Abstract
Tip growth is a common strategy for the rapid elongation of cells to forage the environment and/or to target to long-distance destinations. In the model tip growth system of Arabidopsis pollen tubes, several small-molecule hormones regulate their elongation, but how these rapidly diffusing molecules control extremely localized growth remains mysterious. Here we show that the interconvertible salicylic acid (SA) and methylated SA (MeSA), well characterized for their roles in plant defense, oppositely regulate Arabidopsis pollen tip growth with SA being inhibitory and MeSA stimulatory. The effect of SA and MeSA was independent of known NPR3/NPR4 SA receptor-mediated signaling pathways. SA inhibited clathrin-mediated endocytosis in pollen tubes associated with an increased accumulation of less stretchable demethylated pectin in the apical wall, whereas MeSA did the opposite. Furthermore, SA and MeSA alter the apical activation of ROP1 GTPase, a key regulator of tip growth in pollen tubes, in an opposite manner. Interestingly, both MeSA methylesterase and SA methyltransferase, which catalyze the interconversion between SA and MeSA, are localized at the apical region of pollen tubes, indicating of the tip-localized production of SA and MeSA and consistent with their effects on the apical cellular activities. These findings suggest that local generation of a highly diffusible signal can regulate polarized cell growth, providing a novel mechanism of cell polarity control apart from the one involving protein and mRNA polarization.
Keywords: SA (salicylic acid), MeSA (methyl salicylic acid), endocytosis, FM4-64, CRIB4-GFP, ROP activity
INTRODUCTION
Salicylic acid (SA) is an important phytohormone involved in the regulation of plant defense, growth, and development (Yan and Dong, 2014). NPR1 was found to be an SA receptor by using a method of equilibrium dialysis (Wu et al., 2012). In the meantime, Fu et al. (2012) demonstrated that NPR3 and NPR4 are SA receptors, but did not find evidence for NPR1 as an SA receptor by applying nonequilibrium methods. The discrepancy on NPR1 likely resulted from the difference in the methodologies used. In plant defense, two SA receptors, NPR3 and NPR4, act in the Cullin3 ubiquitin E3 ligase complex with different SA binding affinities to mediate NPR1 degradation (Fu et al., 2012; Moreau et al., 2012; Yan and Dong, 2014). In the absence of SA (or extremely low cellular SA concentrations), NPR1 interacts with NPR4 and is targeted for degradation to suppress auto-defense. Lower concentrations (nanomolar range) of SA disrupt the interaction between NPR1 and NPR4 to remove nuclear NPR1 and suppress auto-defense. Increased concentrations of SA interrupt the interaction to stabilize NPR1 and promote downstream activation. At high concentrations of SA (micromolar range), NPR1 is degraded through the interaction with NPR3 to promote cell death (Fu et al., 2012; Moreau et al., 2012; Shi et al., 2013; Yan and Dong, 2014).
Apart from its importance in plant defense, SA has also been suggested to regulate plant growth and development (Lee and Park, 2010; Rivas-San Vicente and Plasencia, 2011). NahG (a gene encoding salicylic acid hydrolase) overexpressing transgenic plants or sid2 mutant with low levels of endogenous SA show increased growth rates and leaf biomasses (Abreu and Munné-Bosch, 2009; Rivas-San Vicente and Plasencia, 2011). On the contrary, plants with constitutively high levels of SA, such as cpr5 (constitutive expresser of PR5) or acd6–1 (accelerated cell death6–1) and agd2 (aberrant growth and death2), show reduced growth rates in both roots and aerial parts (Bowling et al., 1997; Rivas-San Vicente and Plasencia, 2011). Moreover, hydroxycinnamoyl transferase (HCT) mutant resulting in lower lignin and growth production may be attributed to the higher level of SA, since it can be restored by overexpression of NahG or by sid2 mutations (Gallego-Giraldo et al., 2011). The siz mutant (defective in the SUMO E3 ligase), showing a dwarflike shoot and increased accumulation of SA, can also be suppressed by overexpressing NahG (Miura et al., 2010; Ling et al., 2012). HAHB10, a sunflower HD-Zip 11 transcription factor, promotes flowering when upregulated by SA treatment (Dezar et al., 2011). Moreover, SA inhibits seed germination in favorable conditions, but promotes growth under abiotic stress (Guan and Scandalios, 1995; Rajjou et al., 2006; Alonso-Ramirez et al., 2009; Rivas-San Vicente and Plasencia, 2011). SA also affects leaf and chloroplast structures, stomata closure, and RubisCO activity (Rao et al., 1997; Norman et al., 2004; Xie et al., 2007; Lee et al., 2014). In addition, SA regulates root bending and waving (Du et al., 2013; Zhao et al., 2015). The effect of SA on root waving, though independent of the NPR3 and NPR4 SA receptors, appears to involve NPR1, calcium, and changes in the PIN2 localization to the plasma membrane (PM) (Zhao et al., 2015). Interestingly, SA is reported to inhibit clathrin-mediated endocytosis independent of the NPR3/4-dependent transcriptional regulation. SA treatments reduced the distribution of clathrin heavy chain (CHC) to the PM, while the bending angle after gravity stimulation was reduced in the chc2–2 mutant compared with wild-type. None-theless, the mechanisms by which SA regulates growth and developmental processes remain poorly characterized.
Pollen tube tip growth depends on polarized exocytosis and endocytosis at the cell apex (Yang, 2008). Exocytosis delivers nascent cell membrane and cell wall materials to the tip, allowing for tip growth (Wang et al., 2013; Rounds et al., 2014). Polar exocytosis has also been implicated in the regulation of signaling at the tip by delivering key regulators of ROP GTPase signaling (Hwang et al., 2008; Kato et al., 2010). ROP GTPases are activated at the apical PM of pollen tubes (Hwang et al., 2005) and are thought to be the master regulator of pollen tube tip growth by regulating calcium signaling, actin dynamics, and polar exocytosis that requires both calcium signaling and actin dynamics (Lin et al., 1996; Lin and Yang, 1997; Li et al., 1999; Fu et al., 2001; Gu et al., 2005; Hwang et al., 2008; Yong et al., 2008; Chang et al., 2013). ROP regulates polar exocytosis by two counteracting pathways that modulate actin dynamics: RIC3 (activating Ca2+ signaling and promoting F-actin disassembly) and RIC4 (promoting F-actin assembly) (Gu et al., 2005). Polar vesicle accumulation was regulated by the RIC4-dependent actin assembly, while vesicle exocytosis was facilitated by the RIC3-mediated actin disassembly (Yong et al., 2008). However, signals that regulate ROP GTPases at the tip of pollen tubes remain elusive.
Exocytosis delivers the major cell wall component at the pollen tube tip, methylated pectin, which is required for cell growth and tip-localized expansion. Methylated pectin is also known as soft pectin, as it can be extensively stretched to allow turgor-driven cell expansion. Methylated pectin is converted into demethylated pectin by pectin methylesterase (PME). In contrast, demethylated pectin, also known as hard pectin, is much more resistant to turgor-driven stretch. Demethylated pectin is primarily distributed at the shank to hold the turgor pressure, and is largely excluded from the tip in growing pollen tubes (Bosch et al., 2005; Röckel et al., 2008; Dardelle et al., 2010; Chebli et al., 2012; Mollet et al., 2013). PECTIN METHYLESTERASE 48 plays an important role in pollen grain germination. Knocking out PME48 resulted in delayed germination and double-tip phenotype in nearly 20% of pollen tubes (Leroux et al., 2015). Another pectin methylesterase, VANGUARD1 (VGD1), plays an important role in the regulation of pollen tube wall integrity, as knocking out VGD1 induced frequent pollen tube bursting in vitro (Jiang et al., 2005). Overexpressing PME1 in tobacco pollen tubes resulted in growth inhibition, while overexpression of a gene encoding a PME inhibitor protein, PMEI2, promoted pollen tube growth (Röckel et al., 2008). Thus, a balance between methylated and demethylated pectin levels is critical for proper pollen tube growth.
In addition to exocytosis-mediated pectin distribution, distribution of demethylated pectin may also be regulated by endocytosis. There is evidence that clathrin-mediated endocytosis (CME) internalizes demethylated pectin but not methylated pectin (Yu et al., 2002; Baluska et al., 2005). This is consistent with emerging evidence that supports a critical role for CME in pollen tube tip growth. Defects in AP2 (Adaptor Protein complex2), a conserved protein complex critical for CME, impaired pollen tube growth with less uptake of FM4–64 (N-(3-triethylammoniumpropyl)-4-(p-diethylaminophenyl-hexa-trienyl) pyridinium dibromide), which is inserted into the PM and internalized as a result of endocytosis (Kim et al., 2013). However, how CME is regulated in pollen tubes is completely unknown.
Here, we found that SA and MeSA antagonistically regulated Arabidopsis pollen tube growth by affecting CME. SA inhibited CME while MeSA promoted CME. A decrease in CME is associated with increased hard pectin composition in the pollen tube cell wall. Interestingly, two key enzymes that catalyze the interconversion between SA and MeSA are both localized to the tip of pollen tubes, indicating that the tip of pollen tube-localized subcellular production of these hormones plays an important role in the regulation of polar cell growth by regulating CME.
RESULTS
SA and MeSA Act Antagonistically to Regulate Pollen Tube Growth
Tip growth relies on the localized exocytosis to the apical region of cells, allowing for polar growth of cell membrane and walls to generate tubular cells such as pollen tubes and root hairs. Pollen tube growth is promoted by several plant hormones including auxin (Chen and Zhao, 2008; Wu et al., 2008), brassinosteroids (Vogler et al., 2014), and gibberellin (Singh et al., 2002; Chhun et al., 2007). Interestingly the same hormones also promote the expansion of plant cells undergoing diffuse growth, which is thought to be independent of localized exocytosis. Thus tip growth and diffuse growth may share some common regulatory mechanisms. To further test this notion, we examined the effect of various exogenously applied hormones on in vitro growth of Arabidopsis pollen tubes. We tested two concentrations, 1 µM and 10 µM, with ecotype Columbia-0 (Col-0) pollen tubes cultured on agar media at 28°C for 3 h. Both indole-3-acetic acid and gibberellic acid stimulated pollen tube elongation at 1 µM, as previously reported (Singh et al., 2002; Chhun et al., 2007; Chen and Zhao, 2008; Wu et al., 2008). Jasmonic acid also had a similar effect. In contrast, SA greatly inhibited pollen tube growth (Supplemental Figure 1). At 1–50 µM, SA inhibited pollen tube elongation and pollen grain germination in a concentration-dependent manner. At 20–30 µM, exogenous SA reduced pollen tube length by about 25% (Figure 1A) and the pollen germination rate by about 40% (Figure 1B). Dose-response analysis showed that SA concentrations at 1 µM or lower did not significantly affect Arabidopsis pollen tubes (Supplemental Figure 2), but SA concentrations at 20 µM or higher strongly inhibited pollen germination (Figure 1F). We used 20 µM in our subsequent experiments unless otherwise noted.
Figure 1. SA Inhibits Arabidopsis Pollen Tube Growth while MeSA Promotes Its Growth In Vitro.
