Abstract
Background and aim
Vitamin D deficiency is a pandemic disorder affecting over 1 billion of subjects worldwide. Calcitriol (1,25(OH)2D) represents the perpetrator of the several systemic effects of vitamin D, including the anti-inflammatory, antithrombotic and anti-atherosclerotic actions, potentially preventing acute cardiovascular ischemic events. Variability in the transformation of vitamin D into 1,25(OH)2D has been suggested to modulate its cardioprotective benefits, however, the determinants of the levels of calcitriol and their impact on the cardiovascular risk have been seldom addressed and were, therefore, the aim of the present study.
Methods and results
A consecutive cohort of patients undergoing coronary angiography for acute coronary syndrome (ACS) were included. The levels of 25 and 1,25(OH)2 D were assessed at admission by chemiluminescence immunoassay kit LIAISON® Vitamin D assay (Diasorin Inc) and LIAISON® XL. Hypovitaminosis D was defined as 25(OH)D < 10 ng/ml, whereas calcitriol deficiency as 1,25(OH)2D < 19.9 pg/ml.
We included in our study 228 patients, divided according to median values of 1,25(OH)2D (<or ≥ 41.5 pg/ml). Lower calcitriol was associated with age (p = 0.005), diabetes (p = 0.013), renal failure (p < 0.0001), use of diuretics (p = 0.007), platelets (p = 0.019), WBC (p = 0.032), 25(0H)D (p = 0,046), higher creatinine (p = 0.011), and worse glycaemic and lipid profile.
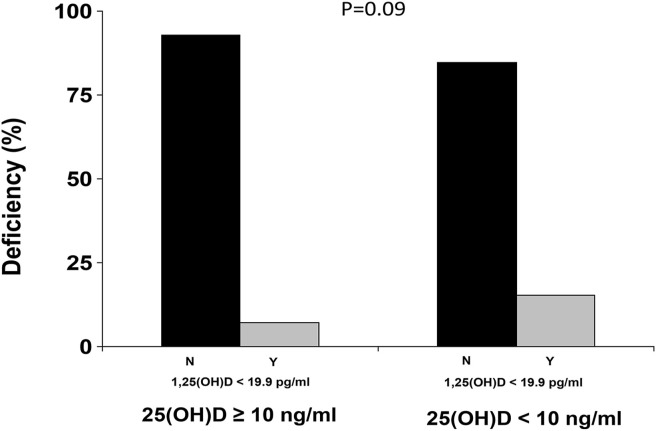
A total of 53 patients (23.2%) had hypovitaminosis D, whereas 19 (9.1%) displayed calcitriol deficiency (15.1% among patients with hypovitaminosis D and 7.1% among patients with normal Vitamin D levels, p = 0.09).
The independent predictors of 1,25(OH)2D above the median were renal failure (OR[95%CI] = 0.242[0.095–0.617], p = 0.003) and level of vitamin D (OR[95%CI] = 1.057[1.018–1.098], p = 0.004).
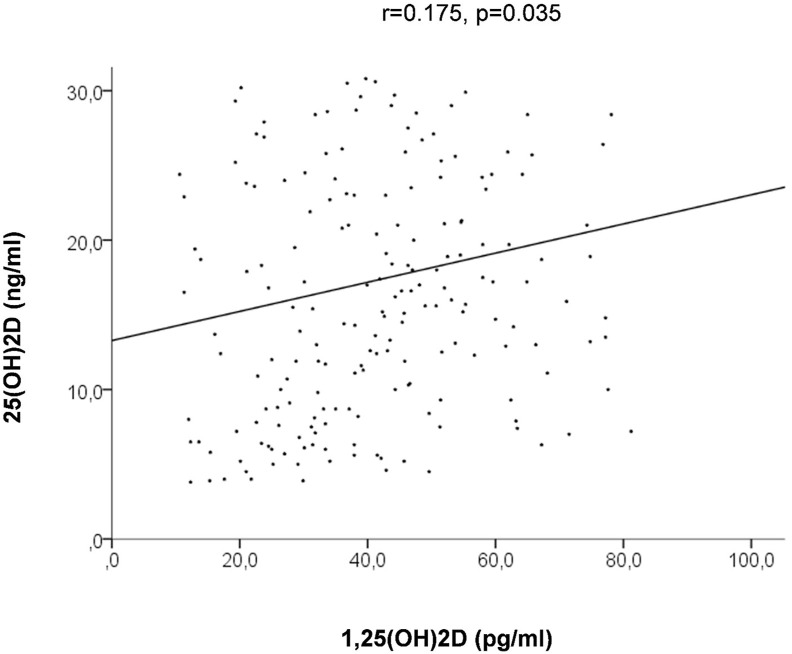
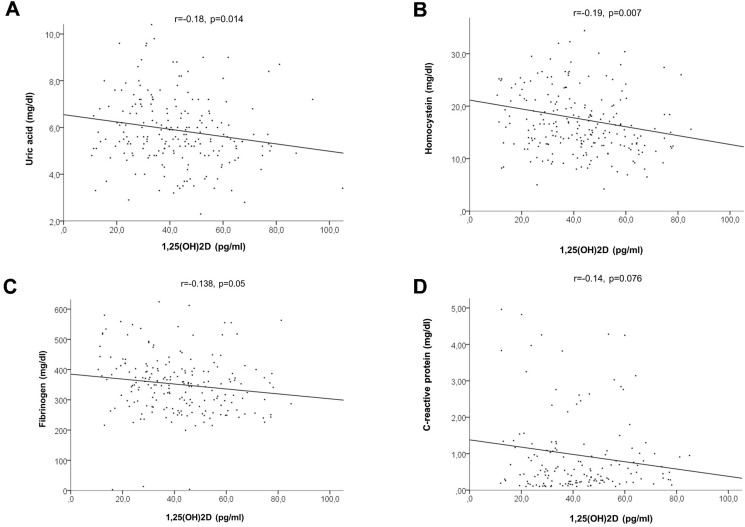
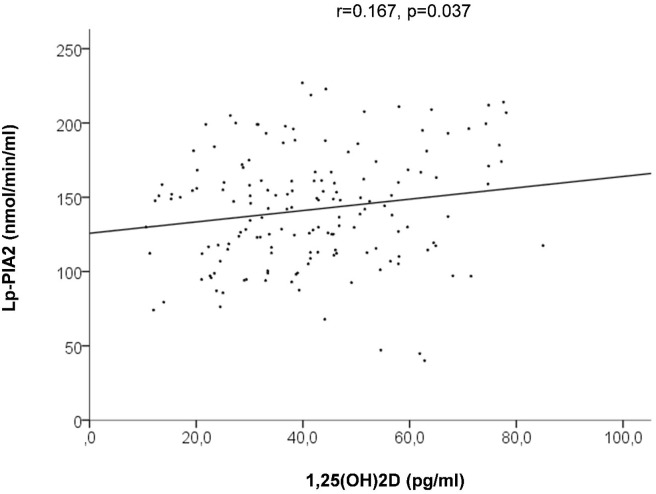
Calcitriol levels, in fact, directly related with the levels of vitamin D (r = 0.175, p = 0.035), whereas an inverse linear relationship was observed with major inflammatory and metabolic markers of cardiovascular risk (C-reactive protein: r = −0.14, p = 0.076; uric acid: r = −0.18, p = 0.014; homocysteine: r = −0.19, p = 0.007; fibrinogen: r = −0.138, p = 0.05) and Lp-PLA2 (r = −0.167, p = 0.037), but not for leukocytes.
Conclusion
