Abstract
Auxin is well known to control pattern formation and directional growth at the organ/tissue levels via the nuclear TIR1/AFB receptor-mediated transcriptional responses. Recent studies have expanded the arena of auxin actions as a trigger or key regulator of cell polarization and morphogenesis. These actions require non-transcriptional responses such as changes in the cytoskeleton and vesicular trafficking, which are commonly regulated by ROP/Rac GTPase-dependent pathways. These findings beg for the question about the nature of auxin receptors that regulate these responses and renew the interest in ABP1 as a cell surface auxin receptor, including the work showing auxin-binding protein 1 (ABP1) interacts with the extracellular domain of the transmembrane kinase (TMK) receptor-like kinases in an auxin-dependent manner, as well as the debate on this auxin binding protein discovered about 40 years ago. This review highlights recent work on the non-transcriptional auxin signaling mechanisms underscoring cell polarity and shape formation in plants.
Introduction
Cell polarity, structural and functional asymmetry within a cell, is fundamental to cell functions in all cellular organisms [1,2]. Plant cells display a diverse array of polarity, e.g. asymmetric distribution of molecules or structures and asymmetric cell growth/expansion and division. Therefore, cell polarization is a fundamental cellular mechanism for developmental regulation and growth modulation in plants, such as cell morphogenesis, cell fate specification, early embryogenesis, and pattern formation [2–5].
In most cases, cell polarization needs a specific signal, either internal or external, to break cell symmetry and initiate polarization events [1,2]. Asymmetric auxin distribution patterns (so-called auxin gradients) provide positional cues for establishing cell polarity and this initial cue can be reinforced by its downstream signaling events, which consequently trigger a multitude of responses. Polar auxin transport plays a crucial role in the generation of diverse polarity systems, such as polar positioning of root hairs in the trichoblast. Polar auxin transport is largely mediated by the PIN-FORMED (PIN) auxin efflux carriers [6,7]. It has been proposed that the polarity of auxin efflux proteins and auxin gradients form a positive feedback relationship: efflux protein polarity determines the direction of auxin flow, which in turn induces continued efflux protein polarization [5,8,9]. A key to validating this hypothesis is to determine how the distribution and the activity of PIN proteins are spatially regulated. PIN polarization and PIN-mediated auxin efflux is also proposed to form a positive feedback loop that is critical for polarized growth during leaf pavement cell (PC) morphogenesis [10,11]. In addition to PIN polarization, auxin has also been shown to promote polar cell growth in single cell model systems, such as root hairs and pollen tubes. These auxin-mediated cell polarization processes appear to be regulated by a common signaling module (RLK-ROP GTPase) [12].
Auxin is a multi-functional phytohormone that modulates nearly all aspects of plant growth and development [13]. It has been well established that a great number of auxin responses is associated with TRANSPORT INHIBITOR RESPONSE1/AUXIN SIGNALING F-BOX (TIR1/AFB)-based nuclear signaling that triggers large scale reprogramming of gene expression [14,15]. However, increasing studies show that auxin also activates ROP GTPase signaling that directly regulates cytoskeletal dynamics and organization, which is required for cell polarization in the cell systems mentioned above. This auxin signaling is thought to require a cell surface auxin receptor that is distinct from the nuclear TIR1/AFB receptors. This is strongly supported by a large battery of data, such as rapid activation of ROPs by auxin, the localization of the ROP signaling modules to the cell surface, the requirement of the ROP-dependent processes for PIN-mediated auxin export to the cell surface, and the auxin-dependent interaction of the auxin-binding protein 1 (ABP1) with a transmembrane kinase (TMK) receptor-like kinase that regulates ROP signaling [10,16–18].
In the present review, we discuss these new roles of auxin and our recent progress in understanding the mechanisms by which auxin regulates cell polarization and morphogenesis.
Auxin regulates polar targeting of PIN proteins and polar auxin transport
The pattern of auxin distribution regulates a diverse array of processes during plant growth and development. The polar auxin transport that generates auxin patterns is critically determined by polar localization of PIN proteins at a specific side of the plasma membrane (PM). It has been observed that PIN proteins cycle between the PM and intracellular compartments through different trafficking pathways [19]. This polar trafficking of PINs from and to the PM can be tightly regulated by the coordination of the clathrin-dependent endocytosis [20] and the GNOM ADP-ribosylation factor-guanine-nucleotide exchange factor (GNOM ARF-GEF)-mediated endosomal recycling [21,22]. An intriguing question is what mechanisms enable plants to maintain the residence of PIN proteins at the correct side of the PM? Given that auxin has been known for its ability to regulate its own transport [5,9], it is no surprise to find that auxin itself is involved in modulating PIN distribution. It has been shown that short auxin treatment promotes the expression of different PIN genes [23–25]. Apart from transcriptional regulation, several studies reported that auxin enhances PIN abundance at the PM by blocking the clathrin-mediated internalization, thus promoting its own efflux from cells [11,26,27]. Using Arabidopsis leaf PCs, several recent studies from our group led to a detailed mechanistic model for this non-transcriptional positive feedback model for auxin-mediated PIN polarization: auxin triggers the activation of the ROP2 signaling pathways, which in turn promotes the formation of F-actin in the lobe region and locally inhibits the clathrin-dependent PIN1 endocytosis, resulting in lobe-specific PIN localization [10,11,28]. PIN exports more auxin into the cell wall and consequently forms a positive auxin feedback loop for PIN1 polarization [10,11,28]. Interestingly, this auxin-dependent positive feedback and self-polarizing system has also been illustrated in the root system [29,30], suggesting that it may be commonly present in different plant tissues. Together, these studies also provide novel insights into the coordination between ROP signaling and PIN distribution for auxin-mediated cell polarity establishment.
Interestingly, the phosphorylation status is also a common mechanism for the regulation of PIN polarity in different tissues [31–34,35•,36••]. The phosphorylation status of PIN proteins is proposed to regulate the polar trafficking of PIN proteins [31,32,37,38]. Reversible protein phosphorylation, catalyzed by the counterbalancing activities of kinases and phosphatases, is a highly conserved regulatory mechanism for polar protein distribution from prokaryotes to eukaryotes. It has been proposed that phosphorylation status of PIN proteins might affect its interaction with distinct trafficking pathways, resulting in different PIN localization pattern [33]. So far, three AGC3 kinases [PINOID (PID), WAVY ROOT GROWTH1 (WAG1) and WAG2] and two phosphatases [phosphatase 2A (PP2A) and type-one protein phosphatase 4 (TOPP4)] have been identified in Arabidopsis for modulating PIN polarity through direct phosphorylation/dephosphorylation of PIN proteins [31,34,35•,37,39]. Recently, Zourelidou and colleagues characterized another subfamily of AGC3 kinases, namely D6 PROTEIN KINASES (D6PKs), which is also able to directly phosphorylate PINs [36••,40••]. More interestingly, D6PK proteins do not significantly affect PIN polarity but they can directly activate PINs [36••,40••]. They further found that this PIN activation step is required for PIN-mediated auxin efflux and can be carried out by other kinases including PID/WAG kinases [36••,40••]. These findings indicate that the phosphorylation status of PIN controls not only its polarity but also activity and confirm a previously unrecognized PIN activation step for polar auxin transport. Moreover, Zourelidou and colleagues also discovered that auxin plays a role in regulating PIN phosphorylation, which has not been reported before [36••,40••].