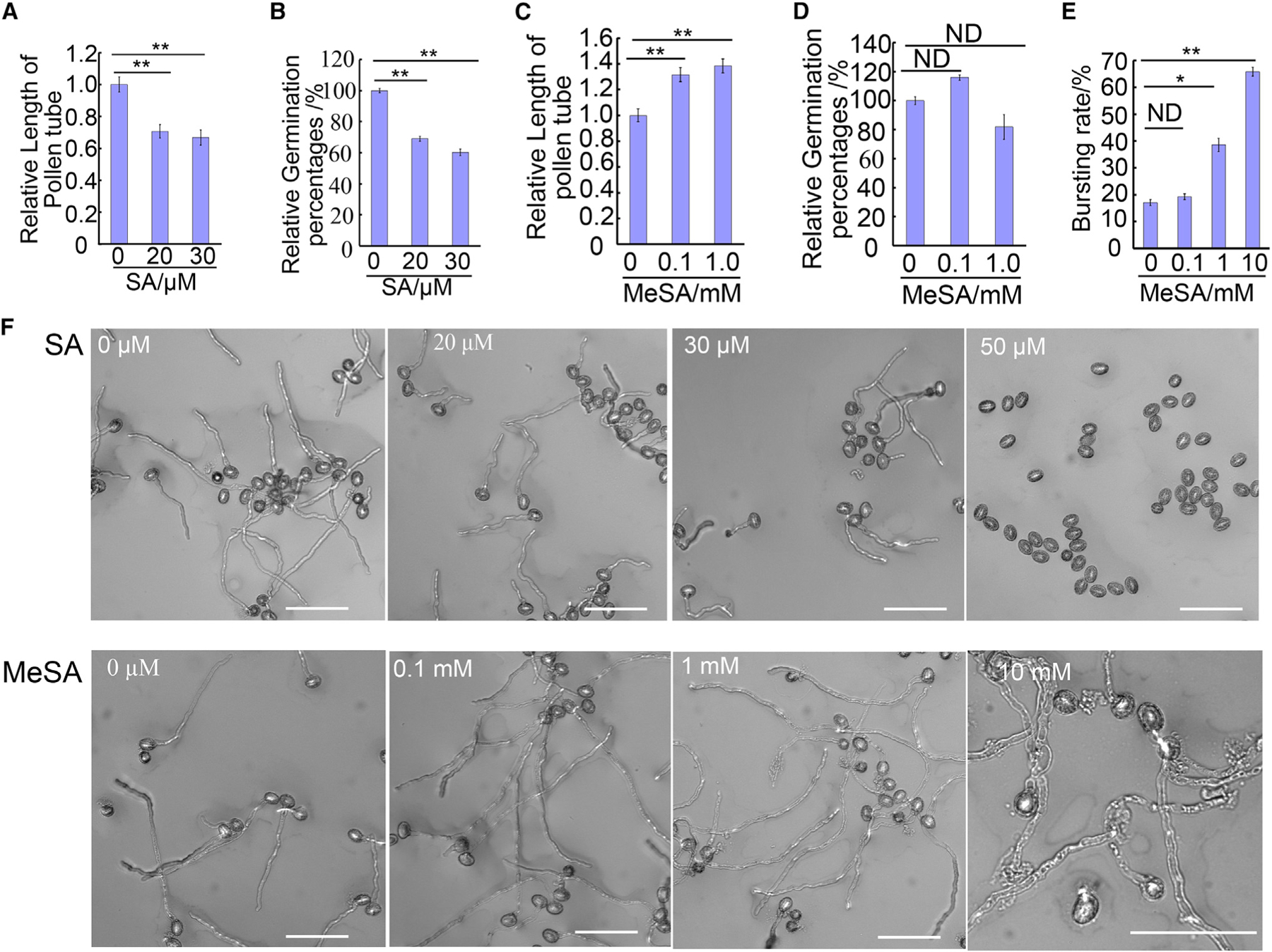
(A–E) Quantitative analysis of pollen tube length (A and C), germination rate (B and D), and bursting rate (E) under different concentrations of exogenously applied SA (A and B) or MeSA (C–E). SA inhibits pollen tube germination and elongation while MeSA promotes pollen tube elongation and causes pollen tubes to burst at 1 mM MeSA or higher.
(F) Representative pollen tube phenotype under different concentrations of SA or MeSA. Pollen tube length and germination rates were normalized to the respective control. Pollen grains were germinated for 3 h at 28°C with pollen germination medium. Scale bar, 100 µm.
Error bars show ±SEM; n > 100. *p < 0.05, **p < 0.01 (Dunnett’s test). ND, no significant difference.
As SA is a weak acid, we assessed whether the inhibitory effect of SA on pollen tube growth was due to changes in pH of media. The control medium without SA had a pH of 5.8, while 20 µM SA lowered the pH to 5.58. However, pollen tube growth did not exhibit any difference on media from pH 5.0 to pH 7.0 (Figure 2A). Thus, we conclude that the effect of SA is independent of the acidification of the media.
Figure 2. SA Suppresses Pollen Tube Growth Independent of Canonical SA Receptors.
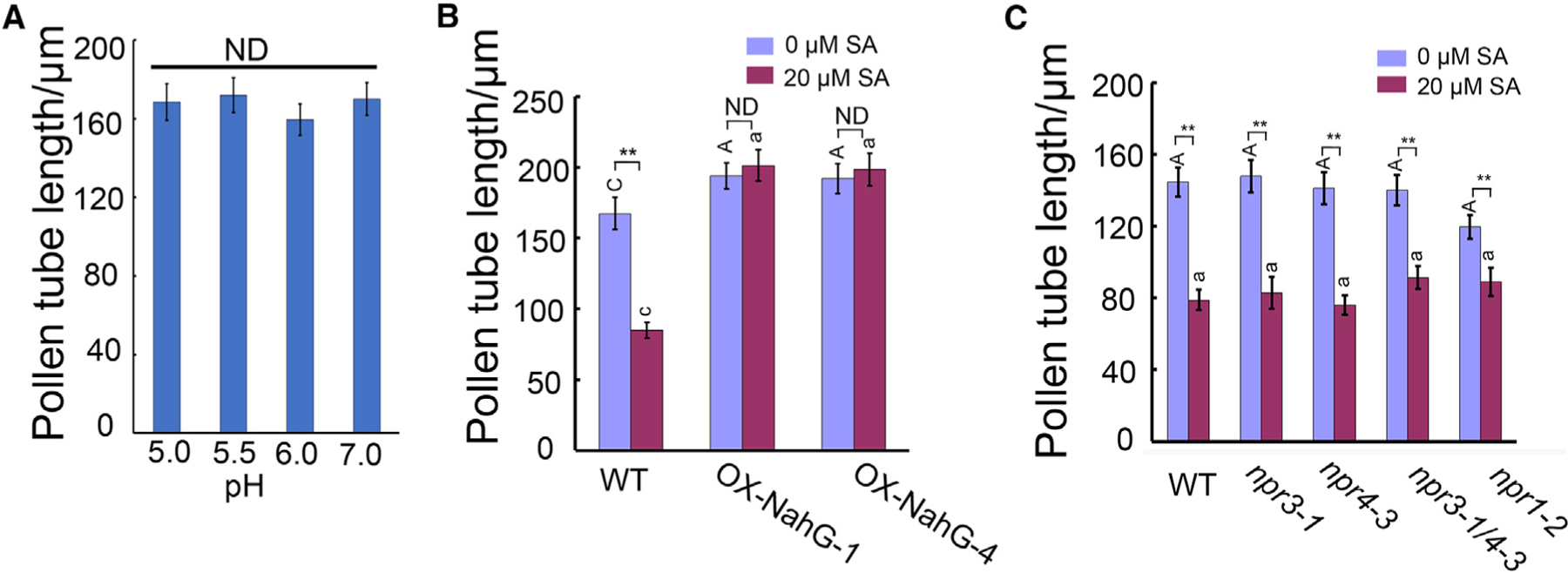
(A) The length of pollen tubes growing on pollen germination media with different pH.
(B) The length of wild-type or LAT52::NahG pollen tubes treated with 0 µM or 20 µM SA for 3 h. SA-induced inhibition of pollen tube growth is suppressed by SA hydroxylase NahG.
(C) Pollen tube length of different SA receptor and signaling mutants treated with 0 µM or 20 µM SA. The results show that npr1–2, npr3–1, npr4–3, and npr3–1 npr4–3 responded to SA in the same manner as wild-type.
Tukey’s test was used in (A) and two-way ANOVA with Tukey’s test in (B and C). A, C represent comparison from different genotypes with 0 µM SA treatment, and a, c comparison from different genotypes with 20 µM SA treatment. Error bars show ±SEM; n > 100. **p < 0.01. ND, no significant difference.
SA is converted to MeSA by SA methyltransferase in the cytoplasm (Dempsey, 2011; Seyfferth and Tsuda, 2014). To assess whether the effect of SA on pollen tube growth was due to its conversion to MeSA, we examined the effect of various levels of MeSA on the growth of pollen tubes. Surprisingly, at a concentration of 100 µM or higher, MeSA stimulated pollen tube elongation by 25%–30% (Figure 1C and 1F). At 1–10 mM, MeSA greatly increased the rate of pollen tube bursting to about 65% compared with about 15% for mock-treated pollen (Figure 1D and 1F). Bursting is often associated with cell wall weakening, a process important for pollen tube elongation (Jiang et al., 2005; Zhang et al., 2010). Thus the bursting effect of MeSA hints that it affects cell wall integrity. Lower concentrations of MeSA (1–1000 nM) did not affect pollen tube growth (Supplemental Figure 3). These results confirm that the inhibitory effect of SA is specific and direct, whereas its metabolic product MeSA may have a promotive effect on pollen tube elongation.
Endogenous SA Regulates Pollen Tube Growth Independent of the Canonical SA Signaling Pathway
We next determined the role of endogenous SA in the regulation of pollen tube growth by generating transgenic lines overexpressing the NahG gene encoding an SA-specific hydrolase (Abreu and Munné-Bosch, 2009) driven by the pollen-specific LAT52 promoter. Out of 21 transgenic plants, seven LAT52:NahG independent lines exhibited longer pollen tubes when cultured in vitro (about 25% longer compared with wild-type tubes) in the absence of exogenous SA. Two of these lines were chosen for further analyses (Figure 2B). As shown in Figure 2B, the LAT52::NahG pollen tubes were resistant to treatment with 20 µM SA. These results suggest that endogenous SA normally suppresses pollen tube elongation and that externally applied SA is taken up to inhibit pollen tube growth.
The concentration of effective SA in pollen tube growth inhibition is much lower compared with the concentration used in triggering defense responses, which is normally 100 µM or higher (Liu et al., 2005; Dempsey, 2011; Yan and Dong, 2014). The effect of low levels (1–20 µM) of exogenous SA strongly supports a “hormonal” role for SA in the regulation of pollen tube growth. Interestingly, a recent report demonstrated that SA inhibited CME in Arabidopsis root cells with an effective dosage similar to that inhibitory to pollen tube growth (Du et al., 2013). They also showed that the inhibitory pathway was independent of the canonical SA signaling pathway involving transcriptional regulation by the known SA receptors NPR3 and NPR4. Thus we tested pollen tube growth in Arabidopsis mutants defective in the canonical SA signaling, npr1–2, npr3–1, npr4–3, and npr3–1 npr4–3. All these mutants exhibited responses identical to that of 20 µM SA to wild-type pollen tubes (Figure 2C). This result suggests that SA’s inhibition to pollen tube growth is independent of the canonical SA signaling pathways that regulate gene transcription and defense responses.
