Abstract
Single-column (batch) preparative chromatography is the technique of choice for purification of biotherapeutics but it is often characterized by an intrinsic limitation in terms of yield-purity trade-off, especially for separations containing a larger number of product-related impurities. This drawback can be alleviated by employing multicolumn continuous chromatography. Among the different methods working in continuous mode, in this paper we will focus in particular on Multicolumn Countercurrent Solvent Gradient Purification (MCSGP) which has been specifically designed for challenging separations of target biomolecules from their product-related impurities. The improvements come from the automatic internal recycling of the impure fractions inside the chromatographic system, which results in an increased yield without compromising the purity of the pool. In this article, steps of the manufacturing process of biopharmaceuticals will be described, as well as the advantages of continuous chromatography over batch processes, by particularly focusing on MCSGP.
Keywords: Continuous chromatography, Preparative chromatography, Purification, Multicolumn platforms, Biopharmaceuticals, Biotherapeutics
1. Introduction
Since the 1980s, biopharmaceuticals have emerged as an innovative class of therapeutics, due to their highly specific activity, a feature that cannot be imitated by traditional drugs. Indeed, they show high specificity towards the target receptors, which makes them very effective even at low concentrations [1,2]. Moreover, most of them are also present in the human body, therefore their side effects are reduced if compared to other chemical drugs. In the last months, their potential has been even more rekindled due to the fact that many of the therapeutics currently under testing for the treatment or prevention of COVID-19 disease are based on biopharmaceuticals (especially monoclonal antibodies or oligonucleotides) [[3], [4], [5]].
In the last years, manufacturing of biopharmaceuticals has been intensively improved. The method chosen to obtain the biomolecule of interest represents the upstream step of the manufacturing process [6,7]. For instance, recombinant technology is the main method to obtain monoclonal antibodies, hormones and blood factors. In this context, continuous bioreactors (e.g., perfusion bioreactors) are getting ever more popular, at the point that they have started to replace traditional batch processes. Alternatively, biopharmaceuticals can be extracted from their natural source or they can be chemically synthesized. The latter strategy, anyway, can be applied only to produce short biopolymeric chains, e.g. polypeptides. These recent innovations in the upstream of biopharmaceuticals have not been followed by similar enhancement in the downstream process, at the point that the latter currently represents a bottleneck in the whole production of biotherapeutics [[8], [9], [10]]. The term downstream in general indicates both the recovery and the purification of a product from a complex mixture [11]. The purification methods of choice must distinguish between molecules that often show only slight variations in size, hydrophobicity or charge. The most versatile, selective and flexible technique to satisfy this need is liquid chromatography. Usually, more than one chromatographic step is required to satisfy the specifications imposed to reach the market [12,13]. Traditionally, these chromatographic purification processes are conducted in batch conditions, often using a single chromatographic column [14].
In general, at least two different purification steps are usually necessary to isolate the target product with the required purity. The first part of the purification process is the removal of process-related impurities, i.e. species that are not chemically similar to the target molecule [6]. They usually include nucleic acids, host cell proteins, lipids, components of the cell culture media, salts, etc. which derive from the manufacturing process. Very often, affinity chromatography in batch conditions is the technique employed, in a bind-and-elute mode [15]. This procedure is called capture step and it consists in loading a large amount of feed into the column until its breakthrough. The product specifically binds to the stationary phase, whereas all the other different species flow through the column and can be discarded. For instance, Staphylococcus Protein A-based stationary phase is largely employed for the purification of monoclonal Antibodies (mAbs), since it allows binding mAbs specifically but reversibly [16]. During this phase, it is important to ensure the recovery of the maximum amount of the target, whereas it is not necessary to satisfy strict purity requirements.
After the capture step, one or more polishing steps are required in order to satisfy the rigorous purity requirements for pharmaceuticals. In order to do that, the product must be separated also from product-related impurities, which are, instead, very often similar to the target molecule (e.g., truncated, deamidated species, etc.) [17]. Most of the time, this is a very challenging task. Affinity chromatography cannot be applied at this stage because of the similarity between the target product and the impurities. Therefore reversed-phase, ion-exchange and hydrophobic interaction chromatography are rather preferred as methods of choice [6]. In order to improve the resolution of the peaks, it is advisable to work in gradient conditions, since the retention of biomolecules is largely dependent on the composition of the mobile phase (e.g., on the salt concentration or on the percentage of organic modifier) [9,[18], [19], [20]].
In preparative chromatography, the similarity between the target and its impurities often result in peaks overlapping, where the target product is intermediate between weakly and strongly adsorbing impurities [21]. Consequently, collecting a considerable amount of pure product is almost impossible. In fact, a widening of the collection window results in an improved yield at expenses of a reduced purity and vice versa. This translates in a yield-purity trade-off, a limit peculiar to batch chromatography [22].
In this frame, multicolumn continuous chromatographic approaches have become increasingly appealing in the field of high value biological products [15], due to the possibility of partially overcoming this limitation. In general, multicolumn continuous chromatography leads to several advantages, especially increased recovery and better resin utilization, but this comes at the expense of the hardware complexity [23].
This paper focuses on Multicolumn Countercurrent Solvent Gradient Purification (MCSGP), one of the most recently developed countercurrent multicolumn techniques specifically designed for challenging separations where many product-related impurities are present. Its operating principles will be discussed and its advantages over traditional single-column techniques will be presented. Method transfer from batch to continuous will be also illustrated, together with a synthetic overview of most interesting applications of MCSGP. In doing this, an effort has been done to describe the process from the viewpoint of analytical chemists (more than that of chemical engineers) in order to make the technology more familiar to this community.
2. Relevant parameters for purification processes
Before describing the fundamentals of batch and continuous processes, some relevant parameters need to be defined. They are usually evaluated by analyzing the eluted fractions by means of a proper analytical high-performance liquid chromatography (HPLC) method.