The maintenance of PIN localization is also regulated by other factors. For example, Feraru et al. reported that cellulose-based connection between the polar domains at the PM and cell wall is required for the maintenance of PIN polarity [41]. Another recent study showed that attachment of endosomes to microtubules can prevent endocytosed PIN proteins from degradation, thus facilitating endocytic recycling of PINs into the PM [42••]. It seems that there is no direct evidence for linking these PIN-regulatory mechanisms to auxin signaling, but there is some indirect evidence, for example, auxin regulates microtubule organization [10,43••].
Auxin-dependent regulation of leaf pavement cell morphogenesis
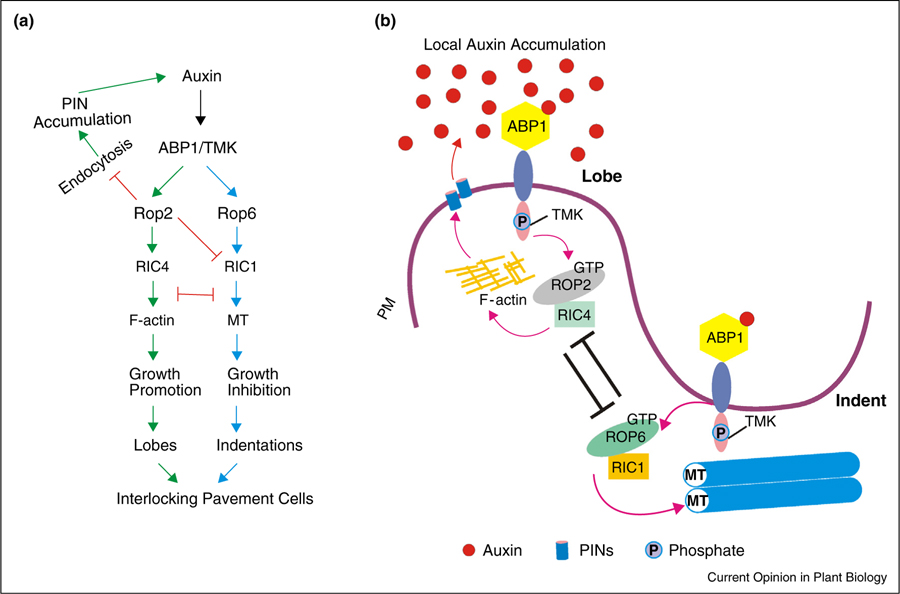
Arabidopsis (Arabidopsis thaliana) leaf epidermis consists primarily of a single cell layer of so-called PCs, which display a fascinating zigzag pattern. Intercalary growth contributes to the formation of interdigitated lobes and indentations of PCs [28]. This precise coordination of complementary lobes and indentations among neighboring cells requires intricate polarity formation (Figure 1). To determine the mechanisms controlling the PC formation, the attention was drawn to auxin, as it was suggested as a positional signal in plant pattern formation [44,45]. The exogenous supply of the synthetic auxin naphthaleneacetic acid (NAA), as well as the use of quadruple yucca mutants with defects in auxin biosynthesis, was successfully used to establish a link between auxin and PC interdigitation [10,34]. The subsequent work suggested that auxin signaling coordinates two mutually exclusive ROP signaling pathways (the ROP2- and ROP6-GTPase pathways), which in turn define lobe-forming and indentation-forming sites to initiate the interdigitation pattern [46••] (Figure 1). The central role of the ROP family in cell polarity is established by virtue of their ability to establish a polar site and to coordinate membrane traffic and cytoskeletal reorganization during polarized cell growth (Figures 1–3). As illustrated in Figure 1, ROP2 preferentially localizes to the lobe regions and induces the accumulation of cortical F-actin through its effector ROP-interacting CRIB-containing protein 4 (RIC4), leading to the lobe outgrowth. On the opposite side, ROP6, through its effector RIC1, promotes the local ordering of cortical microtubules that restrict cell expansion, resulting in indentation.
Figure 1.
Role of auxin signals in leaf pavement cell (PC) interdigitation. (a) A schematic diagram showing the promoting effect of auxin on two antagonistic ROP pathways and PIN1-mediated positive feedback loop in PC interdigitation. (b) A working model of auxin-mediated PC interdigitation. A proposed cell surface ABP1-TMK auxin-sensing complex activates two antagonistic ROP pathways (the ROP2- and ROP6-GTPase pathways) for leaf PC interdigitation. The ROP2-RIC4 interaction promotes lobe outgrowth via actin assembly while the ROP6-RIC1 interaction restricts lobe outgrowth via microtubule organization. PM, plasma membrane; MT, microtubule.
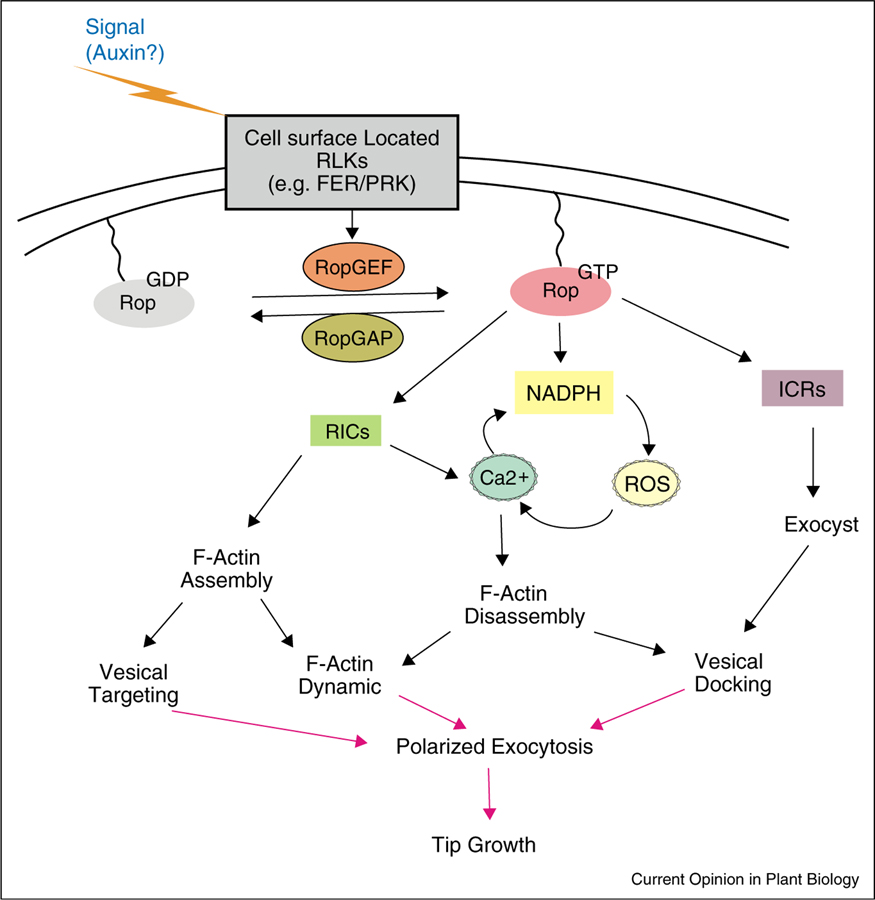
Figure 3.
