Abstract
Because deoxyribonucleoside triphosphates (dNTPs) are the critical substrates for DNA replication and repair, dNTP pools have been studied in context of multiple basic biochemical processes. Over the last 12 years, interest in dNTPs, and specifically the mitochondrial dNTP pools, has expanded to biomedical science because several mitochondrial diseases have been found to be caused by dysfunctions of several enzymes involved in dNTP catabolism or anabolism. Techniques to reliably measure mitochondrial dNTPs should be sensitive and specific to avoid interference caused by the abundant ribonucleotides. Here, we describe detailed protocols to measure mitochondrial dNTPs from two specific samples, cultured skin fibroblasts and mouse liver. The methods can be easily adapted to other types of samples. The protocol follows a polymerase-based method, which is the most widely used approach to measure dNTP pools. Our description is based on the latest update of the technique, which minimizes the potential interference from ribonucleotides.
Keywords: dNTP, Mitochondrial DNA depletion, Mitochondrial DNA replication, PCR, Radio-labeled primer extension
1. Introduction
The deoxyribonucleoside triphosphates (dNTPs) are the essential building blocks for DNA replication and repair, and, for over four decades, measurements of dNTPs in biological samples have been related to basic cell biology and biochemistry. Within the last decade, the study of dNTPs, especially the mitochondrial dNTP pool, has gained increasing interest due to the identification of mutations that alter dNTP metabolism in several severe mitochondrial disorders (1–4). Recent studies have identified intermediate RNA: DNA hybrid fragments during mitochondrial DNA replication (5, 6) suggesting that ribonucleotides may also be incorporated into newly synthesized mitochondrial DNA. Nevertheless, mutations in four different genes disrupt dNTP pathways causing severe mitochondrial diseases with altered dNTP pools (1–4), while only one disease gene has been linked to mitochondrial ribonucleotide metabolism, but not to unbalanced ribonucleotide triphosphate pools (7). Therefore, the studies on the balance of the mtDNA replication substrates have been centered on dNTPs only. These studies have been mainly restricted to experimental animal or cell culture models and aimed at uncovering the molecular bases of mitochondrial dNTP homeostasis and pathomechanisms of genetic diseases with nucleotide pool imbalances.
Methods based on HPLC separation followed by ultraviolet (UV) or mass spectrometry (MS, MS/MS) detection have been employed to resolve dNTPs (dATP, dGTP, dCTP, and dTTP) and ribonucleoside triphosphates (rNTPs; ATP, GTP, CTP, and UTP)(8–11). However, cellular dNTP concentrations are usually between 10- and 1,000-fold lower than those of rNTPs and other partially phosphorylated ribonucleotides, such as ADP and AMP. For this reason, interference from ribonucleotides often impedes reliable quantification of the dNTPs in cell extracts, even when using highly selective methods as HPLC-MS/MS (8, 11). This problem has been often circumvented by chemically removing the ribonucleotides from the extracts (e.g., with boronate or periodate treatment) prior to HPLC injection (11, 12). However, HPLC methods do not achieve the sensitivity needed for the measurement of mitochondrial dNTPs, much less abundant than cytosolic dNTPs.
A different strategy, based on the DNA polymerase-catalyzed incorporation of dNTPs into a template DNA, was described in 1969 (13). Twenty years later, a modified version of this strategy was proposed (14) and adopted, with slight modifications, by many investigators (15–19). The method is based on the incorporation of tritium-labeled dATP (for the determination of dGTP, dCTP, or dTTP) or tritium-labeled dTTP (for the determination of dATP), via primer extension of daughter oligonucleotides from four parental oligonucleotides specifically designed for dATP, dGTP, dCTP, or dTTP determination. The reaction mixture contains radiolabeled dATP (or dTTP) and primed parental oligonucleotide in excess, together with DNA polymerase, such that the limiting reagent for the elongation of the primed oligonucleotide is the endogenous dNTP specie to be measured. After 1 h of reaction, the amount of radioactive nucleotide incorporated depends on the amount of dNTP species in the specimen, which can be calculated from a calibration curve obtained with aqueous standards.
The DNA polymerase most widely used for this assay used to be the Klenow subunit of the E. coli polymerase I. However, a recent report from Dr. Vera Biachi’s group has shown that, under some conditions, Klenow may incorporate ribonucleotide instead of deoxynucleotide, which may lead to overestimations of some dNTPs, especially dGTP and dCTP (20). For this reason, the method described here uses the enzyme Thermo Sequenase, which has been shown to discriminate well between dNTPs and rNTPs even in the presence of 1,000-fold higher ribonucleotides than deoxynucleotides (20).
