Abstract
Saudi Arabia is one of the countries that has been affected by COVID-19. At the beginning of March 2020, it revealed a steadily rising number of laboratory-confirmed cases. By 20th May 2020, 59,854 infected cases had been confirmed, with 329 deaths. To prevent a further outbreak of COVID-19, this article discusses the current understanding of COVID-19 and compares it with the outbreak of Middle East Respiratory Syndrome (MERS) in 2012 in Saudi Arabia. It also discusses the causes, transmission, symptoms, diagnosis, treatments and prevention measures to identify an applicable measure to control COVID-19.
Keywords: Middle East Respiratory Syndrome (MERS), Coronavirus disease-2019 (COVID-19), Causes, Transmission, Symptoms, Diagnosis, Treatments, Prevention measures, Saudi Arabia
1. Introduction
Within the last ten years, there have been two major outbreaks of coronavirus in Saudi Arabia. This first of these was in 2012, with the outbreak of Middle East Respiratory Syndrome-coronavirus (MERS-CoV), which is known to have originated within Saudi Arabia via zoonotic transmission from dromedary camels. The second outbreak, which originated in Wuhan, Hubei province, China, in December 2019, was novel coronavirus-2019 (2019-nCoV), resulting in a disease identified as COVID-19. The latter has claimed and is still claiming the lives of many people around the world, leaving grieving family and friends, a fragile global economy, and frightened people with an uncertain future despite the extensive efforts in each country guided by the world health organization (WHO) (Lai et al., 2020). For example, the first reported case of MERS-CoV in the world was a male Saudi citizen who died of severe pneumonia and multiorgan failure in Jeddah in June 2012. In September 2012, it was identified that the disease was caused by a novel coronavirus, which was given the names nCoV and HCoV-EMC and later also became known as MERS-CoV (Zaki et al., 2012). However, a retrospective study carried out in November 2012 that tested stored respiratory and serum samples of patients who were victims of an outbreak of acute respiratory illness confirmed the presence of MERS-CoV cases as early as April 2012 in Zarqa, Jordan (Hijawi et al., 2013). In 2012, a Qatari patient confirmed to have MERS was taken to the United Kingdom (UK) for treatment, and in 2013, a severe case of MERS was confirmed to be a traveller returning from Pakistan and Saudi Arabia (Farooq et al., 2020). By 2019, the virus had spread not only across the Arabian Peninsula, but also globally, with the number of countries affected totalling 27 (Table 1).
Table 1.
Countries with laboratory-confirmed cases of MERS-CoV infection by Centers for Disease Control and Prevention (CDC, 2019a).
| Countries in or near the Arabian Peninsula that have reported MERS cases | Countries outside the Arabian Peninsula with travel-associated MERS cases |
|---|---|
| Saudi Arabia, Kuwait, Qatar, Oman, Bahrain, Oman, Yemen, United Arab Emirates, Jordan, Lebanon and Iran | Egypt, Tunisia, Algeria, Turkey, Greece, Italy, Netherlands, UK, Austria, France, Germany, Malaysia, China, Philippines, Republic of Korea, Thailand and United States of America (USA) |
Laboratory tests are still being performed for suspected cases, both in the Middle East and all over the world, for travellers returning from Middle Eastern countries owing to the high risk. The number of cases is still increasing, affecting hundreds of people around the world, which can largely be attributed to imported cases from the Middle East. Statistics reveal that the number of people infected and the number of deaths caused by MERS-CoV are continuing to increase. According to WHO, between the outbreak in 2012 and the end of January 2020, MERS-CoV had infected 2519 people globally, resulting in 866 associated deaths, a case fatality rate (CFR) of 34.3%. The highest number of cases was reported by Saudi authorities: 2121 cases with 788 related deaths, and the CFR peaked at 37.1% (WHO, 2020).
