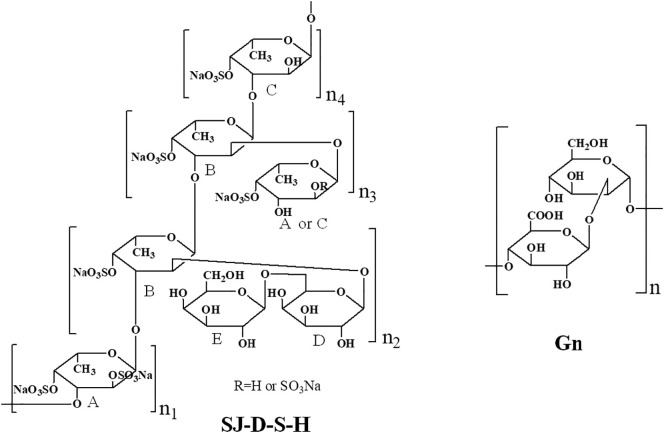
Abstract
The SARS-CoV-2 spike glycoproteins (SGPs) and human angiotensin converting enzyme 2 (ACE2) are the two key targets for the prevention and treatment of COVID-19. Host cell surface heparan sulfate (HS) is believed to interact with SARS-CoV-2 SGPs to facilitate host cell entry. In the current study, a series of polysaccharides from Saccharina japonica were prepared to investigate the structure-activity relationship on the binding abilities of polysaccharides (oligosaccharides) to pseudotype particles, including SARS-CoV-2 SGPs, and ACE2 using surface plasmon resonance. Sulfated galactofucan (SJ-D-S-H) and glucuronomannan (Gn) displayed strongly inhibited interaction between SARS-CoV-2 SGPs and heparin while showing negligible inhibition of the interaction between SARS-CoV-2 SGPs and ACE2. The IC50 values of SJ-D-S-H and Gn in blocking heparin SGP binding were 27 and 231 nM, respectively. NMR analysis showed that the structure of SJ-D-S-H featured with a backbone of 1, 3-linked α-L-Fucp residues sulfated at C4 and C2/C4 and 1, 3-linked α-L-Fucp residues sulfated at C4 and branched with 1, 6-linked β-D-galacto-biose; Gn had a backbone of alternating 1, 4-linked β-D-GlcAp residues and 1, 2-linked α-D-Manp residues. The sulfated galactofucan and glucuronomannan showed strong binding ability to SARS-CoV-2 SGPs, suggesting that these polysaccharides might be good candidates for preventing and/or treating SARS-CoV-2.
Keywords: Glucuronomannan, Sulfated galactofucan, SARS-CoV-2
1. Introduction
COVID-19, caused by the SARS-CoV-2 virus, has now spread worldwide with the tremendous human and economic impact. SARS-CoV-2 is a zoonotic betacoronarirus transmitted through person to person contact by airborne and fecal-oral routes [1]. Approximately 15 million cases of COVID-19 and more than 600,000 deaths have been reported [2]. The absence of vaccines prompts the need for antiviral therapeutics.
Previous studies [[3], [4], [5]] have shown that the host cell surface glycosaminoglycan (GAG), heparan sulfate (HS), interacts with SARS-CoV-2 spike glycoprotein (SGP) and may facilitate host cell entry. Surface plasmon resonance (SPR) has been performed to detect the binding ability between SGP and heparin, a highly sulfated HS analog. Monomeric SARS-CoV-2 SGP was shown to bind more tightly to immobilized heparin (KD = 40 pM) than did SARS-CoV SGP (500 nM) and MERS-CoV SGP (1 nM) [3]. The results of the competition binding assay indicated that the IC50 of heparin, tri-sulfated non-anticoagulant HS and low molecular weight heparin were 0.056 μM, 0.12 μM, and 26.4 μM, respectively [3]. In addition, unbiased computational ligand docking studies suggested that the heparan sulfate (HS) binding domains were at the subunit 1 (S1)/subunit 2 (S2) of spike glycoprotein site and another site (453-459 (YRLFRKS)) [3].
Sulfated polysaccharides from brown seaweeds have similar structures to GAGs. Compared to heparin, the polysaccharide (RPI-27) and low molecular weight polysaccharide (RPI-28), from Saccharina japonica, were shown to exhibit and strongly inhibit the binding of SARS-CoV-2 SGP to immobilized heparin [6]. In addition, RPI-27 and RPI-28 did not show any toxicity using Vero cells using a standard water-soluble tetrazolium salt-1 (WST-1) assay, even at the highest concentrations studied. Moreover, in an in vitro antiviral assays, RPI-28 had an EC50 of 8.3 ± 4.6 μg/mL (corresponding to approximately 83 nM) [6], and was more potent than remdesivir (a drug recently approved for emergency use in severe COVID-19 infections), heparin (2.1 μM), chemo-enzymatically synthesized TriS (a non-anticoagulant heparin analog) [7] (5.0 μM) and non-anticoagulant low molecular weight (LMW) heparin (NACH) [8] (55 μM). These strong interactions were attributed to multivalent binding between the polysaccharides and the SGPs of viral particles [9].
Due to the highly transmissible and pathogenic nature of SARS-CoV-2, a biosafety level 3 (BSL3) containment is required for live-virus studies [10]. A pseudotyping system, which mimics the surface properties of SARS-CoV-2, has been used in biosafety level 2 (BSL2) laboratories for the purpose of vaccine studies, drug inhibition studies and serological screening [10]. Recently, a sulfated fucan from Lytechinus variegatus (sea urchin) and a sulfated galactan from Botryocladia occidentalis (red seaweed) were found to have anti-SARS-CoV-2 activity using these pseudotype SARS-CoV-2 particles [11]. SARS-CoV-2 SGFs could bind ACE2 based on previous studies [12]. In the current study, interactions between pseudotyped particles and polysaccharides from Saccharina japonica have been further investigated to elucidate their structure-activity relationship. In addition, the possible inhibitory activities of polysaccharides on the interaction between SARS-CoV-2 SGPs and ACE2 were also determined.
2. Materials and methods
2.1. Preparation of polysaccharides from Saccharina japonica
Crude polysaccharide from Saccharina japonica (SJ) was obtained according to a previous study by hot water extraction [13]. SJ-100K, SJ-50K, SJ-30K, SJ-10K and SJ-3K were prepared from 0.2 M formic acid catalyzed degradation of SJ using five types of ultrafiltration devices (Ultracel 100 kDa membrane, Ultracel 50 kDa membrane, Ultracel 30 kDa membrane, Ultracel 10 kDa membrane and Ultracel 3 kDa membrane, respectively (Sigma-Aldrich)) [14]. LMW polysaccharide (SJ-D) was prepared by the degradation of SJ polysaccharide using hydrogen peroxide and ascorbic acid [[14], [15], [16], [17]]. SJ and SJ-D were separated by anion-exchange chromatography on a DEAE-Bio Gel agarose FF (6 cm × 40 cm) eluted with water (5 L), 0.5 M NaCl (5 L) (Fraction W: SJ-W and SJ-D-W), 1 M NaCl (5 L) (Fraction I: SJ-I and SJ-D-I) and 2 M NaCl (5 L) (Fraction S: SJ-S and SJ-D-S) [14]. Autohydrolysis was carried out by methods described in previous studies [[18], [19], [20], [21], [22], [23], [24]]. Two types of autohydrolyzed polysaccharides, including higher molecular weight, H-type fractions (SJ-D-W-H, SJ-D-I-H and SJ-D-S-H) and lower molecular weight, L-type fractions (SJ-D-W-L, SJ-D-I-L and SJ-D-S-L) were obtained [14]. Glucuronomannan oligomers (G2, G4 and G6, disaccharide, tetrasaccharide and hexasaccharide, respectively) and glucuronomannan polysaccharide (Gn) with a molecular weight of 7.0 kDa were prepared in our laboratory by previously described methods [[25], [26], [27]]. The desulfated polysaccharides (SJ-I-DS, SJ-S-DS, SJ-D-I-DS and SJ-D-S-DS) and oversulfated polysaccharides (SJ-I-PS, SJ-S-PS, SJ-D-I-PS and SJ-D-S-PS) were prepared according to the previous studies [13,14,28]. Acid degradation was carried out according to the previous study [29]. Three fractions were obtained. Briefly, crude polysaccharide (10 mg/mL) was dissolved in 0.1 M HCl and stirred for 0.5 h at 80 °C. The mixture was neutralized with 5% NH4OH solution in water. The solution was concentrated and precipitated by ethanol. The supernatant (Fraction GX-1: SJ-GX-1 and SJ-D-W-GX-1) was dialyzed and lyophilized. The precipitant was further degraded by 0.5 M HCl and stirred for 2 h at 80 °C. The mixture was neutralized with 5% NH4OH solution in water. The solution was concentrated and precipitated by ethanol. The supernatant (Fraction GX-2: SJ-GX-2 and SJ-D-W-GX-2) was dialyzed and lyophilized. The precipitants (Fraction GX-3: SJ-GX-3 and SJ-D-W-GX-3) were then dialyzed and lyophilized. All samples studied were summarized in Scheme 1 .
