Abstract
The recent coronavirus disease 2019 (COVID-19) pandemic showed a different severity in the disease between males and females. Men have been becoming severely ill at a higher rate than women. These data along with an age-dependent disease susceptibility and mortality in the elderly suggest that sex hormones are the main factors in determining the clinical course of the infection. The differences in aging males versus females and the role of sex hormones in key phenotypes of COVID-19 infection are described in this review. Recommendations based on a dimorphic approach for males and females suggest a sex-specific management the disease.
Keywords: oestrogen, androgen, coronavirus disease 2019, sexual dimorphism, pandemic
Background
Sex is a major determinant in physiology, with phenotypic variability being driven by differences between males and females. Compared to females, males are more susceptible to viral and other respiratory tract infections, with males also outnumbering females in poor prognoses [1].
The response to the ongoing outbreak of the respiratory disease that was recently given the name COVID-19 appears no different. From the early days of the first COVID-19 outbreak in China, although sex-disaggregated data from COVID-19 showed equal cases between men and women, men have been becoming severely ill at a higher rate than women, with a pattern largely repeating itself in other parts of the world, including heavily affected countries, such as Italy [2,3]. Indeed, male sex at any age is independently associated with worse in-hospital outcomes [4,5] (Box 1 ).
Box 1. Men Die from COVID-19 More Than Women Do.
Sex-disaggregated data from COVID-19 show equal cases of infection between men and women, but men were between 10% and 90% more likely than women to die after being diagnosed with COVID-19. Only a minor number of countries provided data broken down by sex. No reliable data exist on the proportion of tests administered to men versus women in any country.
Alt-text: Box 1
A recent review of available data from different studies has confirmed the association between male sex and higher mortality rate [6]. According to the most recent available age-related analysis of mortality from the Italian 'Istituto Superiore di Sanità' (ISS), the vast majority of deceased patients was >70 years old, and slightly less than half of the deaths reported were between 80 and 89 years of age (ISS Report 29, April 2020). Similar to Chinese data, an increased rate of COVID-19-related mortality has been observed in patients with one or more comorbidities, such as hypertension (69%), type 2 diabetes (31%), ischaemic heart disease (28%), renal insufficiency (21%), chronic obstructive pulmonary disease (COPD) (17%), and obesity (12%) (ISS Report 29, April 2020).
In addition to sex dimorphism in severity of the disease, epidemiological studies have shown an age-dependent disease susceptibility, with young individuals experiencing mild illness. With age, along with a reduction in sex hormone production, there is a progressive functional decline and dysregulation in the immune system in both sexes. According to the most recent ISTAT report among people living in Italy as of 1 January 2019, more than 7 million were aged ≥75 years (11.7% of the overall population). More than 60% were women. There were 4.3 million Italians aged >80 years, with a female prevalence of 73% (ISTAT Report 27, April 2020). Therefore, aging of the population per se does not explain the excess COVID-19-related mortality in males versus females.
Altogether, these findings point to a potential role of sex hormones, not just in the sensitivity to infection, but also in the clinical course of the disease. Studies from the 2002–2003 severe acute respiratory syndrome (SARS) epidemic showed both an age-dependent susceptibility and sex-specific differences in both morbidity and mortality [7,8]. Even though an explanation for this is not available, scientists are offering some clues. The higher morbidity in men has been explained by variations in smoking, social behaviour, and delay in medical care between the sexes [9]. These ecological interpretations can be eclipsed by individual genetic, immunological, and hormonal differences.
Finding answers to these questions could help develop more effective prevention and treatment strategies for individual patients, including designing a good vaccine. Certainly, recognising the extent to which disease outbreaks affect women and men differently is a fundamental step in the creation of equitable policies.
In this review, we focus on the role that sex hormones can have in explaining this sex dimorphism.
The Ageing Man versus the Ageing Woman: Hormonal Dimorphism
Is it worth asking what are the distinctive features of elderly men as compared to women of same age that can have an impact in the observed sexual dimorphism of COVID-19 lethality?
Males
During the ageing process, there is a progressive decline in average serum total and free testosterone levels, increase in sex hormone binding globulin (SHBG), and moderate increase in luteinising hormone (LH), with a huge variability among subjects of the same age [10]. Incidence of late-onset hypogonadism (total testosterone <3 ng/ml) has been found to account for 15–20% in men between 49 and 75 years of age [11].
Among the factors associated with decreased testosterone levels, obesity, type 2 diabetes, and COPD were reported to have a significant impact [12].
It has been reported that the angiotensin-converting enzyme 2 (ACE2) (see Glossary), a critical receptor for coronavirus infection, is a constitutive product of adult-type Leydig cells [13], thus suggesting that COVID-19 infection per se may affect testosterone secretion. Furthermore, the association between an increase of proinflammatory state and decline in testosterone is often observed in ageing men [14]. Low testosterone levels may be associated with a reduction of respiratory muscle activity and strength with decreased tolerance to exercise [15,16].
Females
In postmenopausal women oestradiol levels sharply decline with concomitant reduction in total and free testosterone and SHBG. The most represented oestrogen hormone is oestrone. High levels of follicle-stimulating hormone (FSH) and LH are observed [17]. ACE2 has also been described in ovarian granulosa cells and its expression increases with a rise in LH [18].