Purity is the first parameter that is essential for pharmaceutical scopes. It is defined as the ratio between the area of the product peak and the total area of the HPLC chromatogram: purity is calculated as the mean of the purities of the pools at the steady state.
| (1) |
Also, recovery (or yield) of the target at the end of the process needs to be carefully evaluated. This is particularly important when very expensive Active Pharmaceutical Ingredients (APIs) are purified. It is defined as the mass fraction of the product recovered in the eluted stream with respect to the mass of the product dissolved in the feed injected into the column.
| (2) |
Moreover, also productivity can be defined; it is expressed as the mass of target product collected in the eluent stream per total volume of stationary phase and per time. Thus, this parameter indicates how much product is produced per minute and per column volume (Vcol):
| (3) |
where Vcol is calculated as the geometrical volume of the column (in case of multicolumn processes the geometrical volume of all the columns must be considered), whereas the time considered is the duration of a run in batch conditions or a cycle in MCSGP (see later on).
3. Limits of batch chromatography
The outcome of the separation (i.e. resolution of the main peak from the impurities) has a high impact on the performance of the whole process.
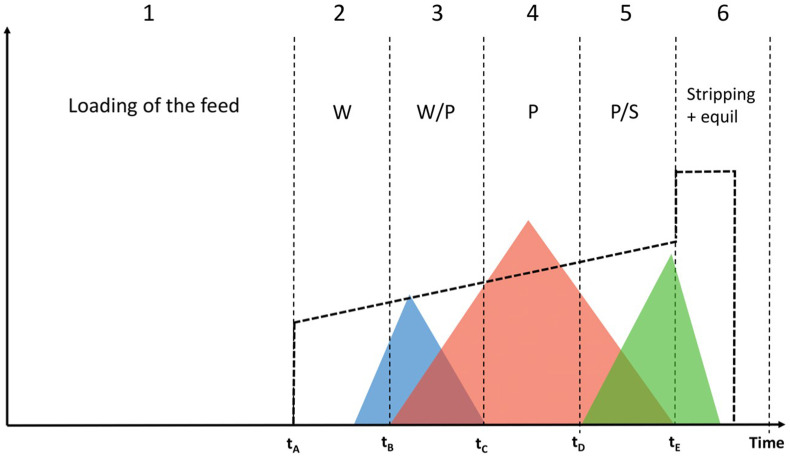
As mentioned before, it frequently happens that batch purifications, especially when many product-related impurities are present, are affected by a yield-purity trade-off. This situation is schematically represented in Fig. 1 . If the overlapping regions are completely discarded, the purity in the pool will be elevated. However, a considerable amount of product still underlies the overlapping portions of the peak. If the collection window is broadened, yield will increase but at the same time purity will decrease since portions of the peak contaminated with impurities are collected. This trade-off is a limit intrinsic to batch chromatography. The difficulty of reaching a good purity and a good yield at the same time makes traditional batch chromatography often impractical [24].
Fig. 1.
Schematic representation of a batch chromatogram.
One could think of decreasing the loading of the feed or the gradient slope, but this would lead to longer times and in turn to higher solvent consumption and lower productivity. Alternatively, more efficient columns can be used but smaller particles would lead to higher backpressures. Therefore, none of these options can effectively be a solution to the problem [25,26].
4. Multicolumn Countercurrent Solvent Gradient Purification (MCSGP)
An appealing possibility to overcome the limit of batch chromatography described above is to replace the single column process with a continuous (or semi-continuous) countercurrent chromatographic process, where the chromatographic system is continuously fed with the crude mixture. To realize the continuous (or semi-continuous) mode, the instrument must be equipped with two or more (identical) columns connected through a series of valves. The term countercurrent refers to a class of chromatographic processes in which the stationary and the mobile phase move into two opposite directions. The movement of the stationary phase is not real but simulated through the switching of the inlet and outlet valves of the columns [6,16].
The use of continuous chromatography operations has considerable advantages not only in terms of recovery of the product (as it will be illustrated in the following) but also in terms of automatization of the purification process.
The first continuous countercurrent chromatography setup was Simulated Moving Bed (SMB), introduced in 1950 to separate two different components under isocratic conditions [27,28]. Since then, many improved versions of the technique have been proposed, but essentially SMB has been limited to the separation of binary mixtures. A dozen years ago, some researchers connected two SMB units in series to purify ternary mixtures [6,29]. In the first SMB process, one compound can be separated from the two remaining species, which enter into the second unit to be further separated. An advantage of this setup compared to MCSGP is that the chromatographic conditions (column, mobile phase, etc.) can be chosen independently for the two units. This, e.g., can improve resolution. On the opposite, not only the experimental setup (connecting tubings, valves, etc.) is much more complex in SMB than in MCSGP but also SMB separations are limited to only isocratic operations.
Recently, two appealing alternatives to SMB have been introduced that can be applied to both capture and polishing steps. Indeed, in the first case, captureSMB can be efficiently used to isolate the target product from its impurities exploiting affinity chromatography interactions. For the sake of space, this technique will not be described in this paper, therefore the interested reader is addressed to other recent papers on the subject [6,23,[30], [31], [32], [33], [34], [35]].
On the other hand, in this work we will focus in particular on the description of MCSGP, a countercurrent technique that can be used for the polishing step. It is practically based on the same principles of SMB, but it allows to manage ternary separations (i.e. separations of target products from co-eluting impurities in the front and in the rear part of the target peak). Moreover, it allows to work under linear gradient conditions which is extremely advantageous when dealing with biomolecules [36,37]. In its first setup, MCSGP was based on the use of six identical columns [19,38]; later on, the equipment has been more and more simplified until arriving at the final version with only two columns [22,25], which is characterized by a reduced complexity in tubing, valves and connections.
4.1. Starting point: the design batch chromatogram
In order to understand the principles and the great potential of MCSGP, let us consider again the batch chromatogram schematically represented in Fig. 1. It represents the case of a center-cut or ternary separation carried out under gradient elution conditions, where the main compound elutes between weak and strong impurities, and their peaks partially overlap [6,39,40]. As it can be observed, it is divided in different zones.
-
•
Zone 1: the column (previously equilibrated with the eluent) is loaded with some fresh feed. Once the analyte is adsorbed onto the stationary phase, the modifier gradient can start (at time tA).
-
•
Zone 2: weakly adsorbing impurities (from now on called W), which are less retained than the target product, start eluting from the column.
-
•
Zone 3: product (P) starts eluting from the column, but the weakly adsorbing impurities are still eluting. Since W and P are not well resolved, their peaks overlap. The product in this zone obviously does not fulfill the purity requirement, because it is contaminated by species W, but at the same time it cannot be wasted and needs to be recovered to obtain a good process yield.