SA and MeSA Antagonistically Affect Clathrin-Mediated Endocytosis
We next assessed the mechanisms by which SA and MeSA regulate pollen tube elongation through analyzing various cellular events that are known to be important for pollen tube tip growth. Given the reported effects of SA on CME (Du et al., 2013) and ROP function as a key regulator in pollen tube growth (Lin and Yang, 1997; Li et al., 1999; Gu et al., 2004; Nibau et al., 2006; Yang and Fu, 2007; Zhang and McCormick, 2007; Qin and Yang, 2011; Craddock and Yang, 2012; Nagawa et al., 2012), we speculated that SA and MeSA may regulate one or both of these two pathways. We first determined whether SA affected endocytosis by using FM4–64 staining (Bolte et al., 2004). Col-0 pollen grains were germinated on regular growth media, and 3 h later a small sample of pollen tubes was treated with 5 µM FM4–64 with or without 50 µM SA. A higher concentration of SA was used to ensure that a short treatment (3 min) was effective. Samples were visualized under confocal microscopy for 3 min after treatments, and endocytosis was measured as average cytosolic signal intensity divided by the PM signal intensity (4 pixels wide) from a line profile crossing the subapical region of pollen tubes (Figure 3A).
Figure 3. SA and MeSA Act Antagonistically to Regulate Pollen Tube Endocytosis.
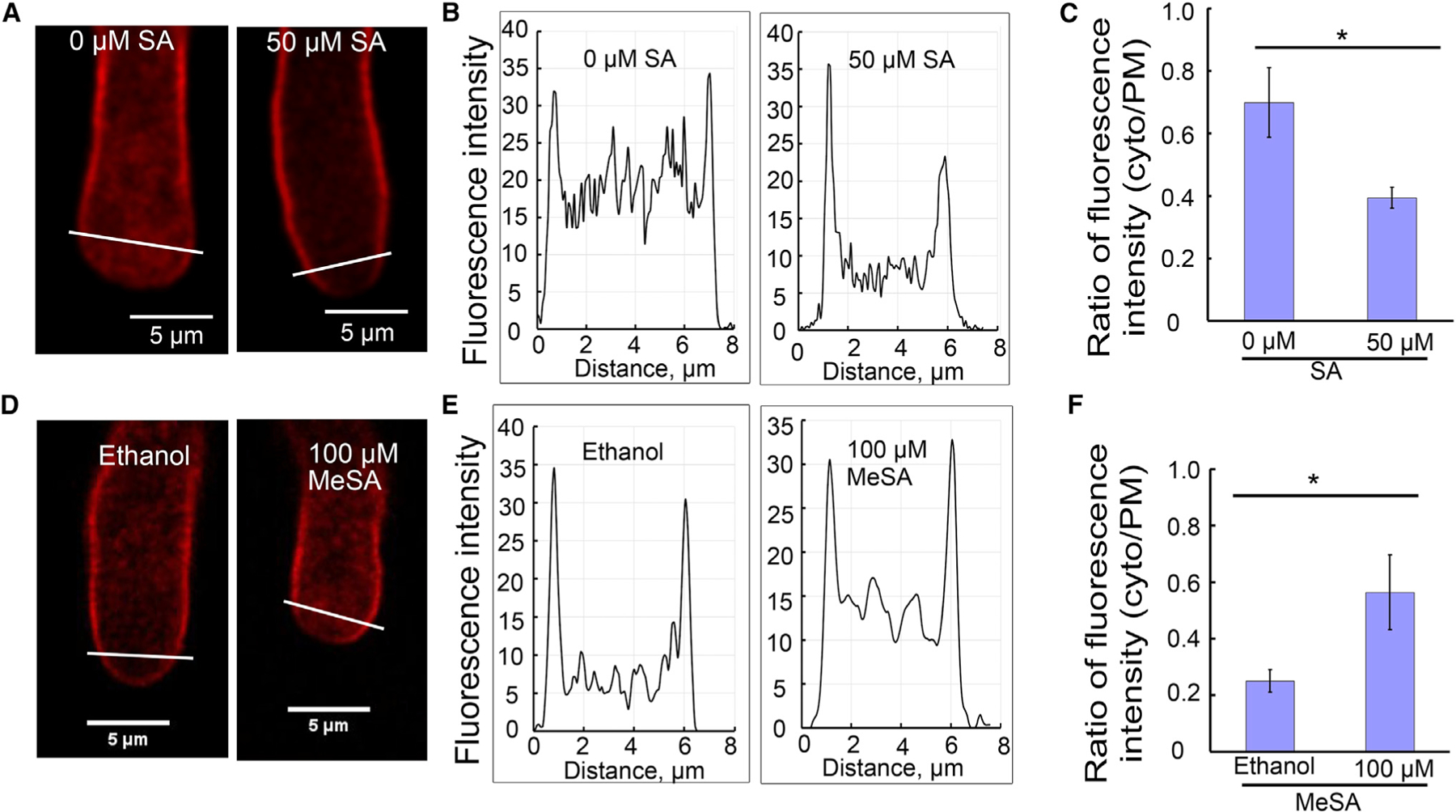
FM4–64 staining of pollen tubes treated with SA for 3 min or MeSA for 2 min.
(A and D) Representative visualization of endocytic tracer FM4–64 in pollen tubes, respectively treated with SA (A) and MeSA (D). The white lines across the pollen tube apical region shows the position of endocytosis measured. Scale bars, 5 µm.
(B and E) Representative graphs of fluorescence intensity of FM4–64 in the subapical region of pollen tubes shown in (A), (B), (D), and (E).
(C and F) Average ratio of FM4–64 (5 µM) fluorescence intensity (cytosol/PM) co-treated with SA (t = 3 min, n = 10) or MeSA (t = 2 min, n = 10) in wild-type pollen tubes.
Error bars show ±SEM. *p < 0.05 (Student’s t-test).
Representative images are shown in Figure 3A and 3D, and their line profile measurements in Figure 3B and 3E. At 50 µM, SA clearly inhibited endocytosis in pollen tubes (Figure 3B and 3C). From the line profile measurement, the high spikes on the leftmost and rightmost of the diagram were extracted as membrane signal and the relatively low spikes in between were measurements of cytosolic signals. The cytosolic FM4–64 signal was much lower in SA-treated pollen tubes (Figure 3C), compared with that of the control tubes. Signal quantification from about 10 pollen tubes in each treatment revealed approximately 40% reduction in the internalization of the membrane FM4–64 signal in SA-treated samples, as measured by the average cytosol/PM signal intensity ratio (Figure 3C). On the contrary, 100 µM MeSA promoted endocytosis by about two-fold, based on the same signal quantification method using images taken 2 min after treatment (Figure 3D–3F). A shorter time point was used to best represent the difference in MeSA-treated pollen tubes and ethanol-treated control pollen tubes. It was imperative that the analysis be carried out shortly after FM dye addition, because this would minimize the change in the cytosol/PM signal ratio due to the recycling of the internalized membrane to the PM. As a result, it was only valuable to compare endocytosis from images taken at earlier time points. In addition, we noticed a relatively weak membrane signal at the tip of pollen tubes compared with the shank region in all tubes. This may be due to rapid exocytosis at the tip, diluting the FM dye-stained membrane, and/or rapid endocytosis at the tip. For this reason, only the flank region was analyzed in this study. The cytosolic FM4–64 signal was not punctuated, but mostly diffuse, consistent with observations from other publications (Sousa et al., 2008; Zhao et al., 2010).
To test whether SA and MeSA affected the internalization of FM dye through CME, we made use of the chc2–2 mutant (Kitakura et al., 2011). Pollen tubes of the chc2–2 mutant grew to be much shorter compared with Col-0 (Figure 4A). Indeed, chc2–2 pollen tube elongation was not affected by 20 µM SA or 100 µM MeSA treatment (Figure 4A and 4B). To further test the involvement of CME, we used FM4–64 to measure endocytosis in chc2–2 mutant with SA or MeSA treatment. The uptake of FM4–64 in chc2–2 was remarkably reduced compared with wild-type (Figure 4C–4E). Consistent with the chc2–2 growth phenotype, neither SA nor MeSA affected FM4–64 internalization in chc2–2 pollen tubes (Figure 4F–4K). These results imply that SA and MeSA regulate CME in pollen tubes.
Figure 4. SA and MeSA Affect Clathrin-Mediated Endocytosis in Arabidopsis Pollen Tubes.
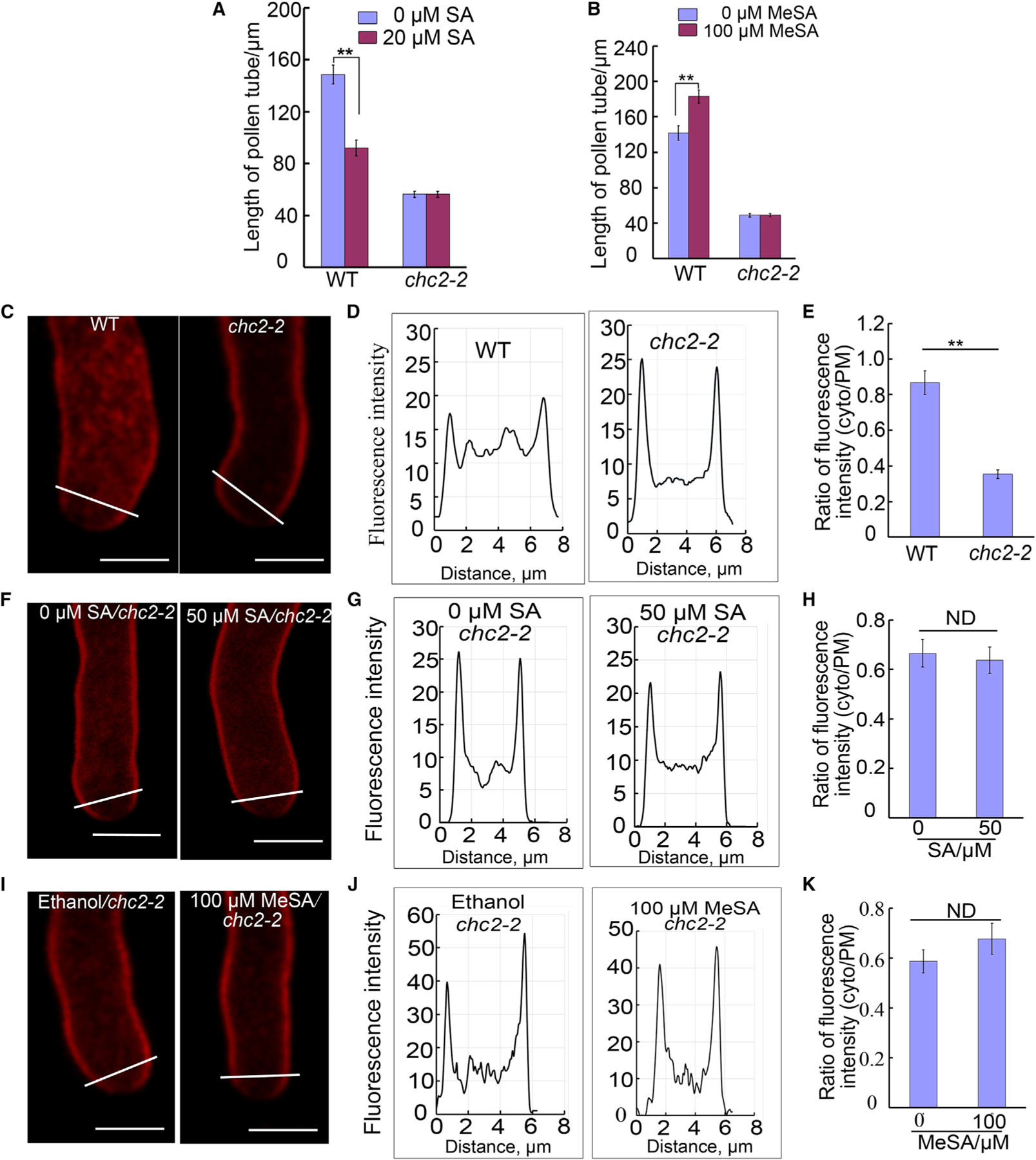
(A and B) The length of Col-0 and chc2–2 mutant pollen tubes treated with SA (A) or MeSA (B) for 3 h.