The present study shows that among ACS patients, calcitriol deficiency is frequent and can occur even among patients with adequate vitamin D levels. We identified renal failure and vitamin D levels as independent predictors of 1,25(OH)2D deficiency. Furthermore, we found a significant inverse relationship of calcitriol with inflammatory and metabolic biomarkers, suggesting a potential more relevant and accurate role of calcitriol, as compared to cholecalciferol, in the prediction of cardiovascular risk. Future trials should certainly investigate the potential role of calcitriol administration in the setting of acute coronary syndromes as much as in other inflammatory disorders, such as the SARS-CoV2 infection.
Keywords: Vitamin D, Calcitriol, Inflammation, Biomarkers, Acute coronary syndrome
Introduction
Vitamin D deficiency represents a rising social problem, affecting a huge part of the population of every age and ethnicity and even over 50% of healthy subjects [1].
In the last years, evidence has emerged for several non-calcium dependent hormonal effects of vitamin D, linking its deficiency to increased mortality and to various chronic pathological conditions, such as cancer, neurological disorders, as well as autoimmune and inflammatory diseases, hypertension, diabetes and atherothrombotic cardiovascular events [2,3].
However, vitamin D supplementation for the prevention of cardiovascular risk has not provided so far clear evidence of benefit, both due to the lack of well conducted dedicated trials and for the potential interference of several conditions that could modulate the response to the treatment [4,5]. In particular, the systemic effects of vitamin D are mediated by the interaction of calcitriol (1,25(OH)2D) with vitamin D receptor (VDR), therefore genetic variants of the VDR, as much as factors regulating the transformation of vitamin D into its active hydroxylated hormone, calcitriol, have been addressed for defining the interindividual variability of the effect of vitamin D [6,7]. Even though the evaluation of the precursor (25(OH)D) is generally preferred above the assessment of calcitriol levels, for its longer plasma half-life, previous studies have instead associated the levels of 1,25(OH)2D to cardiovascular disease and worse prognosis, being more subject to transient variations in the context of acute events, as potentially in the settings of an acute coronary syndrome (ACS) [8,9]. In addition, although the efficiency of calcitriol hydroxylation has been strictly related to renal function [10], representing the primary site for its transformation, extra-renal production of 1,25(OH)2D has also been documented [11]. In fact, 1α-hydroxylase enzyme has been identified in several tissues and districts, including the cardiovascular system, and its exact role is largely undefined, especially in patients without severe renal disease.
Therefore, the aim of the present study was to evaluate the determinants of calcitriol levels in a high-cardiovascular risk population of ACS patients and to evaluate their relationship with inflammatory biomarkers.