Scheme 1.
The flow chart of sample preparation.
2.2. Solution competition SPR analysis
Solution competition SPR measurements were performed on a BIAcore 3000 (GE Healthcare, Uppsala, Sweden). A heparin chip was prepared based on previous studies [5] by the immobilization of biotinylated heparin on a streptavidin (SA) chip. Pseudotype particles were prepared as in previous studies [10,11]. Briefly, HEK293T cells (2 × 106) were plated in a 100-mm tissue culture dish and transfected the next day with a combination of the following plasmids: 9 μg of pLV-eGFP, 9 μg of psPAX2, and 3 μg of pCAGGS-S (SARS-CoV-2)(Catalog No. NR-52310: BEI-Resources). The supernatant from cell culture was harvested, spun in a tabletop centrifuge for 5 min at 2000 ×g to pellet the residual cells and then passed through a 0.45 μm syringe filter. Virus titers were calculated by plating on new HEK293T cells and enumerating GFP positive cells. Pseudotype particles were pre-mixed with different concentrations of polysaccharides or oligosaccharides in HBS-EP buffer and injected over the heparin chip at 30 μL/min to measure the inhibition of pseudotype particle binding to heparin surface. After each run, there was a dissociation period of 3 min and a regeneration period of 1 min using 2 M NaCl.
SARS-CoV-2 spike protein was purchased from Sino Biological Inc. (catalog #: 40591-V02H). SARS-CoV-2 spike protein was immobilized covalently to a CM5 sensor chip using manufacturer methods. Human angiotensin converting enzyme 2 (ACE2 was a generous gift from Professor Jason McLellan from the University of Texas at Austin) at 500 nM with or without polysaccharides or oligosaccharides was injected over the SARS-CoV-2 spike protein chip at 30 μL/min to measure the inhibition of polysaccharides or oligosaccharides on ACE2 binding to SARS-CoV-2 spike protein chip. After each run there was a dissociation period of 3 min and regeneration period of 1 min using with 2 M NaCl.
2.3. Compositional analysis and nuclear magnetic resonance (NMR) spectroscopy
The molar ratio of monosaccharides, fucose (Fuc) and galactose (Gal) contents, were determined based on a modified method from a previous study [30]. Sulfate content was determined by the paper of Dodgson and Price [31]. The molecular weights of the polysaccharides were determined using gel permeation chromatography-high performance liquid chromatography (GPC-HPLC) on TSK G3000 PWxl column (7 μm, 7.8 × 300 mm) with elution in 0.05 M Na2SO4 at a flow rate of 0.5 mL/min at 40 °C with refractive index detection. Ten different molecular weight dextrans, purchased from the National Institute for the Control of Pharmaceutical and Biological Products (China), were used as molecular weight standards.
For NMR analysis, polysaccharides (30 mg) were co-evaporated with deuterium oxide (99.9%) twice before dissolving in deuterium oxide. One-and two-dimensional spectra, including 1H NMR, 13C NMR and 1H— 13C heteronuclear single quantum coherence spectroscopy (HSQC), were recorded at a Hudson-Bruker SB 800 MHz spectrometer (Bruker BioSpin, Billerica, MA, USA) at 25 °C.
3. Results
3.1. SPR solution competition study of different polysaccharides obtained by anion exchange chromatography on heparin chip
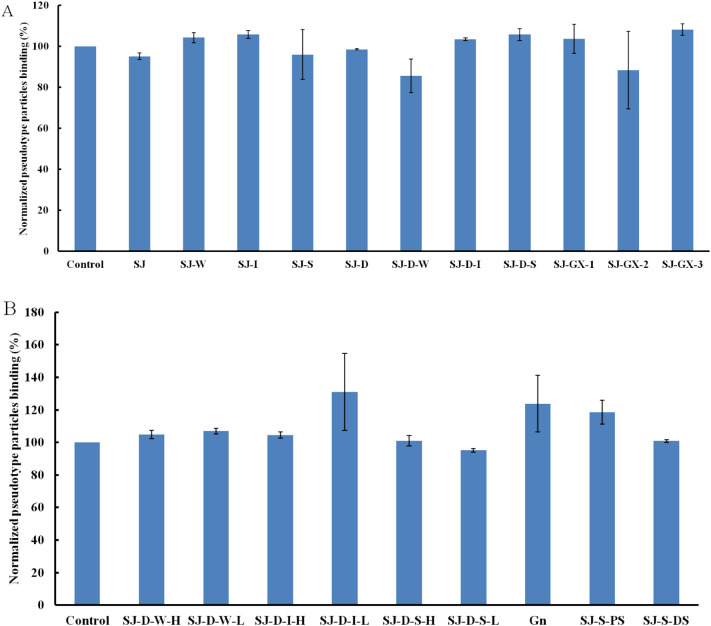
According to a previous study [6], polysaccharide (SJ) from Saccharina japonica has anti-SARS-CoV-2 virus activity and exhibits stronger inhibitory activity binding to SARS-CoV-2 SGPs. Therefore, SJ and its derivatives were screened using SPR to determine binding affinity to the pseudotype particles for the purpose of structure-activity relationship. SJ showed the strongest inhibitory activity, which was consistent with the previous study (Fig. 1A). Molecular weight influenced the binding ability, however, SJ-D still exhibited a strong inhibitory activity (>80%) (Fig. 1B). Anion exchange chromatography was carried out to separate SJ and SJ-D and SJ-I, SI-S and SJ-D-S showed strong inhibitory activity (>80%). Previous studies [[15], [16], [17]] showed that SJ-I, SI-S and SJ-D-S were sulfated galactofucans.
Fig. 1.
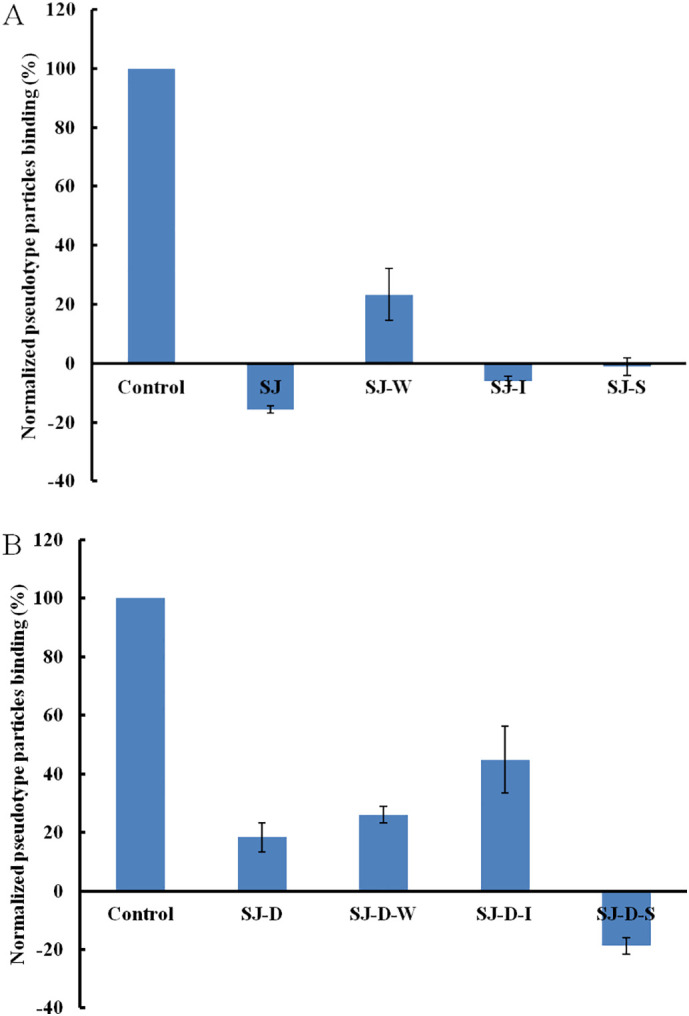
Bar graphs of normalized pseudotype particles binding preference to surface heparin by competing with SJ and its fractions (A) and SJ-D and its fractions (B). Concentration of pseudotype particles was 0.085 mg/mL and concentrations of different polysaccharides were 1000 nM. All bar graphs with standard deviations were based on triplicate experiments.