Similarly, the current outbreak of 2019-nCoV, which originated in Wuhan, has spread across the world. While the swift decision by China to implement a lockdown meant that the country had managed to control the spread, it seems that fresh waves of infection are still a possibility. Furthermore, the disease is continuing to spread in many other countries. As of 20th May 2020, 4,761,559 confirmed cases and 317,525 deaths had been reported in 215 countries and territories, translating into a CFR of 6.66% (WHO, 2020c). A total of 59,854 cases and 329 deaths (CFR of 0.55%) have been reported by Saudi Arabia, which is far more than the total number of worldwide cases reported for MERS (WHO, 2020a). This substantial difference in the incidence of disease is probably due to the lower infection rate (R0) of MERS, potentially owing to its greater severity and mortality rate. 2019-nCoV is a mild upper respiratory illness that may develop into severe interstitial pneumonia and finally into Acute Respiratory Distress Syndrome (ARDS) (Song et al., 2019). Despite the similarity among different coronaviruses, COVID-19 shows different pathogenetic, epidemiological and clinical features to MERS-CoV, a phenomenon that is not yet fully understood. In response to the recent COVID-19 outbreak, this paper reviews the latest data available for MERS and evaluates the preventative measures described in the literature. The aim of this paper is to obtain knowledge and apply it to the current COVID-19 outbreak in Saudi Arabia. Since Saudi Arabia attracts a high number of visitors and is the place of pilgrimage for Muslims around the world, poor control and a lack of preventative measures may lead to the disease spreading locally and internationally. Therefore, this paper will discuss in depth the origin, risk factors, clinical and pathological features, laboratory characteristics, prevention measures and current developments in the treatment of MERS and COVID-19.
2. History and emergence of human coronaviruses (HCoVs)
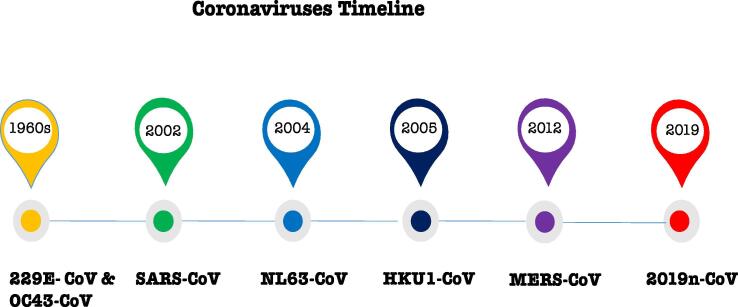
HCoVs develop crown-like spikes on the surface and belong to the Coronaviridae family and subfamily. They are a group of highly diverse, single-stranded, positive-sense ribonucleic acid viruses with an envelope (Shereen et al., 2020). These viruses are responsible for respiratory, hepatic, gastrointestinal and neurological diseases of varying severity in a wide range of animal species, including humans. There are four different genera of coronaviruses: αCoV, βCoV, γCoV, and δCoV (Zumla et al., 2016). The history of HCoVs can be traced back to the 1960s, when 229E and OC43 were some of the first HCoVs confirmed to cause respiratory infections in humans. Despite several reports identifying that these viruses cause lower respiratory tract infection, it was generally believed that coronaviruses had low pathogenicity until the emergence of severe acute respiratory syndrome coronavirus (SARS-CoV) in 2002. The SARS outbreak in Guangdong province, China, in November 2002 resulted in more than 8400 cases with a fatality rate more than 10% (Bleibtreu et al., 2019a).
Later, the HCoVs NL63 and HKU1 were identified in 2004 and 2005 respectively but could not be replicated in the laboratory. Recently, confirmed cases caused by MERS-CoV and 2019-nCoV were identified in 2012 and 2019 respectively (Fig. 1) (Hui, 2017, Song et al., 2019). The phylogenetic analysis of 2019-nCoV revealed that it is closely related to (88–89% similarity) two bat-derived SARS-like coronaviruses, namely bat-SL-CoVZC45 and bat-SL-CoVZXC21. Conversely, it is very dissimilar to SARS-CoV and MERS-CoV, displaying a difference of 79% and 50% respectively.
