of mental health professionals in many areas, although it may also be effective if the pregnant woman is able to share some of her writing with a healthcare professional. Although a quantitative survey of pregnant women found that women preferred video telehealth therapy compared with computer-assisted therapy and self-guided online therapy,74 psychotherapy access is limited for many individuals owing to lack of providers and other barriers, including financial and scheduling logistics. Pregnant women rated computer-based support as acceptable,74 and expressive writing would lend itself well to group visits to learn about the process and then check-ins with a woman’s obstetrical care provider. If it becomes clear that the patient needs greater skill-based support, then web-based therapies such as cognitive behavioral therapy and interpersonal therapy, both evidence-based treatments for perinatal depression, would be reasonable next steps, particularly for women facing logistical barriers in accessing a therapist. These therapies are currently being tested in the format of web-based modules.75, 76, 77 The versatility of writing as therapy allows patients with a variety of disease manifestations who have underlying psychological stress to participate.
Most studies evaluating the effects of expressive writing have been performed in individuals diagnosed with cancer, and positive health effects have been appreciated.39, 40, 41 , 78 , 79 Some preliminary investigation has focused on pregnancy, particularly postpartum and in response to pregnancy complications. Studies done by DiBlasio et al80 and Horsch et al81 explored the use of expressive writing postpartum. In both studies, the intervention group exhibited significant decreases in depressive symptoms from baseline surveys.37 , 38 However, the study by DiBlasio et al80 was limited by the lack of a control (no writing) group and the study by Horsch et al81 found similar results between randomization groups. Qian et al82 qualitatively explored the effectiveness of expressive writing for women undergoing pregnancy termination because of fetal abnormalities. A total of 14 women were interviewed over telephone 1 month after writing expressively for 15 minutes at 3 points during their induced-labor hospitalization: on admission and 1 day and 2 days postdelivery. Women reported that expressive writing helped rationalize their thoughts about pregnancy termination, release emotional distress, and improve general psychological state.82 Some women suggested that this therapy could be more effective if paired with additional psychological support or if used for a longer period.82
Discussion
Meditation and mindfulness, biofeedback, yoga, exercise, and expressive writing have not been explored as possible stress therapy options until recent years. Therefore, research conducted on these interventions is largely preliminary and prospective. Support for these interventions, particularly expressive writing, could benefit from more RCTs with larger sample sizes. Likely, there may be characteristics that make some women more responsive to one over another (eg, anxious depression vs nonanxious depression). In addition, combining some of these might also lead to greater results, such as a mindfulness practice that also includes expressive writing.
Nonpharmacologic interventions, including but not limited to those discussed here, have the potential to be affordable and widely available. In addition, with the rising popularity of online classes and support groups, all of the discussed interventions can be completed at home. This holds great potential for times when leaving one’s home is not possible—a period where situational stress can even be elevated. For example, the methods for stress reduction outlined here provide a safe and potentially effective way for women to reduce stress during pregnancy during a pandemic. Nonpharmacologic interventions also provide patients with a choice of preference; patient autonomy will likely improve participation and engagement. Expressive writing, mindful meditation, biofeedback, yoga, and exercise provide a broad range of options in terms of metacognition and physicality. Finally, these interventions are novel strategies that may be readily available to women of all social and racial/ethnic backgrounds, and the benefits of such could help to close the health disparities gap with respect to maternal and fetal health outcomes. These may also augment pharmacologic treatment of depression and anxiety just as therapy and pharmacotherapy combined can be most effective for those with severe depression or anxiety.83 , 84
Thus, nonpharmacologic interventions for stress reduction provide a viable option available to women during pregnancy. Nevertheless, improvements in the implementation of these options should be explored. Providers must also take into consideration the unique situation and variable social determinants of health that may make some stress-reducing modalities difficult to routinely practice, such as one’s work schedule, caregiver needs, unstable housing and/or internet access, etc. In 1 study, the viability of using expressive writing was studied through agreement of participation and completion of full intervention. Around 8,000 eligible women were contacted, approximately 1400 replied to the study invitation, and 854 agreed to participate.85 However, by the 6-month follow-up, only 290 women remained.84 In addition, <30% of the women in both writing groups fulfilled the intervention conditions completely.85 Most of the women who did not choose to participate marked “too busy” as the reason for their decline. These results reveal that although expressive writing is theoretically more convenient than other interventions, many patients struggled to complete the intervention conditions to the full extent. The study by Horsch et al81 supports this idea as, although 94 of the 105 eligible mothers contacted agreed to participate, only 54 completed the study through to the 3-month follow-up. Further, it is unknown whether combinations of these approaches, such as expressive writing and yoga or exercise, might improve pregnancy outcomes. Finally, incentivizing the use of effective nonpharmacologic therapies for providers could also help with updates of the modalities discussed in this review. In the future, ways to incentivize completion and emphasize the potential importance of writing therapy and meditation should be researched for all stakeholders.