-
•
Zone 4: the target compound does not coelute with any other species and hence purity fulfills the requirement for pharmaceutical scopes.
-
•
Zone 5: this is another overlapping region where the target compound coelutes with the strongly adsorbing impurities (called S).
-
•
Zone 6: the column is stripped with a high percentage of organic modifier, to remove S impurities, and then it is equilibrated again with the eluent composition at the beginning of the gradient.
Fractions of the eluate are periodically collected during the gradient and then analyzed by means of HPLC to obtain a purity profile (zones 2–5).
As reported in Fig. 1, the zones defining the recycling and collection windows in the batch process are delimited by some characteristic times, which are necessary to transfer a chromatographic method from batch to the MCSGP process, as it will be explained in the following.
Also, it must be highlighted that the letter W (or S) does not refer to a single weakly (or strongly) adsorbing species, but rather to a group of impurities which have a similar chromatographic behavior.
The chromatogram obtained in batch is then used to design the MCSGP process, thus it is called design batch chromatogram. It must be calculated on one of the two columns that will be employed for MCSGP.
4.2. Operating principles of MCSGP
Conversely to preparative batch chromatography process, the MCSGP technique, for its intrinsic features, allows to obtain the target product with high purity and high yield at the same time. The main factor enabling an improved performance of MCSGP compared to that of the batch process is the automatic internal recycling of the partially purified side fractions. When working in batch chromatography, the side portions of the main peak, containing both W (or S) and a remarkable amount of P, are discarded from the main collection window but most of the time they are manually reprocessed into the system by the operator, with risk of error and waste of time [41]. In twin-column MCSGP, on the contrary, the recycle is accomplished automatically between the two columns, with no need of intervention by the operator. The two identical columns work either in series (interconnected mode) or in parallel (batch mode), depending on the position of the inlet and outlet column valves [42,43]. When transferring a method from batch to continuous chromatography, this method is performed on both the columns, but shifted of half a cycle [22].
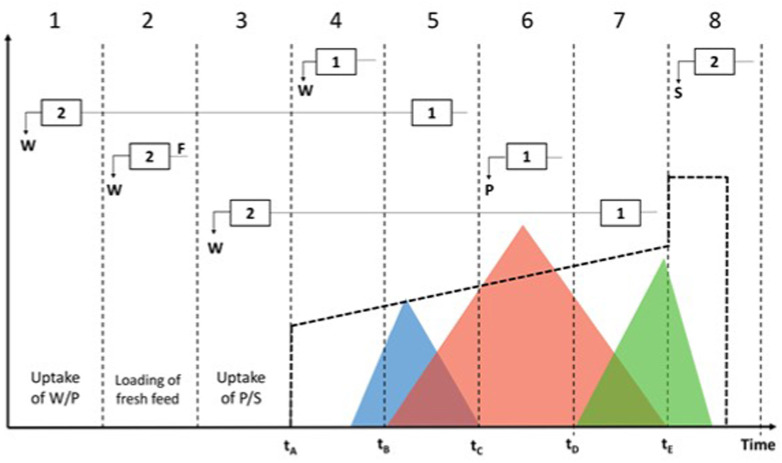
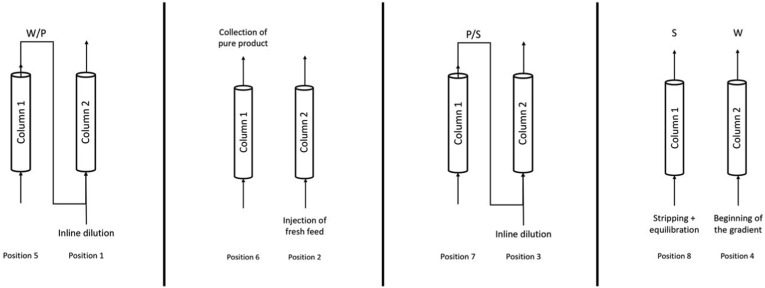
Fig. 2, Fig. 3 represent a case where column-1 is in the upstream position and column-2 is in the downstream position; this means that the recycling regions eluting from column-1 are loaded in column-2:
-
•
Firstly, column-1 is loaded with fresh feed, as in the batch process. When the gradient starts, the first group of analytes to elute is W; this fraction does not contain P (zone 4) and thus it is discarded. At this stage, the columns are disconnected.
-
•
Then, valves switch position and the columns get interconnected. This means that W/P, the overlapping region of W and P, is directly loaded from column-1 (zone 5) into column-2 (zone 1). Inline dilution is applied to ensure that W/P is re-adsorbed on column-2.
-
•
The columns work again in batch mode and a window where product purity satisfies the requirements imposed is recovered from column-1 (zone 6). At the same time, column-2 is loaded with some fresh feed (zone 2).
-
•
After that, the columns get interconnected again to allow the recycling of P/S region from column-1 (zone 7) into column-2 (zone 3). Inline dilution is applied to ensure that P/S is re-adsorbed on column-2.
-
•
Now that column-2 has been fully loaded, it can undergo the solvent gradient: W impurities start eluting (zone 4); on the other side, column-1 is being stripped to remove S and it is also equilibrated (zone 8).
Fig. 2.
Schematic representation of a single switch chromatogram in MCSGP. Reproduced with permissions from Ref. [44].
Fig. 3.
Schematic representation of the path of the eluent stream during the first switch of an MCSGP cycle. The flow direction depends on the position of the inlet and outlet columns valves.