Fig. 1.
The timeline of identified HCoVs that have infected people (CDC, 2020).
3. Causes
3.1. MERS
MERS-CoV is a zoonotic virus, which means it is transmitted between animals and humans. Studies have confirmed that people can be infected via direct or indirect contact with infected dromedary camels. According to WHO (2019), MERS-CoV has been reported in dromedaries in several regions, including the Middle East, Africa, Europe, and South Asia. The outbreak of MERS-CoV is attributed to the dromedary camel trade between numerous Arabian Peninsula regions in addition to other countries such as Kenya, Nigeria, Tunisia, Burkina Faso, Sudan, Egypt Ethiopia and Morocco (Corman et al., 2014, Müller et al., 2014, Miguel, et al., 2017). Although the origin of the virus is not fully clear, it is believed that it may have originated in bats and was later spread to camels sometime in the distant past (Song et al., 2019, Farooq et al., 2020, Killerby et al., 2020). Interestingly, studies on dromedary camel serum collections dating as far back as 1983–1997 indicated that anti-MERS-CoV or anti-MERS-like CoV antibodies were present in the serum, indicating that MERS-CoV may have been widespread in East African countries such as Somalia, Sudan and Egypt as far back as the date of the samples (Müller et al., 2014). Furthermore, a phylogenetic analysis discovered the presence of five different coronavirus lineages in camels, including the recombinant lineage causing the MERS epidemic (Sabir et al., 2016, Bleibtreu, et al., 2019a). Subsequently, in 2012, Saudi Arabia registered the first human death from MERS-CoV: a 60-year-old man who died of respiratory and renal failure only 11 days after being admitted to hospital in Jeddah (Zaki et al., 2012). By September of the same year, a case was confirmed in the UK, where a patient suffered a severe respiratory infection associated with MERS-CoV after travelling to the Middle East (Bermingham et al., 2012). In both cases, it was possible to duplicate the virus in an in vitro tissue culture model, and it was then isolated and identified (Zaki et al., 2012). Furthermore, in April 2013, two healthcare professionals working in an intensive care unit (ICU) died from MERS-CoV infection at Al Zarqa Hospital in Jordan (Hijawi et al., 2013). The human-to-human transmission of MERS continues to spread through community clusters and/or in healthcare settings worldwide (Table 2). Confirmed cases of MERS are still being reported to WHO by many countries (WHO, 2020b).
Table 3.
The key characteristics of 2019-nCoV.
| 2019-nCoV Characteristics |
|---|
|
| Moreover, the transmission of 2019-nCoV via the gastrointestinal route has been confirmed, as was the case for MERS-CoV (Petrosillo et al., 2020). For example, 2019-nCoV was found in gastrointestinal tract specimens (stool and rectal swabs), saliva and urine, and even in oesophageal erosion and the bleeding site of patients with severe peptic ulcer (Xie and Chen, 2020). This was explained by the great binding affinity (~15 nM) of 2019-nCoV with ACE2, which is 10- to 20-fold higher than ACE2′s binding affinity with SARS-CoV (Wrapp et al., 2020). According to the recent reports published by the SMOH and WHO, human-to-human transmission is the main cause of the large-scale transmission of COVID-19 infection. The reproductive number (R0) estimated by WHO for COVID-19 is between 2 and 2.5, which is higher than that of SARS-CoV (1.7–1.9) and MERS (<1). However, other studies have estimated a much higher R0 value of 4 (Chen, 2020). This might explain the rapid development of COVID-19 and its ability to transmit on a global scale at