Conclusion
Expressive writing, meditation, mindfulness, biofeedback, yoga, and exercise are effective therapies for emotional and physical health. Although these methods have not always been considered for medical treatment of individuals, new research is continually revealing the potential of these interventions to improve health outcomes. For example, other nonpharmacologic approaches to reduce stress in pregnancy, including music therapy and a smartphone app specifically designed to reduce stress in pregnancy, have also been evaluated, but more data are needed to determine the effectiveness of these additional modalities.86 , 87 The application of these accessible and widely available interventions hold great potential to effectively reduce stress during pregnancy. In turn, by limiting the negative implications associated with stress in pregnancy, these nonpharmacologic options could help improve birth outcomes. Research should continue to expand on the strengths of each intervention as effective, affordable, and adaptable to make them a more convenient option for stress alleviation in addition to research on how these nonpharmacologic methods could further enhance the effects of pharmacologic therapies to treat psychiatric conditions.
Footnotes
The authors report no conflict of interest.
This study was funded in part by grant numbers R01-MD011609 and K24-ES031131 from the National Institutes of Health.
References
- 1.Rom O., Reznick A.Z. The stress reaction: a historical perspective. Adv Exp Med Biol. 2016;905:1–4. doi: 10.1007/5584_2015_195. [DOI] [PubMed] [Google Scholar]
- 2.McEwen B.S. Stressed or stressed out: what is the difference? J Psychiatry Neurosci. 2005;30:315–318. [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen S., Kessler R.C., Gordon L.U., editors. Measuring stress: a guide for health and social scientists. Oxford University Press; Oxford: 1997. [Google Scholar]
- 4.McEwen B.S. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44. doi: 10.1111/j.1749-6632.1998.tb09546.x. [DOI] [PubMed] [Google Scholar]
- 5.Geronimus A.T., Hicken M., Keene D., Bound J. “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. Am J Public Health. 2006;96:826–833. doi: 10.2105/AJPH.2004.060749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schulkin J., Gold P.W., McEwen B.S. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology. 1998;23:219–243. doi: 10.1016/s0306-4530(97)00099-1. [DOI] [PubMed] [Google Scholar]
- 7.Bower J.E., Greendale G., Crosswell A.D., et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology. 2014;43:20–29. doi: 10.1016/j.psyneuen.2014.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stagl J.M., Lechner S.C., Carver C.S., et al. A randomized controlled trial of cognitive-behavioral stress management in breast cancer: survival and recurrence at 11-year follow-up. Breast Cancer Res Treat. 2015;154:319–328. doi: 10.1007/s10549-015-3626-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antoni M.H., Lutgendorf S.K., Blomberg B., et al. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biol Psychiatry. 2012;71:366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Black D.S., Cole S.W., Irwin M.R., et al. Yogic meditation reverses NF-κB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology. 2013;38:348–355. doi: 10.1016/j.psyneuen.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo B., Bhasin M., Jacquart J., et al. Genomic and clinical effects associated with a relaxation response mind-body intervention in patients with irritable bowel syndrome and inflammatory bowel disease. PLoS One. 2015;10 doi: 10.1371/journal.pone.0123861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qu S., Olafsrud S.M., Meza-Zepeda L.A., Saatcioglu F. Rapid gene expression changes in peripheral blood lymphocytes upon practice of a comprehensive yoga program. PLoS One. 2013;8:e61910. doi: 10.1371/journal.pone.0061910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns E.R., Farr S.L., Howards P.P., Centers for Disease Control and Prevention Stressful life events experienced by women in the year before their infants’ births--United States, 2000–2010. MMWR Morb Mortal Wkly Rep. 2015;64:247–251. [PMC free article] [PubMed] [Google Scholar]