A possible model of auxin-ROP signaling in pollen tube and root hair growth. Auxin may be the polarizing signal that initiates the ROP-dependent tip growth. ROP signaling can be modulated by various upstream regulators and downstream effectors. Tip-localized activated ROP regulates the Ca2+ gradient, actin dynamic as well as exocytic trafficking during tip growth. RLK, receptor-like kinases; GAP, GTPase activating proteins; GEF, guanine nucleotide exchange factor; RIC, Rop-interacting CRIB-containing proteins; ICR, interactor of constitutively active ROPs; ROS, reactive oxygen species
These findings hint the existence of polarly localized auxin efflux carriers, which would cause the local accumulation of extracellular auxin, thus triggering the activation of ROP signaling pathways. This speculation led to the discovery of auxin efflux carrier PIN at the upstream of ROP singling pathways. It was found that PIN1 proteins are asymmetrically distributed at the PM with a preferred localization to lobes instead of indentations [10,46••]. The importance of PIN1 in PC interdigitation was further revealed by phenotypic analysis of Arabidopsis pin1 overexpression and mutant lines. The pin1 mutants display severe PC defects as being devoid of lobes, while PIN1 overexpression leads to the enhanced lobe formation [10]. It appears that PIN1 polarization to the lobe region of PCs is required for PC interdigitation. Apart from the feedback mechanism described above [10,46••], the phosphorylation status-mediated PIN polarization also applies to the formation of multipolar leaf PCs [34,35•]. Loss of PP2A function as well as PID overexpression caused greatly reduced PC interdigitation and shift of PIN1 localization from lobe to indentation regions. Guo et al. (2015) reported that TOPP4 has the capacity to counteract the PID activity via direct dephosphorylation of PIN proteins and is crucial for PC interdigitation [35•]. Overexpression of TOPP4 promoted PC interdigitation while the topp4–1 mutant displayed severe PC defects. Consistently, the lobe-to-indentation shift of PIN polarity was observed in the topp4–1 mutant. It is hypothesized that dephosphorylated PIN proteins may be preferentially recruited into the lobe-targeting pathway, whereas phosphorylated PIN proteins may be sorted into the endocytic pathway. In line with this hypothesis, Guo et al. (2015) showed that TOPP4 is essential for endocytic trafficking of PIN1 in PCs [35•]. The ROP-based vesicular trafficking and phosphorylation-dependent trafficking for PIN distribution is also supported in root cells (Figure 2).
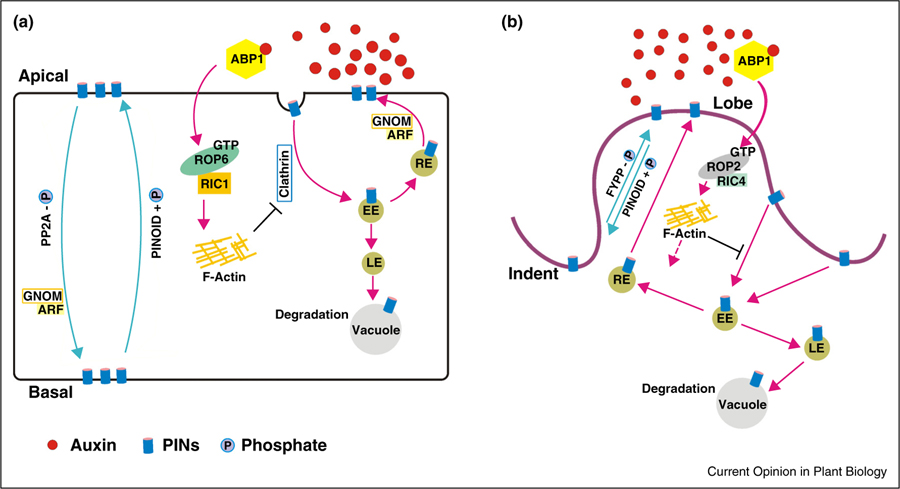
Figure 2.
Auxin inhibition of PIN endocytosis in root (a) and leaf (b) pavement cells (PCs). Auxin binds to its likely receptor ABP1 and inhibits PIN endocytosis through the ROP6/RIC1 pathway in root cells while the ROP2/RIC4 pathway in leaf PCs. PIN proteins are internalized via clathrin-mediated endocytosis and then can follow either the GNOM-dependent recycling route to the PM or the degradation route to the vacuole. Control of PIN polarity also depends on the phosphorylation status of PIN proteins. In root cells, the PIN phosphorylation status is mediated by PINOID kinase and PP2A phosphatase. Hyper-phosphorylated PIN proteins are directed to the apical domain, whereas hypo-phosphorylated PINs are targeted to the basal domain. In leaf PCs, PINOID and FYPP1 regulate the lobe-indentation switch of PIN proteins. Hyper-phosphorylated PINs accumulates at the indentation-forming regions, while hypo-phosphorylated PINs are preferentially sorted to the lobe-forming regions. EE, early endosome; LE, late endosome; RE, recycling endosome.
Mechanical stress also contributes to PC interdigitation [47••]. It was reported that cell-shape derived mechanical forces can alter microtubule assembly, which in turn guides oriented deposition of cell-wall components, thereby reinforcing the tensile stress in the indenting regions. This stress-microtubule feedback loop plays an importance role for the maintenance of the indentation in PCs. Considering that the auxin-triggered ROP6-RIC1 pathway regulates microtubule rearrangement in the indenting regions is also modulated by mechanical stress [10,47••,48•,49], the mechanical signal might be linked to the auxin signal for PC formation. Consistent with this, there is evidence that auxin and mechanical signals interplay in the regulation of PIN polarization during leaf primordia formation [50]. Hence, it would be interesting to investigate whether similar interplays occur in PCs.