a much faster rate than MERS. Furthermore, the School of Public Health of the University of Hong Kong recently confirmed that a patient’s dog was infected with a low level of 2019-nCoV via its nasal and oral cavity, with no prior signs of disease. This might be the first confirmed human-to-animal transmission, which explains the high transmissibility of the disease from and to animals (El Zowalaty and Järhult, 2020). It also highlights the potential need to socially isolate not only from fellow human beings, but also from pets. Despite all the available data on COVID-19, the exact mechanism of 2019-nCoV transmission is not fully understood. Consequently, the world, including Saudi Arabia, has witnessed a lockdown, which was unimaginable by the majority only a few months earlier. Countries have closed their borders, public events have been cancelled, and people have been asked to stay at home and leave only if absolutely necessary. After the initial outbreak in China, when the stringent lockdown measures were implemented, there was a decrease in the number of infected cases in China, with the majority having recovered (Shereen et al., 2020). However, in Saudi Arabia, although the number of cases seems to have plateaued somewhat, it is still not decreasing. The outbreak seems to be concentrated in highly populated cities such as Riyadh, Jeddah, Makkah and Al Madinah (CDC, 2020). On 14th of March, Saudi authorities imposed a 12-hour curfew to fight the outbreak of COVID-19 in all cities and governorates. Following this, on 6th of April, further measures in the form of a 24-hour curfew and a lockdown were imposed on the cities of Riyadh, Tabuk, Dammam, Dhahran and Hofuf, as well as the governorates of Jeddah, Taif, Qatif and Khobar. These actions were taken to support the efforts made by the Kingdom to confront the coronavirus pandemic. To sum up, the current 2019-nCoV seems to have relatively low pathogenicity but high transmissibility compared with earlier HCoVs (Chen, 2020). Table 3 illustrates the main characteristic of 2019-nCoV. The average incubation period for COVID-19 can be as along as 5 days, while the quarantine period should be 14 days from the last exposure date. This is the longest incubation period compared with similar types of HCoVs. However, the incubation period remains a topic of debate in the literature, as incubation periods of up to 27 days or above have been proposed by some studies (Chen, 2020, Shim et al., 2020). |
Table 2.
Epidemiology characteristics of COVID-19 and MERS-CoV across the globe and in the Kingdom of Saudi Arabia (KSA) (up to 20th of May 2020) (WHO, 2020c).
| Epidemiology characteristics | MERS-CoV | COVID-19 |
|---|---|---|
| Original Location | Jeddah, Saudi Arabia | Wuhan, China |
| Outbreak | 12 countries | 215 countries and territories |
| Global Total Cases (KSA) |
2,553 (2,121) up to 31st March 2020 |
4,761,559+ (59,854 + ) |
| Global Total Deaths (KSA) |
876 (788) up to 31st March 2020 |
(317,525 + ) (329 + ) |
| Healthcare Worker Cases (%) | 18.6 | 3.8 |
| Reproductive Number |
<1.00 | greater than3.28 |
| Incubation Period (Days) | 4.5–5.2 | 4.75–6.4 |
| Serial Interval (Days) | 12.6 | 2.6–7.5 |
| Global CFR % (KSA) |
34.30 (37.10) |
6.66 (0.55) |
| CFR with Comorbidities (%) | 60.0 | 73.3 |
3.2. COVID-19
In many ways, COVID-19 is not very different from MERS since it is caused by a zoonotic virus that belongs to the same family of HCoVs. Studies indicate that 2019-nCoV occurred at a live animal market in China, and it was realised that it was transmitted between an animal and a human in late 2019 (Bogoch et al., 2020, Lu et al., 2020a).