Methods
We included in our study a consecutive cohort of patients admitted between November 2016 and January 2018 at the Azienda Ospedaliera-Universitaria, “Maggiore dellaCarità”, Novara, Italy and presenting the following inclusion criteria: 1) acute coronary syndrome (ACS) treated with coronary angiography and percutaneous coronary intervention; 2) availability of 25(OH)D and 1,25(OH)D levels, 3) written informed consent before angiography. No exclusion criteria were applied.
ACS (Unstable Angina, NSTEMI or ST-segment elevation myocardial infarction), was defined by the presence of chest pain at rest with or without cardiac biomarkers elevation > ULN (respectively 0,04 μg/l for Troponin I and 5,00 μg/l for CK-MB) or electrocardiographic modifications (either ST-segment depression or elevation ≥2 mm in at least 2 contiguous leads or new LBB onset or T waves change).
The protocol was approved by our local Ethical Committee and is in accordance to the Declaration of Helsinki statements. All demographic and clinical data were prospectively collected in a dedicated database. Hypertension was defined as a systolic blood pressure (BP) > 140 mmHg and/or a diastolic BP > 90 mmHg or on-treatment with antihypertensive medications. The diagnosis of diabetes was based on previous history of diabetes treated with or without drugs, fasting glycaemia >126 mg/dl or glycosylated haemoglobin >6.5%. Hypercholesterolemia was defined as previous history of hypercholesterolemia, chronic treatment with any cholesterol-lowering agent at admission or fasting total cholesterol >200 mg/dl.
Biochemical measurements
Blood samples were drawn at admission from patients undergoing elective (following a fasting period of 12 h) or urgent coronary angiography. Glucose, creatinine, uric acid levels, blood cells count and lipid profile were determined by standard methods, as previously described [12].
25-hydroxyvitamin D and 1,25(OH)2D measurements were performed by chemiluminescence method through LIAISON® Vitamin D assay (Diasorin Inc) and LIAISON® XL respectively. The normal range for 25-OH D3 levels in our laboratory is from 30 to 100 ng/ml, according to literature reference [13,14]. Hypovitaminosis D was defined, according to the US Endocrine Society guideline (13), as 25-hydroxyvitamin D < 10 ng/ml. Calcitriol deficiency was defined for levels <19.9 pg/ml according to the manufacturer.
Lipoprotein-associated Phospholipase A2 (Lp-PLA2) was analyzed on ADVIA® 1800 Clinical Chemistry Analyzer (Siemens Healthcare Diagnostics). For the quantitative determination of Lp-PLA2 activity, the Diazyme Lp-PLA2 Activity Assay was utilized (Diazyme Laboratories, CA 92064, USA). Diazyme'sLp- PLA2 Activity Assay is an enzymatic assay. Lp-PLA2 in a sample hydrolyzes the acetyl group at the sn-2 position of phospholipids, 1- myristoyl-2-(4-nitrophenylsuccinyl)-sn-glycero-3-phosphocholine (MNP) to generate 4-nitrophenyl group, a colorful product, which can be monitored spectrophotometrically at 405 nm. The activity of Lp-PLA2 in the sample is proportional to the absorbance increase. The instrument calculates the Lp- PLA2 activity of a sample by using the Diazyme Lp-PLA2 Activity Calibrator Set (REF DZ331A-CAL) to generate a calibration curve [15].
Statistical analysis
Statistical analysis was performed using SPSS 22.0 statistical package. Continuous data were expressed as mean ± SD and categorical data as percentage. Analysis of variance and the chi-square test were used for continuous and categorical variables, respectively. Patients were grouped according to median values of 1,25(OH)2D. Forward conditional logistic regression analysis was performed to evaluate the independent predictors of calcitriol deficiency among variables displaying a significant association (p < 0.05) at baseline. Linear regression analysis was applied for evaluating the relationship of calcitriol with other continuous laboratory parameters. Results were considered statistically significant for a two-tailed p < 0.05.
Results
We included in our study 228 patients, divided according to median values of 1,25(OH)2D (<or ≥ 41.5 pg/ml).
Major clinical and demographic features of the included population are displayed in Table 1 . Lower calcitriol was associated to age (p = 0.005), diabetes (p = 0.013), renal failure (p < 0.0001), use of diuretics (p = 0.007), platelets (p = 0.019), WBC (p = 0.032), 25(0H)D (p = 0.046), higher creatinine (p = 0.011), and worse glycaemic and lipid profile.
Table 1.
Clinical characteristics according to median values of 1,25(OH)2D.