Auxin stimulates polar tip growth in root hairs and pollen tubes
An extreme form of polarized cell growth in plants and fungi is tip growth, whereby the growth takes place exclusively in the apical region of the cell. Root hairs and pollen tubes are two well-characterized plant cell types, which display polarized tip growth. Tip growth needs rapid cytoskeletal reorganization and accelerated membrane trafficking to accompany morphological changes and to supply materials required for efficient growth. The mechanisms underlying the polarized growth of root hairs and pollen tubes are highly similar [2,51–53], and it is intriguing that auxin promotes tip growth both in root hairs and pollen tubes [54–57].
Root hair and pollen tube growth is regulated by ROP signaling (Figure 3). Tip-localized active ROP not only determines the tip growth region [58,59], but also regulates the Ca2+ gradient, actin dynamic, exocytic trafficking and NADPH oxidase-mediated reactive oxygen species (ROS) production during tip growth [60,61]. In both systems, ROP signaling is fine-tuned by a plethora of upstream regulators and downstream effectors (Figure 3). ROPs shuttle between the GDP-bound inactive form and the GTP-bound active form. In plants, ROP activation is largely dependent on the plant-specific RopGEFs (guanine nucleotide exchange factors for ROP GTPases) [62], which are cytoplasmic proteins and interact with cell surface-located receptor-like kinases (RLKs). Two pollen-specific RLKs, LePRK1 and LePRK2, have been discovered in tomato (Solanum lycopersicum) and a homolog of LePRKs, AtPRK2 has been identified in Arabidopsis [63,64]. All these PRKs physically interact with RopGEFs [63,64] and their overexpression resulted in obvious morphological phenotypes [65•]. A recent study on AtPRK2 suggests that AtPRK2 may directly phosphorylate and activate RopGEF, which in turn regulates ROP-mediated tip growth [66]. Moreover, the study on LePRK1 revealed that the function of LePRK on tomato pollen tube morphology is closely associated with its effects on actin dynamics [65•]. It appears that KPP (a RopGEF) physically links LePRK1 to the actin binding protein PLIM2a at the PM tip, which promotes membrane-anchored actin bundle formation at the tip, resulting in bleb formation. Taken together, these results support that PRKs function as a bridge to transduce extracellular signals into the ROP-mediated pollen tube growth through its various binding partner, such as RopGEF and PLIM2a. In addition, several effectors have been identified, including a family of CRIB-domain proteins (RICs) and a coil-coil domain protein (ICR1) [67–71].
It was reported that mutations in the receptor-like kinase FERONIA (FER) led to the reduced auxin-stimulated ROS production and root hair elongation [72]. Furthermore FER directly interacts with RopGEFs [72]. These findings indicate that auxin may promote root hair growth through ROP signaling [72]. As discussed above, in the Arabidopsis leaf epidermis auxin activation of ROP signaling during PC formation also requires cell surface receptor-like kinases TMKs, which interact with the ABP1 auxin receptor (see below) [46••]. Given the critical role for RopGEF-interacting RLKs in the regulation of pollen tube and root hair growth, it is attempting to speculate that auxin also regulates tip growth through an RLK-based cell surface receptor.
The ups and downs of the cell surface auxin receptor ABP1
Although different cell types can have distinct mechanisms to coordinate their respective polar growth, it appears that ROP signaling, which enables dynamic regulation of vesicular trafficking (endocytosis and exocytosis) and cytoskeletal reorganization, is a conserved mechanism for auxin-mediated polarity establishment and maintenance across cell types. ROP signaling occurs on the PM and is activated by RLKs. Thus it would make sense that a cell surface auxin receptor instead of the TIR1/AFB nuclear auxin receptors is used for sensing the polarizing auxin signal. This is consistent with the fact that auxin activates ROPs in seconds [10] and the importance of polar auxin export to the cell surface in the regulation of ROP-dependent processes [10]. Furthermore, given the critical importance of PIN and ABC transporter-mediated auxin export in polar auxin transport, it would also make sense that a cell surface auxin sensor is used to monitor extracellular auxin levels for the regulation of polar auxin transport. It has long been proposed that ABP1 acts as a putative cell surface auxin receptor [73]. The first evidence pointing to the function of ABP1 as an auxin receptor was provided by early biochemical studies, which showed that ABP1 has specific auxin-binding properties and that blocking ABP1 function with extracellular applied anti-ABP1 antibodies suppresses auxin promotion of cell expansion [74–76]. Later, numerous genetic studies further showed that ABP1 plays an important role in various auxin-regulated processes [27,29,43••, 77–79].
The complicated subcellular distribution of ABP1 contributed to the early debate about the receptor functionality of ABP1, as the majority of ABP1 is localized in the ER and only a minor portion (10–20%) is secreted to the apoplast [80,81]. The extracellular ABP1 was assumed to be active, because ABP1’s binding to auxin requires a low pH environment as found in the apoplast but does not occur at the neutral pH found in ER [80,81]. The unanswered question why there is an abundant ABP1 in ER has been troublesome to the ABP1 believers. The findings that two early lines containing T-DNA insertions into the ABP1 gene were embryo-lethal impeded the genetic studies on this question [78,82,83]. The discovery of the TIR1/AFB auxin receptors and their broad and critical roles in the regulation of auxin responses [14,84] also helped to cool down the enthusiasm about ABP1. Finally, lack of ABP1-dependent intracellular auxin signaling mechanisms and of an assumed docking protein linking the apoplastic ABP1 to the intracellular signaling events further contributed to the shelfing of ABP1 for nearly two decades.