- 14.Wadhwa P.D., Entringer S., Buss C., Lu M.C. The contribution of maternal stress to preterm birth: issues and considerations. Clin Perinatol. 2011;38:351–384. doi: 10.1016/j.clp.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vianna P., Bauer M.E., Dornfeld D., Chies J.A. Distress conditions during pregnancy may lead to pre-eclampsia by increasing cortisol levels and altering lymphocyte sensitivity to glucocorticoids. Med Hypotheses. 2011;77:188–191. doi: 10.1016/j.mehy.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 16.Corwin E.J., Guo Y., Pajer K., et al. Immune dysregulation and glucocorticoid resistance in minority and low income pregnant women. Psychoneuroendocrinology. 2013;38:1786–1796. doi: 10.1016/j.psyneuen.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holzman C., Eyster J., Kleyn M., et al. Maternal weathering and risk of preterm delivery. Am J Public Health. 2009;99:1864–1871. doi: 10.2105/AJPH.2008.151589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson J.D., Green C.A., Vladutiu C.J., Manuck T.A. Racial disparities in prematurity persist among women of high socioeconomic status. Am J Obstet Gynecol MFM. 2020;2:100104. doi: 10.1016/j.ajogmf.2020.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett E.S., Vitek W., Mbowe O., et al. Allostatic load, a measure of chronic physiological stress, is associated with pregnancy outcomes, but not fertility, among women with unexplained infertility. Hum Reprod. 2018;33:1757–1766. doi: 10.1093/humrep/dey261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hux V.J., Roberts J.M. A potential role for allostatic load in preeclampsia. Matern Child Health J. 2015;19:591–597. doi: 10.1007/s10995-014-1543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell R.B., Green N.S., Steiner C.A., et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120:e1–e9. doi: 10.1542/peds.2006-2386. [DOI] [PubMed] [Google Scholar]
- 22.Manuck T.A., Rice M.M., Bailit J.L., et al. Preterm neonatal morbidity and mortality by gestational age: a contemporary cohort. Am J Obstet Gynecol. 2016;215:103.e1–103.e14. doi: 10.1016/j.ajog.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manuck T.A., Sheng X., Yoder B.A., Varner M.W. Correlation between initial neonatal and early childhood outcomes following preterm birth. Am J Obstet Gynecol. 2014;210:426.e1–426.e9. doi: 10.1016/j.ajog.2014.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Natarajan G., Shankaran S. Short- and long-term outcomes of moderate and late preterm infants. Am J Perinatol. 2016;33:305–317. doi: 10.1055/s-0035-1571150. [DOI] [PubMed] [Google Scholar]
- 25.Bodeau-Livinec F., Marlow N., Ancel P.Y., Kurinczuk J.J., Costeloe K., Kaminski M. Impact of intensive care practices on short-term and long-term outcomes for extremely preterm infants: comparison between the British Isles and France. Pediatrics. 2008;122:e1014–e1021. doi: 10.1542/peds.2007-2976. [DOI] [PubMed] [Google Scholar]
- 26.Vohr B. Long-term outcomes of moderately preterm, late preterm, and early term infants. Clin Perinatol. 2013;40:739–751. doi: 10.1016/j.clp.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Fawcett E.J., Fairbrother N., Cox M.L., White I.R., Fawcett J.M. The prevalence of anxiety disorders during pregnancy and the postpartum period: a multivariate Bayesian meta-analysis. J Clin Psychiatry. 2019;80:18r12527. doi: 10.4088/JCP.18r12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gavin N.I., Gaynes B.N., Lohr K.N., Meltzer-Brody S., Gartlehner G., Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstet Gynecol. 2005;106(5 Pt 1):1071–1083. doi: 10.1097/01.AOG.0000183597.31630.db. [DOI] [PubMed] [Google Scholar]
- 29.Andersson L., Sundström-Poromaa I., Wulff M., Aström M., Bixo M. Depression and anxiety during pregnancy and six months postpartum: a follow-up study. Acta Obstet Gynecol Scand. 2006;85:937–944. doi: 10.1080/00016340600697652. [DOI] [PubMed] [Google Scholar]