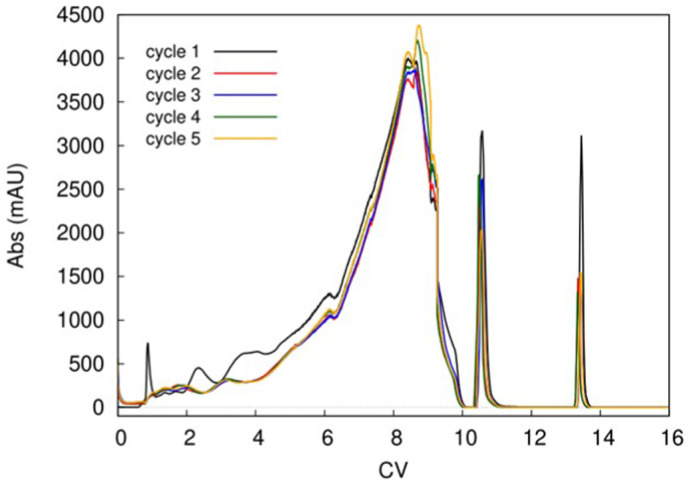
At this point, the columns have switched position. When they exchange position again and return to the initial configurations, a cycle is completed. Thus, one cycle is composed of two switches. Generally, after few switches, the chromatographic system reaches the steady-state, which is demonstrated by the fact that the UV profiles are completely superimposable cycle after cycle. The reader should note that UV profiles are detected at the outlet of the column, before the eluent stream is sent to the waste, to the fractionator or to the other column. Under steady-state conditions, then, very close values of purity and recovery are obtained for every collected pool. Therefore, after the steady state has been reached, the number of cycles to be performed for the entire purification process depends essentially on the amount of fresh feed that must be purified. In order to better understand the meaning of steady-state, an example is reported in Fig. 4 . This picture shows the elution profiles of the first switch of five MCSGP cycles (these experiments were performed by some of the authors of this review in a former study). The biopharmaceutical of interest in that case was a crude synthetic mixture of a therapeutic peptide (Glucagon) [44]. As it can be noted, only the first cycle shows a different UV profile with respect to the others, meaning that cycles from 2 to 5 have reached steady-state conditions.
Fig. 4.
Chromatograms (overlapped) of the first switch of five cycles of a MCSGP run for the purification of crude mixture of glucagon. Sharp peaks on the right correspond to the stripping and equilibration of the column. Reproduced with permission from Ref. [41]. CV: column volume (mL).
The characteristic times of the design batch chromatogram in Fig. 1 correspond to the switching of the inlet and outlet valves of the columns in MCSGP (see Fig. 2), that regulates the path accessible to the eluent stream. Fig. 3 shows in detail the path followed by the mobile phase during the disconnected and interconnected steps. tB indicates the moment where the overlap of W/P starts flowing out, then the product elutes from tC to tD, and finally the overlap of P/S elutes until the time tE. tA represents the moment where the solvent gradient starts.
An important aspect to be considered is that the overlapping regions contain a higher percentage of modifier than at the beginning of the gradient. Therefore, when they are recycled, they need to be diluted with an inline dilution stream, so that the product can be adsorbed on the stationary phase. The fraction containing W/P is diluted to reach the modifier concentration that can be found at tB, so that the product adsorbs on the stationary phase while the weak impurities start moving along the column. The window containing P/S is diluted to reach the percentage of organic modifier at the beginning of the gradient (tA), because both the product and the strong impurities must be retained.
The amount of fresh feed which is injected switch after switch (zone 2 of Fig. 2) is calculated in order to maintain the mass of target compound constant into the system. Therefore, the mass of P to be loaded at every switch is the difference between the quantity of target product loaded in the batch run and the amount of target product which is recycled within the overlapping regions (zone 1 and 3).
4.3. Transfer of a batch method to MCSGP
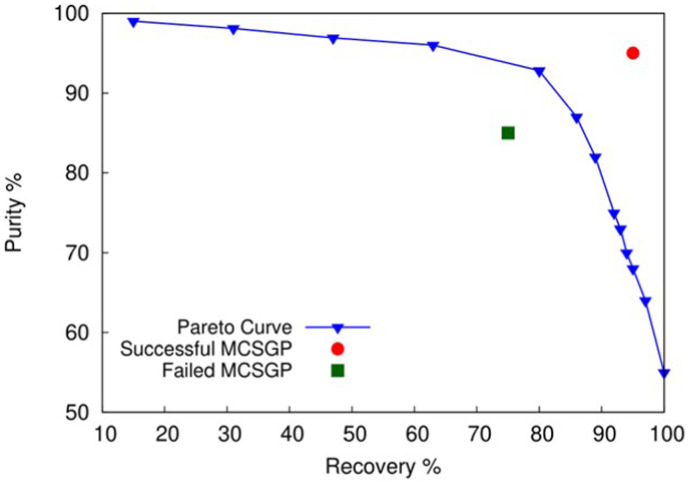
The first thing to do in order to transfer a batch method to MCSGP is to calculate a Pareto curve reporting purity as a function of yield for the batch method (see Fig. 5 ). This is practically done by analyzing through HPLC the fractions of eluate stream collected from the batch column. Purity and yield of the target in each fraction are thus calculated. The result is a purity profile along the gradient which serves to decide which portion of the peak fulfills the purity requirements. This part of the chromatogram will represent the product elution window. Starting from the purest fraction, one needs to imagine pooling it with the neighboring fractions, adding one fraction at a time in order of decreasing purity. By enlarging the pooling window, purity decreases whereas recovery increases. The values of purity and recovery found for every hypothetical pool are then plotted to define the Pareto curve.
Fig. 5.
Blue triangles: Pareto curve of a hypothetical design batch chromatogram. Red and green points: performance of two hypothetical MCSGP processes (red: successful; green: unsuccessful).
In order to make a fair comparison between batch and MCSGP, the Pareto curve should be calculated not only for one of the two columns used in MCSGP but also for a longer column, with a comparable Vcol to the total Vcol of MCSGP. This column will serve as a reference batch. The difference between the reference batch and the design batch is that the first is needed to compare the performance of the processes (at comparable Vcol), whereas the second one is necessary to set the switching times for MCSGP.
During the transfer of a batch method to a MCSGP process, all the operating conditions are kept constant, such as loading of the feed per column, gradient slope and duration of every step of the method. Thus, the only variables that can be changed to modulate the performance of the MCSGP process are the switching times.
The first trial is usually done by choosing the values of tC and tD corresponding to a certain hypothetical pool in which purity fulfills the requirements and recovery is sufficiently high. tB and tE must be set in order to minimize the amount of product eluting in the waste windows. Fig. 2 represents an ideal case where no product is wasted in zones 4 and 8, however in other cases it is preferable to waste a small amount of very impure product in these zones rather than risking the accumulation of impurities in the system during recycling. Since the values of purity and recovery for each cycle are constant after the steady state has been reached, in MCSGP one obtains a point and not a Pareto curve. If the point of the MCSGP lies below the Pareto curve, the MCSGP process can be considered unsuccessful, meaning that it reaches a lower recovery than the batch at the same purity. On the opposite, if the point lies above the Pareto curve, this means that the recovery of MCSGP has overcome that of the batch. From a practical viewpoint it must be said that points on the top right corner of the graphic are an indication of a successful MCSGP. This concept can be better visualized by looking at Fig. 5. This graphic shows from a purely qualitative point of view a Pareto curve related to a batch chromatogram where the purest fraction (99% purity) corresponds to only 15% of recovery, whereas if yield were 100%, purity would decrease to 55%. If the MCSGP is unsuccessful, the set of switching times must be changed. Particularly, it has been proven that the times tB and tE greatly influence recovery. On the other side, the times tC and tD especially impact on purity since they define the product elution window [26].