(C–E) FM4–64 staining of Col-0 or chc2–2 pollen tubes. (C) Representative images of pollen tube staining with endocytic tracer FM4–64 in Col-0 or chc2–2 pollen tubes, respectively. Scale bars, 5 µm. (D) Representative graphs showing fluorescence intensity of FM4–64 in the subapical region of pollen tubes for (C). (E) Average ratio of FM4–64 (5 µM) fluorescence intensity (cytosol/PM) in Col-0 (t = 3 min, n = 12) and chc2–2 (t = 3 min, n = 13) pollen tubes.
(F–H) FM4–64 staining of chc2–2 pollen tubes treated without SA or with 50 µM SA. (F) Representative visualization of endocytic tracer FM4–64 for mock (water) or 50 µM SA. Scale bars, 5 µm. (G) Representative graphs of fluorescence intensity of FM4–64 for (F). (H) Average ratio of FM4–64 (5 µM) fluorescence intensity (cytosol/PM) co-treated with mock (t = 15 min, n = 15) or 50 µM SA (t = 15 min, n = 13) in chc2–2 pollen tubes.
(I–K) FM4–64 staining of chc2–2 pollen tubes treated with mock (ethanol) or 100 µM MeSA. (I) Representative visualization of endocytic tracer FM4–64 for mock or 100 µM MeSA. Scale bars, 5 µm. (J) Representative graphs of fluorescence intensity of FM4–64 in pollen tubes for (I). (K) Average ratio of FM4–64 (5 µM) fluorescence intensity (cytosol/PM) co-treated with mock (ethanol; t = 15 min, n = 15) or 1000 µM MeSA (t = 15 min, n = 13) in chc2–2 pollen tubes. Error bars show ±SEM. **p < 0.01 (Student’s t-test), ND, no significant difference.
SA and MeSA Differentially Affect the Accumulation of Methylesterified and Demethylesterifed Pectins in Pollen Tubes
Pectin is a key component of the pollen tube cell wall and plays a crucial role in the regulation of tip growth and cell wall integrity at the tip of pollen tubes. Pectin exists in two forms, with differential extensibility under the turgor pressure: methylesterified pectin (also known as soft pectin) that is deformable under turgor pressure and de-esterified pectin (also known as hard pectin) that is capable of resisting turgor pressure. Soft pectin is the raw form of pectin, containing an ester group that blocks intermolecule interaction, and accumulates at the tip of the pollen tubes by exocytosis. Soft pectins are gradually demethylesterified by pectin methyl esterase (PME), and form ionic bonds with Ca2+ through the exposed COO— group to become hard pectin. Hard pectin accumulates at the shank and rest of the tube to hold against the internal turgor pressure (Bosch et al., 2005). Although secreted PME catalyzes the hardening of pectin, soft pectin at the tip may be protected by PMEIs (PME inhibitors), which are polarly localized at the tip of pollen tubes. Soft pectin, PME, and PMEI all rely on exocytosis to reach the cell surface. Evidence supports that CME is involved in the differential internalization of demethylated pectin but not methylated pectin (Yu et al., 2002; Baluska et al., 2002, 2005), and thus SA- and MeSA-regulated endocytosis might affect pectin distribution and the soft–hard pectin balance along the pollen tubes.
We employed immunostaining to visualize the soft and hard pectin distribution. JIM5 antibody specifically binds to hard pectin (weakly methylesterified homogalacturonan), while JIM7 binds specifically soft pectin (Clausen et al., 2003). Different batches of pollen tubes were fixed and immune-stained with JIM5 and JIM7 respectively, and images were taken by confocal microscopy for quantification using ImageJ. Signals on a line profile starting from the vertex of the pollen tube to either side along the cell wall were measured. Data from 10 tubes in each treatment were analyzed to plot the signal intensity against distance to the vertex (Figure 5). SA clearly increased the hard pectin level throughout the pollen tubes (Figure 5A and 5B), consistent with the earlier report that supports the internalization of hard pectin by CME (Baluska et al., 2002; Yu et al., 2002). On the contrary, MeSA reduced the hard pectin level (Figure 5C and 5D). JIM7 staining was reduced by 20 µM SA treatment (Figure 5E and 5F), but increased upon 100 µM MeSA treatment (Figure 5G and 5H). These results, at least in part, explain how SA inhibits pollen tube growth while MeSA promotes pollen tube growth and bursting.
Figure 5. Pectin Immunostaining of Col-0 Pollen Tubes Treated with SA or MeSA.
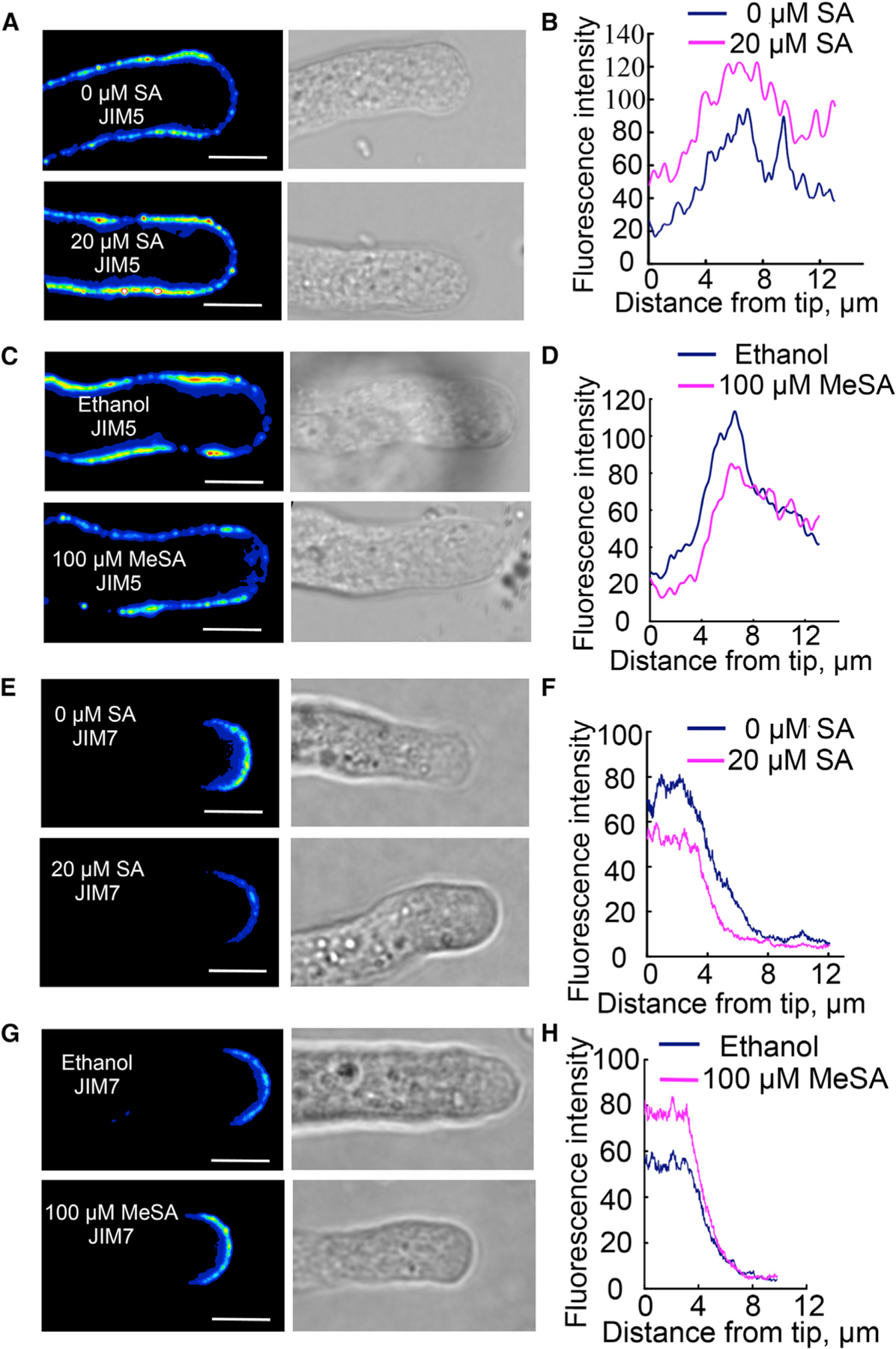
(A, C, E, and G) Representative images of pectin staining with JIM5 antibody to detect de-esterified pectin (hard pectin) (A and C) and with JIM7 antibody to detect esterified pectin (soft pectin) (E and G) in pollen tubes treated with 20 µM SA (A and E) or 100 µM MeSA (C and G). All images are in the same scale (bar = 5 µm).
(B, D, F, and H) Quantitative data of JIM5 (B and D) and JIM7 (F and H) staining in pollen tubes treated with 20 µM SA (B and F) or 100 µM MeSA (D and H).
Note that 20 µM SA treatment reduced esterified pectin and increased de-esterified pectin, while 100 µM MeSA increased the amount of esterified pectin and decreased de-esterified pectin. The data were the average from images taken at the medial sections from 10 tubes in each treatment.
SA and MeSA Have Antagonistic Effects on ROP Activity in Pollen Tubes
We next tested whether SA and MeSA affected ROP activity in pollen tubes, because ROPs have been shown to be a key regulator of pollen tube growth, exhibiting dynamic activation at the growing region of pollen tubes (Kost et al., 1999; Li et al., 1999; Hwang et al., 2005; Gu et al., 2005; Craddock et al., 2012; Chen and Friml, 2014). ROP1, ROP3, and ROP5 in Arabidopsis were believed to function redundantly at the pollen tube tip to regulate polarity and tip growth. CRIB4-GFP, a fusion protein of green fluorescence protein with the CRIB domain (Cdc42/Rac interactive binding domain) of RIC4, has recently been developed as a marker of ROP activity in transgenic Arabidopsis pollen tubes (Supplemental Figure 4A) (N.L. and Z.Y., unpublished results). We treated pollen tubes from LAT52::CRIB4-GFP transgenic plants with 20 µM SA (water as control) and 100 µM MeSA for 30 min (ethanol solution as control), respectively. Healthy pollen tubes were recorded by time-lapse imaging for 80 frames at 0.82 s per frame, and about 10 tubes in each treatment were recorded.
Videos were analyzed with an algorithm we recently developed (Tombo et al., submitted). The algorithm processes each frame independently and identifies the pollen tube shape and pollen tube vertex by a series of image filtering, mask generation, and best ellipses fitting. The algorithm then selected a 5-pixel-thick edge within 10 µm from the vertex as the region of interest for tip PM fluorescence signal. Signal intensity was output as a one-dimensional array along the distance to the vertex, and arrays from different frames of a given video were stacked together for a heatmap representation of membrane CRIB4 fluorescence intensity plotted by time and distance from the vertex (Supplemental Figure 4B). The heatmap was used to present the general dynamic of CRIB4 signal and the oscillation of CRIB4 signal intensity.