2. Materials
2.1. Reagents
-
Mitochondrial isolation buffer: 210-mM d -mannitol, 70-mM sucrose, 10-mM Tris–HCl pH 7.5, 0.2-mM EGTA, and 0.5% (w/v) bovine serum albumin (BSA).
Dissolve 0.121 g of Tris-base (M = 121.14 g/mol) in 85 ml of water and adjust the pH to 7.5 by adding concentrated HCl. Add 3.83 g of d -mannitol (M = 182.2 g/mol), 2.40 g of sucrose (M = 342.3 g/mol), and 7.6 mg of EGTA (380.4 g/mol). Once dissolved, adjust the total volume up to 100 ml with water, and sterilize by filtering through a 0.2- μm pore nylon membrane. Store at −20°C in 10-ml aliquots. Before use, add BSA up to 0.5% (w/v) to the volume needed for the experiment.
1-M Tris–HCl, pH 7.5. Dissolve 12.1 g of Tris-base (M = 121.14 g/mol) in 90 ml of water, and adjust the pH to 7.5 by adding HCl. Store in 10-ml aliquots at −20°C.
0.1-M MgCl2. Dissolve 0.203 g of magnesium chloride hexa-hydrate (MgCl2·6H2O, M = 203.3 g/mol) in 10 ml of water. Store frozen at −20°C in aliquots.
0.5-M dithiothreitol (DTT). Dissolve 0.772 g of DTT (M = 154.3 g/mol) in 10 ml of water. Store frozen at −20°C in aliquots.
-
Oligonucleotides (sequences are written from 5′ to 3′)(see Note 1):
Oligo A: AAATAAATAAATAAATAAATGGCGGTGGAGGCGG
Oligo G: TTTCTTTCTTTCTTTCTTTCGGCGGTGGAGGCGG
Oligo C: TTTGTTTGTTTGTTTGTTTGGGCGGTGGAGGCGG
Oligo T: TTATTATTATTATTATTAGGCGGTGGAGGCGG
Common primer: CCGCCTCCACCGCC
To make stock solutions of the oligonucleotides, reconstitute with sterile water to a final concentration of 100 μM as follows: Add the appropriate volume of sterile water to the vial containing the solid oligonucleotide, allow rehydration at room temperature for 30 min, and then vortex to ensure complete and homogeneous dissolution. Store the stock solutions at −20°C.
Primed oligonucleotides: Mix 50 μl of 100-μM oligo A with 50 μl of 100-μM common primer; make the same separate mixtures for oligo G, oligo C, and oligo T. In each mixture, the final concentrations of both oligos (specific and common) are 50 μM. In a beaker, heat 2 L of water to 70°C, and then, incubate the four tubes in this bath while letting cold the water to room temperature (1–2 h), which will allow the oligos to hybridize to obtain the following primed oligonucleotides (Fig. 1).
Once the mixtures have reached room temperature, dilute each 50-μM primed oligonucleotide solution (100 μl) by adding 900 μl of sterile water (1:10 dilution) to bring the primed oligonucleotides to 5 μM. Make aliquots and store at −20°C until needed.
Tritium-labeled dATP ([8-3H]dATP, tetrasodium salt, at a specific activity of 10–25 Ci/mmol, 1 mCi/ml) for the determination of dGTP, dCTP, and dTTP. This reagent comes in ethanol:water (1:1) solution. Store at −20°C (see Note 2). On the day of the assay, take the volume of radiolabeled reagent that contains the amount of 3H-dATP needed for all the samples to be analyzed (15 pmol of radiolabeled dATP are needed for every reaction tube). Lyophilize by speed vacuum. Once completely dry, add the amount of water needed to achieve a concentration of 15 μM. Let the pellet rehydrate in the tube for 5 min on ice, then vortex and keep the tube on ice until the reagent is used (see Note 3).
Tritium-labeled dTTP ([methyl-3H]dTTP, tetrasodium salt, specific activity 10–25 Ci/mmol, 1 mCi/ml) for the determination of dATP. This reagent comes in ethanol:water (1:1) solution. Store at −20°C. On the day of the assay, dry and reconstitute as indicated for tritium-labeled dATP (see above).
Thermo Sequenase. Keep as indicated by the manufacturer until use. Use preparations with catalytic concentrations around 30 U/μl.
95% ethanol (see Note 4).
60% methanol (v/v) in water: This reagent can be stored at room temperature but will have to be at −20°C before use.
5% (w/v) Na2HPO4: Dissolve 100 g of Na2HPO4 (M = 142.0 g/mol) in 2 L of water.