Based on the previously-mentioned hormonal differences, it can be hypothesised that ageing-related decrease in testosterone may have a role in the progression of COVID-19 infection in males, whereas in postmenopausal women, either the presence of oestrone or of high gonadotropin levels may exert a protective effect. Also, it may be considered that the imprinting of testosterone and oestrogens in younger males and females may play a role in the dimorphic lethality of COVID-19 later in age.
Alternatively, it may be thought that testosterone itself may instead favour the progression of the disease [19]. In fact, the androgen receptor activates the transcription of a transmembrane protease serine 2 (TMPRSS2), the activity of which appears key to SARS coronavirus (SARS-CoV)-2 virus spread and aggressivity in the infected hosts, through the priming of the viral spike protein [20]. An interesting aspect to be evaluated is also the peripheral synthesis of potent androgens from adrenal precursors through the 3β-hydroxysteroid dehydrogenase-1 (HSD3B1) encoded by the HSD3B1 gene, whose the 1245C adrenal-permissive allele encoding a hyperactive enzyme is at its highest frequency in European countries [21] (Box 2 ).
Box 2. Etiology of Sex Dimorphism in COVID-19 Infection.
Comorbidities for noncommunicable disorders are reported more in men than in women, thus favouring complications in males versus females.
In males with hypogonadism (i.e., increased inflammatory response, reduced muscular strength) as well hyperandrogenism (i.e., activation of virus entrance in the cells, increased VTE) can favour complications in men infected with COVID-19.
Oestrogen potentiates the immune response in women, with a lower inflammatory phenotype than observed in men. LH levels in postmenopausal women can stimulate the production of ACE2, suggesting menopausal hormonal therapy (MHT) to prevent COVID-19 infection and its complications. However the use of synthetic oestrogen, either as contraceptive measure or as MHT, should be offered with caution for the increased risk of VTE. The use of oestradiol is, therefore, preferable.
Alt-text: Box 2
The Ageing Man versus the Ageing Woman: Clinical Dimorphism
Comorbidities have been repeatedly reported as key clinical prognostic factors in COVID-19 infection and a sexually dimorphic phenotypical expression of the main underlying disease could possibly have a role in explaining the different outcomes between sexes in this pandemic.
Hypertension
According to the latest available data from ISS (2008–2012), hypertension in frail elderly people has been shown to be an independent predictor of cardiovascular events and mortality. However, similar prevalence of hypertension is reported in Italian people of both sexes between 74 and 79 years of age (78.3% in males vs 80.6% in females). In the Lombardy Region, by far the Italian region most affected by COVID-19, mean blood pressure in males aged 74–79 years (136/75 mmHg) is lower than in females (145/81 mmHg) (ISS Progetto Cuore).
Type 2 Diabetes and Obesity
Type 2 diabetes in Italians aged 75–79 years is more prevalent in males (28%) than in females (17%). In the Lombardy Region, in the same population age range, mean blood glucose is 102 mg/dl in males versus 92 mg/dl in females. In Italy, males are more frequently overweight (52%) versus females (40%), whereas females are more frequently obese (38%) than males (25%). In Lombardy, females between 75 and 79 years of age have on average a higher BMI than males (28 versus 27 kg/m2) (ISS Progetto Cuore).
Ischaemic Heart Disease
In Italy, in the wide age range of 34–79 years, myocardial infarction is more prevalent in males (2%) than in females (0.9%), and revascularisation procedures are largely more frequent in males than in females (5% vs 1%) (ISS Progetto Cuore). However, these data are not corrected for the expected differences between pre-and postmenopausal women.
COPD
In an observational, population-based retrospective study, COPD was found to be present in 508 patients, with a prevalence of 58.9% in males aged 65–80 years, and high frequency of smoking (68% with 26% current smokers), hypertension (66%), type 2 diabetes, and obesity (18%) [22]. In Italy, according to the ISS, smoking between 75 and 79 years of age is largely more frequent in males (62.5% with 10% of current smokers) than in females (23%, with current smokers 7.5%). In Lombardy, current male smokers smoke on average 17 cigarettes per day versus 12 in females (ISS Progetto Cuore).
Renal Insufficiency
In an Italian cohort, part of a European multinational evaluation, only a slightly higher male prevalence (51%) was reported for chronic kidney disease [23]. Based on the previously-mentioned epidemiological data, it appears that only ischemic heart disease and COPD may have a consistently more expressed phenotype in elderly males than females, associated with increased exposure to smoking in males.
The Ageing Man versus the Ageing Woman: Therapeutic Dimorphism
The prevalence of venous thromboembolism (VTE) in COVID-19 patients has been reported as 25%, and VTE is associated with an unfavourable prognosis [24]. Testosterone replacement may be associated with a higher risk of VTE in both eugonadal and hypogonadal people [25]. Some hypogonadal patients, such as those with Klinefelter syndrome, are already at an increased risk of VTE [26]. The use of conjugated oestrogen in postmenopausal women or of contraceptives in the reproductive age could expose them to the same risk.