The developed pneumonia was interpreted as the emergence of novel coronavirus, and it was first called SARS-CoV-2. On 30th of January 2020, WHO declared the outbreak of COVID-19 to be a ‘public health emergency of international concern’. On 2nd of March 2020, just 32 days after the WHO declaration, the Saudi Ministry of Health (SMOH) confirmed the first COVID-19 case in the country in a traveller who had returned from the Islamic Republic of Iran. Initially, the cases recorded in Saudi Arabia occurred in the east of the country in people who had a recent travel history to Iran via Bahrain. A week later, the number of laboratory-confirmed cases amplified and has been rising since then. The majority of the cases were non-Saudi residents who had returned from abroad. On 24th of March, the SMOH reported the first death from COVID-19 in Al-Madinah Al-Menorah in Saudi Arabia, with 767 cases confirmed. The person who died was an elderly resident who was a Pakistani national (Wegaya, 2020).
4. The trend of the MERS outbreak
The outbreak of MERS-CoV spread rapidly in many cities in Saudi Arabia. Owing to its high infectivity rate, many healthcare professionals who had been in contact with the infected patients were infected with it. Furthermore, the MERS-CoV outbreak was observed in nearby countries, such as Kuwait, Jordan, Qatar and Bahrain, and as far as Tunisia. Healthcare systems in the above-mentioned countries responded quickly to the reports of these epidemics. Indeed, although a few patients developed mild infections, the MERS fatality rate was high a >34.3% (Bleibtreu et al., 2019a). Although the MERS epidemic had spread to over 27 countries, none were affected as severely as Saudi Arabia, which had reported more than 71% of the total confirmed cases, as shown in Table 2. Unlike SARS-CoV that disappeared less than two years after evolving, MERS-CoV has not disappeared, and seven years later, it still poses a threat. For example, the SMOH reported to WHO that ten new cases of MERS-CoV infections, which included one fatality, occurred between 1st and 30th of November 2019. These cases were found in different regions, including Riyadh (four), Madinah (two), Al-Qassim (one), Assir (one), Taif (one), and Makkah (one). To date, WHO continues to receive reports of hospital outbreaks among healthcare workers, patients and visitors (WHO, 2020). It can be said that MERS-CoV has the ability to spread among humans after direct or indirect contact with dromedary camels. MERS-CoV causes severe pneumonia that increases the rate of mortality (Song et al., 2019, Killerby et al., 2020). However, the government of Saudi Arabia continues to monitor the outbreak and epidemiological situation of MERS and conducts risk assessments based on the latest available information provided by WHO.
5. The trend of the COVID-19 outbreak
As mentioned above, the outbreak of COVID-19 started in Wuhan, China, in late December 2019. Subsequently, the virus spread rapidly throughout the globe, including Saudi Arabia, where the first case was confirmed on 2nd of March 2020. With the world having become a global village, this puts into perspective the ease of the spread of the virus. The fast growth and ease of commercial air travel and limited specific cures or vaccines available for HCoV infections led to its rapid spread (Lai et al., 2020). To date, COVID-19 has caused tens of thousands (>317,529) of fatalities, which include both the young and the old. With the current lack of antivirals for COVID-19, the vital factors that determine the recovery of a patient include the immune system of the individual and the availability of artificial respiratory devices. As mentioned earlier, COVID-19 is on the rise in Saudi Arabia. The authorities have identified returning travellers as the carriers of COVID-19 and the main reason for its outbreak. Moreover, gatherings of these visitors with local residents, such as in family gatherings and holly places, have been a major contributor to the spread of the disease within the population. The data in Table 2 suggests that 2019-nCoV was able to rapidly spread among the population despite the strong measures implemented by the authorities. The national dashboard of data for the number of confirmed cases still displays a daily increasing trend of confirmed cases in all regions, especially around the centre and west of the country. For example, on 31st of March, the SMOH reported 1563 confirmed cases with ten deaths. Ten days later, the number of confirmed cases increased to 3651 with 47 deaths, while on 21st of April, the number of people infected and the number of deaths increased dramatically to 11,631 and 109 respectively. To date, despite the 24-hour early quarantine and lockdown policies applied (on 15th of March), the number of confirmed cases are still growing.