3.2. SPR solution competition study on modified polysaccharides (mainly sulfated galactofucans) using a heparin chip
After autohydrolysis, two fractions (H-type fraction with high molecular weight and L-type fraction with low molecular weight) were obtained. The results of SPR (Fig. 2A) indicated that H-type fractions (SJ-D-W-H, SJ-D-I-H and SJ-D-S-H) had stronger inhibitory activities than L-type fractions (SJ-D-W-L, SJ-D-I-L and SJ-D-S-L). SJ-D-I-H and SJ-D-S-H showed strong inhibitory activity (> 80%). The impact of sulfate was examined using desulfated polysaccharides (SJ-I-DS, SI-S-DS, SJ-D-I-DS and SJ-D-S-DS) and chemically oversulfated polysaccharides (SJ-I-PS, SI-S-PS, SJ-D-I-PS and SJ-D-S-PS). Oversulfated polysaccharides exhibited very strong inhibitory activities, which were stronger than the native polysaccharides (Fig. 2B and C). Desulfated polysaccharides exhibited only very weak or no inhibitory activities and were weaker inhibitors than the native polysaccharides. We conclude that the presence of sulfate groups a one-factor influencing polysaccharide binding to pseudotype particles.
Fig. 2.
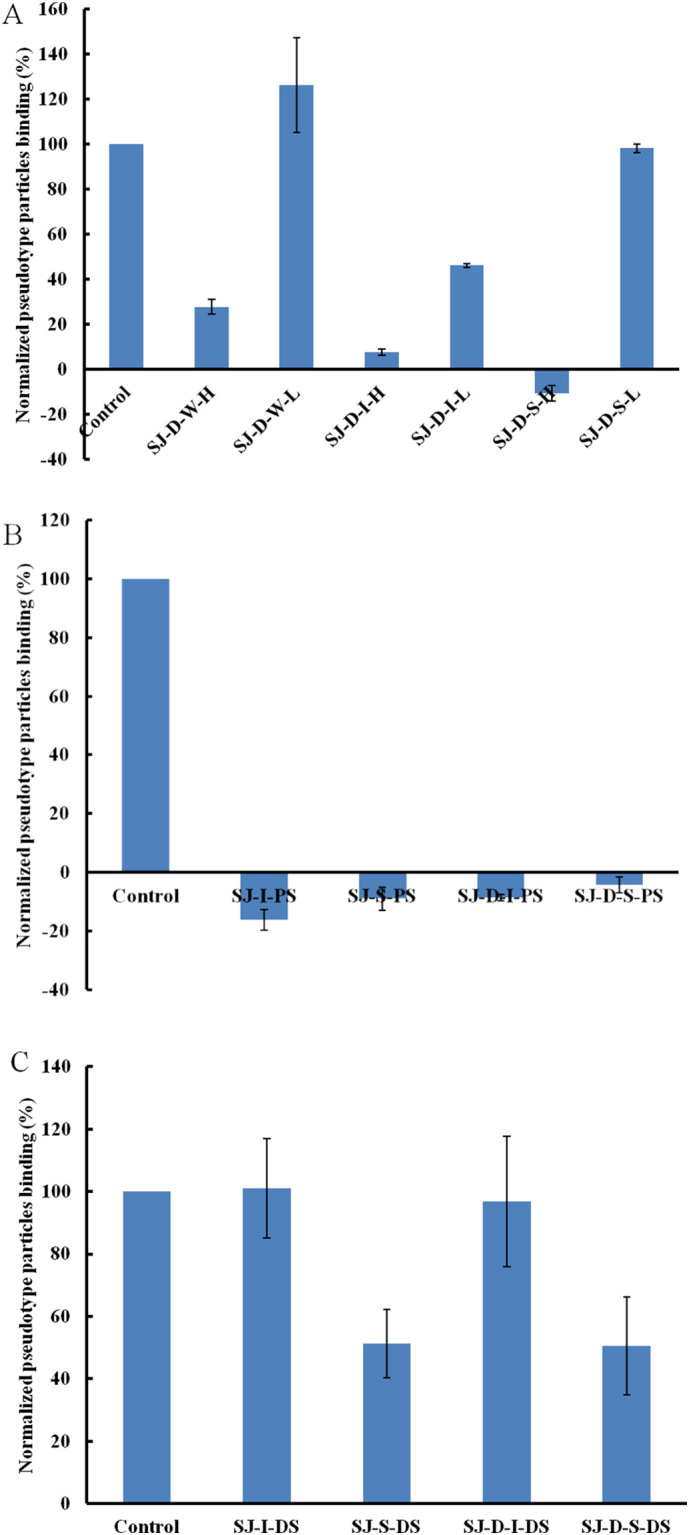
Bar graphs of normalized pseudotype particles binding preference to surface heparin by competing with different modified polysaccharides (mainly on sulfated galactofucan). Concentration of pseudotype particles was 0.085 mg/mL and concentrations of different polysaccharides were 1000 nM. All bar graphs with standard deviations were based on triplicate experiments.
3.3. SPR solution competition study on modified polysaccharides (mainly on glucuronomannan) on heparin chip
Five MWCO membranes (100 K, 50 K, 30 K, 10 K and 3 K) were used to separate the partially degraded polysaccharides by 0.2 M formic acid. SJ-100 K showed the strongest inhibitory activity (Fig. 3A). In addition, 0.1 M HCl and 0.5 M HCl were used sequentially to degrade SJ and SJ-D-W to obtain three fractions: (1) dilute acid-sensitive fractions (SJ-GX-1 and SJ-D-W-GX-1); (2) acid-sensitive fractions (SJ-GX-2 and SJ-D-W-GX-2); and (3) acid-stable fractions (SJ-GX-3 and SJ-D-W-GX-3), respectively. Acid-stable fractions (SJ-GX-3 and SJ-D-W-GX-3) exhibited stronger inhibitory activities than the other two corresponding fractions (Fig. 3B). Monosaccharide analysis indicated that acid-stable fractions (SJ-GX-3 and SJ-D-W-GX-3) were glucuronomannan derivatives, suggesting that glucuronomannan might be one active component to bind the pseudotype particles. A low molecular weight glucuronomannan (Gn) with 7.0 k Da and three glucuronomannan-oligomers were also examined. Gn exhibited very strong inhibitory activity (>90%) (Fig. 3C). It is interesting to note that glucuronomannan-tetramer showed 78% inhibitory activity.
Fig. 3.