ABP1 has resurfaced in recent years, owing to the search for an extracellular auxin receptor that activates ROP signaling in PCs and PIN distribution to the PM, as well as the use of a non-embryo-lethal abp1–5 tilling allele and inducible expression of an ABP1 antibody and antisense construct [10,74,76,85,86]. A series of recent studies using these materials have implicated ABP1 in the regulation of auxin-dependent PC morphogenesis, ROP signaling, PIN trafficking, cell division, gene expression, and microtubule reorientation [10,17,27,29, 46••,79,87]. Importantly, Xu and colleagues [46••] showed that ABP1 is anchored to the PM by the interaction with the extracellular domain of PM-localized TMK1, one of the four member clade of RLKs required for ROP2 and ROP6 activation and PC morphogenesis and many other auxin responses [46••,88•]. This interaction occurs in an auxin-dependent manner, shown by co-immunoprecipitation in Arabidopsis and tobacco leaves where both Arabidopsis TMK1 and ABP1 are transiently expressed [46••]. Notably, there was no interaction between TMK1 and abp1–5 mutant protein, which has a point mutation in the auxin-binding pocket, indicating that auxin binding, per se, is required for this interaction. The discovery of this long-sought docking protein provides the strongest support for the role of ABP1 as a cell surface auxin receptor [12,46••,89,90]. Taken together all these 40 years’ findings provide overwhelming evidence for the involvement of ABP1 in the perception of extracellular auxin. However, a new curse on ABP1 came from two latest reports on the genetic studies of ABP1. Gao et al. (2015) shows that abp1 null mutants lack obvious growth and developmental phenotypes, which seems to contradict these previously described roles of ABP1 [91•]. Various explanations for the contradiction were provided in recent reviews [48•,92]. Another study shows that the reported abp1–5 line contains many background mutations, and at least the reported role of ABP1 in the regulation of phytochrome response in Arabidopsis hypocotyls was actually due to one of these background mutations [93]. Various cellular and biochemical phenotypes reported using the previous abp1 genetic tools need to be thoroughly characterized in the new null abp1 mutants and a cleanup version of the abp1–5 mutant. Given lack of severe phenotypes in the new abp1 null mutants [91•], the existence of potential additional cell surface auxin receptors that may compensate for the loss of ABP1 needs to be considered. Indeed, some members of the cupin family, to which ABP1 belongs, have been shown to have auxin-binding properties [94,95]. It remains to be found out whether these cupin members are able to compensate for the loss of ABP1.
Concluding remarks
Research in recent years has greatly expanded our understanding of the roles of the small molecule auxin in plants. Evidence supports a role for auxin as a common polarizing signal through the conserved ROP GTPase signaling that regulate cytoskeletal organization and vesicular trafficking. However, these findings raise many new questions, for example, how highly diffusible auxin can be translated into the polarity cues for cell polarization and how PIN proteins interplay with auxin signaling to regulate cell polarity. In addition, the interactions between auxin signaling with other hormones or other cues not discussed in this review create a complicated network of cellular events that instruct cell polarization. Moreover, it remains a challenge for the future to determine how auxin acts as a fairly universal signal to activate or modulate cell polarization and polar cell growth in different cell types. Finally the continuing debate on ABP1’s roles will fuel much more intense research on this protein and others proteins that are predicted to act as cell surface auxin receptors. These future studies will build a more comprehensive picture of the role of auxin action in orchestrating the cell polarity and tissue morphogenesis in plant development.
Acknowledgements
This work is supported by the funding from Horticultural Plant Biology and Metabolomics Center, Haixia Institute of Science and Technology, Fujian Agriculture and Forestry University (XP and JC), and by the US National Institute of General Medical Sciences to ZY (R01GM081451 and R01GM100130).
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Cove DJ: The generation and modification of cell polarity. J Exp Bot 2000, 51:831–838. [PubMed] [Google Scholar]
- 2.Yang Z: Cell polarity signaling in Arabidopsis. Annu Rev Cell Dev Biol 2008, 24:551–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lecuit T, Pilot F: Developmental control of cell morphogenesis: a focus on membrane growth. Nat Cell Biol 2003, 5:103–108. [DOI] [PubMed] [Google Scholar]
- 4.Abrash EB, Bergmann DC: Asymmetric cell divisions: a view from plant development. Dev Cell 2009, 16:783–796. [DOI] [PubMed] [Google Scholar]
- 5.Sachs T: Cell polarity and tissue patterning in plants. Development 1991, 112:83–93.1769343 [Google Scholar]
- 6.Blakeslee JJ, Bandyopadhyay A, Ok RL, Mravec J, Titapiwatanakun B, Sauer M, Makam SN, Cheng Y, Bouchard R, Adamec J et al. : Interactions among PIN-FORMED and P-glycoprotein auxin transporters in Arabidopsis. Plant Cell 2007, 19:131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Titapiwatanakun B, Murphy AS: Post-transcriptional regulation of auxin transport proteins: cellular trafficking, protein phosphorylation, protein maturation, ubiquitination, and membrane composition. J Exp Bot 2009, 60:1093–1107. [DOI] [PubMed] [Google Scholar]
- 8.van Berkel K, de Boer RJ, Scheres B, ten Tusscher K: Polar auxin transport: models and mechanisms. Development 2013, 140:2253–2268. [DOI] [PubMed] [Google Scholar]
- 9.Sachs T: The control of the patterned differentiation of vascular tissues. Adv Bot Res 1981, 9:151–262. [Google Scholar]
- 10.Xu T, Wen M, Nagawa S, Fu Y, Chen J, Wu M, Perrot-Rechenmann C, Friml J, Jones AM, Yang Z: Cell surface- and Rho GTPase-based auxin signaling controls cellular interdigitation in Arabidopsis. Cell 2010, 143:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagawa S, Xu T, Lin D, Dhonukshe P, Zhang X, Friml J, Scheres B, Fu Y, Yang Z: ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS Biol 2012:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyawaki KN, Yang Z: Extracellular signals and receptor-like kinases regulating ROP GTPases in plants. Front Plant Sci 2014:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Teale WD, Paponov IA, Palme K: Auxin in action: signalling, transport and the control of plant growth and development. Nat Rev Mol Cell Biol 2006, 7:847–859. [DOI] [PubMed] [Google Scholar]
- 14.Dharmasiri N, Dharmasiri S, Estelle M: The F-box protein TIR1 is an auxin receptor. Nature 2005, 435:441–445. [DOI] [PubMed] [Google Scholar]
- 15.Kepinski S, Leyser O: The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 2005, 435:446–451. [DOI] [PubMed] [Google Scholar]