The last parameter to consider when comparing purification processes is productivity. In some cases, MCSGP gives similar results as that of the batch [25] or slightly lower [44], but this is only partially a concerning point. Indeed, when dealing with very costly biotherapeutics, it is preferable, from an economic viewpoint, to maximize the recovery of the product rather than productivity of the process. Just to make an example, the cost of raw glucagon is declared to be around some thousand dollars per gram [45]. It is evident that an increase in recovery is reflected in a great economic advantage. Moreover, the typical definition of productivity given in Eq. (3), usually considered when comparing the processes, does not consider the economic advantage coming from the automatization of the process, which is, instead, a very important point.
4.4. Applications of MCSGP
The main field where MCSGP has been successfully applied is the purification of biomolecules (such as protein, antibodies and peptides), where a wide variety of mobile and stationary phases were tested.
The interest towards antibodies, especially mAbs, as therapeutics is increasing, and thus their demand. At the same time, mAbs are produced as a mixture of different isomers, which must be separated to ensure a good quality of the product and meet the market specifications. The MCSGP process has been proven to be a successful method for this scope and for this class of biomolecules [16,[46], [47], [48]].
MCSGP process allowed to reach a higher yield and better productivity than the batch also in the case of a mono-PEGylated protein, the α-Lactalbumin. The mixture of proteins with different degrees of PEGylation was separated using anion exchange chromatography [17].
Beside proteins, also mixtures of peptides have been purified through MCSGP process. The very first cases of application of a 6-column or 3-column MCSGP to an industrial sample were related to the separation of Calcitonin, a peptidic hormone made of 32 amino acids, from its impurities [19,21,49]. Lately, some of the authors of this review have successfully investigated the purification of an industrial mixture of Glucagon (29-amino acids peptide) using a 2-column MCSGP equipment. In that case, the yield was 23% higher than the batch, with a purity of nearly 90% [44].
Another class of biotherapeutics for which the MCSGP has been proven to be a good purification strategy is that of oligonucleotides. This technique applied to a mixture of oligonucleotides allowed to increase the mass recovered by 50% at a target purity of 92% [50].
MCSGP can be applied also in the case of cannabinoids identification and purification. Cannabidiols (CBD), for instance, are a group of cannabinoids, natural compounds extracted from Cannabis Sativa L. CBD is under investigation for their therapeutic properties; anyway, the regulation imposes strict limitations for the concentration of tetrahydrocannabinol (THC) in the CBD mixtures, since THC is a psychoactive substance. MCSGP has been successfully applied to obtain a THC-free product [51].
Table 1 reports a comparison between the performance obtained in batch and in MCSGP for the purification of different target molecules. As can be noted, MCSGP results to be a successful process when it comes to tricky ternary separations of expensive biomolecules and biopharmaceuticals, especially if their batch purification shows a strong yield-purity trade-off. In those cases, MCSGP can lead to an increase in yield and consequently to a benefit also with respect to the economics of production [41].
Table 1.
Comparison between the performance of batch and MCSGP processes for different purification cases found in literature.
| Compound | Batch |
MCSGP |
Ref. | ||||
|---|---|---|---|---|---|---|---|
| Purity % | Recovery % | Productivity (g/L/h) | Purity % | Recovery % | Productivity (g/L/h) | ||
| Oligonucleotide | 91.6% | 55.7% | 11.9 | 91.9% | 91.2% | 5.89 | [50] |
| Cannabidiol | THC < 100 ppm | 52% | 8 | >99.5% (THC < 100 ppm) | 94% | 60 | [51] |
| Peptide (glucagon) | 89.3% | 71.2% | 9.9 | 89.2% | 88% | 6.1 | [44] |
| Peptide | 98.7% | 19.3% | 3 | 98.7% | 94.3% | 28 | [22] |
| Monoclonal antibody | 92% | 85% | 1.8 | 92% | 94% | 2.6 | [25] |
5. Conclusions and outlook
Thanks to nonstop technological improvements, continuous, or semi-continuous (periodic), countercurrent preparative liquid chromatography has nowadays reached the stage of mature technology. These techniques are increasingly gaining importance from the industrial viewpoint and they are considered a promising candidate that can revolutionize the purification of biomolecule at a manufacturing level. The current increasing interest towards continuous purification processes is primarily driven by the improved quality, which directly translate into drug safety and efficacy, of the final products in addition to economic advantages, related to the high automation degree and improved yields. This is particularly so when the goal is to maximize product-recovery rather than process-productivity, that is the case of, e.g., highly molecularly active molecules. Many of today and tomorrow targeted therapeutics belong to this class. From a wider perspective, thus, the technology has the potential to act as driver for the shift to precision medicine [52].
There are, however, still many challenges to overcome. From a theoretical viewpoint, there is room for studies focusing on the modeling of the process [9,[53], [54], [55], [56], [57], [58], [59], [60], [61]]. Even though this relies essentially on the well-known theory of nonlinear chromatography, robust, validated models able to simulate the process in all its stages are not yet available. This will favor the optimization of purification conditions and, in the meantime, it will improve the confidence to use the technology. The availability of robust and reliable models will also favor the introduction of automation and digitalization. Using model-based algorithms, derived also from machine learning techniques, it is possible to control the operation of these units in terms of both rejecting disturbances, so as to keep the product under specifications, and keeping optimal operating conditions in terms of minimal production costs, i.e., productivity and buffer consumption [62]. The application of model predictive control techniques appears perfectly suitable for this purpose as already done in the frame of the chiral SMB continuous process [63].