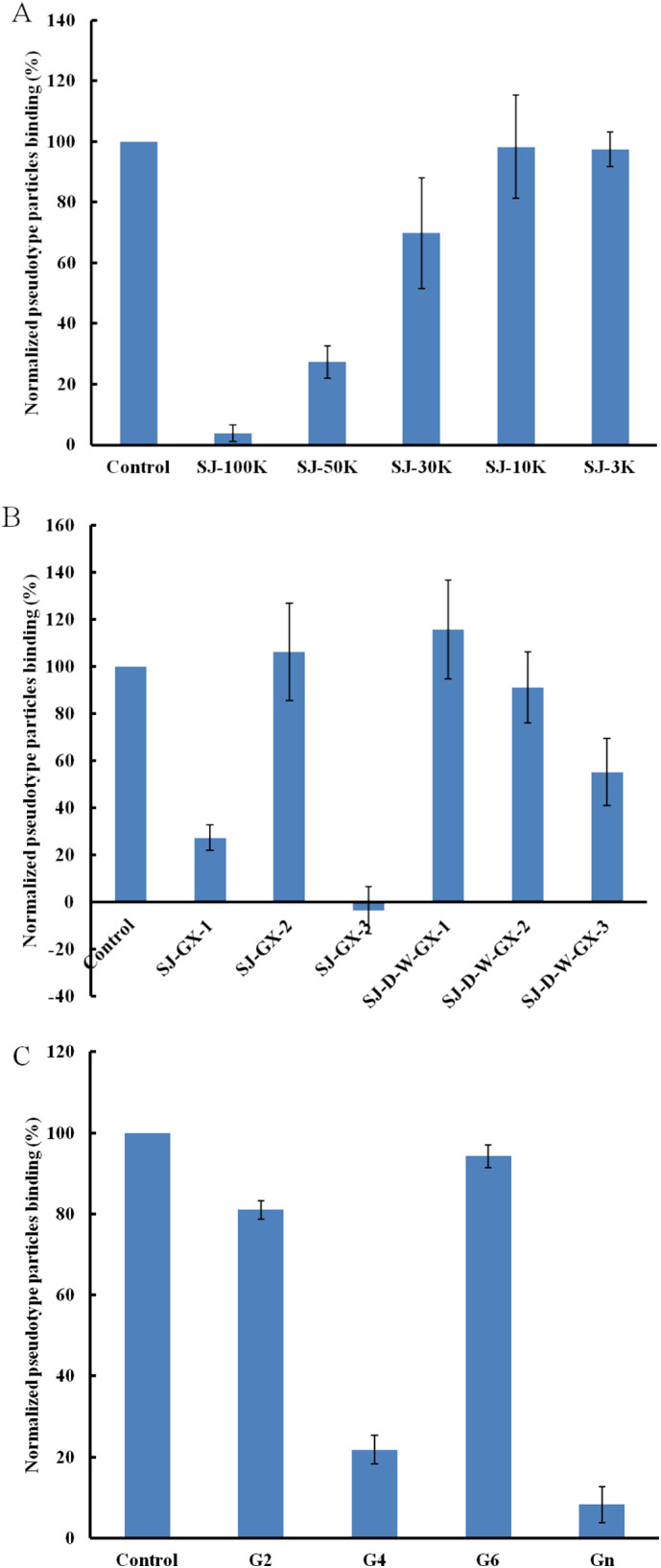
Bar graphs of normalized pseudotype particles binding preference to surface heparin by competing with different modified polysaccharides (mainly on glucuronomannan). Concentration of pseudotype particles was 0.085 mg/mL and concentrations of different polysaccharides were 1000 nM. All bar graphs with standard deviations were based on triplicate experiments.
3.4. Kinetics measurements of pseudotype particles interaction with SJ-D-S-H and Gn on heparin chip
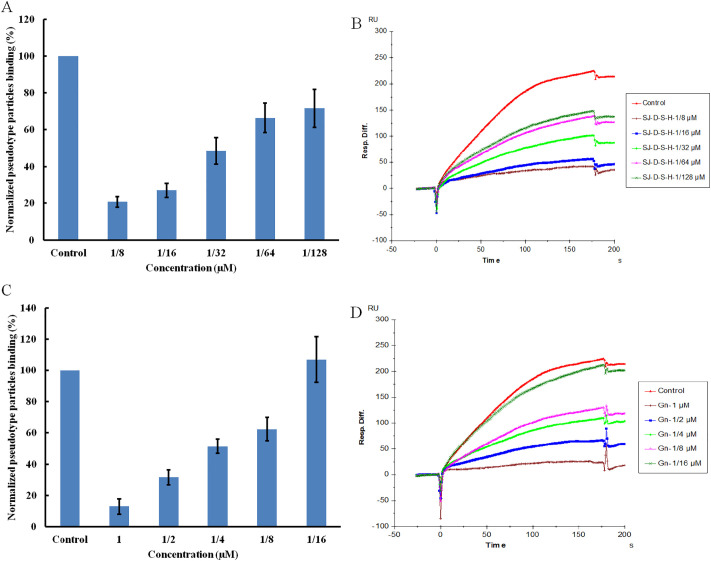
Based on the types of polysaccharides from fucose-containing polysaccharides from brown algae [32], a sulfated galactofucan (SJ-D-S-H) and a glucuronomannan (Gn) were further studied. The yields of SJ-D-S-H and Gn from SJ were 3.4% and 0.5%, respectively. We determined the kinetic measurements of the interaction of SJ-D-S-H and Gn with pseudotype particles by solution-based affinities (Ki) and calculated from IC50 values from SPR competition experiments. The IC50 values of SJ-D-S-H and Gn binding to pseudotype particles are shown in Fig. 4 . According to a previous study [11], the dissociation constant (KD) for heparin and pseudotype particles was 0.85 nM (The estimated molecular weight of pseudotype particles is 2.5 × 105 kDa). Therefore, the Ki of samples were calculated using the equation: Ki = IC50/(1 + [C]/KD), where [C] is the concentration of pseudotype particles (0.34 nM) used in the competition SPR, and for pseudotype particles binding affinity (KD) for heparin [14,33]. It was shown that the Ki of SJ-D-S-H and Gn binding to pseudotype particles were calculated to be 19 nM and 165 nM, respectively.
Fig. 4.
SJ-D-S-H competes with heparin for pseudotype particles (A and B) binding with a IC50 of 27 nM and Gn competes with heparin for pseudotype particles (C and D) binding with an IC50 of 231 nM. Concentrations were 0.085 mg/mL for pseudotype particles. All bar graphs with standard deviations were based on triplicate experiments.
3.5. Structure features of SJ-D-S-H and Gn
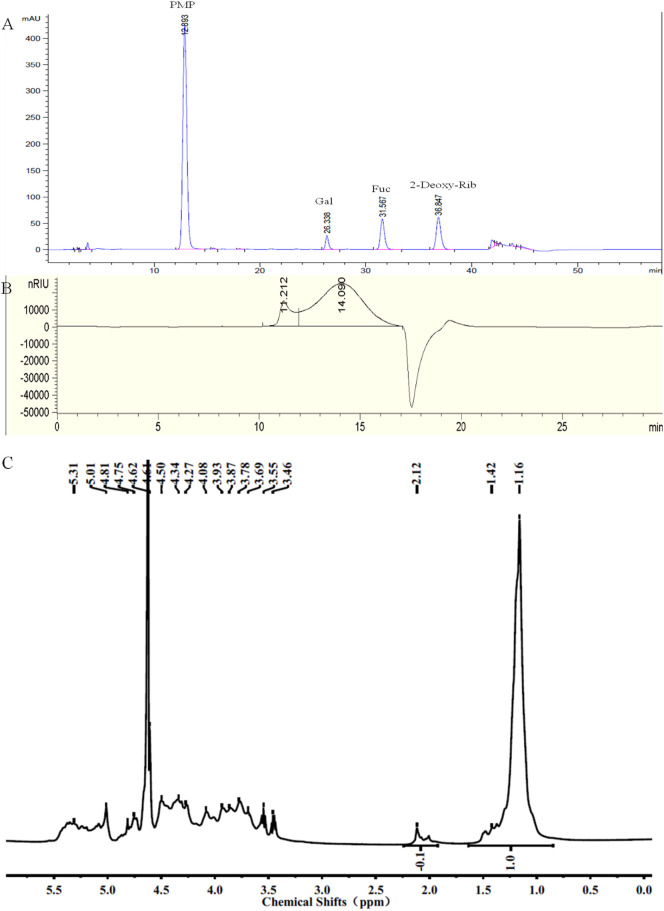
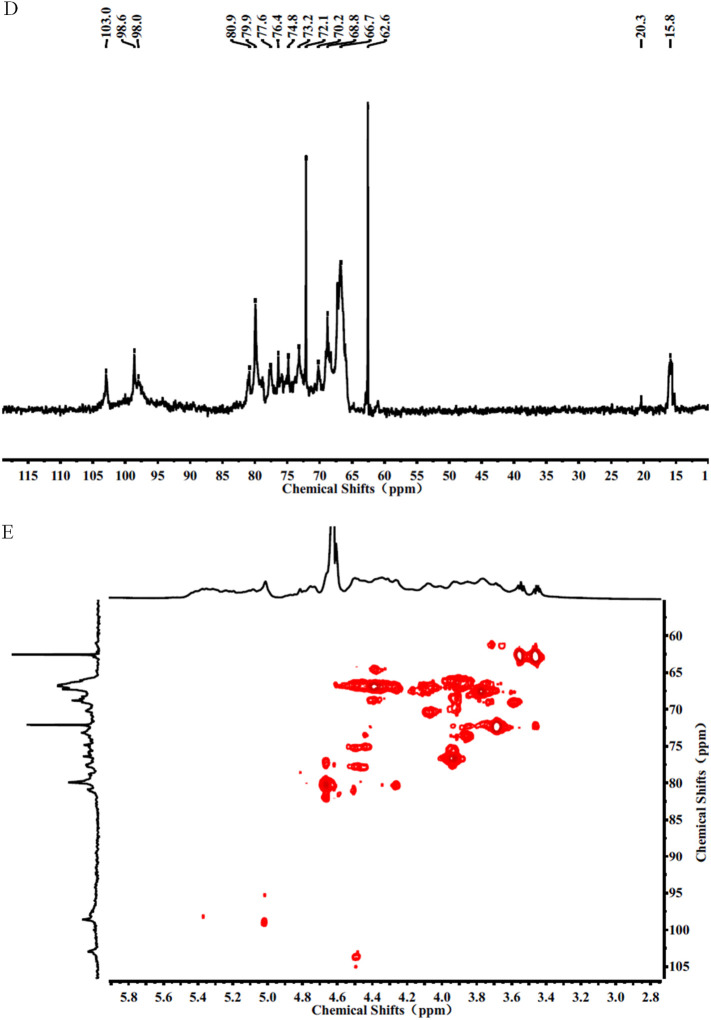
The chemical composition analysis in Fig. 5A indicate that SJ-D-S-H had 21% sulfate, 36% Fuc and 10% Gal, suggesting that the molar ratio of Gal to Fuc in SJ-D-S-H was 0.25: 1. The molar ratio of sulfate residues to Fuc residues was 0.83, using the equation: (sulfate content/103)/(Fuc content/146), where 103 was calculated as SO3Na and 146 was Fuc-H2O. The molar ratio of sulfate to hexose, including Fuc and Gal was 0.66 calculated using the equation: (sulfate content/103)/(Fuc content/164 + Gal content/162), where 103 was calculated as SO3Na, 162 was Gal-H2O and 146 was Fuc-H2O. GPC-HPLC shows that SJ-D-S-H had a major peak at 14.090 min, corresponding to the molecular weight of 13.7 kDa and an accompanying shoulder peak (The area ratio of the major peak to the shoulder peak was 1:8) at 11.212 min corresponding to a molecular weight of 195.0 kDa (Fig. 5B). NMR spectroscopy was performed to elucidate the structure of SJ-D-S-H (Fig. 5). Resonances with chemical shifts for anomeric carbons, at 103.0 ppm, are characteristic peaks for 1, 6-linked β-D-Gal residues and β-D-Gal residues, assigned to the residue D and E. These are also confirmed by the presence of two peaks at 62.6 and 72.1 ppm, corresponding to the C6 of β-D-Gal residues and 1, 6-linked β-D-Gal residues, respectively. The chemical shifts at approximately 98.6 ppm are characteristic peaks for 1, 3-linked α-L-Fuc4S residues, assigned to the residue C. The chemical shifts at approximately 98.0 ppm are characteristic peaks for 1, 3-linked α-L-Fuc2, 4S residues and 1, 3-linked α-L-Fuc4S residues branched at C2 with galacto-biose, corresponding to the residues A and B [22,[34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44]]. In terms of the 1H NMR spectrum, resonances with chemical shifts of anomeric protons, at approximately 5.31 ppm, are characteristic peaks for 1, 3-linked α-L-Fuc2, 4S residues (Residue A) and 1,3-linked α-L-Fuc4S residues (Residue B) branched at C2, respectively. The chemical shifts at approximately 5.01 ppm are characteristic peaks for 1, 3-linked α-L-Fuc4S residues (Residue C). The chemical shifts at approximately 