Hypovitaminosis D has been shown to be independently associated with increased risk of viral acute respiratory infection [27]. A meta-analysis of clinical trials on vitamin D supplementation has shown moderate protective effects of these infections [28]. Moreover, in patients with acute illness in intensive care units (ICUs), poor vitamin D status may aggravate outcome, and correction with (high doses) of vitamin D could decrease morbidity and mortality [29]. Moreover, hypovitaminosis D was found to segregate with diabetes [30], obesity [31], and high inflammatory status [32].
Vitamin D balance largely depends on sun exposure [27]. Lack of food fortification and change in lifestyle (increased by home confinements) account for the large prevalence of low circulating vitamin D levels in Italy, with increased susceptibility to complications and mortality due to COVID-19 infection [33]. Vitamin D is still prevalently used in patients with osteoporosis, the vast majority of whom are postmenopausal women and this can represent another predisposing factor to an increased aggressiveness of COVID-19 in older men versus older postmenopausal women [34].
Sex Hormones Targets in COVID-19
Sex Hormones and the Immune Response
Sex differences in human immunity are profound, as males and females respond differently to infection and therapy, showing also different propensity to autoimmunity [35]. Sex differences in the prevalence, intensity and outcome of viral infections are well conserved across species, from humans to other mammals, to Drosophila melanogaster [36,37], resulting in different incidence, duration, severity, and case fatality rates following infection [38]. While the precise incidence of how influenza differs between males and females is difficult to ascertain, the rates of hospitalisation and general morbidity from seasonal influenza viruses are consistently higher in males than females at all ages [39].
Females exhibit elevated humoral and cell-mediated immune response to antigenic stimulation, vaccination, and infection than males, with sex differences in the innate detection of viruses by pattern recognition receptors [40., 41., 42., 43., 44.]. Several genes that encode for immunological proteins are on the X chromosome and may escape X inactivation, resulting in biallelic expression in females versus males; a condition linked also to a higher risk for autoimmune disorders in women than in men [45]. Studies in both humans and rodents have shown that inflammatory immune responses are generally higher in females than males, with both basal levels of immunoglobulins and antibody responses to viruses and vaccines being consistently higher in females than males [46,47].
The prevailing hypothesis for immunological differences between the sexes is that sex steroids, particularly androgen and oestrogen, influence the immune system, reflecting endocrine–immune interactions [48]. Immune responses to viruses can vary with changes in hormone concentrations caused by natural fluctuations during the menstrual cycle, or due to contraception use or pregnancy [49]. After menopause, the abrupt reduction of oestrogen and progesterone in women is followed by an acute dysregulation of the immune function that is corrected with the administration of oestrogen replacement therapy [50]. Moreover, in women the production of inflammatory IL-6 after viral infection is lower than in males and is often correlated with a better longevity [51]. Certainly, receptors for sex steroids are expressed in several immune cells, with androgen suppressing the activity of these cells and elevated levels of oestrogen attenuating both the production of chemokines and the leukocyte/monocyte recruitment in many tissues, including lungs [36]. These sex hormonal functions can lead to either a proinflammatory or an anti-inflammatory (or tolerogenic) phenotype, and are potentially relevant in diseases associated with highly pathogenic viruses characterised by fever, pneumonia, and acute respiratory distress syndrome; complications hypothesised to be mediated by a true cytokine storm in response to infection [52]. Indeed, the beneficial effect of immunosuppressive intervention is now being explored in the hyperinflammatory COVID-19 respiratory distress syndrome.
The recent discovery of a sexual dimorphism in the human immune system has clearly revealed a shared epigenomic signature of ageing, including increasing monocytes and cytotoxic cell functions, with changes being greater in magnitude than anticipated in men, who with time exhibit a higher innate and proinflammatory activity [53].
Sex Hormones and Cardiovascular Health
Underlying cardiovascular disease (CVD) is associated with an increased risk of in-hospital death among patients hospitalised with COVID-19 [54]. In addition to the virus binding and entering through ACE2, the cardiovascular system is also affected with myocardial injury, dysrhythmias, and VTE [55]. Emergency clinicians should consider these complications in order to avoid interactions of current therapies for COVID-19 with cardiovascular medications. In contrast, several studies have suggested that endogenous circulating androgen and oestrogen have a neutral or beneficial effect on CVD [56]. However, data on the administration of androgen in males are conflicting [56].
The importance of ovarian hormones as a risk factor for stroke is evident, as to the incidence of female strokes is lower before than after menopause [57]. Premenopausal women have a lower incidence of stroke as compared to young males, but at the menopause transition, the incidence of stroke is twice that of men [57]. Data regarding oestrogen therapy and stroke risk are more ambiguous. The Women’s Health Initiative study, which had a significant impact on menopause medicine, concluded that conjugated oestrogen use increased stroke risk, mostly in the 60–69 years of age group [58]. Overall, the evidence linking oestrogen loss to increased stroke risk is strong, but data on oestrogen therapy and stroke risk are controversial. The increase in gonadotropins and the altered ratio of androgen to oestrogen after menopause may be important modifiers of stroke risk and stroke outcomes.