-
Phosphate buffered saline (PBS): 140-mM NaCl, 10 mM Na2HPO4, pH 7.4 (see Note 5).
Dissolve 8.2 g of NaCl (M = 58.5 g/mol) and 1.42 g of Na2HPO4 (M = 141.96 g/mol) in nearly 1 L of water. Adjust to pH 7.4 with HCl. Then, complete the volume to 1 L with water.
Fig. 1.
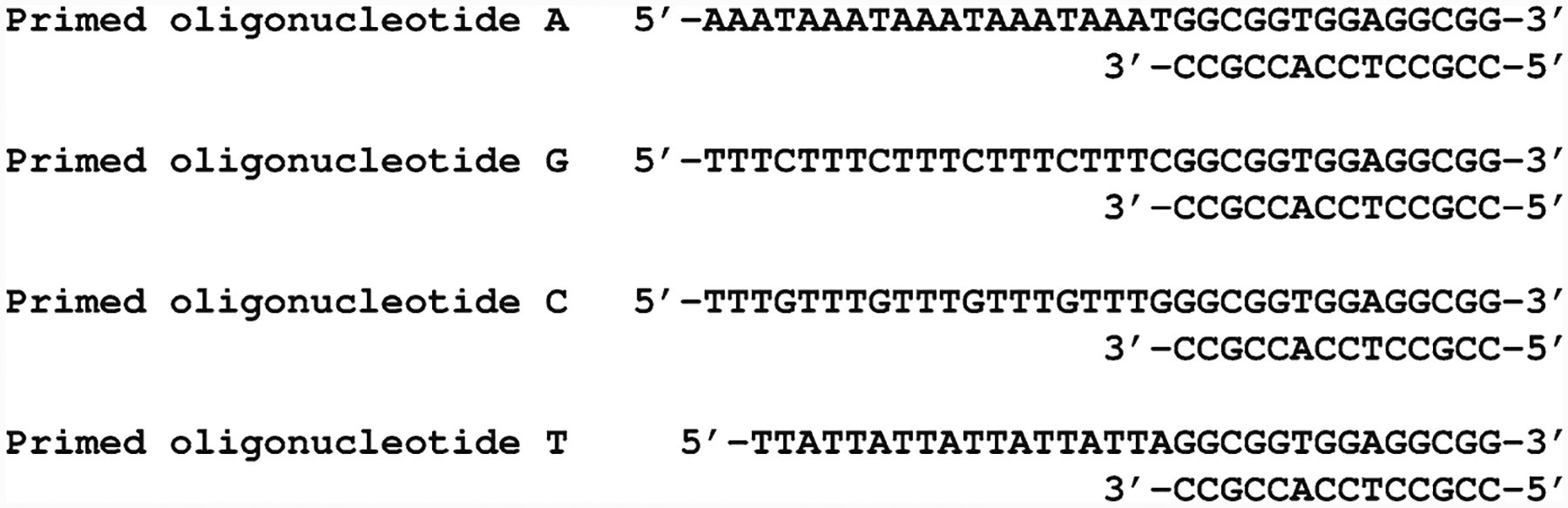
Primed oligonucleotides used for measurement of dNTPs. Primed oligonucleotides, as designed by Sherman and Fyfe (14). See the text for the details on preparation.
2.2. Aqueous dNTP Standards
From a stock solution of dATP, dGTP, dCTP, and dTTP, 10 mM each (see Note 6), make serial dilutions with water to obtain the following concentrations for the aqueous standards to be included in the assay: 400, 200, 100, 50, 25, and 5 nM. The standard zero (pure water) should be also included in the calibration curve.
2.3. Other Materials and Equipment
Syringes and needles (22 gauge × 1¼″)(0.70 × 30 mm)(for homogenization of cells).
Glass–glass homogenizer (for homogenization of tissues).
Whatman DE81 discs (2.3-cm diameter).
Scintillation cocktail.
Beta counter.
Scintillation vials.
Scrappers (for cultured cells).
Speed vacuum concentrator.
3. Methods (see Note 7)
3.1. Isolation of Mitochondria from Cultured Skin Fibroblasts
(see Note 8)
Culture a minimum of 30 × 106 cells in Petri dishes (see Note 9) for collection and use under testing conditions (see Note 10). If the final results are to be expressed per million of cells, an additional plate should be cultured in the same conditions for counting purposes.
Once the cells are in the desired conditions, put the plates on ice, and carry out the following steps in a cold chamber.
Remove the culture medium, and wash all the plates with cold PBS three times.
After the last wash, eliminate as much PBS as possible, and then, add 2 ml of isolation buffer to the first plate.