- 16.Shi J, Yang Z: Is ABP1 an auxin receptor yet? Mol Plant 2011, 4:635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Yang Z: Novel ABP1-TMK auxin sensing system controls ROP GTPase-mediated interdigitated cell expansion in Arabidopsis. Small GTPases 2014, 5:e29711. [DOI] [PubMed] [Google Scholar]
- 18.Lin D, Ren H, Fu Y: ROP GTPase-mediated auxin signaling regulates pavement cell interdigitation in Arabidopsis thaliana. J Integr Plant Biol 2015, 57:31–39. [DOI] [PubMed] [Google Scholar]
- 19.Geldner N, Friml J, Stierhof YD, Jürgens G, Palme K: Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 2001, 413:425–428. [DOI] [PubMed] [Google Scholar]
- 20.Dhonukshe P, Aniento F, Hwang I, Robinson DG, Mravec J, Stierhof Y, Friml J: Clathrin-mediated constitutive endocytosis of PIN auxin efflux carriers in Arabidopsis. Curr Biol 2007, 17:520–527. [DOI] [PubMed] [Google Scholar]
- 21.Geldner N, Anders N, Wolters H, Keicher J, Kornberger W, Muller P, Delbarre A, Ueda T, Nakano A, Jürgens G: The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 2003, 112:219–230. [DOI] [PubMed] [Google Scholar]
- 22.Adamowski M, Friml J: PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 2015, 27:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS: Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with auxin transport in Arabidopsis thaliana. Plant Cell 2004, 16:1898–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heisler MG, Ohno C, Das P, Sieber P, Reddy GV, Long JA, Meyerowitz EM: Patterns of auxin transport and gene expression during primordium development revealed by live imaging of the Arabidopsis inflorescence meristem. Curr Biol 2005, 15:1899–1911. [DOI] [PubMed] [Google Scholar]
- 25.Vieten A, Vanneste S, Wisniewska J, Benková E, Benjamins R, Beeckman T, Luschnig C, Friml J: Functional redundancy of PIN proteins is accompanied by auxin-dependent cross-regulation of PIN expression. Development 2005, 132:4521–4531. [DOI] [PubMed] [Google Scholar]
- 26.Paciorek T, Zažímalová E, Ruthardt N, Petrášek J, Stierhof Y-, Kleine-Vehn J, Morris DA, Emans N, Jürgens G, Geldner N, Friml J: Auxin inhibits endocytosis and promotes its own efflux from cells. Nature 2005, 435:1251–1256. [DOI] [PubMed] [Google Scholar]
- 27.Robert S, Kleine-Vehn J, Barbez E, Sauer M, Paciorek T, Baster P, Vanneste S, Zhang J, Simon S, Covanová M et al. : ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis. Cell 2010, 143:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y, Gu Y, Zheng Z, Wasteneys G, Yang Z: Arabidopsis interdigitating cell growth requires two antagonistic pathways with opposing action on cell morphogenesis. Cell 2005, 120:687–700. [DOI] [PubMed] [Google Scholar]
- 29.Chen X, Naramoto S, Robert S, Tejos R, Lӧfke C, Lin D, Yang Z, Friml J: ABP1 and ROP6 GTPase signaling regulate clathrin-mediated endocytosis in Arabidopsis roots. Curr Biol 2012, 22:1326–1332. [DOI] [PubMed] [Google Scholar]
- 30.Lin D, Nagawa S, Chen J, Cao L, Chen X, Xu T, Li H, Dhonukshe P, Yamamuro C, Friml J et al. : A ROP GTPase-dependent auxin signaling pathway regulates the subcellular distribution of PIN2 in Arabidopsis roots. Curr Biol 2012, 22:1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Michniewicz M, Zago MK, Abas L, Weijers D, Schweighofer A, Meskiene I, Heisler MG, Ohno C, Zhang J, Huang F et al. : Antagonistic regulation of PIN phosphorylation by PP2A and PINOID directs auxin flux. Cell 2007, 130:1044–1056. [DOI] [PubMed] [Google Scholar]
- 32.Friml J, Yang X, Michniewicz M, Weijers D, Quint A, Tietz O, Benjamins R, Ouwerkerk PBF, Ljung K, Sandberg G et al. : A PINOID-dependent binary switch in apical–basal PIN polar targeting directs auxin efflux. Science 2004, 306:862–865. [DOI] [PubMed] [Google Scholar]
- 33.Ganguly A, Sasayama D, Cho HT: Regulation of the polarity of protein trafficking by phosphorylation. Mol Cells 2012, 33:423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Lin D, Dhonukshe P, Nagawa S, Chen D, Friml J, Scheres B, Guo H, Yang Z: Phosphorylation switch modulates the interdigitated pattern of PIN1 localization and cell expansion in Arabidopsis leaf epidermis. Cell Res 2011, 21:970–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.•.Guo X, Qin Q, Yan J, Niu Y, Huang B, Guan L, Li Y, Ren D, Li J, Hou S: Type-one protein phosphatase4 regulates pavement cell interdigitation by modulating PIN-FORMED1 polarity and trafficking in Arabidopsis. Plant Physiol 2015, 167:1058–1075.Using genetic and biochemical approaches, the authors revealed a unique function of a type-one protein phosphatase, TOPP4, which can directly dephosphorylate PIN1 and thus affect PIN1 polar localization and endocytic trafficking in pavement cells.
- 36.••.Zourelidou M, Absmanner B, Weller B, Barbosa ICR, Willige BC, Fastner A, Streit V, Port SA, Colcombet J, van Bentem SF et al. : Auxin efflux by PIN-FORMED proteins is activated by two different protein kinases, D6 PROTEIN KINASE and PINOID. eLife 2014.This study confirmed, for the first time in heterologous Xenopus oocyte system and in Arabidopsis thaliana inflorescence stems, that D6 protein kinase and PINOID (PID)/WAG kinases mediate the activation of PIN proteins, which is a prerequisite for PIN-mediated auxin transport.
- 37.Kleine-Vehn J, Huang F, Naramoto S, Zhang J, Michniewicz M, Offringa R, Friml J: PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell 2009, 21:3839–3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang J, Nodzynski T, Pencík A, Rolcík J, Friml J: PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proc Natl Acad Sci U S A 2010, 107:918–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dhonukshe P, Huang F, Galvan-Ampudia CS, Mähönen AP, Kleine-Vehn J, Xu J, Quint A, Prasad K, Friml J, Scheres B, Offringa R: Plasma membrane-bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS(N/S) motifs to direct apical PIN recycling. Development 2010, 137:3245–3255. [DOI] [PubMed] [Google Scholar]
- 40.••.Barbosa ICR, Zourelidou M, Willige BC, Weller B: D6 PROTEIN KINASE activates auxin transport-dependent growth and PINFORMED phosphorylation at the plasma membrane. Dev Cell 2014, 29:674–685.The authors explored the cell biological behaviour of D6 PROTEIN KINASE with the aim to uncover its role in auxin transport and proposed that D6PK polar targeting at the plasma membrane may control not only PIN polarity but also its activity.
- 41.Feraru E, Feraru MI, Kleine-Vehn J, Martiniére A, Mouille G, Vanneste S, Vernhettes S, Runions J, Friml J: PIN polarity maintenance by the cell wall in Arabidopsis. Curr Biol 2011, 21:338–343. [DOI] [PubMed] [Google Scholar]
- 42.••.Ambrose C, Ruan Y, Gardiner J, Tamblyn LM, Catching A, Kirik V, Marc J, Overall R, Wasteneys GO: CLASP interacts with sorting nexin 1 to link microtubules and auxin transport via PIN2 recycling in Arabidopsis thaliana. Dev Cell 2013, 24:649–659.The authors revealed that the interaction between the microtubule-associated protein CLASP and the retromer component sorting nexin1 can stabilize the association between PIN2-carrying endosomes and microtubules, thus preventing PIN2 degradation and promoting its recycling into the plasma membrane.