- 30.Halbreich U. Women’s reproductive related disorders (RRDs) J Affect Disord. 2010;122(1-2):10–13. doi: 10.1016/j.jad.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Tandon S.D., Cluxton-Keller F., Leis J., Le H.N., Perry D.F. A comparison of three screening tools to identify perinatal depression among low-income African American women. J Affect Disord. 2012;136:155–162. doi: 10.1016/j.jad.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Biaggi A., Conroy S., Pawlby S., Pariante C.M. Identifying the women at risk of antenatal anxiety and depression: a systematic review. J Affect Disord. 2016;191:62–77. doi: 10.1016/j.jad.2015.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Osborne S., Biaggi A., Chua T.E., et al. Antenatal depression programs cortisol stress reactivity in offspring through increased maternal inflammation and cortisol in pregnancy: the Psychiatry Research and Motherhood - Depression (PRAM-D) Study. Psychoneuroendocrinology. 2018;98:211–221. doi: 10.1016/j.psyneuen.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Field T. Prenatal anxiety effects: a review. Infant Behav Dev. 2017;49:120–128. doi: 10.1016/j.infbeh.2017.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Leff Gelman P., Mancilla-Herrera I., Flores-Ramos M., et al. The cytokine profile of women with severe anxiety and depression during pregnancy. BMC Psychiatry. 2019;19:104. doi: 10.1186/s12888-019-2087-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hogue C.J., Bremner J.D. Stress model for research into preterm delivery among black women. Am J Obstet Gynecol. 2005;192(Suppl5):S47–S55. doi: 10.1016/j.ajog.2005.01.073. [DOI] [PubMed] [Google Scholar]
- 37.Rankin K.M., David R.J., Collins J.W., Jr. African American women’s exposure to interpersonal racial discrimination in public settings and preterm birth: the effect of coping behaviors. Ethn Dis. 2011;21:370–376. [PubMed] [Google Scholar]
- 38.McDonald S.W., Kingston D., Bayrampour H., Dolan S.M., Tough S.C. Cumulative psychosocial stress, coping resources, and preterm birth. Arch Womens Ment Health. 2014;17:559–568. doi: 10.1007/s00737-014-0436-5. [DOI] [PubMed] [Google Scholar]
- 39.Salisbury A.L., O’Grady K.E., Battle C.L., et al. The roles of maternal depression, serotonin reuptake inhibitor treatment, and concomitant benzodiazepine use on infant neurobehavioral functioning over the first postnatal month. Am J Psychiatry. 2016;173:147–157. doi: 10.1176/appi.ajp.2015.14080989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox E.Q., Sowa N.A., Meltzer-Brody S.E., Gaynes B.N. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77:1189–1200. doi: 10.4088/JCP.15r10174. [DOI] [PubMed] [Google Scholar]
- 41.Rush A.J., Trivedi M.H., Wisniewski S.R., et al. Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR∗D report. Am J Psychiatry. 2006;163:1905–1917. doi: 10.1176/ajp.2006.163.11.1905. [DOI] [PubMed] [Google Scholar]
- 42.Kircanski K., Williams L.M., Gotlib I.H. Heart rate variability as a biomarker of anxious depression response to antidepressant medication. Depress Anxiety. 2019;36:63–71. doi: 10.1002/da.22843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pinter A., Szatmari S., Jr., Horvath T., et al. Cardiac dysautonomia in depression - heart rate variability biofeedback as a potential add-on therapy. Neuropsychiatr Dis Treat. 2019;15:1287–1310. doi: 10.2147/NDT.S200360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Viljoen M., Claassen N. Allostatic load and heart rate variability as health risk indicators. Afr Health Sci. 2017;17:428–435. doi: 10.4314/ahs.v17i2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizuno T., Tamakoshi K., Tanabe K. Anxiety during pregnancy and autonomic nervous system activity: a longitudinal observational and cross-sectional study. J Psychosom Res. 2017;99:105–111. doi: 10.1016/j.jpsychores.