Most importantly, the trend of the COVID-19 outbreak in Saudi Arabia and the rest of the world has had other devastating effects. These include economic instability and challenges related to sustainability, especially in countries like the USA, the UK, and other countries in Europe. Consequently, on 11th of March 2020, WHO declared that this COVID-19 outbreak was the sixth public health emergency of international concern, following H1N-2009, polio-2014, Ebola-2014 in West Africa, Zika-2016, and Ebola-2019 in the Democratic Republic of Congo. Therefore, governments, healthcare professionals, leaders and the public need to cooperate on a global level to develop effective strategies to stop the COVID-2019 outbreak (Yoo, 2020).
6. Transmission
6.1. MERS
Although several field surveys on many types of animals, both domestic and wild, including dromedary camels (Camelus dromedarius), Bactrian camels (Camelus bactrianus), cattle, sheep, chickens, swine, ducks, bats, buffalo, goats and equids, have been conducted, the exact mode of transmission of MERS-CoV to humans is not yet fully understood. However, epidemiologic, genetic and phenotypic studies suggest that dromedary camels might be the main intermediary reservoirs of MERS-CoV (Azhar et al., 2014, Reusken et al., 2014, Conzade et al., 2018). It has been reported that MERS-CoV can survive in camel’s milk for long periods, although no viable virus is detected after pasteurisation at 63 °C for longer than half an hour. Therefore, it is believed that camels are the intermediary host species for MERS-CoV. However, to date, the origin and the mode of transmission in many primary MERS cases remain uncertain. Although some people support the hypothesis that bats are the primary source of transmission of MERS-CoV infections to humans, not enough data supports this hypothesis. For example, studies have found MERS-CoV-specific antibodies in dromedary camels in many countries, with the exception of the Netherlands (Reusken et al., 2013a), Australia (Hemida et al., 2014) and Kazakhstan (Miguel et al., 2016). Moreover, a study of more than 1000 samples from Taphozous bats found only one fragment of the virus that closely matched the human isolate (Memish et al., 2013).
The human-to-human transmission of MERS-CoV can occur within households and communities, and especially within hospitals. The largest MERS-CoV outbreaks within healthcare settings occurred in Saudi Arabia, followed by the United Arab Emirates and the Republic of Korea (Azhar et al., 2019). According to the WHO report on MERS in December 2019, among a total of 17 new laboratory-confirmed cases globally, 15 cases were reported by Saudi Arabia, with five associated deaths (WHO, 2020). Although studies indicate that MERS-CoV does not appear to transmit easily from person to person unless there is close contact, such as providing clinical care (Drosten et al., 2014, Korea Centers for Disease Control and Prevention, 2015, Zumla et al., 2015, Kim et al., 2017, Azhar et al., 2019), <50% of infected patients can transmit the virus to other individuals they come into contact with (Hui et al., 2018). The strong measures implemented to prevent MERS-CoV infection in hospital settings and nosocomial outbreaks is highly important. Despite the available knowledge, more studies need to be conducted to determine whether MERS-CoV is transmitted via direct or indirect contact, airborne droplets or ingestion (Azhar et al., 2014). WHO expects further cases of MERS-CoV infection to emerge in the Middle East and that the infection will continue to be exported to other countries by individuals who might develop the infection after exposure to dromedary camels or an infected individual. Until an effective vaccine is discovered, it is vital to increase public awareness of the disease and educate them on the prevention measures to limit the risk of its transmission and thus avoid another outbreak of MERS-CoV (Killerby et al., 2020).