Collect thoroughly the cells from the surface with a scraper, and carefully transfer the whole volume of the suspension to the second plate of cells using a Pasteur pipet.
Repeat the cell collection with scraper and transfer to the third plate of cells. Repeat the same procedure with all the plates needed for the determination.
The final suspension should contain all the cells in the 2 ml of isolation buffer initially poured in the first plate (see Note 11).
Break the cells by passing the suspension through a 22-gauge 1¼-inch needle (0.70 × 30 mm) in a 5-ml syringe (ten strokes). Further steps can be performed outside of the cold chamber, by keeping the samples always on ice.
Centrifuge the homogenate at 20,000 × g at 4°C for 20 min.
Remove the supernatant (cytosolic fraction), which can be stored at −80°C for further use (see Note 12)
Wash the pellet (combined nuclear + mitochondrial fraction) with 0.5 ml of isolation buffer and centrifuge at 20,000 × g for 20 min.
Discard the supernatant and resuspend the mitochondrial pellet in 1 ml of isolation buffer without BSA. Use one portion of this suspension for the determination of protein (see Note 13). Centrifuge the rest of the suspension at 20,000 × g at 4°C for 20 min.
Discard the supernatant and save the pellet on ice for dNTP extraction (Subheading 3.3)
3.2. Isolation of Mitochondria from Mouse Liver
Fresh whole liver, rapidly excised from a recently sacrificed mouse, must be rapidly washed in cold PBS to eliminate the excess of blood and transferred to cold isolation buffer (4-ml/g tissue). All further steps should be carried out on ice in a cold room.
Cut the liver into small pieces using dissection scissors and homogenize in a glass–glass homogenizer (four up-and-down strokes).
Centrifuge the homogenate at 1,000 × g for 5 min at 4°C to eliminate the nuclei and remains of undisrupted tissue.
Transfer the supernatant to a tube of the appropriate volume and centrifuge at 13,000 × g at 4°C for 2 min (see Note 14).
Discard the supernatant, wash the pellet with 1 ml of isolation buffer, and then, centrifuge at 13,000 × g at 4°C for 2 min.
Resuspend the mitochondrial pellet in 1 ml of isolation buffer without BSA, and save one portion of this suspension for the determination of protein (see Note 13). Centrifuge the rest of the suspension at 13,000 × g at 4°C for 2 min.
Discard the supernatant and save the pellet on ice for dNTP extraction (Subheading 3.3).
3.3. dNTP Extraction from the Mitochondrial Pellets
Add 2 ml of 60% methanol at −20°C to the mitochondrial pellet, vortex and keep at −20°C for 2 h.
Centrifuge at 25,000 × g for 10 min and transfer the supernatant to a new tube, and discard the pellet.
Incubate the supernatant in boiling water for 3 min, and centrifuge at 25,000 × g for 10 min. Transfer the supernatant to a new tube, and discard the pellet (see Note 15).
Evaporate the solvent (60% methanol) of the supernatant with a speed vacuum concentrator.
Add the appropriate volume of sterile distilled water to the dry residue (see Note 16), let redissolve on ice for 10 min followed by vortexing. Keep the extract at −80°C until analysis.
3.4. DNA Polymerase Assay (20)
Label 0.5-ml (or 0.2-ml) capped tubes according to the number of samples to be processed, including duplicates and different dilutions. In addition, label tubes for the standard curves with duplicate or triplicate of the expected concentrations. The standard zero (water) should be included in the standard curve (see Note 17).
Table 1 shows the reaction mixture for each individual tube.
Prepare four master mixes (for determination of dATP, dGTP, dCTP, and dTTP, respectively) containing the following reagents in the proportions indicated in Table 1 : Tris–HCl buffer, MgCl2, DTT, the specific primed oligonucleotide, the radiolabeled nucleotide (3H-dATP for dGTP, dCTP, and dTTP determinations, and 3H-dTTP for dATP determination), water, and Thermo Sequenase. Keep the master mixes on ice all the time (see Note 19).
Put 15 μl of each master mix in the corresponding tubes, previously labeled for samples and standards. Maintain the tubes constantly on ice.
Add 5 μl of standards, undiluted extracts, and diluted extracts to the corresponding tubes, and mix with several up-and-downs using the pipette.
Incubate at 48°C for 1 h.
Label with a pencil as many DE81 Whatman discs as reaction tubes are in process. The identification of the disc should be clearly visible to make easier further steps of the protocol and to minimize the chances of losing the label (see Note 20). Distribute the discs individually on a parafilm surface or aluminum foil sheet (see Note 21).
After 1 h of reaction at 48°C, spot 18 μl of the reaction mixtures on the corresponding discs. Air-dry the discs.