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 46.Goyal M., Singh S., Sibinga E.M., et al. Meditation programs for psychological stress and well-being: a systematic review and meta-analysis. JAMA Intern Med. 2014;174:357–368. doi: 10.1001/jamainternmed.2013.13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dimidjian S., Goodman S.H., Felder J.N., Gallop R., Brown A.P., Beck A. Staying well during pregnancy and the postpartum: a pilot randomized trial of mindfulness-based cognitive therapy for the prevention of depressive relapse/recurrence. J Consult Clin Psychol. 2016;84:134–145. doi: 10.1037/ccp0000068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lyddy C.J., Good D.J. Being while doing: an inductive model of mindfulness at work. Front Psychol. 2017;7:2060. doi: 10.3389/fpsyg.2016.02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lever Taylor B., Cavanagh K., Strauss C. The effectiveness of mindfulness-based interventions in the perinatal period: a systematic review and meta-analysis. PLoS One. 2016;11 doi: 10.1371/journal.pone.0155720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dhillon A., Sparkes E., Duarte R.V. Mindfulness-based interventions during pregnancy: a systematic review and meta-analysis. Mindfulness (N Y) 2017;8:1421–1437. doi: 10.1007/s12671-017-0726-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pan W.L., Chang C.W., Chen S.M., Gau M.L. Assessing the effectiveness of mindfulness-based programs on mental health during pregnancy and early motherhood - a randomized control trial. BMC Pregnancy Childbirth. 2019;19:346. doi: 10.1186/s12884-019-2503-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azam M.A., Latman V.V., Katz J. Effects of a 12-minute smartphone-based mindful breathing task on heart rate variability for students with clinically relevant chronic pain, depression, and anxiety: protocol for a randomized controlled trial. JMIR Res Protoc. 2019;8:e14119. doi: 10.2196/14119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goessl V.C., Curtiss J.E., Hofmann S.G. The effect of heart rate variability biofeedback training on stress and anxiety: a meta-analysis. Psychol Med. 2017;47:2578–2586. doi: 10.1017/S0033291717001003. [DOI] [PubMed] [Google Scholar]
- 54.Kudo N., Shinohara H., Kodama H. Heart rate variability biofeedback intervention for reduction of psychological stress during the early postpartum period. Appl Psychophysiol Biofeedback. 2014;39:203–211. doi: 10.1007/s10484-014-9259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siepmann M., Hennig U.D., Siepmann T., et al. The effects of heart rate variability biofeedback in patients with preterm labour. Appl Psychophysiol Biofeedback. 2014;39:27–35. doi: 10.1007/s10484-013-9238-1. [DOI] [PubMed] [Google Scholar]
- 56.Herbell K., Zauszniewski J.A. Reducing psychological stress in peripartum women with heart rate variability biofeedback: a systematic review. J Holist Nurs. 2019;37:273–285. doi: 10.1177/0898010118783030. [DOI] [PubMed] [Google Scholar]
- 57.van der Zwan J.E., Huizink A.C., Lehrer P.M., Koot H.M., de Vente W. The effect of heart rate variability biofeedback training on mental health of pregnant and non-pregnant women: a randomized controlled trial. Int J Environ Res Public Health. 2019;16:1051. doi: 10.3390/ijerph16061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Clarke T.C., Barnes P.M., Black L.I., Stussman B.J., Nahin R.L. Use of yoga, meditation, and chiropractors among U.S. adults aged 18 and over. NCHS Data Brief. 2018;(325):1–8. [PubMed] [Google Scholar]
- 59.Cramer H., Lauche R., Langhorst J., Dobos G. Is one yoga style better than another? A systematic review of associations of yoga style and conclusions in randomized yoga trials. Complement Ther Med. 2016;25:178–187. doi: 10.1016/j.ctim.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 60.Field T., Diego M., Hernandez-Reif M., Medina L., Delgado J., Hernandez A. Yoga and massage therapy reduce prenatal depression and prematurity. J Bodyw Mov Ther. 2012;16:204–209. doi: 10.1016/j.jbmt.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen P.J., Yang L., Chou C.C., Li C.C., Chang Y.C., Liaw J.J. Effects of prenatal yoga on women’s stress and immune function across pregnancy: a randomized controlled trial. Complement Ther Med. 2017;31:109–117. doi: 10.1016/j.ctim.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 62.Jiang Q., Wu Z., Zhou L., Dunlop J., Chen P. Effects of yoga intervention during pregnancy: a review for current status. Am J Perinatol. 2015;32:503–514. doi: 10.1055/s-0034-1396701. [DOI] [PubMed] [Google Scholar]