| Baseline demographic and clinical characteristics | <41.5 pg/ml (n = 111) | ≥41.5 pg/ml (n = 117) | P value |
|---|---|---|---|
| Age (mean ± SD) | 68.9 ± 12,8 | 64.4 ± 11,3 | 0.005 |
| Weight (Kg±SD) | 78 ± 17 | 74 ± 14.3 | 0.14 |
| Male Sex (%) | 76,6% | 69.2 | 0.24 |
| BMI (mean ± SD) | 27.0 ± 4,9 | 26.6 ± 4.2 | 0.54 |
| Hypercholesterolemia (%) | 48.3 | 59.3 | 0.11 |
| Diabetes mellitus (%) | 46.2 | 27.7 | 0.013 |
| Renal failure (%) | 29.3 | 9.3 | <0.001 |
| Active smokers (%) | 28.4 | 26.3 | 0.62 |
| Hypertension (%) | 73.3 | 61.0 | 0.05 |
| History of MI (%) | 21.6 | 19.8 | 0.87 |
| Previous PCI (%) | 25.0 | 22.2 | 0.65 |
| Previous CABG (%) | 8.6 | 9.3 | 1.00 |
| ACS type (%) | 0.14 | ||
| NSTEMI/UA (%) | 65.3 | 77.8 | |
| STEMI (%) | 34.7 | 22.2 | |
| CAD (%) | |||
| Left Main disease or 3-vessel CAD (%) | 52.5 | 44.6 | 0.46 |
| Concomitant medications | |||
| ACE inhibitors (%) | 22.1 | 23.7 | 0.8 |
| ARB (%) | 28.1 | 17.9 | 0.085 |
| Beta blockers (%) | 44.7 | 40.7 | 0.6 |
| Nitrates (%) | 18.3 | 13.7 | 0.37 |
| Statins (%) | 36.5 | 40.2 | 0.59 |
| ASA (%) | 45.2 | 42.7 | 0.79 |
| Clopidogrel (%) | 10.6 | 6.0 | 0.24 |
| Calcium antagonists (%) | 28.1 | 17.8 | 0.085 |
| Diuretics (%) | 33.3 | 18.1 | 0.007 |
| Vitamin D (%) | 3.6 | 6.0 | 0.54 |
| Antidiabetic therapy | 0.02 | ||
| Oral hypoglycemic drugs (%) | 13.6 | 5.2 | |
| Insulin (%) | 14.4 | 4.3 | |
| Biochemistry parameters | |||
| Platelets (10ˆ6/ml; mean ± SD) | 222.82 ± 69.7 | 244.24 ± 68.9 | 0.019 |
| Haemoglobin (g/dl) | 13.3 ± 2.1 | 13.8 ± 3.1 | 0.16 |
| WBC (10ˆ3/ml; mean ± SD) | 10.2 ± 4.2 | 9.2 ± 2.8 | 0.032 |
| HDL cholesterol (mg/dL) | 43.9 ± 19.5 | 47.4 ± 20.7 | 0.2 |
| LDL cholesterol (mg/dl) | 98.3 ± 34 | 112.3 ± 40 | 0.03 |
| Glycaemia (mg/dL) | 135.3 ± 61 | 114.2 ± 29.8 | 0.01 |
| HbA1c (%) | 6.2 ± 1.1 | 5.9 ± 0.9 | 0.068 |
| 25(OH)D (ng/ml) | 16.5 ± 10.3 | 19.1 ± 8.8 | 0.046 |
| Creatinine (mg/dL) | 1.04 ± 0.6 | 0.86 ± 0.497 | 0.011 |
| C reactive protein (mg/dL) | 1.2 ± 2.2 | 0.7 ± 1.4 | 0.09 |
| Uric acid (mg/dl) | 6.1 ± 1.6 | 5.7 ± 1.6 | 0.056 |
CAD = Coronary Artery Disease; MI = Myocardial Infarction; PCI = Percutaneous Coronary Interventions; CABG = Coronary Artery Bypass Grafting; STEMI = ST-Elevation Myocardial Infarction; ACS = Acute Coronary Syndrome; ACE = Angiotensin Converting Enzyme; ARB = Angiotensin Receptor Blockers.
A total of 53 patients (23.2%) had hypovitaminosis D, whereas 19 (9.1%) displayed calcitriol < 19.9 pg/ml (15.1% among patients with hypovitaminosis D and 7.1% among patients with normal cholecalciferol levels, p = 0.09), as shown in Fig. 1 .
Figure 1.
Prevalence of 25 and 1,25(OH)2D in included population.
The independent predictors of 1,25(OH)2D above the median were renal failure (OR[95%CI] = 0.242[0.095–0.617], p = 0.003) and vitamin D levels (OR[95%CI] = 1.057[1.018–1.098], p = 0.004).
Calcitriol levels, in fact, directly related with the levels of 25(OH)2D (r = 0.175, p = 0.035, Fig. 2 ) whereas an inverse linear relationship was observed with major inflammatory and metabolic markers of cardiovascular risk (C-reactive protein: r = −0.14, p = 0.076; uric acid: r = −0.18, p = 0.014; homocysteine: r = −0.19, p = 0.007; fibrinogen: r = −0.138, p = 0.05), as shown in Fig. 3 A, B, C and D respectively, and with Lp-PLA2 (r = −0.167, p = 0.037, Fig. 4 ).
Figure 2.
Linear relationship between 25 and 1,25(OH)2D.
Figure 3.
Linear relationship between 1,25(OH)2D, uric acid (A, upper left), homocystein (B, upper right), fibrinogen (C, lower left) and C-reactive protein (D, lower right).
Figure 4.
Linear relationship between 1,25(OH)2D and lipoprotein-associated phospholipase A2 (Lp-PlA2).
No relationship was observed for calcitriol with white blood cells count (r = −0.08; p = 0.29), neutrophils (r = 0.06, p = 0.37) and lymphocytes (r = 0.008, p = 0.91).