Although, as seen above, MCSGP can be applied very conveniently down-stream to batch or fed-batch bioreactors, we believe that it will play a major role also in the establishment of continuous and integrated processes for the manufacturing of therapeutic proteins [64]. The most important pharma regulatory Agencies look positively at these developments and are active in the definition of Quality Aspects (QA) and ad-hoc regulatory actions for continuous manufacturing [65,66]. The time is therefore ripe for change.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank ChromaCon AG- A YMC Company (Zurich, Switzerland), in particular Dr. Thomas Müller-Späth, for technical support. Prof. Walter Cabri (University of Bologna, Bologna Italy and Fresenius Kabi iPSUM, Villadose, Rovigo, Italy), Dr. Antonio Ricci and Dr. Marco Macis from Fresenius Kabi iPSUM (Villadose, Rovigo, Italy) are also acknowledged. Finally, the authors are grateful to the Italian University and Scientific Research Ministry (grant PRIN2017Y2PAB8_003, title: “Cutting edge analytical chemistry methodologies and bio-tools to boost precision medicine in hormone-related diseases”) for financial support.
References
- 1.de Castro R.J.S., Sato H.H. Biologically active peptides: processes for their generation, purification and identification and applications as natural additives in the food and pharmaceutical industries. Food Res. Int. 2015;74:185–198. doi: 10.1016/j.foodres.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 2.Uhlig T., Kyprianou T., Martinelli F.G., Oppici C.A., Heiligers D., Hills D. The emergence of peptides in the pharmaceutical business: from exploration to exploitation. EuPA Open Proteomics. 2014;4:58–69. [Google Scholar]
- 3.Nicastri E., Petrosillo N., Bartoli T.A., Lepore L., Mondi A., Palmieri F., D'Offizi G., Marchioni L., Muratelli S., Ippolito G., Antinori A. National institute for the infectious diseases “L. Spallanzani”, IRCCS. Recommendations for COVID-19 clinical management. Infect. Dis. Rep. 2020;12 doi: 10.4081/idr.2020.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morse J.S., Lalonde T., Xu S., Liu W.R. Learning from the past: possible urgent prevention and treatment options for severe acute respiratory infections caused by 2019-ncov. Chembiochem. 2020;21:730–738. doi: 10.1002/cbic.202000047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li G., Clercq E.D. Therapeutic options for the 2019 novel coronavirus (2019-ncov) Nat. Rev. 2020;150:149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 6.Pfister D., Nicoud L., Morbidelli M. Cambridge University Press; 2018. Continuous Biopharmaceutical Processes - Chromatography, Bioconjugation and Protein Stability. [Google Scholar]
- 7.Wolf M., Bielser J.M., Morbidelli M. Cambridge University Press; 2020. Perfusion Cell Culture Processes for Biopharmaceuticals: Process Development, Design, and Scale-Up. [Google Scholar]
- 8.Steinebach F., Müller-Späth T., Morbidelli M. Continuous counter-current chromatography for capture and polishing steps in biopharmaceutical production. Biotechnol. J. 2016;11:1126–1141. doi: 10.1002/biot.201500354. [DOI] [PubMed] [Google Scholar]
- 9.De Luca C., Felletti S., Macis M., Cabri W., Lievore G., Morbidelli M., Cavazzini A., Catani M., Ricci A. Modeling the nonlinear behavior of a bioactive peptide in reversed-phase gradient elution chromatography. J. Chromatogr. A. 2020;1616:460789. doi: 10.1016/j.chroma.2019.460789. [DOI] [PubMed] [Google Scholar]
- 10.Carta G., Jungbauer A. Wiley-VCH Verlag GmbH & Co.; Weinheim, Germany: 2010. Protein Chromatography: Process Development and Scale-Up. [Google Scholar]
- 11.Buyel J.F., Twyman R.M., Fischer R. Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol. Adv. 2015;33:902–913. doi: 10.1016/j.biotechadv.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 12.Tarafder A., Aumann L., Morbidelli M. The role of ion-pairing in peak deformations in overloaded reversed-phase chromatography of peptides. J. Chromatogr. A. 2010;1217:7065–7073. doi: 10.1016/j.chroma.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 13.Boi C. Membrane adsorbers as purification tools for monoclonal antibody purification. J. Chromatogr. B. 2007;848:19–27. doi: 10.1016/j.jchromb.2006.08.044. [DOI] [PubMed] [Google Scholar]
- 14.Bernardi S., Gétaz D., Forrer N., Morbidelli M. Modeling of mixed-mode chromatography of peptides. J. Chromatogr. A. 2013;1283:46–52. doi: 10.1016/j.chroma.2013.01.054. [DOI] [PubMed] [Google Scholar]
- 15.Zydney A.L. Continuous downstream processing for high value biological products: a review. Biotechnol. Bioeng. 2016;113:465–475. doi: 10.1002/bit.25695. [DOI] [PubMed] [Google Scholar]
- 16.Müller-Späth T., Aumann L., Ströhlein G., Kornmann H., Valax P., Delegrange L., Charbaut E., Baer G., Lamproye A., Jöhnck M., Schulte M., Morbidelli M. Two step capture and purification of IgG2 using multicolumn countercurrent solvent gradient purification (MCSGP), Biotech. Bioengineering. 2010;107:974–984. doi: 10.1002/bit.22887. [DOI] [PubMed] [Google Scholar]