As COVID-19 shows different morbidity and mortality rates in men versus women, sex hormones, and in particular oestrogen and the response to oestrogen (through oestrogen receptor α, oestrogen receptor β, and G protein-coupled receptor), could have an impact on the outcomes of COVID-19 infection. Different targets of oestrogen in cardiovascular physiology are recognised in the anti-inflammatory effect, in the dilatation of the arterial vascular wall, in the endothelial function, and in the intravascular coagulation [59].
While the anti-inflammatory role of oestrogen in atherosclerosis has been well established [60., 61., 62.], oestrogen-induced vasodilation is beginning to be cemented. The vasorelaxant direct effect of endogenous oestrogen on the arterial wall has been documented through several molecular pathways, including nitric oxide (NO) [63,64]. The survival rate of SARS-CoV-infected cells was greatly increased in vitro by treatment with NO donors [65]. Findings from the 2004 SARS-CoV outbreak suggest the potential role of inhaled NO as a supportive measure for treating pulmonary complications [66].
Coagulation dysfunction is related to development of the acute respiratory distress syndrome typical of COVID-19 [2] and any intervention that affects the coagulation phenotype can became important in prevention or intervention in COVID-19 infection. Oestrogen-containing medications, prescribed either for contraception in women in reproductive age or for prevention of osteoporosis and alleviating symptoms related to menopause, are associated with changes in the haemostatic balance and contribute to increased risk of VTE [67,68]. However, low doses of oestradiol or its transcutaneous administration in menopausal women do not seem to be linked to these complications [69].
Interestingly, natural plant phytoestrogens, able to interact preferentially with oestrogen receptor β, can reduce proinflammatory cytokines [70]. The flavonoid genistein has the function of alleviating and treating disseminated intravascular coagulation caused by lipopolysaccharide [71].
Finally, the hypotensive ACE2 enzyme is a critical receptor for coronavirus infections [13]. ACE2 protein is present in alveolar lung cells, heart, kidneys, blood vessels and intestines, justifying the multiorgan disease in the COVID-19. Interestingly, also the gene encoding for ACE2 is on chromosome X and this could offer to women another way to respond to COVID-19 infection better than men. Finally, soluble ACE2 has been evaluated in phase I and II studies, as potentially therapeutic for coronavirus infection [72].
Sex Hormones and Lungs
Lungs represent the most important target system for COVID-19 infection in humans. Consequently, any environmental or endogenous factor involved in lung development and function must be taken into consideration. Smoking and vaping have been indicated as risk factors for susceptibility to COVID-19 [73]. Among endogenous factors to be evaluated, sex hormones represent an interesting area of investigation.
Sex determination and differentiation occur during prenatal development and involve genetic and hormonal factors that cause the primordial reproductive system to become either feminised or masculinised. These biological sex differences are present at the time of birth and probably impact the development of the respiratory system and susceptibility to respiratory tract infections.
In adult mammals, it is well known that oestrogen receptors are expressed and functionally active in most nonreproductive tissues, including lungs [74]. It is conceivable that sexual dimorphism typical of several chronic conditions, including respiratory diseases, may originate from a sex-specific architecture in the course of embryonal development, also through the expression and activity of the oestrogen receptors.
Oestrogen has been implicated in sex-related differences in the development and clinical outcomes of asthma, although the published data are conflicting [75]. In a recent study, asthma susceptibility and severity in the offspring increases in a sex-specific manner during prenatal stress challenges, with the disruption of the airway epithelium and consequent increased permeability orchestrating the foetal origin of asthma [76].
Interestingly, the gene encoding the TMPRSS2 enzyme is regulated by androgen in a lung-derived cell line model [77], but it is not known if this protease is regulated by androgen in physiological settings. In the case of a positive answer, inhibition of the androgen response could suppress the TMPRSS2 enzyme and, therefore, the viral entry into the cells.
Concluding Remarks and Future Perspectives
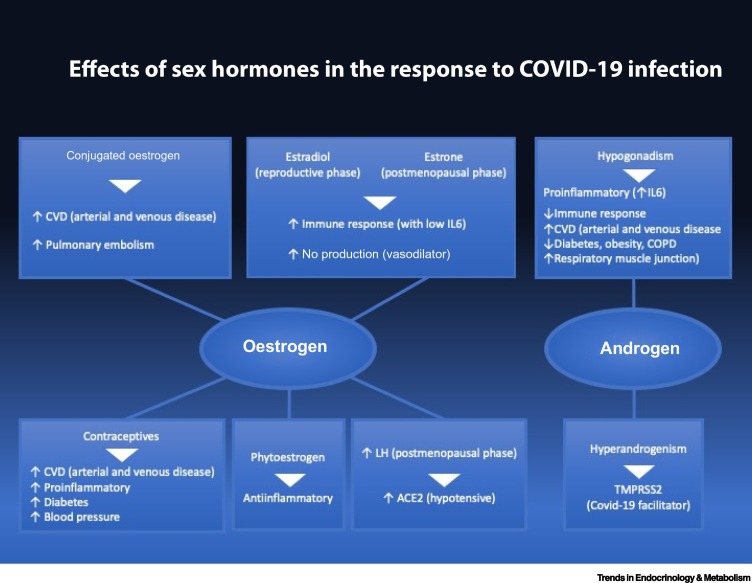
Several lines of evidence point to specific roles that oestrogen and androgen seem to have in the response to viral infections (Figure 1 ). Even though published data point to a higher severity in males than in females, no sex- or gender-specific recommendations have been reported regarding the management of COVID-19 patients [78]. Based on the considerations made in this paper, we would like to suggest that sex-specific measures be considered in the comprehensive management plan for COVID-19 (Table 1 ).