Discussion
This is the first study conducted so far to evaluate the clinical determinants of calcitriol levels and their relationship with inflammatory and cardiovascular risk biomarkers among ACS patients undergoing early invasive management. Even though we observed a significant independent association of 1,2(OH)2D with vitamin D levels, calcitriol deficiency was much less frequent as compared to hypovitaminosis D, suggesting a potential “reserve” of vitamin D activation despite a deficiency of the substrate. Moreover, the prevalence of calcitriol deficiency was non-significantly lower in patients with hypovitaminosis D, therefore suggesting the relevance of factors modulating the transformation of calcitriol and the importance of the direct dosing of the activated hormone rather than vitamin D. In addition, despite the inclusion of high-risk ACS population, calcitriol related with several parameters mirroring the pro-inflammatory status and the cardiovascular risk, therefore suggesting a more complex disease and potentially worse long-term outcomes.
Even though calcitriol represents the perpetrator of the effects of vitamin D, few studies have so far addressed the impact of its deficiency on the maintenance of the systemic homeostasis and on the pathogenesis of the several disorders linked to hypovitaminosis D [16,17]. In fact, the relatively shorter plasmatic half-life of 1,25(OH)2D, only 12–36 h, in the human circulation as compared to its precursor has often been considered a barrier for its clinical application [18], therefore accounting for the large amount of literature providing evidence for the relationship of vitamin D and cardiovascular disorders [[19], [20], [21], [22]].
However, 25(OH)D and 1,25(OH)2D cannot be considered equivalent measurements, since several genetic and metabolic factors, as much as comorbidities, can prevent its transformation into the active hormone [23]. Indeed, circulating calcitriol can be considered in a relatively steady state in healthy subjects [24] and, based on the development of more accurate methods for its measurement, raising interest has been addressed towards its evaluation, and especially after the inconclusive results of the studies with 25(OH)D supplementation.
In fact, in a recent study on dialysis patients no change in the intestinal absorption of Ca++ was obtained after 3months of 20,000 IU/week of cholecalciferol therapy before the normalisation of 25D and improvement of 1,25D levels [25]. In further studies, no effect on bone metabolism parameters was evident following cholecalciferol supplementation of dialysis patients [26].
A similar superiority of calcitriol above vitamin D has been documented in the cardioprotective, pleiotropic effect. In a recent study, Zitterman et al. [24] documented among over 500 patients with heart failure that lower calcitriol was associated with a higher rate of coronary artery disease and increased mid-term mortality. Moreover, in vitro studies have shown that the active form of vitamin D induces a complex dual upregulation of endothelin and nitric oxide in cultured endothelial cells, reduces the expression of the angiotensin-1 receptor, improving endothelial function and preventing reactive oxygen species overproduction and downregulates hyperinsulinism and insulin resistance, lowering also the concentrations of various inflammatory markers including TNF-a, CRP and IL-6 in an overweight animal model [[27], [28], [29]].
In addition, calcitriol has been shown to raise the levels of IL-12, that is relevant to macrophage activity and atherogenesis [30] and to modulate the coagulation cascade, increasing thrombomodulin and down-regulating tissue factor [31]. However, its role in coronary thrombosis has never been assessed.
Indeed, it could be hypothesized that the direct assessment of the levels of calcitriol could provide a more strict correlation with the pathogenesis of atherosclerotic lesions and that their transient variations could favour plaques rupture, which represents the well-established causal mechanisms for acute coronary syndromes. Nevertheless, such hypotheses have never been considered, so far, in a dedicated clinical study.
We firstly report the prevalence and predictors of calcitriol deficiency in a consecutive cohort of ACS patients undergoing early invasive management. We documented that lower calcitriol levels were associated with main established cardiovascular risk factors, and in particular with hypertension, diabetes and worse lipid profile, but also with renal function, and with a non-significant trend for presentation with ST-segment elevation MI.
However, at multivariate analysis, only renal failure and baseline levels of 25(OH)D emerged as independent predictors of calcitriol inadequate levels, whereas no impact was played by age or gender, in accordance to the previous reports by Levin et al. [32] in an unselected cohort of 1814 subjects. However, in this study, 1,25(OH)2D levels were related to diabetic status and parameters of renal function, as glomerular filtration and albumin-to-creatinine levels, although they recruited a large proportion of patients with kidney disease. In addition, oppositely to our results, in the study by Levin et al. the prevalence of calcitriol deficiency was much higher than cholecalciferol deficiency. However, in the above study [32], about 35–40% of the study cohort was receiving a multivitamin supplementation, thus potentially accounting for the lower prevalence of hypovitaminosis D, as compared to our data.
In fact, similarly to our conclusion, in a previous study over 500 patients with different degrees of renal dysfunction (from no chronic kidney disease-CKD to haemodialysis), Pasquali et al. [33] documented that both vitamin D levels, renal function and parathyroid hormone could modulate the efficiency of the hydroxylation of 25(OH)D to calcitriol. In fact, the lowest absolute values were observed in haemodyalisis patients without cholecalciferol deficiency, as these patients cannot be expected to substantially increase circulating levels of calcitriol from damaged kidneys simply because of substrate availability. In comparison, Vitamin D-replete CKD patients exhibited a hydroxylation efficiency that, on average, did not significantly differ from haemodyalisis patients, suggesting that the system is blunted in spite of some residual renal function.