4.50 ppm are characteristic peaks for 1, 6-linked β-D-Gal residues (Residue D and E), respectively [[34], [35], [36], [37], [38]]. Moreover, the chemical shifts at 2.12 and 20.3 ppm can be assigned to acetyl groups, indicating that SJ-D-S-H was also acetylated. Two methyl groups at 1.16/1.42 and 2.12 ppm were characteristic of the C6 methyl group of fucose and CH3 of acetyl group, respectively. By comparing these two peak area, it was suggested that the molar ratio of acetyl group to the methyl group of Fuc residues was 0.1 in Fig. 5C. Therefore, based on the above results, we conclude that the primary structure of SJ-D-S-H is 1, 3-linked α-L-Fucp residues sulfated at C4 and C2/C4 and 1, 3-linked α-L-Fucp residues sulfated at C4 and branched with 1, 6-linked β-D-galacto-biose (Fig. 3). In addition, SJ-D-S-H was also randomly substituted with acetyl group and sulfate groups on a small fraction of the Gal residues. The primary structure of SJ-D-S-H is proposed in Fig. 6 .
Fig. 5.
The PMP derivatization-HPLC spectrum (A), GPC-HPLC (B), 1H NMR spectrum (C), 13C NMR spectrum (D) and HSQC spectrum (E) for SJ-D-S-H.
Fig. 6.
The proposed primary structures of SJ-D-S-H and Gn.
Based on previous studies [[25], [26], [27],29,45], glucuronomannan (Gn) consists of alternating 1, 4-linked β-D-GlcAp residues and 1, 2-linked α-D-Manp residues and has a molecular weight of 7.0 kDa. The primary structure of Gn proposed is shown in Fig. 6.
3.6. SPR solution competition study on SARS-CoV-2 spike protein chip of polysaccharides with ACE2
SARS-CoV-2 SGF binds to ACE2 with high affinity [12]. In our previous study [11], we proposed a model of SARS-CoV-2 attachment and entry, suggesting that virus at first bound to HS in the nasal epithelium glycocalyx, increasing the local concentration of virus, leading to increased infection rates. The HS-virus complex then bound to ACE2 and was endocytosed. In the current study, we show that the sulfated polysaccharides from S. japonica exhibited the inhibitory activity between heparin and pseudotype particles containing the SARS-CoV-2 SGPs. Here, we examined whether sulfated polysaccharides could inhibit the interaction between SARS-CoV-2 SGFs and ACE2. SARS-CoV-2 SGFs was immobilized to a CM5 sensor chip to answer this question. ACE2 with or without polysaccharides (or oligosaccharides) was injected over the SARS-CoV-2 spike protein chip to measure the inhibition of polysaccharides on ACE2 binding to SARS-CoV-2 spike protein. ACE2 binds to the SARS-CoV-2 SGP at 140 nM KD (Fig. 7 and Fig. S1), which is lower than the KD (15 nM) between SARS-CoV RBD-SD1 and ACE2 [12]. SPR results for polysaccharides showed no or low inhibitory activity on the interaction between SARS-CoV-2 SGPs and ACE2, suggesting that polysaccharides could not inhibit the interaction between SARS-CoV-2 SGPs and ACE2.
Fig. 7.
Bar graphs of normalized ACE2 binding preference to surface SARS-CoV-2 spike protein by competing with different polysaccharides. Concentration of ACE2 was 500 nM and concentrations of different polysaccharides were 1000 nM. All bar graphs with standard deviations were based on triplicate experiments.
4. Discussion and conclusion
COVID-19 could invade host cell by interacting with host cell surface GAGs through its SGPs [3]. SPR studies confirmed that both monomeric and trimeric SARS-CoV-2 SGPs show very strong binding affinity to heparin, suggesting that heparin had multiple potential targets could be used in COVID-19 therapy [5]. Fucose-containing sulfated polysaccharides (FCSPs) are polysaccharides obtained from brown algae [32,40,[46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62]]. Sulfated polysaccharides from marine seaweeds have been suggested to have potential antiviral therapeutic activities [[63], [64], [65]]. FCSPs from Adenocystis utricularis, Undaria pinnatifida, Stoechospermum marginatum, Cystoseira indica, Cladosiphon okamuranus or Fucus vesiculosus reportedly exhibit anti-HSV-1, HSV-2, HCMV, VSV, Sindbis virus or HIV-1 by stimulating innate and adaptive immune defense, inhibition of cell adhesion (Stage I), viral replication (Stage II), blockage of the reverse transcriptase (Stage III) [58,62,63,[66], [67], [68], [69], [70], [71], [72], [73]].