For comparison of CIRB4-GFP signal intensity among different treatment groups, membrane fluorescence signal intensity (as above) was divided by that of its immediate cytosolic neighbor, and recorded as an array of values representing different distances to the vertex. Arrays of values were averaged within samples of 10th to 50th frames of each given video, and about 10 videos in each treatment group were used for statistical analysis. The “relative intensity” used here was robust against photo-bleaching errors. Treatments and control plots were compared in the same axis sets, and we noted that SA increased CRIB4 signal in pollen tube tips (Figure 6A) while MeSA decreased CRIB4 signal in pollen tube tips (Figure 6B).
Figure 6. Antagonistic Effects of SA and MeSA on ROP Activity in Pollen Tubes.
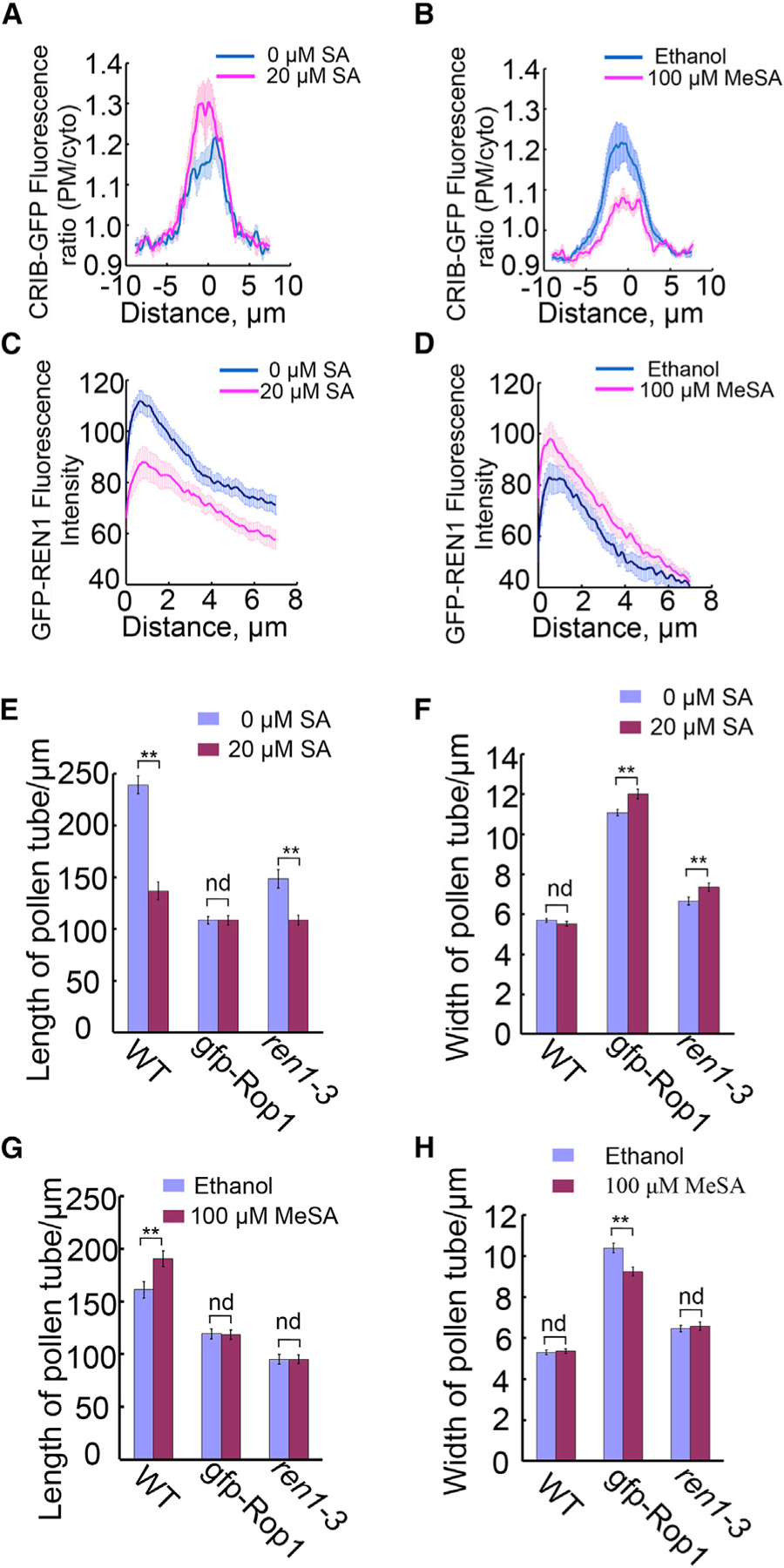
(A and B) Average relative intensity of CRIB4-GFP along the plasma membrane at the tip of pollen tubes treated with 20 µM SA (n = 13) and water control (n = 11) for 30 min (A), or 100 µM MeSA (n = 14) and ethanol control (n = 13) for 30 min (B). Note that SA increased CRIB4-GFP intensity while MeSA decreased it.
(C and D) Effects of SA or MeSA on GFP-REN1 distribution in cytosol of pollen tubes. Distribution of GFP-REN1 in pollen tubes treated with 20 µM SA (n = 11), 100 µM MeSA (n = 20), or controls (water n = 11, ethanol n = 12).
(E) SA inhibited ren1–3 pollen tube elongation but not GFP-ROP1.
(F) SA enhanced GFP-ROP1 and ren1–3 pollen tube width.
(G) MeSA did not affect the length of GFP-ROP1 or ren1–3 pollen tubes.
(H) MeSA decreased the width of GFP-ROP1 pollen tubes but not ren1–3 pollen tubes.
All graphs are presented as means ± SEM. **p < 0.01 (two-way ANOVA with Tukey’s test). nd, no significant difference.
Consistent with the changes in the CRIB4-GFP distribution, we found that SA and MeSA treatment affected REN1 distribution to the pollen tube tip. REN1, a RopGAP that deactivates ROPs, is localized to the apical cap and exocytic vesicles in the pollen tube tip (Supplemental Figure 4C) and is involved in maintaining the dynamic of the tip-localized ROP1 activity (Hwang et al., 2008). Thus an increase in the distribution of REN1 to the apical PM, where active ROP1 is localized, will lead to reduced ROP1 activity. We visualized REN1 distribution using the REN1 marker line (LAT52::GFP-REN1/ren1-1) (Hwang et al., 2008). Col-0 pollen tubes germinated for 2 h were treated with 20 µM SA or 100 µM MeSA for 30 min and then were observed using time-lapse confocal microscopy at 1.2-s intervals. Results were analyzed using the algorithm similar to that for CRIB4 measurement. A line along the PM within 4 µm with the vertex in the middle was drawn in the cytosol starting directly behind the pollen tube apical PM and ending 8 µm deep into the cytosol. The average fluorescence signal intensity on the line was stacked together by each frame in a video. About 15 videos in each treatment were averaged and the final data were plotted as shown in Figure 6C and 6D. SA deceased REN1 intensity in general with 8-µm depth in the pollen tube (Figure 6C), while MeSA increased it (Figure 6D). Taken together, these results suggest that SA promoted ROP1 activation while MeSA suppressed ROP1 activation at the tip of pollen tubes.
To further verify the hypothesis that SA and MeSA directly or indirectly regulate ROP1 activity at the tip of pollen tubes, we investigated whether SA and MeSA altered growth polarity in pollen tubes with increased ROP activity. We previously showed that increasing apical ROP activity over a threshold by either ROP1 overexpression or ren1 mutations caused growth depolarization (Hwang et al., 2008). Pollen tubes from a LAT52::GFP-ROP1 line with moderate ROP1 overexpression exhibit wider and shorter tubes (Hwang et al., 2008). Similar phenotypes are found in ren1–3, a weak ren1 allele with a T-DNA insertion in the sequences encoding the C-terminal region of REN1 protein (Hwang et al., 2008). Both of these two lines have higher ROP activity. SA treatment enhanced the width of GFP-ROP1 transgenic pollen tubes and ren1–3 tubes (Figure 6E and 6F). On the contrary, MeSA decreased the width of GFP-ROP1 pollen tubes, though not that of ren1–3 pollen tubes (Figure 6G and 6H). These results are consistent with the changes in ROP activity (Figure 6A and 6B) and REN1 distribution induced by SA and MeSA treatments (Figure 6C and 6D), and thus further support the hypothesis that SA or MeSA regulates the apical ROP activity in an antagonistic manner.
MeSA Methylesterase and SA Methyltransferase Localized to the Tip Region of Pollen Tubes
Because SA and MeSA regulate pollen tube growth apparently independent of the transcriptional SA signaling pathway, we hypothesize that SA and MeSA directly modulate tip-localized activities such as ROP activation and CME. How can highly diffusible MeSA and SA regulate these tip-localized activities? One possibility would be that SA and MeSA are somehow compartmentalized to regulate the apical activities. We thus tested whether enzymes involved in the generation of SA and MeSA are distributed to the apical region of pollen tubes. Methyl salicylate esterase (MES) converts MeSA to SA, while benzoic acid and SA carboxyl methyltransferase (BSMT) catalyzes SA methyl ester to form methyl salicylate (Forouhar et al., 2005; Koo et al., 2007; Park et al., 2007; Yang et al., 2008; An and Mou, 2011). We chose MES6 and BSMT1, which are preferentially expressed in pollen tubes (Koo et al., 2007; An and Mou, 2011).
To assess the subcellular localization of MES6 and BSMT1, we generated transgenic plants in which expressing LAT52::MES-GFP or LAT52::BSMT1-GFP. MES6-GFP was localized to the apical cortex of pollen tubes (Figure 7A–7C), a pattern highly similar to that of ICR1/RIP1 (Li et al., 2008), which is presumably associated with the exocyst complex that is involved in the tethering of vesicles to the PM. On the other hand, BSMT1-GFP is enriched in the apical region, similar to the distribution of exocytotic vesicles (Figure 7D). These localization patterns support the hypothesis that the interconversion of SA and MeSA takes place at the tip of pollen tubes to generate tip-localized SA and MeSA for the regulation of pollen tube tip growth, and are consistent with our finding that SA regulates pollen tube growth independent of the NPR1/NPR3/NPR4-dependent transcriptional pathways. Thus these hormones may act locally through a cytoplasmic receptor that directly regulates subcellular activities such as ROP activation and endocytosis at the PM.
Figure 7. Subcellular Localization of MES6-GFP and BSMT1-GFP in Arabidopsis Pollen Tubes.