Figure 1.
Effects of Sex Hormones in the Response to Coronavirus Disease 2019 Infection.
Table 1.
Potential Dimorphic Approach in the Management of COVID-19 Patients.
| Therapeutic approaches |
|---|
| Males |
| Hypogonadic patients under treatment with androgens should be evaluated for the right dosage, avoiding the proinflammatory response of hypogonadism and the increased VTE typical of hypergonadism. |
| Men in therapy with anti-hormonal treatment for prostate cancer should be carefully evaluated |
| Females |
| Contraceptive pills should be stopped |
| Use of MRT in postmenopausal women should be continued, giving the preference to transcutaneous administration of E2 |
| Women on therapy with aromatase inhibitors should be put under active surveillance |
Special attention should be paid to patients who take hormonal therapy, like hypogonadal men, women under therapy with contraceptives, postmenopausal women taking menopausal hormonal therapy, and cancer patients under treatment with inhibitors of sex hormones synthesis and release.
In hypogonadal men testosterone should be administered at the right dose, in order to avoid the increased proinflammatory cytokines observed in hypogonadism [12,79] and the increased VTE risk seen in patients treated for hypogonadism (particularly in those with Klinefelter syndrome). If testosterone levels are high for age, therapeutic and prophylactic potential of drugs that temporarily target androgen activity, such as androgen receptor inhibitors, steroidogenesis inhibitors, and 5-α reductase inhibitors, should be tested clinically [80]. Men on therapy with sex hormone blockers for prostate cancer should be put under active surveillance.
Use of hormonal therapy with oestradiol in postmenopausal women should be continued (https://clinicaltrials.gov.ct2/show/NTC04359329), while conjugated oestrogen or contraceptives should be stopped. Women on therapy with aromatase inhibitors should be put under active surveillance.
Maintaining vitamin D treatment in persons already diagnosed with hypovitaminosis D is recommended [81,82], and supplementation with vitamin D of elderly comorbid persons in home confinement [31] should be considered. The issue of universal supplementation with vitamin D due to high risk of complicated COVID-19 infection in Italy or in other countries, including those hospitalised, in and not in ICU, remains open [31].
The sex disparities observed in COVID-19 vulnerability support the need for understanding the role of sex in case fatality, leading to a future tailoring of treatment according to sex (see Outstanding Questions). Data should be disaggregated for age and sex. Moreover, analyses should include interventions and any confounding factor for COVID-19 infections. Basic and animal studies will help to understand the reciprocal regulation by sex hormones and on sex hormonal production of COVID-19. Until this information becomes available, we can only hypothesise possible causal mechanisms for sex dimorphism in COVID-19 infection.
Outstanding Questions.
COVID-19: Men seem to be dying more than women. Why?
Sex and age disaggregated data on epidemics have been requested by WHO. We expect more data in the future.
The availability of data disaggregated for noncommunicable diseases will help to develop a risk card for COVID-19.
Research in animals and cells will uncover effects of COVID-19 infection on sex hormones secretion.
Reciprocally sex hormones should be tested in vitro and in vivo for potential direct effects on viral infection and lethality.
In-depth knowledge of the mechanisms previously described will make possible a personalised and smart use of the most effective therapies.
Breakthroughs in searching for the best management of these patients are foreseen. An example is the recent identification of raloxifene, a selective oestrogen receptor regulator that preferentially binds to oestrogen receptor β, as one of the 40 molecules out of 400 000 tested capable of blocking virus replication in cells.
Alt-text: Outstanding Questions
Acknowledgements
This work was supported by the Fondazione Italiana Ricerca sulle Malattie dell’Osso (F.I.R.M.O.).
Glossary
- 3β-hydroxysteroid dehydrogenase-1
an enzyme belonging to the family of the oxidoreductases expressed in placenta and peripheral tissues and capable to contribute to the extragonadal androgen synthesis.
- Angiotensin-converting enzyme 2
an enzyme attached to the cell membrane in several vital organs and able to catalyse the hydrolysis of angiotensin II (a vasoconstrictor peptide) into angiotensin (1–7) ( a vasodilator peptide).
- Transmembrane protease serine 2
an androgen-dependent 492-amino-acid type II transmembrane serine protease expressed in the cell surface of normal and diseased tissues (including prostate and lung) and thus ideally located to regulate cell–cell and cell–matrix interactions.