In our study, the rate of patients with inadequate calcitriol levels was less than 50% of the patients displaying severe cholecalciferol deficiency, suggesting the possibility to recruit a synthetic “reserve” for preserving the levels and function of the active hormone. In fact, the efficiency of 1,25(OH)2 D hydroxylation, however, can be rapidly increased by paracrine and auto-regulatory mechanisms, stimulating a local activity of the 1-α-hydroxylase in presence of high PTH, low calcium or inflammatory factors and cytokines. In fact, Pasquali et al. [33] showed in subjects with no CKD, that the efficiency of calcitriol production was linearly related to the levels of substrate, but became an exponential increment when 25(OH)D levels declined below 20 ng/ml. On the contrary, such reserve was not observed in presence of renal dysfunction, confirming that kidney activity and vitamin D levels represent the principal determinants of calcitriol production.
Indeed, vitamin D transformation is a tubular process, therefore being potentially less related with the levels of renal clearance and glomerular filtration rate (GFR). Consistently, in the previous report of Prince et al. [34] and Pasquali et al. [33] vitamin D hydroxylation was maintained in moderate renal dysfunction and not completely abolished in hemodialysis patients, furthermore pointing at a potential role of extra-renal production of calcitriol.
Such paracrine activation of vitamin D could play an intriguing role in preventing the acute inflammatory processes leading to the instabilization and rupture of a plaque and therefore to the onset of acute coronary syndromes. In fact, we observed a direct linear correlation of calcitriol with several biomarkers of the inflammatory and thrombotic process, and the levels of Lp-PlA2 even in a selected population of high-risk ACS patients, where an elevation of such parameters could be expected in all patients. Nevertheless, such hypothesis certainly needs further confirmations in larger dedicated studies, aiming at the exact definition of the pathophysiological mechanisms and the prognostic relevance of our observations, potentially offering new targets for the treatment and risk stratification of ACS patients.
Due to the high prevalence of low calcitriol levels, our findings certainly pave the way for future investigations on the potential role of acute calcitriol supplementation in the setting of ACS or in special acute inflammatory settings, including those patients suffering from acute pulmonary inflammatory disease, especially in light of recent studies showing that the COVID-19 is associated with panvascular inflammation [35,36], rather than an isolated pulmonary disease, that may strongly condition the survival of these patients.
Study limitations
The first limitation to our study is the relatively small sample size, with subsequent low statistical power. In fact, our study represents the first exploratory investigation in ACS. Therefore, in the absence of specific references, we could not appropriately calculate a priori the sample size.
Indeed, the differential distribution of cardiovascular risk factors could have partially represented a confounder, and especially for diabetes. In fact, diabetic status and glycaemic control could have impacted on vitamin D levels and activation, but also on inflammation markers. However, we could not evaluate the duration of diabetes on the impact of the different oral hypoglycaemic drugs.
Neither, we assessed the levels of parathyroid hormone or cytokines such as TNF alpha and IL6, that would have given more insight about the modulation of inflammation by vitamin D and its metabolites in patients with ACS.
Moreover, we restricted our analysis to higher-risk ACS patients, in absence of a control population. Nevertheless, such recruitment was planned on purpose, since no data have been so far reported for calcitriol in an acute cardiovascular setting, representing on the contrary a subset of patients that could achieve the greatest benefits from the restoration of the cardioprotective effects of vitamin D.
Finally, we did not collect follow-up data, especially in patients undergoing coronary angioplasty, and thus we cannot exclude an impact of 1,25(OH)2D of the progression of CAD, recurrent cardiovascular events and the risk of adverse events after PCI.
Conclusions
The present study shows that among ACS patients, calcitriol deficiency is frequent and can occur even among patients with adequate vitamin D levels. We identified renal failure and vitamin D levels as independent predictors of 1,25(OH)2D deficiency. We observed a significant inverse relationship of calcitriol with inflammatory and metabolic biomarkers, therefore pointing at a potential more relevant and accurate role of calcitriol, as compared to vitamin D in the prediction of cardiovascular risk. Future trials should certainly investigate the potential role of acute calcitriol administration in the setting of acute coronary syndromes as much as in other inflammatory disorders.
Data availability statement
No shared data to report. The data belong to the Authors of the manuscript.
Declaration of competing interest
No conflict of interest to disclose.