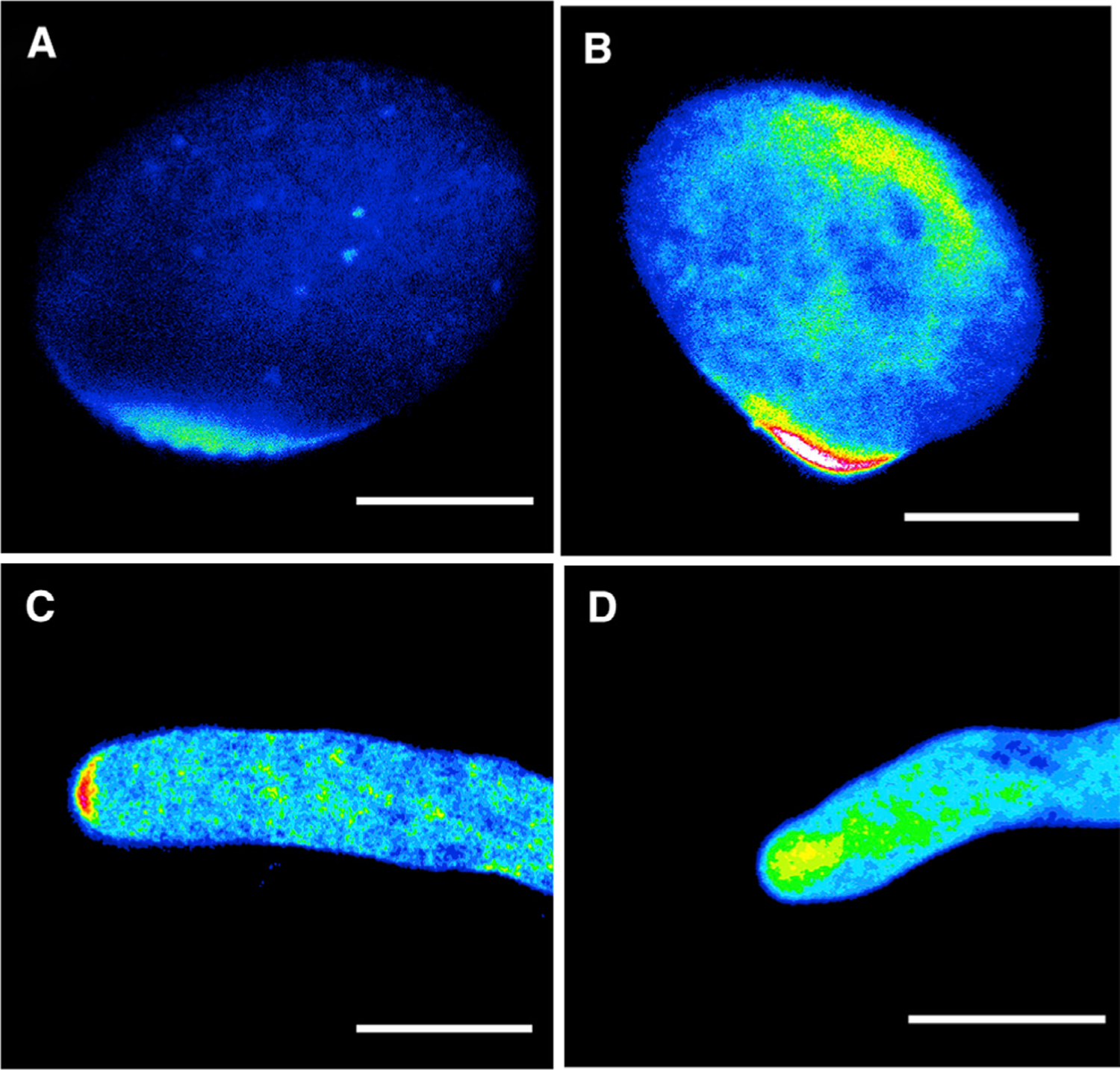
(A–C) MES6-GFP localization in pollen before germination (A), during germination (B), and after germination (C).
(D) BSMT1-GFP localization in growing pollen tubes.
Scale bars, 10 µm.
DISCUSSION
Localized subcellular distribution of proteins and mRNAs and localized protein activation provide important mechanisms for the control of cell polarization (Hwang et al., 2008; Yang, 2008; Johnson et al., 2011; Kitakura et al., 2011; Lau and Bergmann, 2012; Nagaoka et al., 2012; Thompson, 2013; Feigin et al., 2014; Singer-Krüger and Jansen, 2014; Treuner-Lange and Søgaard-Andersen, 2014; Fenix and Burnette, 2015; Gandalovilová et al., 2016). In this report, we present evidence that tip-localized synthesis and metabolism of small-molecule hormones modulate polar growth in pollen tubes. Our data suggest tip localization of the enzymes for the interconversion between SA and MeSA, which modulate pollen tube tip growth by acting on tip-localized endocytosis and ROP activation. Thus we propose that local generation of these highly diffusible signals provides an important new mechanism for the regulation of polar cell growth.
Local distribution and activity regulation of large molecules such as proteins can be readily achieved by vesicular trafficking and association with existing localized molecules, but how highly diffusible signals can achieve subcellular localization in their control of cell polarity processes has been a conundrum. Calcium, which is also highly diffusible, forms a tip-high gradient, but the mechanism by which the calcium gradient is generated is unknown. Several small-molecule plant hormones such as auxin, gibberellin, and brassinosteroids have been shown to regulate polarized tip growth in pollen tubes, but their mode of action has remained a mystery. The polarization of PIN auxin efflux carriers is thought to be regulated by auxin through a positive feedback loop, but subcellular distribution of auxin has not been detected (Adamowski, 2015). Similarly, cytokinin has also been shown to regulate PIN polarization (Marhavy et al., 2014), but there is no evidence that cytokinin is locally distributed within a cell. Our results suggest a novel mechanism for the regulation of tip growth by locally synthesizing SA and MeSA. It would be interesting to know whether SA and MeSA also form a tip-high gradient like calcium gradients, but no methodology is currently available for the measurement of intracellular distribution of these molecules. We propose that the localized synthesis of SA and MeSA lead to local accumulation of these signals that regulate tip-localized activities. This is consistent with our findings that SA and MeSA antagonistically affect the apical ROP activity and CME.
The effects of SA and MeSA on pollen tube growth are independent of the NPR3/4 SA receptors and NPR1 that regulates nuclear gene expression. In contrast, SA and MeSA respectively inhibit and promote CME, which is thought to localize to the shoulder of the pollen tube apex. SA has also been shown to inhibit clathrin-mediated endocytosis of PIN proteins in roots, suggesting that SA regulation of CME is a common action of SA (Yang, 2008; Kitakura et al., 2011; Nagawa et al., 2012; Du et al., 2013; Adamowski, 2015; Zhao et al., 2015). Indeed we found that SA increased the amount of demethylated pectin in the apical wall while MeSA did the opposite, consistent with their regulation of pollen tube tip growth. We also found that the apical ROP activity increased by SA treatment. This could be the indirect effect of SA through its regulation of CME, as CME also appears to promote ROP1 activation by internalizing a negative regulator of ROP1 signaling (Li et al., unpublished results). These findings regarding NPR3/4-independent roles of SA raise some exciting questions to be addressed in the future: (1) Which SA/MeSA receptors regulate CME and how do they regulate CME? (2) Does the NPR3/4-independent SA signaling also affect defense responses?
Our results showing that SA and MeSA have antagonistic effects also raise some interesting questions. First of all, what is the mechanism behind the antagonistic effects? MeSA may act as a competitive inhibitor of SA for binding to the receptor. These two chemicals share similar structures and can easily interconvert in vivo. This could explain why much higher concentrations of MeSA were needed to promote pollen tube growth. Furthermore, this possible mode of action is consistent with the finding that MeSA could not significantly stimulate pollen tube growth in SA-depleted mutants (Supplemental Figure 5). Alternatively, it is possible that both act as signals to activate two antagonistic pathways regulating CME. The fact that both enzymes involved in the interconversion are localized to the tip supports this hypothesis. Further experiments, such as identification of MeSA receptors, will be necessary to test this hypothesis. In either case, the SA-to-MeSA ratio is expected to play a critical role in the control of pollen tube growth. This is the first hormone known to exhibit such a balancing act involving the substrate and the product of an interconvertible reaction. This raises another interesting question: why the SA/MeSA ratio is used as a mechanism to modulate tip growth. This may be related to the defense role of SA in plants. Plants have to create a mechanism to balance growth and defense. For example, auxin can promote cell division and expansion in favorable conditions, but induces tumor formation if it is overproduced due to infection by agrobacteria (Ludwig-Müller, 2015). Pollen tube growth rate in vivo is variable depending on the paths where pollen tubes grow, and it is important to rapidly regulate the rate of growth when pollen tubes arrive at a specific path. For instance, pollen tube growth rate is less than half of the initial growth rate on stigma papilla cells (Cheung, 2010). The SA/MeSA ratio may be an elegant mechanism for the fine-tuning of tip growth rate for pollen tubes in vivo. It will be interesting to assess the significance of this ratio in the regulation of pollen tube growth during pollination.
METHODS
Plant Growth and Pollen Germination Conditions
Arabidopsis thaliana ecotype Col-0 wild-type, mutant, or transgenic plants were grown at 22°C in a controlled growth room with a 16-h light/8-h dark cycle. For pollen germination, Arabidopsis flowers were collected and pollen grains germinated on semi-solid agar medium with 18% sucrose, 1 µM CaCl2, 1 µM Ca(NO3)2, 1 mM MgSO4, 0.01% H3BO3, and 0.05% noble agar at 28°C for 2–3 h (Li et al., 1999).
Plasmid Construction and Plant Transformation
The NahG cDNA was cloned using PCR primers (attb-NahG-F: GTGGG GACAAGTTTGTACAAAAAAGCAGGCTTCATGAAAAACAATAAACTTGCT, attb-NahG-R: GTGGGGACCACTTTGTACAAGAAAGCTGGGTCCCCTTGACGTAGCGCACCCCC), which were designed according to the NahG sequence on the NCBI website and the GATEWAY system. Amplified NahG fragment was recombined into pDoner-Gateway vector (Clontech) by BP reaction, then sequenced with M13F and M13R primers. To create the Lat52:GFP-Lat52:NahG construct, we recombined the pDoner vector with NahG into the destination vector that had been modified to contain Lat52:GFP-Lat52:Gateway.
To generate AtMES6-GFP and AtBSMT1-GFP constructs, we cloned AtMES6 and AtBSMT1 from cDNA with the primers (MES6-F: CAAA AAAGCAGGCTTCATGGAGAATAAGAACCAGAAGCG; MES6-R: CAAGA AAGCTGGGTCATTTTTGCAATATTTATTTGCAATC) (BSMT1-F: CAAAAA AGCAGGCTTCATGGATCCAAGATTCATCAACAC; BSMT1-R: CAAGAAA GCTGGGTCCTTCTTAGTCAAGGAAACGACAAG) into Lat52:Gateway-GFP vector (Clontech) using the method described above. Transgenic Arabidopsis plants were generated by the floral dipping method with Agrobacterium strain (GV3101) carrying the above binary vectors (Clough and Bent, 1998). Basta resistant T1 plants were obtained and T2 homozygous plants were used for pollen tube observations.
Drug Treatments
For pollen tube length, germination, and bursting rate analysis, pollen grains were dusted on pollen germination medium with different concentrations of SA (same volume of water as control) or MeSA (same volume of ethanol as control) (Li et al., 1999). Three hours after germination, images were taken using a Nikon microscope equipped with Metamorph software. ImageJ was used to quantify pollen tube length, germination, and burst rates. SA stock solution was dissolved in sterilized water, and MeSA was dissolved in ethanol. To analyze the effects of SA or MeSA on various subcellular events, we applied a droplet of liquid germination medium containing 20 µM SA or 100 µM MeSA to the surface of pollen tubes that had been growing on agar media for 3 h on normal germination medium. Images or videos were taken 20 min after treatments using a Leica SP5 confocal microscope.