Wash the discs three times (10 min each) with 5% (w/v) Na2HPO4, once (10 min) with water, and once (10 min) with 95% ethanol. Distribute the discs on a surface and air-dry (see Note 22).
Place the discs in scintillation vials (previously labeled), add the appropriate volume of scintillation cocktail, and measure the dpm in a program set up for 3H (see Note 23).
Generate a calibration curve for each dNTP by plotting the standard concentration (independent variable) against the dpm obtained for the standards (dependent variable). Adjust to a linear regression y = Ax + B, where y represents dpm, x represents the dNTP concentration of the standard (in nmol/L), A is the slope in dpm·L/nmol, and B is the offset in dpm (see Note 24)
Calculate the concentration of each dNTP in the extracts using the calibration curves. The values may be expressed as for millions of cells (in the case of cell culture) or per milligram of protein.
Table 1.
Composition of the reaction mixture for the determination of dNTPs (see Note 18)
| Reagent | Volume (μl) | Final concentration |
|---|---|---|
| 1-M Tris-HCl, pH 7.5 | 0.8 | 40 mM |
| 0.1-M MgCl2 | 2 | 10 mM |
| 0.5-M DTT | 0.2 | 5 mM |
| 5-μM specific primed oligonucleotide | 1 | 0.25 μM |
| 15-μM radiolabeled deoxynucleotide | 1 | 0.75 μM |
| Thermo sequenase (32 U/μl) | 0.016 | 0.025 U/μl |
| Water | 10 | - |
| Extract (or standard) | 5 | 1:4 |
| Total volume | 20 | - |
4. Notes
Synthetic oligonucleotides can be purchased from several companies that offer custom designed oligos. We recommend to order HPLC-purified oligos to ensure the accurate length of most molecules received in the material purchased, which will improve the performance of the assay (e.g., it will enhance the sensitivity by minimizing the background signal).
There are different preparations of tritium-labeled dNTPs, including different cationic salts and different shipping solvents. The water:ethanol (1:1) solution does not freeze at −20°C and manufacturers indicate that, in these conditions, the radiolabeled compound is more stable than in solid state or in aqueous solution. The chemical concentration (μmol/L) of the solution received should be calculated for every batch from the activity per volume (e.g., 1 mCi/ml) to the specific radioactivity (we usually purchase at 10–25 Ci/mmol). From these particular values, the range of concentrations results to be 40–100 μmol/L.
Since the radiolabeled reagents are in ethanol:water (1:1) solution, the time for the solvent evaporation is not expected to be very long (around 1–1.5 h for 50 μl, depending on the speed vacuum device). The temperature of the solution should not exceed 20°C during the drying process, and the compound should not remain in the solid state any longer than necessary. The protocol described here does not involve dilution of the radiolabel; thus, the specific radioactivity remains unchanged. Theoretically, the higher specific radioactivity, the better sensitivity of the assay, but in our experience, acceptable sensitivities for most purposes can be achieved after a radiolabel 1:3 dilution by adding cold dATP (or cold dTTP) to the radiolabeled dATP (or dTTP). If this is done, it should be taken in account that the chemical concentration of the reagent to be used should be 15 μM in any case.
The ethanol is only needed for a washing step (Subheading 3.4, step 9 in the protocol). Many descriptions of the method indicate to use absolute ethanol, which is more expensive than the common 95% ethanol. However, the protocol works perfectly with 95% ethanol.
Although the most common and physiological formulation of PBS includes potassium cation, for the purposes of this protocol both formulations of PBS are acceptable. We have described here a formulation with sodium salts only. Moreover, it is also possible to purchase inexpensive preformulated PBS tablets to be dissolved in an appropriate volume of water to obtain PBS ready to use.
Premade dNTP aqueous solutions containing the four dNTP, usually at 10 mM, can be purchased from several companies. This is the most convenient way to obtain a concentrated stock dNTP solution, and then to prepare the standards at the concentrations needed. Separate 10-mM stock solutions of dATP, dGTP, dCTP, and dTTP are also available. Alternatively, the solid compounds can be purchased to prepare the solutions.
The protocols described here for the isolation of mitochondrial dNTPs involve two steps: (1) isolation of a mitochondria-enriched fraction by differential centrifugation and (2) extraction of dNTPs from the mitochondria-enriched fraction by treatment with 60% methanol. The protocol to obtain a mitochondria-enriched fraction may vary depending on the type of cultured cells or the tissue (detailed protocols can be found in refs. 21 and 22). Once this fraction has been obtained, the steps to extract the dNTPs are identical, regardless of what was the original sample. We describe here the protocols for cultured human skin fibroblasts and for mouse liver.