- 17.Subramanian G., editor. Continuous Processing in Pharmaceutical Manufacturing. Wiley-VCH; 2014. [Google Scholar]
- 18.Ströhlein G., Aumann L., Mazzotti M., Morbidelli M. A continuous, counter-current multi-column chromatographic process incorporating modifier gradients for ternary separations. J. Chromatogr. A. 2006;1126:338–346. doi: 10.1016/j.chroma.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 19.Aumann L., Ströhlein G., Morbidelli M. Parametric study of a 6-column countercurrent solvent gradient purification (MCSGP) unit. Biotechnol. Bioeng. 2007;98:1029–1042. doi: 10.1002/bit.21529. [DOI] [PubMed] [Google Scholar]
- 20.Åsberg D., Leśko M., Enmark M., Samuelsson J., Kaczmarski K., Fornstedt T. Fast estimation of adsorption isotherm parameters in gradient elution preparative liquid chromatography. I: the single component case. J. Chromatogr. A. 2013;1299:64–70. doi: 10.1016/j.chroma.2013.05.041. [DOI] [PubMed] [Google Scholar]
- 21.Aumann L., Morbidelli M. A continuous multicolumn countercurrent solvent gradient purification (MCSGP) process. Biotechnol. Bioeng. 2007;98:1043–1055. doi: 10.1002/bit.21527. [DOI] [PubMed] [Google Scholar]
- 22.Müller-Späth T., Ströhlein G., Lyngberg O., Maclean D. Enabling high purities and yields in therapeutic peptide purification using multicolumn countercurrent solvent gradient purification. Chem. Today. 2013;31:56–60. [Google Scholar]
- 23.Baur D., Angarita M., Müller-Späth T., Steinebach F., Morbidelli M. Comparison of batch and continuous multi-column protein a capture processes by optimal design. Biotechnol. J. 2016;11:920–931. doi: 10.1002/biot.201500481. [DOI] [PubMed] [Google Scholar]
- 24.Chibério A.S., Policarpo G.F.M., Antunes J.C., Santos T.P., Ribeiro R.P.P.L., Mota J.P.B. Batch chromatography with recycle lag. II—physical realization and experimental validation. J. Chromatogr. A. 2020;1623:461211. doi: 10.1016/j.chroma.2020.461211. [DOI] [PubMed] [Google Scholar]
- 25.Steinebach F., Ulmer N., Decker L., Aumann L., Morbidelli M. Experimental design of a twin-column countercurrent gradient purification process. J. Chromatogr. A. 2017;1492:19–26. doi: 10.1016/j.chroma.2017.02.049. [DOI] [PubMed] [Google Scholar]
- 26.Vogg S., Ulmer N., Souquet J., Broly H., Morbidelli M. Experimental evaluation of the impact of intrinsic process parameters on the performance of a continuous chromatographic polishing unit (MCSGP) Biotechnol. J. 2019;14 doi: 10.1002/biot.201800732. [DOI] [PubMed] [Google Scholar]
- 27.Mazzotti M., Storti G., Morbidelli M. Optimal operation of simulated moving bed units for nonlinear chromatographic separations. J. Chromatogr. A. 1997;769:3–24. [Google Scholar]
- 28.Schramm H., Kaspereit M., Kienle A., Seidel-Morgenstern A. Simulated moving bed process with cyclic modulation of the feed concentration. J. Chromatogr. A. 2003;1006:77–86. doi: 10.1016/s0021-9673(03)00327-3. [DOI] [PubMed] [Google Scholar]
- 29.Seok Hur J., Wankat P.C. New design of simulated moving bed for ternary separations. Ind. Eng. Chem. Res. 2005;44:1906–1913. [Google Scholar]
- 30.Vogg S., Müller-Späth T., Morbidelli M. Current status and future challenges in continuous biochromatography. Curr. Opin. Chem. Eng. 2018;22:138–144. [Google Scholar]
- 31.Pfister D., David L., Holzer M., Nicoud R.M. Designing affinity chromatographic processes for the capture of antibodies. Part I: a simplified approach. J. Chromatogr. A. 2017;1494:27–39. doi: 10.1016/j.chroma.2017.02.070. [DOI] [PubMed] [Google Scholar]
- 32.Baur D., Angarita M., Müller-Späth T., Morbidelli M. Optimal model-based design of the twin-column captureSMB process improves capacity utilization and productivity in protein A affinity capture. Biotechnol. J. 2016;11:920–931. doi: 10.1002/biot.201500223. [DOI] [PubMed] [Google Scholar]
- 33.Behere K., Yoon S. Chromatography bioseparation technologies and in-silico modelings for continuous production of biotherapeutics. J. Chromatogr. A. 2020;1627:461376. doi: 10.1016/j.chroma.2020.461376. [DOI] [PubMed] [Google Scholar]
- 34.Ulmer N., Müller-Späth T., Neunstoecklin B., Aumann L., Bavand M., Morbidelli M. Affinity capture of f(ab’)2 fragments: usin twin-column countercurrent chromatography. Bioproc. Int. 2020;13 [Google Scholar]
- 35.Angarita M., Müller-Späth T., Baur D., Lievrouw R., Lissens G., Morbidelli M. Twin-column captureSMB: a novel cyclic process for protein A affinity chromatography. J. Chromatogr. A. 2015;1389:85–95. doi: 10.1016/j.chroma.2015.02.046. [DOI] [PubMed] [Google Scholar]
- 36.Grossman C., Ströhlein G., Morari M., Morbidelli M. Optimizing model predictive control of the chromatographic multicolumn solvent gradient purification (MCSGP) process. J. Process Contr. 2010;20:618–629. [Google Scholar]
- 37.Krättli M., Müller-Späth T., Morbidelli M. Multifraction separation in countercurrent chromatography. Biotechnol. Bioeng. 2013;110:2436–2444. doi: 10.1002/bit.24901. [DOI] [PubMed] [Google Scholar]
- 38.L. Aumann, M. Morbidelli, EU Patent Application Publ., EP 1877769 B1 (2006).