FM4–64 Dye Staining and Analysis of Endocytosis
FM4–64 (Invitrogen, Grand Island, NY, USA) was dissolved in water purified with Millipore system to 10 mM in stock. FM4–64 dye staining was performed at 2–3 h after pollen germination. A droplet (10 µl) of liquid pollen germination medium containing 5 µM FM4–64 dye and 50 µM SA or 100 µM MeSA was applied onto thin layers of solid pollen germination medium with growing pollen tubes. The videos were taken at 2 min (MeSA) or 3 min (SA) after the application of FM4–64 dye using a confocal microscope (Leica SP5). An excitation argon laser (excitation wavelength 488 nm) was used with emission wavelength between 550 and 700 nm. The data were analyzed by using open-source ImageJ. A parameter k = cytosol/PM (the ratio of FM4–64 intensity between cytosol and PM), derived by drawing a line across the tube at the subapical region, was used to indicate endocytosis rate.
Visualization and Analysis of Active ROPs in Pollen Tube
The cDNA of a fragment of RIC4 (AT5G16490) containing the CRIB domain (CRIB4, amino acids 64–130 of RIC4) was fused with GFP at the C terminus and subcloned into a binary vector, pCL, which was constructed by inserting the pollen tube-specific LAT52 promoter into pCAMBIA1300 using SalI and XbaI restriction sites. The pCL-CRIB4-GFP construct was introduced into wild-type A. thaliana plants using the Agrobacterium-mediated floral-dip method (Clough and Bent, 1998) to visualize the distribution of active ROP1 in the PM of pollen tubes.
Pollen tubes expressing CRIB4-GFP were observed under a Leica SP5 confocal microscope (488 nm excitation, 498–560 nm emission), and the median planes of pollen tube tips were imaged. The images were analyzed using MatLab. The relative signal intensity of CRIB4-GFP is defined as the ratio of the fluorescence intensity at the PM to the immediately cytosolic fluorescence intensity.
GFP-REN1 Fluorescence Intensity Measurement and Data Analysis
The subcellular localization of REN1 was analyzed using pollen tubes expressing Lat52::GFP-REN1 in the ren1–1 background as described previously (Hwang et al., 2008). Three hours after germination on a normal medium, pollen tubes were treated with 20 µM SA or 100 µM MeSA for 30 min. Videos were taken with the same confocal setting as for CRIB4-GFP (using argon-ion laser with 488 nm excitation and 498–520 nm emission wavelengths). The corresponding algorithm was used for data analysis.
Pectin Immunostaining
Pollen tubes were fixed with 4% paraformaldehyde (4% paraformaldehyde, 3 mM MgSO4, 2 mM CaCl2, 18% sucrose, and 50 mM PIPES buffer [pH 6.9]) after germinating on solid pollen germination medium with 20 µM SA or 100 µM MeSA for 2–3 h as mentioned above. Fixed pollen tubes were gently washed with PBS buffer (137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4 [pH 7.2]) three times for 10 min each wash. Pollen tubes were blocked with 1% (w/v) BSA diluted in PBS buffer at room temperature for 1 h. The primary antibody JIM5 (1:700 dilution) or JIM7 (1:500 dilution) was then used to stain weakly and highly methylated pectin, respectively, for 2 h. After washing the primary antibody with PBS, fluorescein isothiocyanate-conjugated secondary antibody (1:1000 dilution) was added and incubated for 2 h at room temperature. After washing with PBS three times for 10 min, pollen tubes were observed with the SP5 confocal microscope with 488 nm excitation and 500–600 nm emission, and analyzed using ImageJ.
Supplementary Material
ACKNOWLEDGMENTS
We thank Xinnian Dong and John Withers of Duke University and members of the Yang laboratory and the laboratory of Natasha Raikhel for helpful comments and discussion, and Dr. David Carter at the Center for Plant Cell Biology in the University of California-Riverside for assistance with microscopy work. We thank Thomas Eulgem (University of California, Riverside) for sharing published materials of 35S::NahG transgenic plants.
FUNDING
This study was supported by a grant from the National Institute of General Medical Sciences to Z.Y. (GM100130) and the Cooperative Innovation Center of Engineering and New Products for Developmental Biology of Hunan Province (20134486) to X.L. D.R. was partially supported by the China Scholarship Council.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information is available at Molecular Plant Online.
No conflict of interest declared.
REFERENCES
- Abreu ME, and Munné-Bosch S (2009). Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J. Exp. Bot 60:1261–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamowski M (2015). PIN-dependent auxin transport: action, regulation, and evolution. Plant Cell 27:20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Ramirez A, Rodriguez D, Reyes D, Jimenez J, Nicolas G, Lopez-Climent M, Gomez-Cadenas A, and Nicolas C (2009). Evidence for a role of gibberellins in salicylic acid modulated early plant responses to abiotic stress in Arabidopsis thaliana seeds. Plant Physiol 150:1335–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An C, and Mou Z (2011). Salicylic acid and its function in plant immunity. J. Integr. Plant Biol 53:412–428. [DOI] [PubMed] [Google Scholar]
- Baluska F, Hlavacka A, Samaj J, Palme K, Robinson DG, Matoh T, McCurdy DW, Menzel D, and Volkmann D (2002). F-actin-dependent endocytosis of cell wall pectins in meristematic root cells. Insights from brefeldin A-induced compartments. Plant Physiol 130:422–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baluska F, Liners F, Hlava A, Schlicht M, Van Cutsem P, Mccurdy DW, and Menzel D (2005). Cell wall pectins and xyloglucans are internalized into dividing root cells and accumulate within cell plates during cytokinesis. Protoplasma 225:141–155. [DOI] [PubMed] [Google Scholar]
- Bolte S, Talbot C, Boutte Y, Catrice O, Read ND, and Satiat-Jeunemaitre B (2004). FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J. Microsc 214: 159–173. [DOI] [PubMed] [Google Scholar]
- Bosch M, Cheung AY, and Hepler PK (2005). Pectin methylesterase, a regulator of pollen tube growth. Plant Physiol 138:1334–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling SA, Clarke JD, Liu Y, Klessig DF, Dongag X, and Carolina N (1997). The cpr5 mutant of Arabidopsis expresses both NPRl -dependent and NPRl −1ndependent resistance. Plant Cell 9:1573–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang F, Gu Y, Ma H, and Yang Z (2013). AtPRK2 promotes ROP1 activation via RopGEFs in the control of polarized pollen tube growth. Mol. Plant 6:1187–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebli Y, Kaneda M, Zerzour R, and Geitmann A (2012). The cell wall of the Arabidopsis pollen tube—spatial distribution, recycling, and network formation of polysaccharides. Plant Physiol 160:1940–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, and Friml J (2014). Rho-GTPase-regulated vesicle trafficking in plant cell polarity. Biochem. Soc. Trans 42:212–218. [DOI] [PubMed] [Google Scholar]
- Chen D, and Zhao J (2008). Free IAA in stigmas and styles during pollen germination and pollen tube growth of Nicotiana tabacum. Physiol. Plant 134:202–215. [DOI] [PubMed] [Google Scholar]
- Cheung AY (2010). The pollen tube journey in the pistil and imaging the in vivo process by two-photon microscopy the pollen tube journey in the pistil and imaging the in vivo process by two-photon microscopy. J. Exp. Bot 61:1907–1915. [DOI] [PubMed] [Google Scholar]
- Chhun T, Aya K, Asano K, Yamamoto E, Morinaka Y, Watanabe M, Kitano H, Ashikari M, Matsuoka M, and Ueguchi-Tanaka M (2007). Gibberellin regulates pollen viability and pollen tube growth in rice. Plant Cell 19:3876–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clausen MH, Willats WGT, and Paul J (2003). Synthetic methyl hexagalacturonate hapten inhibitors of anti- homogalacturonan monoclonal antibodies LM7, JIM5 and JIM7. Carbohydr. Res 338: 1797–1800. [DOI] [PubMed] [Google Scholar]
- Clough SJ, and Bent AF (1998). Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16:735–743. [DOI] [PubMed] [Google Scholar]
- Craddock C, and Yang Z (2012). Endocytic signaling pathways in leaves and roots; same players different rules. Front. Plant Sci 3:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craddock C, Lavagi I, and Yang Z (2012). New insights into Rho signaling from plant ROP/Rac GTPases. Trends Cell Biol 22:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardelle F, Lehner A, Ramdani Y, Bardor M, Lerouge P, Driouich A, and Mollet J-C (2010). Biochemical and immunocytological characterizations of Arabidopsis thaliana pollen tube cell wall. Plant Physiol 153:1563–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dempsey. (2011). Salicylic acid biosynthesis and metabolism Arabidopsis Book, 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dezar CA, Giacomelli JI, Manavella PA, Re DA, Alves-Ferreira M, Baldwin IT, Bonaventure G, and Chan RL (2011). HAHB10, a sunflower HD-Zip II transcription factor, participates in the induction of flowering and in the control of phytohormone-mediated responses to biotic stress. J. Exp. Bot 62:1061–1076. [DOI] [PubMed] [Google Scholar]
- Du Y, Tejos R, Beck M, Himschoot E, Li H, Robatzek S, Vanneste S, and Friml J (2013). Salicylic acid interferes with clathrin-mediated endocytic protein trafficking. Proc. Natl. Acad. Sci. USA 110:7946–7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feigin ME, Akshinthala SD, Araki K, Rosenberg AZ, Muthuswamy LB, Martin B, Lehmann BD, Berman HK, Pietenpol JA, Cardiff RD, et al. (2014). Mislocalization of the cell polarity protein scribble promotes mammary tumorigenesis and is associated with basal breast cancer. Cancer Res 74:3180–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenix AM, and Burnette DT (2015). A small part of myosin IIB takes on a big role in cell polarity. J. Cell Biol 209:11–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forouhar F, Yang Y, Kumar D, Chen Y, Fridman E, Park SW, Chiang Y, Acton TB, Montelione GT, Pichersky E, et al. (2005). Structural and biochemical studies identify tobacco SABP2 as a methyl salicylate esterase and implicate it in plant innate immunity. Proc. Natl. Acad. Sci. USA 102:1773–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Wu G, and Yang Z (2001). Rop GTPase-dependent dynamics of tip-localized F-actin controls tip growth in pollen tubes. J. Cell Biol 152:1019–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, et al. (2012). NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486:228–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego-Giraldo L, Escamilla-Trevino L, Jackson LA, and Dixon RA (2011). Salicylic acid mediates the reduced growth of lignin down-regulated plants. Proc. Natl. Acad. Sci. USA 108:20814–20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandalovilová A, Tomáš V, Daniel R, and Jan B (2016). Cell polarity signaling in the plasticity of cancer cell invasiveness. Oncotarget 7:25022–25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Wang Z, and Yang Z (2004). ROP/RAC GTPase: an old new master regulator for plant signaling. Curr. Opin. Plant Biol 7:527–536. [DOI] [PubMed] [Google Scholar]