This method for isolation of mitochondrial dNTP pools is based on the protocol described in ref. 23. Most protocols to prepare mitochondria-rich fraction use differential centrifugation of the cell homogenates, with a first low speed centrifugation (~300 × g) to pellet and discard the nuclei, and a second high speed centrifugation (~20,000 × g) to pellet the mitochondria-enriched fraction. However, a substantial amount of mitochondria can be present in the nuclear fraction discarded. This may constitute an important loss of material from samples, e.g., cultured cells, which do not usually produce enough mitochondria to allow dNTP determinations. The method established for cells by Pontarin et al. (23) and described here uses a single 20,000 × g centrifugation, which sediments a combined nuclear + mitochondrial fraction. Nuclear dNTPs are eliminated by a single wash because nuclei (but not mitochondria) are freely permeable for nucleotides. The combined mitochondria plus dNTP-free nuclei are pelleted from the wash at 20,000 × g and used for the dNTP extraction.
The cells should be cultured on a surface accessible to collection using scraper; cell collection using trypsin is not recommended as even the limited proteolysis produced by this enzyme may enhance the leakage of dNTPs, thus affecting the final result. In our experience, human skin fibroblasts reach high confluence at around 20,000 cells/cm2, but this value may present some variability depending on several factors, including the culture conditions and interindividual variations.
The amounts of mitochondrial and cytosolic dNTPs depend on the cell cycle status; in cultured cells, it has been shown that cycling cells have up to 50 times more dNTPs than quiescent cells (24). Moreover, the mitochondria-rich fraction should be prepared rapidly to avoid dNTP leakage from mitochondria and to minimize ATP breakdown, which also influences negatively the dNTP content (25).
The number of plates needed for mitochondrial dNTP measurement depends on the particular purposes of the experiment. In our experience, around 1,500 cm2 (i.e., ten plates of 13.7-cm diameter) are advisable when mitochondrial pools from confluent skin fibroblasts are to be measured. In this case, 2 ml of isolation buffer initially poured in the first plate should be sufficient to obtain a final suspension of between 2 and 4 ml due to the additional volume of the cells and residual PBS from the plates. Every plate should be carefully scraped, and the cell suspension should be fully recovered and passed successively from every plate to the next one. For experiments with cycling fibroblasts (therefore, contact inhibition should be avoided), the number of plates needed should be estimated taking in account that more surfaces will be needed to get the same number of cells. However, cycling cells contain more dNTPs than quiescent cells, and so, less than 30 million cells may be sufficient.
The cytosolic fraction can be used for dNTP measurement or for other purposes, depending on the particular objectives of the specific experiments. If dNTPs are to be measured in this fraction, one aliquot of the cytosolic fraction should be taken, pure methanol added up to a final concentration of 60%, and then follow the protocol as from the Subheading 3.3, step 1. Because the amounts of cytosolic dNTPs per million of cells are expected to be 10- to 50-fold higher than those expected of mitochondria, the volumes and the dilutions used in the final steps (see Subheading 3.3, step 5 and Notes 16 and 17) should be rescaled accordingly.
The protein concentration of the mitochondrial suspension can be determined using the Bradford method (26) using bovine serum albumin to prepare the calibration curve or with the method routinely used in your laboratory.
Alternatively, the supernatant can be aliquoted in eppendorf tubes, centrifuged as indicated, and then combine all the pellets together in the next wash.
Boiling the extract after the first methanol precipitation helps inactivate any remaining enzyme activity in the extracts (23). The pellet after the second precipitation may be barely visible, but most versions of this protocol include this boiling step to minimize dNTP catabolism in the extract.
The dry extract should be redissolved in the appropriate volume of water, which will depend on the type of sample and the initial amount of mitochondria. We recommend 100 μl for the mitochondrial extract obtained from ~30 × 106 cultured cells and 200 μl for the mitochondrial extract obtained from the whole mouse liver. It is expected that the resulting dNTP concentrations, as well as those of a 1:3 dilution, are quantifiable in the conditions of the assay described here. If different amounts of mitochondria are processed, the final volumes will have to be adjusted accordingly to ensure quantifiable concentrations in the final extract. Adding too much water in this step may lead to undetectable or barely quantifiable dNTPs.
Each nucleotide is determined in a different reaction. In addition, we recommend processing each sample with a duplicate of the undiluted extract and a duplicate with a dilution of the extract (e.g., 1:3 or 1:5 dilution, trying to ensure that the dNTP concentration in the diluted sample is reliably quantifiable). Because this is a long technique that can be influenced by many factors, it is necessary to include as many controls as possible to ensure the accuracy of the results. Processing two different dilutions of the same extract will allow verification that the result is reproducible at both concentrations.