Handling Editor: P. Strazzullo
References
- 1.Pilz S., Trummer C., Pandis M., Schwetz V., Aberer F., Grübler M., et al. Vitamin D: current guidelines and future outlook. Anticancer Res. 2018;38(2):1145–1151. doi: 10.21873/anticanres.12333. [DOI] [PubMed] [Google Scholar]
- 2.Mozos I., Marginean O. Links between vitamin D deficiency and CardiovascularDiseases. BioMed Res Int. 2015;2015:109275. doi: 10.1155/2015/109275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kunadian V., Ford G.A., Bavamia B., Qiu W., Manson J. Vitamin D deficiency and coronary artery disease: a review of the evidence. Am Heart J. 2014;167:283–291. doi: 10.1016/j.ahj.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Elamin M.B., Abu Elnour N.O., Elamin K.B., Fatourechi M.M., Alkatib A.A., Almandoz J.P., et al. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metabol. 2011;96:1931–1942. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 5.Theodoratou E., Tzoulaki I., Zgaga L., Ioannidis J.P. Vitamin D and multiplehealth outcomes: umbrella review of systematic reviews and meta-analyses ofobservational studies and randomised trials. BMJ. 2014;348:2035. doi: 10.1136/bmj.g2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schneider A.L.C., Lutsey P.L., Selvin E. Vitamin D, vitamin D binding protein gene polymorphisms, race and risk of incident stroke: the Atherosclerosis Risk in Communities (ARIC) study. Eur J Neurol. 2015;22:1220–1227. doi: 10.1111/ene.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daffara V., Verdoia M., Rolla R., Nardin M., Marino P., Bellomo G., et al. Novara Atherosclerosis Study Group (NAS) Impact of polymorphism rs7041and rs4588 of Vitamin D Binding Protein on the extent of coronary artery disease. Nutr Metabol Cardiovasc Dis. 2017;27(9):775–783. doi: 10.1016/j.numecd.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 8.Zittermann A., Schleithoff S.S., Frisch S., Götting C., Kuhn J., Koertke H., et al. Circulating calcitriol concentrations and totalmortality. Clin Chem. 2009;55(6):1163–1170. doi: 10.1373/clinchem.2008.120006. [DOI] [PubMed] [Google Scholar]
- 9.Mawer E.B., Schaefer K., Lumb G.A., Stanbury S.W. The metabolism of isotopically labelled vitaminD3 in man: the influence of the state of vitamin Dnutrition. Clin Sci. 1971;40:39–53. doi: 10.1042/cs0400039. [DOI] [PubMed] [Google Scholar]
- 10.Juttman, Buurman cj, De Kam e. Visser ti, Birkenhagerjc: serum concentrations of metabolites of vitamin D in patientswith chronic renal failure (CRF). Consequences for the treatment with l-a-hydroxy-derivatives. C/in Endocrinol. 1981;14:225–236. doi: 10.1111/j.1365-2265.1981.tb00191.x. [DOI] [PubMed] [Google Scholar]
- 11.Richart T., Li Y., Staessen J.A. Renal versus extrarenal activation of vitamin D in relation to atherosclerosis, arterial stiffening, and hypertension. Am J Hypertens. 2007;20:1007–1015. doi: 10.1016/j.amjhyper.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 12.De Luca G., Secco G.G., Santagostino M., Venegoni L., Iorio S., Cassetti E., et al. Novara Atherosclerosis Study Group (NAS) Uric acid does not affect the prevalence and extent of coronary artery disease. Results from a prospective study. NutrMetab Cardiovasc Dis. 2012;22:426–433. doi: 10.1016/j.numecd.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Holick M.F. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 14.Verdoia M., Schaffer A., Sartori C., Barbieri L., Cassetti E., Marino P., et al. Vitamin D deficiency is independently associated with the extent of coronary artery disease. Eur J Clin Invest. 2014;44(7):634–642. doi: 10.1111/eci.12281. [DOI] [PubMed] [Google Scholar]
- 15.Davidson M.H.1, Corson M.A., Alberts M.J., Anderson J.L., Gorelick P.B., Jones P.H., et al. Consensus panel recommendation for incorporating lipoprotein-associated phospholipase A2 testing into cardiovascular disease risk assessment guidelines. Am J Cardiol. 2008 16;101(12A):51F–57F. doi: 10.1016/j.amjcard.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 16.Dong J., Wong S.L., Lau C.W., Lee H.K., Ng C.F., Zhang L., et al. Calcitriol protects renovascular function in hypertension by down-regulating angiotensin II type 1 receptors and reducing oxidative stress. Eur Heart J. 2012;33:2980–2990. doi: 10.1093/eurheartj/ehr459. [DOI] [PubMed] [Google Scholar]
- 17.Takeda M., Yamashita T., Sasaki N., Nakajima K., Kita T., Shinohara M., et al. Oral administration of an active form of vitamin D3 (calcitriol)decreases atherosclerosis in mice by inducing regulatory T cells and immaturedendritic cells with tolerogenic functions. Arterioscler Thromb Vasc Biol. 2010;30:2495–2503. doi: 10.1161/ATVBAHA.110.215459. [DOI] [PubMed] [Google Scholar]
- 18.Jongen M.J., Bishop J.E., Cade C., Norman A.W. Effect of dietary calcium, phosphate and vitamin D deprivation on the pharmacokinetics of 1,25- dihydroxyvitamin D3 in the rat. Horm Metab Res. 1987;19:481. doi: 10.1055/s-2007-1011858. [DOI] [PubMed] [Google Scholar]