- 43.••.Chen X, Grandont L, Li H, Hauschild R, Paque S, Abuzeineh A, Rakusová H, Benkova E, Perrot-Rechenmann C, Friml J: Inhibition of cell expansion by rapid ABP1-mediated auxin effect on microtubules. Nature 2014, 516:90–93.This study provided biochemical and genetic evidence supporting that ABP1 is required for transmitting auxin signals to the ROP6-RIC1-KTN1 pathway for microtubule rearrangement to regulate cell expansion.
- 44.Uggla C, Moritz T, Sandberg G, Sundberg B: Auxin as a positional signal in pattern formation in plants. Proc Natl Acad Sci U S A 1996, 93:9282–9286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uggla C, Mellerowicz EJ, Sundberg B: Indole-3-acetic acid controls cambial growth in scots pine by positional signaling. Plant Physiol 1998, 117:113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.••.Xu T, Dai N, Chen J, Nagawa S, Cao M, Li H, Zhou Z, Chen X, De Rycke R, Rakusová H et al. : Cell surface ABP1-TMK auxin-sensing complex activates ROP GTPase signaling. Science 2014, 343:1025–1028.This study identified the long-sought ABP1 docking protein by demonstrating that ABP1 interacts with the extracellular domain of the TMK1 receptor-like kinase in an auxin-dependent manner and that TMK1 together with three other functionally overlapping TMK1 homologs is required for auxin-mediated ROP signaling in Arabidopsis.
- 47.••.Sampathkumar A, Krupinski P, Wightman R, Milani P, Berquand A, Boudaoud A, Hamant O, Jönsson H, Meyerowitz EM: Subcellular and supracellular mechanical stress prescribes cytoskeleton behavior in Arabidopsis cotyledon pavement cells. eLife 2014, 3:e01967.Using live imaging and modeling of cell mechanic, the authors illustrated that a positive feedback loop between mechanical stress and microtubule arrangement is essential for shaping leaf pavement cells.
- 48.•.Chen J, Wang F, Zheng S, Xu T, Yang Z: Pavement cells: a model system for non-transcriptional auxin signalling and crosstalks. J Exp Bot 2015, 66:4957–4970.This review provides an overall view of recent findings on the role of non-transcriptional auxin signalling in pavement cell morphogenesis. In addition, the interplay of auxin and cytokinin and the possible link between auxin and mechanical signals in the regulation of PC formation are also discussed.
- 49.Lin D, Cao L, Zhou Z, Zhu L, Ehrhardt D, Yang Z, Fu Y: Rho GTPase signaling activates microtubule severing to promote microtubule ordering in Arabidopsis. Curr Biol 2013, 23:290–297. [DOI] [PubMed] [Google Scholar]
- 50.Sampathkumar A, Yan A, Krupinski P, Meyerowitz EM: Physical forces regulate plant development and morphogenesis. Curr Biol 2014, 24:R475–R483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen X, Friml J: Rho-GTPase-regulated vesicle trafficking in plant cell polarity. Biochem Soc Trans 2014, 42:212–218. [DOI] [PubMed] [Google Scholar]
- 52.Hepler PK, Vidali L, Cheung AY: Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 2001, 17:159–187. [DOI] [PubMed] [Google Scholar]
- 53.Campanoni P, Blatt MR: Membrane trafficking and polar growth in root hairs and pollen tubes. J Exp Bot 2007, 58:65–74. [DOI] [PubMed] [Google Scholar]
- 54.Chen D, Zhao J: Free, IAA in stigmas and styles during pollen germination and pollen tube growth of Nicotiana tabacum. Physiol Plant 2008, 134:202–215. [DOI] [PubMed] [Google Scholar]
- 55.Wu J, Lin Y, Zhang X, Pang D, Zhao J: IAA stimulates pollen tube growth and mediates the modification of its wall composition and structure in Torenia fournieri. J Exp Bot 2008, 59:2529–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jones AR, Kramer EM, Knox K, Swarup R, Bennett MJ, Lazarus CM, Leyser HMO, Grierson CS: Auxin transport through non-hair cells sustains root-hair development. Nat Cell Biol 2009, 11:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee RD, Cho H: Auxin, the organizer of the hormonal/ environmental signals for root hair growth. Front Plant Sci 2013:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin Y, Wang Y, Zhu J, Yang Z: Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell 1996, 8:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Li H, Wu G, Ware D, Davis KR, Yang Z: Arabidopsis Rho-related GTPases: differential gene expression in pollen and polar localization in fission yeast. Plant Physiol 1998, 118:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cheung AY, Wu H: Structural and signaling networks for the polar cell growth machinery in pollen tubes. Annu Rev Plant Biol 2008, 59:547–572. [DOI] [PubMed] [Google Scholar]
- 61.Foreman J, Demidchik V, Bothwell JHF, Mylona P, Miedema H, Angel Torres M, Linstead P, Costa S, Brownlee C, Jones JDG et al. : Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 2003, 422:442–446. [DOI] [PubMed] [Google Scholar]
- 62.Berken A, Thomas C, Wittinghofer A: A new family of RhoGEFs activates the Rop molecular switch in plants. Nature 2005, 436:1176–1180. [DOI] [PubMed] [Google Scholar]
- 63.Kaothien P, Sung HO, Shuai B, Wengier D, Cotter R, Kelley D, Kiriakopolos S, Muschietti J, McCormick S: Kinase partner protein interacts with the LePRK1 and LePRK2 receptor kinases and plays a role in polarized pollen tube growth. Plant J 2005, 42:492–503. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, McCormick S: A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc Natl Acad Sci U S A 2007, 104:18830–18835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.•.Gui C, Dong X, Liu H, Huang W, Barberini ML, Wang S, Muschietti J, Gao X, Muschietti J, McCormick S, Tang W: Overexpression of the tomato pollen receptor kinase LePRK1 rewires pollen tube growth to a blebbing mode. Plant Cell 2014, 26:3538–3555.The authors observed that LePRK is able to change the pollen tube growth mode from tubular to blebbing. Using in vitro pull-down, yeast two-hybrid, in vivo BiFC, and pollen tube coexpression assays, the authors further discovered that a solid linkage between a plasma membrane-localized receptor like kinase, a RopGEF and actin filaments contributes to this growth mode transition.