References
- 1.Casimiro J.F., et al. Sex and inflammation in respiratory diseases: a clinical viewpoint. Biol. Sex Differ. 2013;4:16. doi: 10.1186/2042-6410-4-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen N., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grasselli G., et al. Network C-LI Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Palaiodimos L., et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108 doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jian-Min J., et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front. Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges do Nascimento I.J., et al. Novel coronavirus infection (COVID-19) in humans: a scoping review and meta-analysis. J. Clin. Med. 2020;9:941. doi: 10.3390/jcm9040941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholls J.M., et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karlberg J., et al. Do men have a higher case fatality rate of severe acute respiratory syndrome than women do? Am. J. Epidemiol. 2004;159:229–231. doi: 10.1093/aje/kwh056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zyl-Smith van, et al. Tobacco smoking and COVID-19 infection. Lancet Respir. Med. 2020;8:664–665. doi: 10.1016/S2213-2600(20)30239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu F.C., et al. European Male Aging Study Group. Hypothalamic-pituitary-testicular axis disruptions in older men are differentially linked to age and modifiable risk factors: the European Male Aging Study. J. Clin. Endocrinol. Metab. 2008;93:2737–2745. doi: 10.1210/jc.2007-1972. [DOI] [PubMed] [Google Scholar]
- 11.Wu F.C., et al. EMAS Group Identification of late-onset hypogonadism in middle-aged and elderly men. N. Engl. J. Med. 2010;363:123–135. doi: 10.1056/NEJMoa0911101. [DOI] [PubMed] [Google Scholar]
- 12.Balasubramanian V., Naing S. Hypogonadism in chronic obstructive pulmonary disease: incidence and effects. Curr. Opin. Pulm. Med. 2012;18:112–117. doi: 10.1097/MCP.0b013e32834feb37. [DOI] [PubMed] [Google Scholar]
- 13.Douglas G.C., et al. The novel angiotensin-converting enzyme (ACE) homolog, ACE2, is selectively expressed by adult Leydig cells of the testis. Endocrinology. 2004;145:4703–4711. doi: 10.1210/en.2004-0443. [DOI] [PubMed] [Google Scholar]
- 14.Maggio M., et al. The relationship between testosterone and molecular markers of inflammation in older men. J. Endocrinol. Investig. 2005;28:116–119. [PubMed] [Google Scholar]
- 15.Montanõ L.M., et al. Androgens are bronchoactive drugs that act by relaxing airway smooth muscle and preventing bronchospasm. J. Endocrinol. 2014;222:1–13. doi: 10.1530/JOE-14-0074. [DOI] [PubMed] [Google Scholar]
- 16.Mohan S.S., et al. Higher serum testosterone and dihydrotestosterone, but not oestradiol, are independently associated with favourable indices of lung function in community-dwelling men. Clin. Endocrinol. 2015;83:268–276. doi: 10.1111/cen.12738. [DOI] [PubMed] [Google Scholar]
- 17.Minkin M.J. Menopause: hormones, lifestyle, and optimizing aging. Obstet. Gynecol. Clin. North Am. 2019;46:501–514. doi: 10.1016/j.ogc.2019.04.008. [DOI] [PubMed] [Google Scholar]
- 18.Honorato-Sampaio K., et al. Evidence that angiotensin-(1–7) is an intermediate of gonadotrophin-induced oocyte maturation in the rat preovulatory follicle. Exp. Physiol. 2012;97:642–650. doi: 10.1113/expphysiol.2011.061960. [DOI] [PubMed] [Google Scholar]
- 19.Pozzilli P., Lenzi A. Testosterone, a key hormone in the context of COVID-19 pandemic. Metab. Clin. Exp. 2020;108 doi: 10.1016/j.metabol.2020.154252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharifi N., Ryan C.J. Androgen hazards with COVID-19. End. Rel. Cancer. 2020;27:E1–E3. doi: 10.1530/ERC-20-0133. [DOI] [PubMed] [Google Scholar]
- 22.Pinna P., et al. COPD-HF Study Group. Prevalence and management of COPD and heart failure comorbidity in the general practitioner setting. Respir. Med. 2017;131:1–5. doi: 10.1016/j.rmed.2017.07.059. [DOI] [PubMed] [Google Scholar]
- 23.Bruck K., et al. European CKD Burden Consortium. CKD prevalence varies across the European general population. J. Am. Soc. Nephrol. 2016;27:2135–2147. doi: 10.1681/ASN.2015050542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui S., et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J. Thromb. Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker R.F., et al. Association of testosterone therapy with risk of venous thromboembolism among men with and without hypogonadism. JAMA Intern. Med. 2019;18:190–197. doi: 10.1001/jamainternmed.2019.5135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang S., et al. Klinefelter syndrome and testosterone treatment: a national cohort study on thrombosis risk. Endocr. Connect. 2020;9:34–43. doi: 10.1530/EC-19-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bouillon R., et al. Skeletal and extraskeletal actions of vitamin D: current evidence and outstanding questions. Endocr. Rev. 2019;40:1109–1151. doi: 10.1210/er.2018-00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martineau A.R., et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356 doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christopher K.B. Vitamin D and critical illness outcomes. Curr. Opin. Crit. Care. 2016;22:332–338. doi: 10.1097/MCC.0000000000000328. [DOI] [PubMed] [Google Scholar]