Our previous study [6] showed that SJ exhibited promising anti-COVID-19 viral activity in vitro. However, SJ was a crude heteropolysaccharide from brown alga Saccharina japonica. The structure-activity relationship was studied to find the active components of these polysaccharides. According to the previous studies [32], polysaccharides from brown algae contain sulfated fucan, sulfated galactofucan, fucoglucuronan and fucoglucuronomannan. Anion exchange chromatography was performed to separate SJ and SJ-D into weak (W-type fraction), intermediate (I-type fraction) and strongly (S-type fraction) charged fractions. SPR results indicated that SJ-I and SJ-S had very strong inhibitory activities while only SJ-D-S had very strong inhibitory activity, suggesting that molecular weight also is important for antiviral activity. Selective C2-desulfation was accomplished by autohydrolysis, which is accompanied by partial debranching. Higher molecular weight components (SJ-D-W-H, SJ-D-I-H and SJ-D-S-H) showed stronger inhibitory activities than corresponding low molecular weight components (SJ-D-W-L, SJ-D-I-L and SJ-D-S-L), confirming the above hypothesis. In addition, SJ-D-I-H and SJ-D-S-H showed very strong inhibitory activities. Sulfate content was also examined by preparing desulfated polysaccharides and oversulfated polysaccharides, suggesting that sulfate content was also an important factor. Therefore, we conclude that molecular weight and sulfation level are two important factors, which is consistent with the previous studies [70]. SJ was also degraded using formic acid and separated using five types of ultrafiltration membranes to obtain five different molecular weight fractions. SPR results indicated that the largest molecular weight component ST-100 K, an acid-stable fraction, had the strongest inhibitory activity. Therefore, two concentrations of acid, 0.1 M and 0.5 M HCl, were used to degrade SJ and SJ-D-W to obtain three fractions: (1) dilute acid-sensitive fractions (SJ-GX-1 and SJ-D-W-GX-1); (2) acid-sensitive fractions (SJ-GX-2 and SJ-D-W-GX-2); and (3) acid-stable fractions (SJ-GX-3 and SJ-D-W-GX-3). We found that acid-stable fractions had stronger inhibitory activities than other fractions. Monosaccharide analysis indicated that SJ-GX-3 and SJ-D-W-GX-3 were glucuronomannan derivatives. Three glucuronomannan oligomers (G2, G4 and G6) and one glucuronomannan (Gn) with 7.0 kDa were prepared and their binding to pseudotype particles were determined. The results showed that Gn might be the active component.
In conclusion, polysaccharides from marine seaweeds (Gn and SJ-D-S-H) might represent a good candidate for treatment of COVID-19 based on our systematic study of structure-activity relationship of interaction with SARS-CoV-2 SGPs but not ACE2.
The following is the supplementary data related to this article.
SPR sensorgrams for binding kinetics measurements for human ACE2 and immobilized SARS-CoV-2 spike protein interactions. The concentrations of ACE2 (from top to bottom) were 1000, 600, 400, 200, 100 nM, respectively. The black curves are the fitting curves using a 1:1 L model from BIAevaluation 4.0.1.
CRediT authorship contribution statement
Weihua Jin: Conceptualization, Methodology, Data curation, Formal analysis, Funding acquisition, Resources, Writing-Original draft preparation, Project administration; Wenjing Zhang: Methodology, Writing-Original draft preparation, Funding acquisition; Dipanwita Mitra: Conceptualization, Methodology; Martin G. McCandless: Conceptualization, Methodology; Poonam Sharma: Conceptualization, Methodology; Ritesh Tandon: Conceptualization, Methodology; Fuming Zhang: Methodology, Writing Reviewing and Editing; Robert J. Linhardt: Writing-Reviewing and Editing.
Acknowledgements
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (No. LY19D060006), the National Natural Science Foundation of China (No. 41906095) and China Scholarship Council (W.J.). R.T. received funding from National Aeronautics and Space Administration (80NSSC19K1603) and the University of Mississippi Medical Center. The National Institutes of Health Grants DK111958, CA231074, NS088496 and AG062344 to R.J.L.
References
- 1.Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.COVID-19 case tracker. 2020. https://coronavirus.jhu.edu/#covid-19-basics
- 3.Kim S.Y., Jin W., Sood A., Montgomery D.W., Grant O.C., Fuster M.M., Fu L., Dordick J.S., Woods R.J., Zhang F., Linhardt R.J. Characterization of heparin and severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) spike glycoprotein binding interactions. Antivir. Res. 2020;181 doi: 10.1016/j.antiviral.2020.104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mycroft-West C., Su D., Elli S., Li Y., Guimond S., Miller G., Turnbull J., Yates E., Guerrini M., Fernig D., Lima M., Skidmore M. The 2019 coronavirus (SARS-CoV-2) surface protein (Spike) S1 Receptor Binding Domain undergoes conformational change upon heparin binding. BioRxiv. 2020 doi: 10.1101/2020.02.29.971093. [DOI] [Google Scholar]
- 5.Lindahl U., Li J.P. Heparin - an old drug with multiple potential targets in Covid-19 therapy. J. Thromb. Haemost. 2020 doi: 10.1111/jth.14898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwon P.S., Oh H., Kwon S.-J., Jin W., Zhang F., Fraser K., Hong J.J., Linhardt R.J., Dordick J.S. Sulfated polysaccharides effectively inhibit SARS-CoV-2 in vitro. Cell Discovery. 2020;6:50. doi: 10.1038/s41421-020-00192-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cress B.F., Bhaskar U., Vaidyanathan D., Williams A., Cai C., Liu X., Fu L., V M.C., Zhang F., Mousa S.A., Dordick J.S., Koffas M.A.G., Linhardt R.J. Heavy heparin: a stable isotope-enriched, chemoenzymatically-synthesized, poly-component drug. Angew. Chem. Int. Ed. Eng. 2019;58:5962–5966. doi: 10.1002/anie.201900768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin Y.P., Yu Y., Marcinkiewicz A.L., Lederman P., Hart T.M., Zhang F., Linhardt R.J. Non-anticoagulant heparin as a pre-exposure prophylaxis prevents lyme disease infection. ACS Infect. Dis. 2020;6:503–514. doi: 10.1021/acsinfecdis.9b00425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nemanichvili N., Tomris I., Turner H.L., McBride R., Grant O.C., van der Woude R., Aldosari M.H., Pieters R.J., Woods R.J., Paulson J.C., Boons G.J., Ward A.B., Verheije M.H., de Vries R.P. Fluorescent trimeric hemagglutinins reveal multivalent receptor binding properties. J. Mol. Biol. 2019;431:842–856. doi: 10.1016/j.jmb.2018.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tandon R., Mitra D., Sharma P., Stray S.J., Bates J.T., Marshall G.D. Effective screening of SARS-CoV-2 neutralizing antibodies in patient serum using lentivirus particles pseudotyped with SARS-CoV-2 spike glycoprotein. MedRxiv. 2020 doi: 10.1101/2020.05.21.20108951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tandon R., Sharp J.S., Zhang F., Pomin V.H., Ashpole N.M., Mitra D., Jin W., Liu H., Sharma P., Linhardt R.J. Effective inhibition of SARS-CoV-2 entry by heparin and enoxaparin derivatives. BioRxiv. 2020 doi: 10.1101/2020.06.08.140236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wrapp D., Wang N., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin W.H., Zhang W.J., Liang H.Z., Zhang Q.B. The structure-activity relationship between marine algae polysaccharides and anti-complement activity. Mar. Drugs. 2016;14:3. doi: 10.3390/md14010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jin W., Jiang D., Zhang W., Wang C., Xia K., Zhang F., Linhardt R.J. Interactions of fibroblast growth factors with sulfated galactofucan from Saccharina japonica. Int. J. Biol. Macromol. 2020;160:26–34. doi: 10.1016/j.ijbiomac.2020.05.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin W.H., Wang J., Jiang H., Song N., Zhang W.J., Zhang Q.B. The neuroprotective activities of heteropolysaccharides extracted from Saccharina japonica. Carbohydr. Polym. 2013;97:116–120. doi: 10.1016/j.carbpol.2013.04.055. [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Zhang Q., Zhang Z., Li Z. Antioxidant activity of sulfated polysaccharide fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2008;42:127–132. doi: 10.1016/j.ijbiomac.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 17.Wang J., Zhang Q., Zhang Z., Song H., Li P. Potential antioxidant and anticoagulant capacity of low molecular weight fucoidan fractions extracted from Laminaria japonica. Int. J. Biol. Macromol. 2010;46:6–12. doi: 10.1016/j.ijbiomac.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 18.Jin W., Tang H., Zhang J., Wei B., Sun J., Zhang W., Zhang F., Wang H., Linhardt R.J., Zhong W. Structural analysis of a novel sulfated galacto-fuco-xylo-glucurono-mannan from Sargassum fusiforme and its anti-lung cancer activity. Int. J. Biol. Macromol. 2020;149:450–458. doi: 10.1016/j.ijbiomac.2020.01.275. [DOI] [PubMed] [Google Scholar]