Several alternative methods are possible as described in the modified protocol to avoid interference caused by ribonucleotides (20). For example, Taq DNA polymerase can be used instead of Thermo Sequenase, and Klenow enzyme can be used, with some restrictions, for dATP and dTTP determinations. Here, we detail the protocol with the particular conditions that we use for dNTP determinations, but modifications of this protocol according to ref. 20 are also possible.
The master mixes should be prepared immediately before use and kept on ice before starting the reaction. The simultaneous presence of primed oligonucleotides, radiolabeled dNTP and Thermo Sequenase, in the absence of the sample, may promote nonspecific incorporation of radiolabel into the primed oligonucleotides, thus increasing the background signal and affecting the detection limit of the assay.
Label with pencil. Do not use ink because a further washing step with ethanol would erase the label.
It is advisable to include the following controls for every assay: Measure the specific activity of the [3H]-dATP and [3H]-dTTP reagents in the beta counter. In addition, process DE81 discs with master mixes spotted, omitting the washing steps, to measure the total radioactivity loaded for every master mix.
The discs can be washed in four pools, one for each dNTP to be measured. Each wash can be done in 200–300 ml of washing solution in beakers or other recipients of the appropriate volume, using a horizontal shaker.
Since readings below 100 cpm are not expected (not even for the zero standard, which will have the lowest radiolabel), a reading time of 1 min in the beta counter is sufficient to obtain reliable counts.
In our experience, the calibration curves are acceptably linear within the range of 5–200 nmol/L. The slopes and offset of the curves depend on the specific activity of the 3H-dATP and 3H-dTTP used. The offset values should be similar to the dpm obtained for the standard zero.
Acknowledgment
Dr. Martí is supported by a grant from the Spanish Instituto de Salud Carlos III (PS09/01591). Dr. Hirano is supported by NIH grants R01 HD056103 (cofunded by NICHD and the NIH Office of Dietary Supplements), R01 HD057543, and RC1 NS070232; MDA grant 115567; and the Marriott Mitochondrial Disorder Clinical Research Fund.
References
- 1.Bourdon A, Minai L, Serre V, Jais JP, Sarzi E, Aubert S, et al. (2007). Mutation of RRM2B, encoding p53-controlled ribonucleotide reductase (p53R2), causes severe mitochondrial DNA depletion. Nat Genet 39:776–780. [DOI] [PubMed] [Google Scholar]
- 2.Mandel H, Szargel R, Labay V, Elpeleg O, Saada A, Shalata A, et al. (2001). The deoxyguanosine kinase gene is mutated in individuals with depleted hepatocerebral mitochondrial DNA. Nat Genet 29:337–341. [DOI] [PubMed] [Google Scholar]
- 3.Nishino I, Spinazzola A, Hirano M (1999). Thymidine phosphorylase gene mutations in MNGIE, a human mitochondrial disorder. Science 283:689–692. [DOI] [PubMed] [Google Scholar]
- 4.Saada A, Shaag A, Mandel H, Nevo Y, Eriksson S, Elpeleg O (2001). Mutant mitochondrial thymidine kinase in mitochondrial DNA depletion myopathy. Nat Genet 29:342–344. [DOI] [PubMed] [Google Scholar]
- 5.Pohjoismaki JL, Holmes JB, Wood SR, Yang MY, Yasukawa T, Reyes A, et al. (2010). Mammalian mitochondrial DNA replication intermediates are essentially duplex but contain extensive tracts of RNA/DNA hybrid. J Mol Biol 397:1144–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasukawa T, Reyes A, Cluett TJ, Yang MY, Bowmaker M, Jacobs HT, et al. (2006). Replication of vertebrate mitochondrial DNA entails transient ribonucleotide incorporation throughout the lagging strand. Embo J 25:5358–5371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaukonen J, Juselius JK, Tiranti V, Kyttala A, Zeviani M, Comi GP, et al. (2000). Role of adenine nucleotide translocator 1 in mtDNA maintenance. Science 289:782–785. [DOI] [PubMed] [Google Scholar]