6.2. COVID-19
2019-nCoV is the third zoonotic coronavirus to cause an outbreak in the 21st century. It is a highly contagious virus that is transmitted rapidly among people, which led to a global pandemic. Similar to the previous outbreaks of coronaviruses, SARS-CoV and MERS-CoV, which were transmitted from bats to palm civets or dromedary camels and finally to humans, 2019-nCoV is also believed to have an intermediate host in its route from bats to humans (Xie and Chen, 2020). It has been suggested that pangolins are a potential intermediate host since they have high similarity to the virus genome, 85.5% to 92.4%, representing two sub-lineages of novel coronavirus in the phylogenetic tree (Lam et al., 2020). In another study, it was found that 2019-nCoV is a recombinant of bat and snake coronaviruses when compared with relative synonymous codon among different animal species (Ji et al., 2020). Further confirmation that 2019-nCoV was transmitted to humans through animals comes from its link to fish and wild animal markets (CDC, 2019b). Nevertheless, after having infected a human, the recent outcomes and new cases of infections confirm that 2019-nCoV can be transmitted from human to human through direct contact (Shereen et al., 2020, Shim et al., 2020). Studies also showed that the virus is capable of spreading via respiratory droplets from a distance of 2 m or even via contaminated surfaces (Lai et al., 2020). The Saudi data centre reveals a daily increase in the number of new confirmed COVID-19 cases being transmitted within families and within the healthcare setting to healthcare workers.
7. Clinical features
The unique symptoms of any infection make the identification and detection of the disease easier for differential diagnosis with other viral diseases. However, the absence of specific clinical features for MERS-CoV infection makes this task a major challenge (Garbati et al., 2016, Mohd et al., 2016). This leads to delays in deploying the precautions that would prevent potential secondary contamination (Bleibtreu et al., 2019a). Along with this, it can result in medical confusion and inappropriate patient management (Bleibtreu et al., 2018). The clinical features of MERS are very variable, which makes it difficult for differential diagnosis with other viral respiratory diseases. These features range from no symptoms to a flu-like syndrome, pneumonia and ARDS (Mohd et al., 2016, Aleanizy et al., 2017). Likewise, the clinical features of COVID-19 greatly resemble viral pneumonia caused by SARS and MERS. The majority of COVID-19 cases are mild (81%) with self-limiting features and those infected usually recover in two weeks (Wu and McGoogan, 2020). Most people (about 80%) recover from the disease without needing special treatment. Approximately 1 out of 6 COVID-19 patients become seriously ill and have breathing difficulties (WHO, 2020). The most common symptoms of MERS and COVID-19 include fever, cough, fatigue, sore throat and dyspnoea. Digestive system disorders (diarrhoea) and acute renal failure are associated with MERS, whereas rhinorrhoea, sneezing, ground-glass opacity, blood disorders and bilateral pneumonia are more likely to be symptoms of COVID-19 (Xie and Chen, 2020). Patients with COVID-19 suffer more respiratory symptoms than patients with MERS (Xie and Chen, 2020, Yi et al., 2020, Yoo, 2020). Table 4 summarises the systemic and respiratory disorders related to MERS and COVID-19.
Table 4.
The main symptoms of MERS and COVID-19.
| MERS | COVID-19 | |
|---|---|---|
| Systemic Disorders | Cough, Fever, Headache, Tiredness, Sputum production, Myalgia, Diarrhoea, Digestive system signs, Chills, Arthralgia, Odynophagia, Haemoptysis, Acute renal failure, Multi-organ failure. | Dry cough, Fever, Headache, Tiredness, Sputum production, Myalgia, Diarrhoea (2% of patients), Haemoptysis, Lymphopenia, Blood disorders. |
| Respiratory Disorders | Sore throat, Dyspnoea, Haemoptysis, Rapid development of pneumonia, ARDS | Sore throat, Rhinorrhoea and Sneezing, Pharyngalgia, Dyspnoea, Haemoptysis, Bilateral pneumonia, Ground-glass opacity, Pneumonia, ARDS. |
8. Diagnosis
The optimal test for the diagnosis of HCoV infections is the reverse transcription polymerase chain reaction test (RT-PCR). Other medical investigations such as serology tests and computed tomography (CT) scans can also be used as supporting diagnostic tests in most cases, but are often used for second-line diagnostic investigation.