- 63.Gong H., Ni C., Shen X., Wu T., Jiang C. Yoga for prenatal depression: a systematic review and meta-analysis. BMC Psychiatry. 2015;15:14. doi: 10.1186/s12888-015-0393-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ng Q.X., Venkatanarayanan N., Loke W., et al. A meta-analysis of the effectiveness of yoga-based interventions for maternal depression during pregnancy. Complement Ther Clin Pract. 2019;34:8–12. doi: 10.1016/j.ctcp.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 65.Deslandes A.C. Exercise and mental health: what did we learn in the last 20 years? Front Psychiatry. 2014;5:66. doi: 10.3389/fpsyt.2014.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mendinueta A., Esnal H., Arrieta H., et al. What accounts for physical activity during pregnancy? A study on the sociodemographic predictors of self-reported and objectively assessed physical activity during the 1st and 2nd trimesters of pregnancy. Int J Environ Res Public Health. 2020;17:2517. doi: 10.3390/ijerph17072517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Oliveira C., Imakawa T.D.S., Moisés E.C.D. Physical activity during pregnancy: recommendations and assessment tools. Rev Bras Ginecol Obstet. 2017;39:424–432. doi: 10.1055/s-0037-1604180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Parad A., Leonard E., Handler L. FPIN’s Clinical Inquiries. Exercise and pregnancy loss. Am Fam Physician. 2015;91:437–438. [PubMed] [Google Scholar]
- 69.da Silva S.G., Ricardo L.I., Evenson K.R., Hallal P.C. Leisure-time physical activity in pregnancy and maternal-child health: a systematic review and meta-analysis of randomized controlled trials and cohort studies. Sports Med. 2017;47:295–317. doi: 10.1007/s40279-016-0565-2. [DOI] [PubMed] [Google Scholar]
- 70.Watson S.J., Lewis A.J., Boyce P., Galbally M. Exercise frequency and maternal mental health: parallel process modelling across the perinatal period in an Australian pregnancy cohort. J Psychosom Res. 2018;111:91–99. doi: 10.1016/j.jpsychores.2018.05.013. [DOI] [PubMed] [Google Scholar]
- 71.Sinclair I., St-Pierre M., Elgbeili G., et al. Psychosocial stress, sedentary behavior, and physical activity during pregnancy among Canadian women: relationships in a diverse cohort and a nationwide sample. Int J Environ Res Public Health. 2019;16:5150. doi: 10.3390/ijerph16245150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perales M., Refoyo I., Coteron J., Bacchi M., Barakat R. Exercise during pregnancy attenuates prenatal depression: a randomized controlled trial. Eval Health Prof. 2015;38:59–72. doi: 10.1177/0163278714533566. [DOI] [PubMed] [Google Scholar]
- 73.Pennebaker J.W., Beall S.K. Confronting a traumatic event: toward an understanding of inhibition and disease. J Abnorm Psychol. 1986;95:274–281. doi: 10.1037//0021-843x.95.3.274. [DOI] [PubMed] [Google Scholar]
- 74.Hantsoo L., Podcasy J., Sammel M., Epperson C.N., Kim D.R. Pregnancy and the acceptability of computer-based Versus traditional mental health treatments. J Womens Health (Larchmt) 2017;26:1106–1113. doi: 10.1089/jwh.2016.6255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bright K.S., Mughal M.K., Wajid A., et al. Internet-based interpersonal psychotherapy for stress, anxiety, and depression in prenatal women: study protocol for a pilot randomized controlled trial. Trials. 2019;20:814. doi: 10.1186/s13063-019-3897-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Danaher B.G., Milgrom J., Seeley J.R., et al. Web-based intervention for postpartum depression: formative research and design of the MomMoodBooster program. JMIR Res Protoc. 