- 19.Jin W., Wu W., Tang H., Wei B., Wang H., Sun J., Zhang W., Zhong W. Structure analysis and anti-tumor and anti-angiogenic activities of sulfated galactofucan extracted from Sargassum thunbergii. Mar. Drugs. 2019;17:52. doi: 10.3390/md17010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ciancia M., Satob Y., Nonamic H., Cerezod A.S., Erra-Balsellsd R., Matulewicz M.C. Autohydrolysis of a partially cyclized mu/nu-carrageenan and structural elucidation of the oligosaccharides by chemical analysis, NMR spectroscopy and UV-MALDI mass spectrometry. Arkivoc. 2005;xii:319–331. [Google Scholar]
- 21.Rodriguez-Jasso R.M., Mussatto S.I., Pastrana L., Aguilar C.N., Teixeira J.A. Extraction of sulfated polysaccharides by autohydrolysis of brown seaweed Fucus vesiculosus. J. Appl. Phycol. 2013;25:31–39. [Google Scholar]
- 22.Menshova R.V., Anastyuk S.D., Ermakova S.P., Shevchenko N.M., Isakov V.I., Zvyagintseva T.N. Structure and anticancer activity in vitro of sulfated galactofucan from brown alga Alaria angusta. Carbohydr. Polym. 2015;132:118–125. doi: 10.1016/j.carbpol.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 23.Shevchenko N.M., Anastyuk S.D., Menshova R.V., Vishchuk O.S., Isakov V.I., Zadorozhny P.A., Sikorskaya T.V., Zvyagintseva T.N. Further studies on structure of fucoidan from brown alga Saccharina gurjanovae. Carbohydr. Polym. 2015;121:207–216. doi: 10.1016/j.carbpol.2014.12.042. [DOI] [PubMed] [Google Scholar]
- 24.Anastyuk S.D., Imbs T.I., Shevchenko N.M., Dmitrenok P.S., Zvyagintseva T.N. ESIMS analysis of fucoidan preparations from Costaria costata, extracted from alga at different life-stages. Carbohydr. Polym. 2012;90:993–1002. doi: 10.1016/j.carbpol.2012.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Jin W., Ren L., Liu B., Zhang Q., Zhong W. Structural features of sulfated glucuronomannan oligosaccharides and their antioxidant activity. Mar. Drugs. 2018;16:291. doi: 10.3390/md16090291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin W., Wang J., Ren S., Song N., Zhang Q. Structural analysis of a heteropolysaccharide from Saccharina japonica by electrospray mass spectrometry in tandem with collision-induced dissociation tandem mass spectrometry (ESI-CID-MS/MS) Mar. Drugs. 2012;10:2138–2152. doi: 10.3390/md10102138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin W., He X., Zhu J., Fang Q., Bin W., Sun J., Zhang W., Zhang Z., Zhang F., Linhardt R.J., Wang H., Zhong W. Inhibition of glucuronomannan hexamer on the proliferation of lung cancer through binding with immunoglobulin G. Carbohydr. Polym. 2020;248 doi: 10.1016/j.carbpol.2020.116785. [DOI] [PubMed] [Google Scholar]
- 28.Jin W., Zhang W., Liu G., Yao J., Shan T., Sun C., Zhang Q. The structure-activity relationship between polysaccharides from Sargassum thunbergii and anti-tumor activity. Int. J. Biol. Macromol. 2017;105:686–692. doi: 10.1016/j.ijbiomac.2017.07.089. [DOI] [PubMed] [Google Scholar]
- 29.Wu J.D., Lv Y.J., Liu X.X., Zhao X.L., Jiao G.L., Tai W.J., Wang P.P., Zhao X., Cai C., Yu G.L. Structural study of sulfated Fuco-oligosaccharide branched glucuronomannan from Kjellmaniella crassifolia by ESI-CID-MS/MS. J. Carbohydr. Chem. 2015;34:303–317. [Google Scholar]
- 30.Li J., Gu F., Cai C., Hu M., Fan L., Hao J., Yu G. Purification, structural characterization, and immunomodulatory activity of the polysaccharides from Ganoderma lucidum. Int. J. Biol. Macromol. 2020;143:806–813. doi: 10.1016/j.ijbiomac.2019.09.141. [DOI] [PubMed] [Google Scholar]
- 31.Zhang W. 2nd ed. Zhejiang University Press; Hangzhou: 1999. Biotechnology of Glycoconjugated; pp. 91–92. [Google Scholar]
- 32.Deniaud-Bouet E., Hardouin K., Potin P., Kloareg B., Herve C. A review about brown algal cell walls and fucose-containing sulfated polysaccharides: cell wall context, biomedical properties and key research challenges. Carbohydr. Polym. 2017;175:395–408. doi: 10.1016/j.carbpol.2017.07.082. [DOI] [PubMed] [Google Scholar]
- 33.Zhao J., Liu X., Kao C., Zhang E., Li Q., Zhang F., Linhardt R.J. Kinetic and structural studies of interactions between glycosaminoglycans and langerin. Biochemistry. 2016;55:4552–4559. doi: 10.1021/acs.biochem.6b00555. [DOI] [PubMed] [Google Scholar]
- 34.Nifantiev N., Ustyuzhanina N., Krylov V., Grachev A., Gerbst A. Synthesis, NMR and conformational studies of fucoidan fragments, 8: convergent synthesis of branched and linear oligosaccharides. Synth. 2006;2006:4017–4031. doi: 10.1055/s-2006-950333. [DOI] [Google Scholar]
- 35.Vinnitskiy D.Z., Krylov V.B., Ustyuzhanina N.E., Dmitrenok A.S., Nifantiev N.E. The synthesis of heterosaccharides related to the fucoidan from Chordaria flagelliformis bearing an alpha-L-fucofuranosyl unit. Org. Biomol. Chem. 2016;14:598–611. doi: 10.1039/c5ob02040a. [DOI] [PubMed] [Google Scholar]
- 36.Zhou J., Yang L., Hu W. Stereoselective synthesis of a sulfated tetrasaccharide corresponding to a rare sequence in the galactofucan isolated from Sargassum polycystum. J. Organomet. Chem. 2014;79:4718–4726. doi: 10.1021/jo500503r. [DOI] [PubMed] [Google Scholar]
- 37.Kasai A., Arafuka S., Koshiba N., Takahashi D., Toshima K. Systematic synthesis of low-molecular weight fucoidan derivatives and their effect on cancer cells. Org. Biomol. Chem. 2015;13:10556–10568. doi: 10.1039/c5ob01634g. [DOI] [PubMed] [Google Scholar]
- 38.Zong C., Li Z., Sun T., Wang P., Ding N., Li Y. Convenient synthesis of sulfated oligofucosides. Carbohydr. Res. 2010;345:1522–1532. doi: 10.1016/j.carres.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 39.Bilan M.I., Grachev A.A., Shashkov A.S., Kelly M., Sanderson C.J., Nifantiev N.E., Usov A.I. Further studies on the composition and structure of a fucoidan preparation from the brown alga Saccharina latissima. Carbohydr. Res. 2010;345:2038–2047. doi: 10.1016/j.carres.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 40.Bilan M.I., Grachev A.A., Shashkov A.S., Thuy T.T., Van T.T., Ly B.M., Nifantiev N.E., Usov A.I. Preliminary investigation of a highly sulfated galactofucan fraction isolated from the brown alga Sargassum polycystum. Carbohydr. Res. 2013;377:48–57. doi: 10.1016/j.carres.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 41.Bilan M.I., Ustyuzhanina N.E., Shashkov A.S., Thanh T.T.T., Bui M.L., Tran T.T.V., Bui V.N., Nifantiev N.E., Usov A.I. A sulfated galactofucan from the brown alga Hormophysa cuneiformis (Fucales, Sargassaceae) Carbohydr. Res. 2018;469:48–54. doi: 10.1016/j.carres.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 42.Bilan M.I., Ustyuzhanina N.E., Shashkov A.S., Thanh T.T.T., Bui M.L., Tran T.T.V., Bui V.N., Usov A.I. Sulfated polysaccharides of the Vietnamese brown alga Sargassum aquifolium (Fucales, Sargassaceae) Carbohydr. Res. 2017;449:23–31. doi: 10.1016/j.carres.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 43.Leal D., Mansilla A., Matsuhiro B., Moncada-Basualto M., Lapier M., Maya J.D., Olea-Azar C., De Borggraeve W.M. Chemical structure and biological properties of sulfated fucan from the sequential extraction of subAntarctic Lessonia sp (Phaeophyceae) Carbohydr. Polym. 2018;199:304–313. doi: 10.1016/j.carbpol.2018.07.012. [DOI] [PubMed] [Google Scholar]