- Gu Y, Fu Y, Dowd P, Li S, Vernoud V, Gilroy S, and Yang Z (2005). A Rho family GTPase controls actin dynamics and tip growth via two counteracting downstream pathways in pollen tubes. J. Cell Biol 169:127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan L, and Scandalios JG (1995). Developmentally related responses of maize catalase genes to salicylic acid. Proc. Natl. Acad. Sci. USA 92:5930–5934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J-U, Gu Y, Lee Y-J, and Yang Z (2005). Oscillatory ROP GTPase activation leads the oscillatory polarized growth of pollen tubes. Mol. Biol. Cell 16:5385–5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J-U, Vernoud V, Szumlanski A, Nielsen E, and Yang Z (2008). A tip-localized Rho GTPase-activating protein controls cell polarity by globally inhibiting Rho GTPase at the cell apex. Curr. Biol 18:1907–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Yang S-L, Xie L-F, Puah CS, Zhang X-Q, Yang W-C, Sundaresan V, and Ye D (2005). VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract. Plant Cell 17:584–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J, Jin M, and Lew D (2011). Symmetry breaking and the establishment of cell polarity in budding yeast. Curr. Opin. Genet. Dev 21:740–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato N, He H, and Steger AP (2010). A systems model of vesicle trafficking in Arabidopsis pollen tubes. Plant Physiol 152:590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Xu Z-Y, Song K, Kim DH, Kang H, Reichardt I, Sohn EJ, Friml J, Juergens G, and Hwang I (2013). Adaptor protein complex 2-mediated endocytosis is crucial for male reproductive organ development in Arabidopsis. Plant Cell 25:2970–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitakura S, Vanneste S, Robert S, Löfke C, Teichmann T, Tanaka H, and Friml J (2011). Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell 23:1920–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo YJ, Kim MA, Kim EH, Song JT, Jung C, Moon JK, Kim JH, Seo HS, Song SI, Kim JK, et al. (2007). Overexpression of salicylic acid carboxyl methyltransferase reduces salicylic acid-mediated pathogen resistance in Arabidopsis thaliana. Plant Mol. Biol 64:1–15. [DOI] [PubMed] [Google Scholar]
- Kost B, Lemichez E, Spielhofer P, Hong Y, Tolias K, Carpenter C, and Chua N (1999). Tube growth. J. Cell Biol 145:317–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau OS, and Bergmann DC (2012). Stomatal development: a plant’s perspective on cell polarity, cell fate transitions and intercellular communication. Development 139:3683–3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, and Park C-M (2010). Modulation of reactive oxygen species by salicylic acid in Arabidopsis seed germination under high salinity. Plant Signal. Behav 5:1534–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SY, Damodaran PN, and Roh KS (2014). Influence of salicylic acid on rubisco and rubisco activase in tobacco plant grown under sodium chloride in vitro. Saudi J. Biol. Sci 21:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroux C, Bouton S, Kiefer-Meyer MC, Fabrice TN, Mareck A, Guénin S, Fournet F, Ringli C, Pelloux J, Driouich A, et al. (2015). Pectin METHYLESTERASE48 is involved in Arabidopsis pollen grain germination. Plant Physiol 167:367–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Lin Y, Heath RM, Zhu MX, and Yang Z (1999). Control of pollen tube tip growth by a Rop GTPase-dependent pathway that leads to tip-localized calcium influx. Plant Cell 11:1731–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Gu Y, Yan A, Lord E, and Yang ZB (2008). RIP1 (ROP Interactive Partner 1)/ICR1 marks pollen germination sites and may act in the ROP1 pathway in the control of polarized pollen growth. Mol. Plant 1:1021–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, and Yang Z (1997). Inhibition of pollen tube elongation by microinjected anti-Rop1Ps antibodies suggests a crucial role for Rho-type GTPases in the control of tip growth. Plant Cell 9:1647–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Wang Y, Zhu J, and Yang Z (1996). Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes. Plant Cell 8:293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling Y, Zhang C, Chen T, Hao H, Liu P, Bressan RA, Hasegawa PM, Jin JB, and Lin J (2012). Mutation in SUMO E3 ligase, SIZ1, disrupts the mature female gametophyte in Arabidopsis. PLoS One 7:e29470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Holub EB, Alonso JM, Ecker JR, and Fobert PR (2005). An Arabidopsis NPR1-like gene, NPR4, is required for disease resistance. Plant J 41:304–318. [DOI] [PubMed] [Google Scholar]
- Ludwig-Müller J (2015). Bacteria and fungi controlling plant growth by manipulating auxin: balance between development and defense. Plant Physiol 172:4–12. [DOI] [PubMed] [Google Scholar]
- Marhavy P, Feraru E, Bielach A, and Offringa R (2014). Report cytokinin controls polarity of PIN1-dependent auxin transport during lateral root organogenesis. Curr. Biol 24:1031–1037. [DOI] [PubMed] [Google Scholar]
- Miura K, Lee J, Miura T, and Hasegawa PM (2010). SIZ1 controls cell growth and plant development in Arabidopsis through salicylic acid. Plant Cell Physiol 51:103–113. [DOI] [PubMed] [Google Scholar]
- Mollet J-C, Leroux C, Dardelle F, and Lehner A (2013). Cell wall composition, biosynthesis and remodeling during pollen tube growth. Plants 2:107–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau M, Tian M, and Klessig DF (2012). Salicylic acid binds NPR3 and NPR4 to regulate NPR1-dependent defense responses. Cell Res 22:1631–1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaoka K, Udagawa T, and Richter JD (2012). Cpeb mediated ZO-1 mRNA localization is required for epithelial tight junction assembly and cell polarity. Nat. Commun 29:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagawa S, Xu T, Lin D, Dhonukshe P, Zhang X, Friml J, Scheres B, Fu Y, and Yang Z (2012). ROP GTPase-dependent actin microfilaments promote PIN1 polarization by localized inhibition of clathrin-dependent endocytosis. PLoS Biol 10:e1001299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibau C, Wu HM, and Cheung AY (2006). RAC/ROP GTPases: “hubs” for signal integration and diversification in plants. Trends Plant Sci 11:309–315. [DOI] [PubMed] [Google Scholar]
- Norman C, Howell KA, Millar AH, Whelan JM, and Day DA (2004). Salicylic acid is an uncoupler and inhibitor of mitochondrial electron transport. Plant Physiol 134:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S-W, Kaimoyo E, Kumar D, Mosher S, and Klessig DF (2007). Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318:113–116. [DOI] [PubMed] [Google Scholar]
- Qin Y, and Yang Z (2011). Rapid tip growth: insights from pollen tubes. Semin. Cell Dev. Biol 22:816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajjou L, Belghazi M, Huguet R, Robin C, Moreau A, Job C, and Job D (2006). Proteomic investigation of the effect of salicylic acid on Arabidopsis seed germination and establishment of early defense mechanisms. Plant Physiol 141:910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao MV, Paliyath G, Ormrod DP, Murr DP, and Watkins CB (1997). Influence of salicylic acid on H2O2 production, oxidative stress, and H2O2-metabolizing enzymes. Salicylic acid-mediated oxidative damage requires H2O2. Plant Physiol 115:137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-San Vicente M, and Plasencia J (2011). Salicylic acid beyond defence: its role in plant growth and development. J. Exp. Bot 62:3321–3338. [DOI] [PubMed] [Google Scholar]
- Röckel N, Wolf S, Kost B, Rausch T, and Greiner S (2008). Elaborate spatial patterning of cell-wall PME and PMEI at the pollen tube tip involves PMEI endocytosis, and reflects the distribution of esterified and de-esterified pectins. Plant J 53:133–143. [DOI] [PubMed] [Google Scholar]
- Rounds CM, Hepler PK, and Winship LJ (2014). The apical actin fringe contributes to localized cell wall deposition and polarized growth in the lily pollen tube. Plant Physiol 166:139–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyfferth C, and Tsuda K (2014). Salicylic acid signal transduction: the initiation of biosynthesis, perception and transcriptional reprogramming. Front. Plant Sci 5:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z, Maximova S, Liu Y, Verica J, and Guiltinan MJ (2013). The salicylic acid receptor NPR3 is a negative regulator of the transcriptional defense response during early flower development in Arabidopsis. Mol. Plant 6:802–816. [DOI] [PubMed] [Google Scholar]
- Singer-Krüger B, and Jansen R-P (2014). Here, there, everywhere. mRNA localization in budding yeast. RNA Biol 11:1031–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh D, Jermakow A, and Swain S (2002). Gibberellins are required for seed development and pollen tube growth in Arabidopsis. Plant Cell 14:3133–3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa E, Kost B, and Malhó R (2008). Arabidopsis phosphatidylinositol-4-monophosphate 5-kinase 4 regulates pollen tube growth and polarity by modulating membrane recycling. Plant Cell 20:3050–3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson BJ (2013). Cell polarity: models and mechanisms from yeast, worms and flies. Development 140:13–21. [DOI] [PubMed] [Google Scholar]
- Treuner-Lange A, and Søgaard-Andersen L (2014). Regulation of cell polarity in bacteria. J. Cell Biol 206:7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogler F, Schmalzl C, Englhart M, Bircheneder M, and Sprunck S (2014). Brassinosteroids promote Arabidopsis pollen germination and growth. Plant Reprod 27:153–167. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhuang X, Cai Y, Cheung AY, and Jiang L (2013). Apical F-actin-regulated exocytic targeting of NtPPME1 is essential for construction and rigidity of the pollen tube cell wall. Plant J 76:367–379. [DOI] [PubMed] [Google Scholar]
- Wu JZ, Lin Y, Zhang XL, Pang DW, and Zhao J (2008). IAA stimulates pollen tube growth and mediates the modification of its wall composition and structure in Torenia fournieri. J. Exp. Bot 59:2529–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Zhang D, Chu JY, Boyle P, Wang Y, Brindle ID, De Luca V, and Després C (2012). The Arabidopsis NPR1 protein is a receptor for the plant defense hormone salicylic acid. Cell Rep 1:639–647. [DOI] [PubMed] [Google Scholar]
- Xie Z, Shane ZZ, Cook E, and Shen QJ (2007). Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid-inducible WRKY gene. Plant Mol. Biol 64:293–303. [DOI] [PubMed] [Google Scholar]
- Yan S, and Dong X (2014). Perception of the plant immune signal salicylic acid. Curr. Opin. Plant Biol 20C:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z (2008). Cell polarity signaling in Arabidopsis. Annu. Rev. Cell Dev. Biol 24:551–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, and Fu Y (2007). ROP/RAC GTPase signaling. Curr. Opin. Plant Biol 10:490–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Xu R, Ma C-J, Vlot AC, Klessig DF, and Pichersky E (2008). Inactive methyl indole-3-acetic acid ester can be hydrolyzed and activated by several esterases belonging to the AtMES esterase family of Arabidopsis. Plant Physiol 147:1034–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong JL, Szumlanski A, Nielsen E, and Yang Z (2008). Rho-GTPase-dependent filamentous actin dynamics coordinate vesicle targeting and exocytosis during tip growth. J. Cell Biol 181:1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Hlavacka A, Matoh T, Volkmann D, Menzel D, Goldbach HE, and Baluska F (2002). Short-term boron deprivation inhibits endocytosis of cell wall pectins in meristematic cells of maize and wheat root apices. Plant Physiol 130:415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, and McCormick S (2007). A distinct mechanism regulating a pollen-specific guanine nucleotide exchange factor for the small GTPase Rop in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 104:18830–18835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GY, Feng J, Wu J, and Wang XW (2010). BoPMEI1, a pollen-specific pectin methylesterase inhibitor, has an essential role in pollen tube growth. Planta 231:1323–1334. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Yan A, Feijó JA, Furutani M, Takenawa T, Hwang I, Fu Y, and Yang Z (2010). Phosphoinositides regulate clathrin-dependent endocytosis at the tip of pollen tubes in Arabidopsis and tobacco. Plant Cell 22:4031–4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X, Wang J, Yuan J, Wang X, Zhao Q, Kong P, and Zhang X (2015). NITRIC OXIDE-ASSOCIATED PROTEIN1 (AtNOA1) is essential for salicylic acid-induced root waving in Arabidopsis thaliana. New Phytol 1:1–13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