- 8.Chen P, Liu Z, Liu S, Xie Z, Aimiuwu J, Pang J, et al. (2009). A LC-MS/MS method for the analysis of intracellular nucleoside triphosphate levels. Pharm Res 26:1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Decosterd LA, Cottin E, Chen X, Lejeune F, Mirimanoff RO, Biollaz J, et al. (1999). Simultaneous determination of deoxyribonucleoside in the presence of ribonucleoside triphosphates in human carcinoma cells by high-performance liquid chromatography. Anal Biochem 270:59–68. [DOI] [PubMed] [Google Scholar]
- 10.Di Pierro D, Tavazzi B, Perno CF, Bartolini M, Balestra E, Calio R, et al. (1995). An ion-pairing high-performance liquid chromatographic method for the direct simultaneous determination of nucleotides, deoxynucleotides, nicotinic coenzymes, oxypurines, nucleosides, and bases in perchloric acid cell extracts. Anal Biochem 231:407–412. [DOI] [PubMed] [Google Scholar]
- 11.Hennere G, Becher F, Pruvost A, Goujard C, Grassi J, Benech H (2003). Liquid chromatography-tandem mass spectrometry assays for intracellular deoxyribonucleotide triphosphate competitors of nucleoside antiretrovirals. J Chromatogr B Analyt Technol Biomed Life Sci 789:273–281. [DOI] [PubMed] [Google Scholar]
- 12.Shewach DS (1992). Quantitation of deoxyribonucleoside 5′-triphosphates by a sequential boronate and anion-exchange high-pressure liquid chromatographic procedure. Anal Biochem 206:178–182. [DOI] [PubMed] [Google Scholar]
- 13.Solter AW, Handschumacher RE (1969). A rapid quantitative determination of deoxyribonucleoside triphosphates based on the enzymatic synthesis of DNA. Biochim Biophys Acta 174:585–590. [DOI] [PubMed] [Google Scholar]
- 14.Sherman PA, Fyfe JA (1989). Enzymatic assay for deoxyribonucleoside triphosphates using synthetic oligonucleotides as template primers. Anal Biochem 180:222–226. [DOI] [PubMed] [Google Scholar]
- 15.Bianchi V, Borella S, Rampazzo C, Ferraro P, Calderazzo F, Bianchi LC, et al. (1997). Cell cycle-dependent metabolism of pyrimidine deoxynucleoside triphosphates in CEM cells. J Biol Chem 272:16118–16124. [DOI] [PubMed] [Google Scholar]
- 16.Dorado B, Area E, Akman HO, Hirano M Onset and organ specificity of Tk2 deficiency depends on Tk1 down-regulation and transcriptional compensation. Hum Mol Genet 20:155–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopez LC, Akman HO, Garcia-Cazorla A, Dorado B, Marti R, Nishino I, et al. (2009). Unbalanced deoxynucleotide pools cause mitochondrial DNA instability in thymidine phosphorylase-deficient mice. Hum Mol Genet 18:714–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saada A, Ben-Shalom E, Zyslin R, Miller C, Mandel H, Elpeleg O (2003). Mitochondrial deoxyribonucleoside triphosphate pools in thymidine kinase 2 deficiency. Biochem Biophys Res Commun 310:963–966. [DOI] [PubMed] [Google Scholar]
- 19.Song S, Wheeler LJ, Mathews CK (2003). Deoxyribonucleotide pool imbalance stimulates deletions in HeLa cell mitochondrial DNA. J Biol Chem 278:43893–43896. [DOI] [PubMed] [Google Scholar]
- 20.Ferraro P, Franzolin E, Pontarin G, Reichard P, Bianchi V (2010). Quantitation of cellular deoxynucleoside triphosphates. Nucleic Acids Res 38:e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fernandez-Vizarra E, Ferrin G, Perez-Martos A, Fernandez-Silva P, Zeviani M, Enriquez JA Isolation of mitochondria for biogenetical studies: An update. Mitochondrion 10:253–262. [DOI] [PubMed] [Google Scholar]
- 22.Frezza C, Cipolat S, Scorrano L (2007). Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat Protoc 2:287–295. [DOI] [PubMed] [Google Scholar]
- 23.Pontarin G, Gallinaro L, Ferraro P, Reichard P, Bianchi V (2003). Origins of mitochondrial thymidine triphosphate: dynamic relations to cytosolic pools. Proc Natl Acad Sci USA 100:12159–12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferraro P, Pontarin G, Crocco L, Fabris S, Reichard P, Bianchi V (2005). Mitochondrial deoxynucleotide pools in quiescent fibroblasts: a possible model for mitochondrial neurogastrointestinal encephalomyopathy (MNGIE). J Biol Chem 280:24472–24480. [DOI] [PubMed] [Google Scholar]
- 25.Ferraro P, Nicolosi L, Bernardi P, Reichard P, Bianchi V (2006). Mitochondrial deoxynucleotide pool sizes in mouse liver and evidence for a transport mechanism for thymidine mono-phosphate. Proc Natl Acad Sci USA 103:18586–18591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bradford MM (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]