- 66.Chang F, Gu Y, Ma H, Yang Z: AtPRK2 promotes ROP1 activation via RopGEFs in the control of polarized pollen tube growth. Mol Plant 2013, 6:1187–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lee YJ, Szumlanski A, Nielsen E, Yang Z: Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J Cell Biol 2008, 181:1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hwang J, Wu G, Yan A, Lee Y, Grierson CS, Yang Z: Pollen-tube tip growth requires a balance of lateral propagation and global inhibition of Rho-family GTPase activity. J Cell Sci 2010, 123:340–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li S, Gu Y, Yan A, Lord E, Yang Z: RIP1 (ROP Interactive Partner 1)/ICR1 marks pollen germination sites and may act in the ROP1 pathway in the control of polarized pollen growth. Mol Plant 2008, 1:1021–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, Yang Z: A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J Cell Biol 2005, 169:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H, Lin Y, Heath RM, Zhu MX, Yang Z: Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 1999, 11:1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Duan Q, Kita D, Li C, Cheung AY, Wu H: FERONIA receptor-like kinase regulates RHO GTPase signaling of root hair development. Proc Natl Acad Sci U S A 2010, 107: 17821–17826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Napier RM, David KM, Perrot-Rechenmann C: A short history of auxin-binding proteins. Plant Mol Biol 2002, 49:339–348. [PubMed] [Google Scholar]
- 74.Venis MA, Napier RM, Barbier-Brygoo H, Maurel C, Perrot-Rechenmann C, Guern J: Antibodies to a peptide from the maize auxin-binding protein have auxin agonist activity. Proc Natl Acad Sci U S A 1992, 89:7208–7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thiel G, Blatt MR, Fricker MD, White IR, Millner P: Modulation of K+ channels in Vicia stomatal guard cells by peptide homologs to the auxin-binding protein C terminus. Proc Natl Acad Sci U S A 1993, 90:11493–11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Leblanc N, David K, Grosclaude J, Pradier J-, Barbier-Brygoo H, Labiau S, Perrot-Rechenmann C: A novel immunological approach establishes that the auxin-binding protein, Nt-abp1, is an element involved in auxin signaling at the plasma membrane. J Biol Chem 1999, 274:28314–28320. [DOI] [PubMed] [Google Scholar]
- 77.Tromas A, Paponov I, Perrot-Rechenmann C: Auxin binding protein 1: Functional and evolutionary aspects. Trends Plant Sci 2010, 15:436–446. [DOI] [PubMed] [Google Scholar]
- 78.Chen J, Ullah H, Young JC, Sussman MR, Jones AM: ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev 2001, 15:902–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tromas A, Paque S, Stierlé V, Quettier A, Muller P, Lechner E, Genschik P, Perrot-Rechenmann C: Auxin-binding protein 1 is a negative regulator of the SCF TIR1/AFB pathway. Nat Commun 2013:4. [DOI] [PubMed] [Google Scholar]
- 80.Sauer M, Kleine-Vehn J: AUXIN BINDING PROTEIN 1: the outsider. Plant Cell 2011, 23:2033–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shimomura S: Identification of a glycosylphosphatidylinositol-anchored plasma membrane protein interacting with the C-terminus of auxin-binding protein 1: a photoaffinity crosslinking study. Plant Mol Biol 2006, 60:663–677. [DOI] [PubMed] [Google Scholar]
- 82.Tzafrir I, Pena-Muralla R, Dickerman A, Berg M, Rogers R, Hutchens S, Sweeney TC, McElver J, Aux G, Patton D, Meinke D: Identification of genes required for embryo development in Arabidopsis. Plant Physiol 2004, 135:1206–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meinke D, Muralla R, Sweeney C, Dickerman A: Identifying essential genes in Arabidopsis thaliana. Trends Plant Sci 2008, 13:483–491. [DOI] [PubMed] [Google Scholar]
- 84.Wang R, Estelle M: Diversity and specificity: auxin perception and signaling through the TIR1/AFB pathway. Curr Opin Plant Biol 2014, 21:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Braun N, Wyrzykowsk J, Muller P, David K, Couch D, Perrot-Rechenmann C, Fleming AJ: Conditional repression of AUXIN BINDING PROTEIN1 reveals that it coordinates cell division and cell expansion during postembryonic shoot development in Arabidopsis and tobacco. Plant Cell 2008, 20:2746–2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tromas A, Braun N, Muller P, Khodus T, Paponov IA, Palme K, Ljung K, Lee J, Benfey P, Murray JAH et al. : The AUXIN BINDING PROTEIN 1 is required for differential auxin responses mediating root growth. PLoS ONE 2009:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Paque S, Mouille G, Grandont L, Alabadí D, Gaertner C, Goyallon A, Muller P, Primard-Brisset C, Sormani R, Blázquez MA, Perrot-Rechenmann C: AUXIN BINDING PROTEIN1 links cell wall remodeling, auxin signaling, and cell expansion in Arabidopsis. Plant Cell 2014, 26:280–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.•.Dai N, Wang W, Patterson SE, Bleecker AB: The TMK subfamily of receptor-like kinases in Arabidopsis display an essential role in growth and a reduced sensitivity to auxin. PLOS ONE 2013:8.This study provided genetic evidence showing that the TMK subfamily of receptor-like kinases acts downstream of auxin and affects multiple auxin-mediated processes, such as cell expansion and cell proliferation.
- 89.Rigal A, Ma Q, Robert S: Unraveling plant hormone signaling through the use of small molecules. Front Plant Sci 2014, 5:373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Grones P, Chen X, Simon S, Kaufmann WA, Riet De Rycke RD, Nodzyński T, Zažmalová E, Friml J: Auxin-binding pocket of ABP1 is crucial for its gain-of-function cellular and developmental roles. J Exp Bot 2015. [DOI] [PubMed]
- 91.•.Gao Y, Zhang Y, Zhang D, Dai X, Estelle M, Zhao Y: Auxin binding protein 1 (ABP1) is not required for either auxin signaling or Arabidopsis development. Proc Natl Acad Sci U S A 2015, 112:2275–2280.Using the CRISPR-based technology, the authors generated an Arabidopsis abp1 null mutant. They further found that this CRISPR-induced abp1 mutant and a T-DNA insertional mutant do not show obvious developmental defects compared with wild-type plants and questioned the role of ABP1 in auxin signaling.
- 92.Habets MEJ, Offringa R: Auxin binding protein 1: a red herring after all? Mol Plant 2015, 8:1131–1134. [DOI] [PubMed] [Google Scholar]
- 93.Enders TA, Oh S, Yang Z, Montgomery BL, Strader LC: Genome sequencing of Arabidopsis abp1–5 reveals second-site mutations that may affect phenotypes. Plant Cell 2015, 27:1820–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ohmiya A, Tanaka Y, Kadowaki K, Hayashi T: Cloning of genes encoding auxin-binding proteins (ABP19/20) from peach: significant peptide sequence similarity with germin-like proteins. Plant Cell Physiol 1998, 39: 492–499. [DOI] [PubMed] [Google Scholar]
- 95.Yin K, Han X, Xu Z, Xue H: Arabidopsis GLP4 is localized to the Golgi and binds auxin in vitro. Acta Biochim Biophys Sin 2009, 41:478–487. [DOI] [PubMed] [Google Scholar]