- 44.Usoltseva R.V., Anastyuk S.D., Shevchenko N.M., Surits V.V., Silchenko A.S., Isakov V.V., Zvyagintseva T.N., Thinh P.D., Ermakova S.P. Polysaccharides from brown algae Sargassum duplicatum: the structure and anticancer activity in vitro. Carbohydr. Polym. 2017;175:547–556. doi: 10.1016/j.carbpol.2017.08.044. [DOI] [PubMed] [Google Scholar]
- 45.Wang P., Zhao X., Lv Y., Liu Y., Lang Y., Wu J., Liu X., Li M., Yu G. Analysis of structural heterogeneity of fucoidan from Hizikia fusiforme by ES-CID-MS/MS. Carbohydr. Polym. 2012;90:602–607. doi: 10.1016/j.carbpol.2012.05.084. [DOI] [PubMed] [Google Scholar]
- 46.Nunes C., Coimbra M.A. The potential of fucose-containing sulfated polysaccharides as scaffolds for biomedical applications. Curr. Med. Chem. 2019;26(35):6399–6411. doi: 10.2174/0929867326666181213093718. [DOI] [PubMed] [Google Scholar]
- 47.Berteau O., Mulloy B. Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology. 2003;13:29R–40R. doi: 10.1093/glycob/cwg058. [DOI] [PubMed] [Google Scholar]
- 48.Cumashi A., Ushakova N.A., Preobrazhenskaya M.E., D'Incecco A., Piccoli A., Totani L., Tinari N., Morozevich G.E., Berman A.E., Bilan M.I., Usov A.I., Ustyuzhanina N.E., Grachev A.A., Sanderson C.J., Kelly M., Rabinovich G.A., Iacobelli S., Nifantiev N.E. A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology. 2007;17:541–552. doi: 10.1093/glycob/cwm014. [DOI] [PubMed] [Google Scholar]
- 49.Fitton J.H., Stringer D.N., Karpiniec S.S. Therapies from fucoidan: an update. Mar. Drugs. 2015;13:5920–5946. doi: 10.3390/md13095920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kusaykin M., Bakunina I., Sova V., Ermakova S., Kuznetsova T., Besednova N., Zaporozhets T., Zvyagintseva T. Structure, biological activity, and enzymatic transformation of fucoidans from the brown seaweeds. Biotechnol. J. 2008;3:904–915. doi: 10.1002/biot.200700054. [DOI] [PubMed] [Google Scholar]
- 51.Li B., Lu F., Wei X., Zhao R. Fucoidan: structure and bioactivity. Molecules. 2008;13:1671–1695. doi: 10.3390/molecules13081671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mestechkina N.M., Shcherbukhin V.D. Sulfated polysaccharides and their anticoagulant activity: a review. Appl. Biochem. Microbiol. 2010;46:291–298. [PubMed] [Google Scholar]
- 53.Mourão P.A., Pereira M.S. Searching for alternatives to heparin: sulfated fucans from marine invertebrates. Trends Cardiovasc. Med. 1999;9:225–232. doi: 10.1016/s1050-1738(00)00032-3. [DOI] [PubMed] [Google Scholar]
- 54.Pangestuti R., Kim S.K. Neuroprotective effects of marine algae. Mar. Drugs. 2011;9:803–818. doi: 10.3390/md9050803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pomin V.H. An overview about the structure-function relationship of marine sulfated homopolysaccharides with regular chemical structures. Biopolym. 2009;91:601–609. doi: 10.1002/bip.21200. [DOI] [PubMed] [Google Scholar]
- 56.Senthilkumar K., Manivasagan P., Venkatesan J., Kim S.K. Brown seaweed fucoidan: biological activity and apoptosis, growth signaling mechanism in cancer. Int. J. Biol. Macromol. 2013;60:366–374. doi: 10.1016/j.ijbiomac.2013.06.030. [DOI] [PubMed] [Google Scholar]
- 57.Usov A.I., Bilan M.I. Fucoidans-sulfated polysaccharides of brown algae. Russian Cehem. Rev. 2009;78:785–799. [Google Scholar]
- 58.Wang W., Wang S.-X., Guan H.-S. The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar. Drugs. 2012;10:2795–2816. doi: 10.3390/md10122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wijesekara I., Pangestuti R., Kim S.-K. Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae. Carbohydr. Polym. 2011;84:14–21. [Google Scholar]
- 60.Wijesinghe W.A.J.P., Jeon Y.-J. Biological activities and potential industrial applications of fucose rich sulfated polysaccharides and fucoidans isolated from brown seaweeds: a review. Carbohydr. Polym. 2012;88:13–20. [Google Scholar]
- 61.Jiao G., Yu G., Zhang J., Ewart H. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs. 2011;9:196–223. doi: 10.3390/md9020196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skriptsova A.V. Fucoidans of brown algae: biosynthesis, localization, and physiological role in thallus. Russ. J. Mar. Biol. 2015;41:145–156. [Google Scholar]
- 63.Shi Q., Wang A., Lu Z., Qin C., Hu J., Yin J. Overview on the antiviral activities and mechanisms of marine polysaccharides from seaweeds. Carbohydr. Res. 2017;453-454:1–9. doi: 10.1016/j.carres.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 64.Pereira L., Critchley A.T. The COVID 19 novel coronavirus pandemic 2020: seaweeds to the rescue? Why does substantial, supporting research about the antiviral properties of seaweed polysaccharides seem to go unrecognized by the pharmaceutical community in these desperate times? J. Appl. Phycol. 2020:1–3. doi: 10.1007/s10811-020-02143-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sansone C., Brunet C., Noonan D.M., Albini A. Marine algal antioxidants as potential vectors for controlling viral diseases. Antioxidants (Basel) 2020;9:392. doi: 10.3390/antiox9050392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.de Jesus Raposo M.F., de Morais A.M., de Morais R.M. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs. 2015;13:2967–3028. doi: 10.3390/md13052967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salehi B., Sharifi-Rad J., Seca A.M.L., Pinto D., Michalak I., Trincone A., Mishra A.P., Nigam M., Zam W., Martins N. Current trends on seaweeds: looking at chemical composition, phytopharmacology, and cosmetic applications. Molecules. 2019;24:4182. doi: 10.3390/molecules24224182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mandal P., Mateu C.G., Chattopadhyay K., Pujol C.A., Damonte E.B., Ray B. Structural features and antiviral activity of sulphated fucans from the brown seaweed Cystoseira Indica. Antivir. Chem. Chemother. 2007;18:153–162. doi: 10.1177/095632020701800305. [DOI] [PubMed] [Google Scholar]
- 69.Hayashi K., Nakano T., Hashimoto M., Kanekiyo K., Hayashi T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008;8:109–116. doi: 10.1016/j.intimp.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 70.Wang Y., Xing M., Cao Q., Ji A., Liang H., Song S. Biological activities of fucoidan and the factors mediating its therapeutic effects: a review of recent studies. Mar. Drugs. 2019;17:183. doi: 10.3390/md17030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Aguilar-Briseno J.A., Cruz-Suarez L.E., Sassi J.F., Ricque-Marie D., Zapata-Benavides P., Mendoza-Gamboa E., Rodriguez-Padilla C., Trejo-Avila L.M. Sulphated polysaccharides from Ulva clathrata and Cladosiphon okamuranus seaweeds both inhibit viral attachment/entry and cell-cell fusion, in NDV infection. Mar. Drugs. 2015;13:697–712. doi: 10.3390/md13020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mohammadi Pour P., Fakhri S., Asgary S., Farzaei M.H., Echeverría J. The signaling pathways, and therapeutic targets of antiviral agents: focusing on the antiviral approaches and clinical perspectives of anthocyanins in the management of viral diseases. Front. Pharmacol. 2019;10:1207. doi: 10.3389/fphar.2019.01207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Elizondo-Gonzalez R., Cruz-Suarez L.E., Ricque-Marie D., Mendoza-Gamboa E., Rodriguez-Padilla C., Trejo-Avila L.M. In vitro characterization of the antiviral activity of fucoidan from Cladosiphon okamuranus against Newcastle disease virus. Virol. J. 2012;9:307. doi: 10.1186/1743-422X-9-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SPR sensorgrams for binding kinetics measurements for human ACE2 and immobilized SARS-CoV-2 spike protein interactions. The concentrations of ACE2 (from top to bottom) were 1000, 600, 400, 200, 100 nM, respectively. The black curves are the fitting curves using a 1:1 L model from BIAevaluation 4.0.1.