2012;1:e18. doi: 10.2196/resprot.2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kingston D., Janes-Kelley S., Tyrrell J., et al. An integrated web-based mental health intervention of assessment-referral-care to reduce stress, anxiety, and depression in hospitalized pregnant women with medically high-risk pregnancies: a feasibility study protocol of hospital-based implementation. JMIR Res Protoc. 2015;4:e9. doi: 10.2196/resprot.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu Q., Dong L., Wu I.H.C., You J., Huang J., Hu Y. The impact of an expressive writing intervention on quality of life among Chinese breast cancer patients undergoing chemotherapy. Support Care Cancer. 2019;27:165–173. doi: 10.1007/s00520-018-4308-9. [DOI] [PubMed] [Google Scholar]
- 79.La Marca L., Maniscalco E., Fabbiano F., Verderame F., Schimmenti A. Efficacy of Pennebaker’s expressive writing intervention in reducing psychiatric symptoms among patients with first-time cancer diagnosis: a randomized clinical trial. Support Care Cancer. 2019;27:1801–1809. doi: 10.1007/s00520-018-4438-0. [DOI] [PubMed] [Google Scholar]
- 80.Di Blasio P., Camisasca E., Caravita S.C., et al. The effects of expressive writing on postpartum depression and posttraumatic stress symptoms. Psychol Rep. 2015;117:856–882. doi: 10.2466/02.13.PR0.117c29z3. [DOI] [PubMed] [Google Scholar]
- 81.Horsch A., Tolsa J.F., Gilbert L., du Chêne L.J., Müller-Nix C., Bickle Graz M. Improving maternal mental health following preterm birth using an expressive writing intervention: a randomized controlled trial. Child Psychiatry Hum Dev. 2016;47:780–791. doi: 10.1007/s10578-015-0611-6. [DOI] [PubMed] [Google Scholar]
- 82.Qian J., Yu X., Sun S., Zhou X., Wu M., Yang M. Expressive writing for Chinese women with foetal abnormalities undergoing pregnancy termination: an interview study of women’s perceptions. Midwifery. 2019;79:102548. doi: 10.1016/j.midw.2019.102548. [DOI] [PubMed] [Google Scholar]
- 83.Wisner K.L., Zarin D.A., Holmboe E.S., et al. Risk-benefit decision making for treatment of depression during pregnancy. Am J Psychiatry. 2000;157:1933–1940. doi: 10.1176/appi.ajp.157.12.1933. [DOI] [PubMed] [Google Scholar]
- 84.Kimmel M.C., Cox E., Schiller C., Gettes E., Meltzer-Brody S. Pharmacologic treatment of perinatal depression. Obstet Gynecol Clin North Am. 2018;45:419–440. doi: 10.1016/j.ogc.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 85.Ayers S., Crawley R., Button S., et al. Evaluation of expressive writing for postpartum health: a randomised controlled trial. J Behav Med. 2018;41:614–626. doi: 10.1007/s10865-018-9970-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Corbijn van Willenswaard K., Lynn F., McNeill J., et al. Music interventions to reduce stress and anxiety in pregnancy: a systematic review and meta-analysis. BMC Psychiatry. 2017;17:271. doi: 10.1186/s12888-017-1432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jallo N., Thacker L.R., 2nd, Menzies V., Stojanovic P., Svikis D.S. A stress coping app for hospitalized pregnant women at risk for preterm birth. MCN Am J Matern Child Nurs. 2017;42:257–262. doi: 10.1097/NMC.0000000000000355. [DOI] [PubMed] [Google Scholar]
- 88.Harville E.W., Boynton-Jarrett R., Power C., Hypponen E. Childhood hardship, maternal smoking, and birth outcomes: a prospective cohort study. Arch Pediatr Adolesc Med. 2010;164:533–539. doi: 10.1001/archpediatrics.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mendez-Figueroa H., Chauhan S.P., Tolcher M.C., et al. Peripartum outcomes before and after hurricane Harvey. Obstet Gynecol. 2019;134:1005–1016. doi: 10.1097/AOG.0000000000003522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Oyarzo C., Bertoglia P., Avendano R., Bacigalupo F., Escudero A., Acurio J., Escudero C. Adverse perinatal outcomes after the February 27th 2010 Chilean earthquake. J Matern Fetal Neonatal Med. 2012;25:1868–1873. doi: 10.3109/14767058.2012.678437. [DOI] [PubMed] [Google Scholar]