- 30.Isaia G., et al. High prevalence of hypovitaminosis D in female type 2 diabetic population. Diabetes Care. 2001;24:1496. doi: 10.2337/diacare.24.8.1496. [DOI] [PubMed] [Google Scholar]
- 31.Formenti A.M., et al. Body mass index predicts resistance to active vitamin D in patients with hypoparathyroidism. Endocrine. 2019;66:699–700. doi: 10.1007/s12020-019-02105-6. [DOI] [PubMed] [Google Scholar]
- 32.Boccardi V., et al. Hypovitaminosis D: a disease marker in hospitalized very old persons at risk of malnutrition. Nutrients. 2019;11:128–138. doi: 10.3390/nu11010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giustina A., Formenti A.M. Rapid response to: Preventing a covid-19 pandemic. BMJ. 2020 doi: 10.1136/bmj.m810. Published online February 28, 2020. [DOI] [Google Scholar]
- 34.Orwoll E., et al. Vitamin D deficiency in older men. J. Clin. Endocrinol. Metab. 2009;94:1214–1222. doi: 10.1210/jc.2008-1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klein S.L., Flanagan K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016;16:626–638. doi: 10.1038/nri.2016.90. [DOI] [PubMed] [Google Scholar]
- 36.Klein S.L., et al. Mechanisms of sex disparities in influenza pathogenesis. J. Leukoc. Biol. 2012;92:67–73. doi: 10.1189/jlb.0811427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Belmonte R.L., et al. Sexual dimorphisms in innate immunity and responses to infection in Drosophila melanogaster. Front. Immunol. 2020;10:3075–3093. doi: 10.3389/fimmu.2019.03075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klein S.L., Roberts C.W., editors. Sex Hormones and Immunity to Infection. Springer-Verlag; 2010. [Google Scholar]
- 39.Jensen-Fangel S., et al. Gender differences in hospitalization rates for respiratory tract infections in Danish youth. Scand. J. Infect. Dis. 2004;36:31–36. doi: 10.1080/00365540310017618. [DOI] [PubMed] [Google Scholar]
- 40.Klein S.L. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci. Biobehav. Rev. 2020;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- 41.Schuurs A.H., Verheul H.A. Effects of gender and sex steroids on the immune response. J. Steroid Biochem. 1990;135:157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- 42.Roberts C.W., et al. Sex-associated hormones and immunity for protozoan parasites. Clin. Microbiol. Rev. 2001;14:476–488. doi: 10.1128/CMR.14.3.476-488.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ehlers R., et al. Assessment of vaccine candidates for persons aged 50 and older: a review. BMC Geriatr. 2013;13:32. doi: 10.1186/1471-2318-13-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meier A., et al. Sex differences in the Toll-like receptor-mediated response of plasmacytoid dendritic cells to HIV. Nat. Med. 2009;15:955–959. doi: 10.1038/nm.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pisitkun P., et al. Autoreactive B cell responses to RNA-related antigens d’urto TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 46.Da Silva J.A. Sex hormones, glucocorticoids and autoimmunity: facts and hypotheses. Ann. Rheum. Dis. 1995;54:6–16. doi: 10.1136/ard.54.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cook I.F. Sexual dimorphism of humoral immunity with human vaccines. Vaccine. 2008;26:3551–3555. doi: 10.1016/j.vaccine.2008.04.054. [DOI] [PubMed] [Google Scholar]
- 48.Brabin L. Interactions of the female hormonal environment, susceptibility to viral infections, and disease progression. AIDS Patient Care STDs. 2002;16:211–221. doi: 10.1089/10872910252972267. [DOI] [PubMed] [Google Scholar]
- 49.Kamada M., et al. B cell subsets in postmenopausal women and the effect of hormone replacement therapy. Maturitas. 2001;37:173–179. doi: 10.1016/s0378-5122(00)00180-8. [DOI] [PubMed] [Google Scholar]
- 50.Conti P., Younes A. Coronavirus CoV-19/SARS-CoV-2 affects women less than men: clinical response to viral infection. J. Biol. Regul. Homeost. Agents. 2020;7:34. doi: 10.23812/Editorial-Conti-3. [DOI] [PubMed] [Google Scholar]
- 51.Mehta P., et al. on behalf of the HLH Across Speciality Collaboration, UK COVID-19: consider cytokine storm syndromes and immunosuppressive. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marquez E.J., et al. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020;11:751. doi: 10.1038/s41467-020-14396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mehra M.R., et al. Cardiovascular disease, drug therapy, and mortality in Covid-19. N. Engl. J. Med. 2020;382:972–973. doi: 10.1056/NEJMoa2007621. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Long B., et al. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020;38:1504–1507. doi: 10.1016/j.ajem.2020.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Muller M. Endogenous sex hormones and cardiovascular disease in men. J. Clin. Endocrinol. Metab. 2003;88:5076–5086. doi: 10.1210/jc.2003-030611. [DOI] [PubMed] [Google Scholar]
- 56.Scarabin P.-Y. Endogenous sex hormones and cardiovascular disease in postmenopausal women: new but conflicting data. Am. Transl. Med. 2018;6:448–457. doi: 10.21037/atm.2018.11.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Towfighi A., et al. A midlife stroke surge among women in the United States. Neurology. 2007;69:1898–1904. doi: 10.1212/01.wnl.0000268491.89956.c2. [DOI] [PubMed] [Google Scholar]