- 19.Verdoia M., Ceccon C., Nardin M., Suryapranata H., De Luca G., Novara Atherosclerosis Study Group (NAS) Vitamin D deficiency and periprocedural myocardial infarction in patients undergoing percutaneous coronary interventions. CardiovascRevascMed. 2018;19(7 Pt A):744–750. doi: 10.1016/j.carrev.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Verdoia M., Pergolini P., Rolla R., Nardin M., Schaffer A., Barbieri L., et al. Impact of high-dose statins on vitamin D levels and platelet function in patients with coronary artery disease. Thromb Res. 2017;150:90–95. doi: 10.1016/j.thromres.2016.12.019. [DOI] [PubMed] [Google Scholar]
- 21.Nardin M., Verdoia M., Schaffer A., Barbieri L., Marino P., De Luca G. Vitamin D status, diabetes mellitus and coronary artery disease in patients undergoing coronary angiography. Atherosclerosis. 2016;250:114–121. doi: 10.1016/j.atherosclerosis.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 22.Verdoia M., Schaffer A., Barbieri L., Di Giovine G., Marino P., Suryapranata H., et al. Impact of gender difference on vitamin D status and its relationship with the extent of coronary artery disease. NutrMetabCardiovascDis. 2015;25(5):464–470. doi: 10.1016/j.numecd.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 23.Verdoia M., Daffara V., Pergolini P., Rolla R., Marino P., Bellomo G., et al. Vitamin D Binding Protein rs7041 polymorphism and high-residual platelet reactivity in patients receiving dual antiplatelet therapy with clopidogrel or ticagrelor. VasculPharmacol. 2017;93–95:42–47. doi: 10.1016/j.vph.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Zittermann A., Heer M., Caillot-Augusso A., RettbergP, Scheld K., Drummer C., et al. Microgravity inhibits intestinal calcium absorption as shown by a stablestrontium test. Eur J Clin Invest. 2000;30:1036–1043. doi: 10.1046/j.1365-2362.2000.00682.x. [DOI] [PubMed] [Google Scholar]
- 25.Armas L.A., Zena M., Lund R., Heaney R.P. Calcium absorption response tocholecalciferol supplementation in hemodialysis. Clin J Am Soc Nephrol. 2013;8(6):1003–1008. doi: 10.2215/CJN.08610812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hewitt N.A., O'Connor A.A., O'Shaughnessy D.V., Elder G.J. Effects of cholecalciferolon functional, biochemical, vascular, and quality of life outcomes inhemodialysis patients. Clin J Am Soc Nephrol. 2013 Jul;8(7):1143–1149. doi: 10.2215/CJN.02840312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fryer R.M., Rakestraw P.A., Nakane M., Dixon D., Banfor P.N., Koch K.A., et al. Differential inhibition of renin mRNA expression by paricalcitol and calcitricol in C57/BL6 mice. Nephron Physiol. 2007;106(4):p76–p81. doi: 10.1159/000104875. [DOI] [PubMed] [Google Scholar]
- 28.Alkharfy K.M., Al-Daghri N.M., Yakout S.M., Ahmed M. Calcitriol attenuates weight-related systemic inflammation and ultrastructural changes in the liver in a rodent model. Basic Clin Pharmacol Toxicol. 2013 Jan;112(1):42–923. doi: 10.1111/j.1742-7843.2012.00936.x. [DOI] [PubMed] [Google Scholar]
- 29.Muller K., Haahr P.M., Diamant M., Rieneck K., Kharazmi A., Bendtzen K. 1,25-dihydroxyvitaminD3 inhibits cytokine production by human blood monocytes at the post-transcriptional level. Cytokine. 1992;4:506–512. doi: 10.1016/1043-4666(92)90012-g. [DOI] [PubMed] [Google Scholar]
- 30.Uyemura K., Demer L.L., Castle S.C., Jullien D., Berliner J.A., Gately M.K., et al. Cross-regulatory roles of interleukin (IL)-12 andIL-10 in atherosclerosis. J Clin Invest. 1996 May 1;97(9):2130–2138. doi: 10.1172/JCI118650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martinez-Moreno J.M., Herencia C., Montes de Oca A., Muñoz-Castañeda J.R., Rodríguez-Ortiz M.E., Díaz-Tocados J.M., et al. Vitamin D modulates tissue factor and protease-activated receptor 2 expression in vascular smooth muscle cells. FASEB (Fed Am Soc Exp Biol) J. 2016;30(3):1367–1376. doi: 10.1096/fj.15-272872. [DOI] [PubMed] [Google Scholar]
- 32.Levin A., Bakris G.L., Molitch M., Smulders M., Tian J., Williams L.A., et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]
- 33.Pasquali M., Tartaglione L., Rotondi S., Muci M.L., Mandanici G., Farcomeni A., et al. Calcitriol/calcifediol ratio: an indicator of vitamin D hydroxylation efficiency? BBA Clin. 2015;3:251–256. doi: 10.1016/j.bbacli.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prince R.L., Hutchison B.G., Kent J.C., Kent G.N., Retallack R.W. Calcitriol deficiency with retained synthetic reserve in chronic renal failure. Kidney Int. 1988;33(3):722–728. doi: 10.1038/ki.1988.58. [DOI] [PubMed] [Google Scholar]
- 35.Madjid M., Safavi-Naeini P., Solomon S.D., Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020 Mar 27 doi: 10.1001/jamacardio.2020.1286. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review [published online ahead of print, 2020 Mar 19] Eur Radiol. 2020 doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No shared data to report. The data belong to the Authors of the manuscript.