- 39.Ströhlein G., Aumann L., Müller-Späth T., Tarafder A., Morbidelli M. The Multicolumn Countercurrent Solvent Gradient Purification process-A continuous chromatographic process for monoclonal antibodies without using Protein A. Biopharm Int. 2007;22:42–48. [Google Scholar]
- 40.Jungbauer A. Continuous downstream processing of biopharmaceuticals. Trends Biotechnol. 2013;31:479–492. doi: 10.1016/j.tibtech.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 41.Müller-Späth T., Bavand M. Purification of synthetic peptides by countercurrent chromatography (MCSGP) - economic evaluation. Pharmaceut. Eng. 2019;39:68–77. [Google Scholar]
- 42.Papathanasiou M.M., Steinebach F., Ströhlein G., Müller-Späth T., Nascu I., Oberdieck R., Morbidelli M., Mantalaris A., Pistikopoulos E.N. A control strategy for periodic systems – application to the twin-column MCSGP. Computer Aided Chem. Eng. 2015;37:1505–1510. [Google Scholar]
- 43.Papathanasiou M.M., Steinebach F., Morbidelli M., Mantalaris A., Pistikopoulos E.N. Intelligent, model-based control towards the intensification of downstream processes. Comput. Chem. Eng. 2017;105:173–184. [Google Scholar]
- 44.De Luca C., Felletti S., Lievore G., Buratti A., Vogg S., Morbidelli M., Cavazzini A., Catani M., Macis M., Ricci A., Cabri W. From batch to continuous chromatographic purification of a therapeutic peptide through multicolumn countercurrent solvent gradient purification. J. Chromatogr. A. 2020;461304 doi: 10.1016/j.chroma.2020.461304. [DOI] [PubMed] [Google Scholar]
- 45.Rylander D.J. Glucagon in the artificial pancreas: supply and marketing challenges. J Diabetes Sci. Technol. 2014;9:52–55. doi: 10.1177/1932296814546668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Müller-Späth T., Krättli M., Aumann L., Ströhlein G., Morbidelli M. Increasing the activity of monoclonal antibody therapeutics by continuous chromatography (MCSGP), Biotech. Bioengineering. 2010;107:652–662. doi: 10.1002/bit.22843. [DOI] [PubMed] [Google Scholar]
- 47.Müller-Späth T., Ulmer N., Aumann L., Ströhlein G., Bavand M., Hendriks L.J.A., de Kruif J., Throsby M., Bakker A.B.H. Purifying common light-chain bispecific antibodies. Bioproc. Int. 2013;11:36–45. [Google Scholar]
- 48.Gottschalk U., editor. Process Scale Purification of Antibodies. John Wiley & Sons; 2017. [Google Scholar]
- 49.Aumann L., Morbidelli M. A semicontinuous 3-column countercurrent solvent gradient purification (MCSGP) process. Biotechnol. Bioeng. 2008;99:728–733. doi: 10.1002/bit.21585. [DOI] [PubMed] [Google Scholar]
- 50.Purification of a Therapeutic Oligonucleotide Using Twin-Column Chromatography (MCSGP), Application Note P2-V1 by ChromaCon. 2019. [Google Scholar]
- 51.Highly Pure Cannabidiol (CBD) by Twin-Column Chromatography (MCSGP), Application Note P2-V1 by ChromaCon. 2019. [Google Scholar]
- 52.Catani M., De Luca C., Medeiros Garcia Alcântara J., Manfredini N., Perrone D., Marchesi E., Weldon R., Müller-Späth T., Cavazzini A., Morbidelli M., Sponchioni M. Oligonucleotides: current trends and innovative applications in the synthesis, characterization and purification. Biotechnol. J. 2020;15:1900226. doi: 10.1002/biot.201900226. [DOI] [PubMed] [Google Scholar]
- 53.Marchetti N., Dondi F., Felinger A., Guerrini R., Salvadori S., Cavazzini A. Modeling of overloaded gradient elution of nociceptin/orphanin FQ in reversed-phase liquid chromatography. J. Chromatogr. A. 2005;1079:162–172. doi: 10.1016/j.chroma.2005.02.078. [DOI] [PubMed] [Google Scholar]
- 54.Åsberg D., Leśko M., Leek T., Samuelsson J., Kaczmarski K., Fornstedt T. Estimation of nonlinear adsorption isotherms in gradient elution RP-LC of peptides in the presence of an adsorbing additive. Chromatographia. 2017:961–966. doi: 10.1007/s10337-017-3298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samuelsson J., Eiriksson F.F., Åsberg D., Thorsteinsdóttir M., Fornstedt T. Determining gradient conditions for peptide purification in RPLC with machine-learning-based retention time predictions. J. Chromatogr. A. 2019;1598:92–100. doi: 10.1016/j.chroma.2019.03.043. [DOI] [PubMed] [Google Scholar]
- 56.Forssén P., Samuelsson J., Fornstedt T. Relative importance of column and adsorption parameters on the productivity in preparative liquid chromatography II: investigation of separation systems with competitive Langmuir adsorption isotherms. J. Chromatogr. A. 2014;1347:72–79. doi: 10.1016/j.chroma.2014.04.059. [DOI] [PubMed] [Google Scholar]
- 57.Forssén P., Fornstedt T. A model free method for estimation of complicated adsorption isotherms in liquid chromatography. J. Chromatogr. A. 2015;1409:108–115. doi: 10.1016/j.chroma.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 58.Gritti F., Felinger A., Guiochon G. Overloaded gradient elution chromatography on heterogeneous adsorbents in reversed-phase liquid chromatography. J. Chromatogr. A. 2003;1017:45–61. doi: 10.1016/s0021-9673(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 59.Gritti F., Felinger A., Guiochon G. Properties of the adsorption equilibrium isotherms used and measured in RPLC. J. Chromatogr. A Chromatographia. 2004;60:S3–S12. [Google Scholar]
- 60.Qamar S., Rehman N., Carta G., Seidel-Morgenstern A. Analysis of gradient elution chromatography using the transport model. Chem. Eng. Sci. 2020;225:115809. [Google Scholar]
- 61.Creasy A., Lomino J., Carta G. Gradient elution behavior of proteins in hydrophobic interaction chromatography with a U-shaped retention factor curve under overloaded conditions. J. Chromatogr. A. 2018;1578:28–34. doi: 10.1016/j.chroma.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Carta G., Perez-Almodovar E.X. Productivity considerations and design charts for biomolecule capture with periodic countercurrent adsorption systems. Separ. Sci. Technol. 2010;45:149–154. [Google Scholar]
- 63.Abel S., Erdem G., Amanullah M., Mazzotti M., Morari M., Morbidelli M. Optimizing control of simulated moving beds—experimental implementation. J. Chromatogr. A. 2005;1092:2–16. doi: 10.1016/j.chroma.2005.04.101. [DOI] [PubMed] [Google Scholar]
- 64.Karst D.J., Steinebach F., Morbidelli M. Continuous integrated manufacturing of therapeutic proteins. Curr. Opin. Biotechnol. 2018;53:76–84. doi: 10.1016/j.copbio.2017.12.015. [DOI] [PubMed] [Google Scholar]
- 65.Su Q., Ganesh S., Moreno M., Bommireddy Y., Gonzalez M., Reklaitis G.V., Nagy Z.K. A perspective on Quality-by-Control (QbC) in pharmaceutical continuous manufacturing. Comput. Chem. Eng. 2019;125:216–233. doi: 10.1016/j.compchemeng.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee S.L., O'Connor T.F., Yang X., Chatterjee S., Madurawe R.D., Moore C.M.V., Yu L.X., Woodcock J. Modernizing pharmaceutical manufacturing: from batch to continuous production. J. Pharm. Innov. 2015;10:191–199. [Google Scholar]