- 58.Hendrix S.L., et al. WHI Investigators. Effects of conjugated equine estrogen and stroke in the Women’s Health Initiative. Circulation. 2006;113:2425–2434. doi: 10.1161/CIRCULATIONAHA.105.594077. [DOI] [PubMed] [Google Scholar]
- 59.Meyer M.R., Barton M. Estrogens and coronary artery disease: new clinical perspectives. Adv. Pharmacol. 2016;77:307–360. doi: 10.1016/bs.apha.2016.05.003. [DOI] [PubMed] [Google Scholar]
- 60.Xing D., et al. Estrogen and mechanisms of vascular protection. Atheroscler. Thromb. Vasc. Biol. 2009;29:289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barton M., et al. Vascular protection with estrogen. In vitro and in vivo effects – mechanisms and clinical implication. Praxis. 1997;86:129–137. [PubMed] [Google Scholar]
- 62.Lin Z.-H., et al. The effects of estradiol on inflammatory and endothelial dysfunction in rats with preeclampsia. Int. J. Mol. Med. 2020;45:825–835. doi: 10.3892/ijmm.2020.4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Guo X. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J. Biol. Chem. 2005;280:19704–19710. doi: 10.1074/jbc.M501244200. [DOI] [PubMed] [Google Scholar]
- 64.Fukuma N., et al. Estrogen receptor-alpha non nuclear signaling confers cardio protection and is essential to cGMP-PDE5 inhibition efficacy. JACC Basic Transl. Sci. 2020;5:282–295. doi: 10.1016/j.jacbts.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Keyaerts E., et al. Inhibition of SARS-coronavirus infection in vitro by S-nitroso-N-acetylpenicillamine, a nitric oxide donor compound. Int. J. Infect. Dis. 2004;8:223–226. doi: 10.1016/j.ijid.2004.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen L., et al. Inhalation of nitric oxide in the treatment of severe acute respiratory syndrome: a rescue trial in Beijing. Clin. Infect. Dis. 2004;39:1531–1535. doi: 10.1086/425357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gialeraki A., et al. Oral contraceptives and HRT risk of thrombosis. Clin. Appl. Thromb. Hemost. 2018;24:217–225. doi: 10.1177/1076029616683802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dupuis M., et al. Effects of estrogens on platelets and megakaryocytes. Int. J. Mol. Sci. 2019;20:3111–3134. doi: 10.3390/ijms20123111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rovinski D., et al. Risk of venous thromboembolism events in postmenopausal using oral versus non-oral hormone therapy: a systematic review and meta-analysis. Thromb. Res. 2018;168:P83–P95. doi: 10.1016/j.thromres.2018.06.014. [DOI] [PubMed] [Google Scholar]
- 70.Darabi P., et al. Therapeutic potentials of the natural plant flavonoid apigenin In polycystic ovary syndrome in rat model: via modulation of pro-inflammatory cytokines and antioxidant activity. Gynecol. Endocrinol. 2019;30:1–6. doi: 10.1080/09513590.2019.1706084. [DOI] [PubMed] [Google Scholar]
- 71.Meng Z.J., et al. Sodium tanghi one HA sulfonate attenuates cardiac dysfunction and improves survival of rats with cecal ligation and puncture-induced sepsis. J. Nat. Med. 2018;72:846–855. doi: 10.1016/S1875-5364(18)30126-2. [DOI] [PubMed] [Google Scholar]
- 72.Monteil V., et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905–913. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1056. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Walker V.R., Korach K.S. Estrogen receptor knockout mice as a model for endocrine research. ILAR J. 2004;45:455–461. doi: 10.1093/ilar.45.4.455. [DOI] [PubMed] [Google Scholar]
- 75.Nwaru B.I., et al. Exogenous sex steroid hormones and asthma in females: protocol for a population-based retrospective cohort study using a UK primary care database. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2017-020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zazara D.E., et al. A prenatally disrupted airway epithelium orchestrates the fetal origin of asthma in mice. J. Allergy Clin. Immunol. 2020;145:1641–1654. doi: 10.1016/j.jaci.2020.01.050. [DOI] [PubMed] [Google Scholar]
- 77.Mikkonen L., et al. Androgen receptor and androgen-dependent gene expression in lung. Mol. Cell. Endocrinol. 2010;317:14–24. doi: 10.1016/j.mce.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 78.Walter L.A., McGregor A.J. Sex- and gender-specific observations and implications for COVID-19. West. J. Emerg. Med. 2020;21:507–509. doi: 10.5811/westjem.2020.4.47536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mohamad N.V., et al. The relationship between circulating testosterone and inflammatory cytokines in men. Aging Male. 2019;22:129–140. doi: 10.1080/13685538.2018.1482487. [DOI] [PubMed] [Google Scholar]
- 80.Wambier C.G., Goren A. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is likely to be androgen mediated. J. Am. Acad. Dermatol. 2020;83:308–309. doi: 10.1016/j.jaad.2020.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Puig-Domingo M., et al. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marazuela M., et al. Endocrine and metabolic aspects of the COVID-19 pandemic. Rev. Endocr. Metab. Disord. 2020;21:495–507. doi: 10.1007/s11154-020-09569-2. [DOI] [PMC free article] [PubMed] [Google Scholar]