8.1. MERS
The PCR-based detection method is presently the best available technique for the identification of the virus in a respiratory sample, and also for the detection of infections caused by MERS-CoV. Several other clinical specimens, such as sputum, endotracheal aspirate, bronchoalveolar lavage, nasal or nasopharyngeal swabs, faeces, urine, blood and lung tissue can also be used to diagnose the type of infection (Hui et al., 2018, Azhar et al., 2019). Serology tests can also be performed and they are particularly useful for cases that have a high suspicion of MERS-CoV, but have negative PCR results. These include serum neutralisation assays (Müller et al., 2015), microarrays (Reusken et al., 2013b) and newly enzyme-linked immunosorbent assay (ELISA) confirmed by a micro-neutralisation test (Trivedi et al., 2018). These tests are normally able to detect the relevant antibodies ten days after the disease develops (Mohd et al., 2016, Xie et al., 2020). Blood tests can therefore reveal if the person was previously infected, indicated by the presence of MERS-CoV antibodies (Medical News Today, 2020). Additionally, radiographic results can indicate infection with MERS. Significant radiographic features include bilateral hilar infiltration, unilateral or bilateral patchy densities or infiltrates, segmented or lobar opacities, ground-glass opacities and small pleural effusions (Hui, 2017).
8.2. COVID-19
Similar to MERS, RT-PCR is the most widely used technique for COVID-19 detection. Additionally, other possible methods of diagnosing COVID-19 are immune identification technologies, such as point-of-care testing (POCT) of IgM/IgG, and ELISA, but further study is needed (Chen, 2020, Xie and Chen, 2020). Patients infected with COVID-19 often appear to have abnormal chest CT scans, demonstrating bilateral pulmonary parenchymal ground-glass, rounded consolidative pulmonary opacities and a peripheral lung distribution, which can aid in a differential diagnosis (Petrosillo et al., 2020).
9. Treatment
To date, no approved specific antiviral agents have been available for current or previous outbreaks of HCoVs, but convalescent sera or monoclonal antibodies which inhibit SARS-CoV or MERS-CoV are currently under development in vitro and in animal testing (Yi et al., 2020, Zhang et al., 2020). Beside using existing antivirals and other possible drugs, the overall treatment strategies involve: 1) maintaining the internal environment (i.e. water, electrolytes and other internal environment factors), 2) observing vital signs (e.g. heart rate, pulse, respiratory rate, blood pressure and oxygen saturation) and 3) ensuring sufficient energy intake (62.63).
9.1. MERS
Some therapeutic options against various viral elements are currently available, whilst others are under development (McKimm-Breschkin et al., 2018). Examples of different classes of treatments are: 1) immunotherapy with anti-MERS-CoV antibodies, 2) molecules with antiviral activities, 3) symptomatic treatment preventive therapies and 4) vaccines, although these are currently under development (Azhar et al., 2019). Table 5 shows the anti-MERS-CoV agents that are currently being used. Combination treatments have also been used, e.g. Interferon-α (IFN-α) with antiviral or protease inhibitors or mycophenolate mofetil (Hui, 2017). A retrospective study in Saudi Arabia on MERS-CoV patients with refractory respiratory failure showed that, as with other respiratory infections, it is necessary to use extracorporeal membrane oxygenation (ECMO) as salvage treatment for those who suffer respiratory failure. In regards to the use of chloroquine, no supportive studies are available at the present time (12.65).
Table 5.
The potential treatments for MERS and COVID-19.
| Anti-MERS-CoV agents used | Anti-Covid-19 agents used |
|---|---|
| Intravenous Immunoglobulins | Oseltamivir |
| Interferon-α | Interferon-α |
| Ribavirin | Ritonavir |
| Lopinavir | Lopinavir |
| Protease inhibitors | Ganciclovir |
| Mycophenolate mofetil | Remdesivir |
| Nitazoxamide | Ribavirin |
| Alisporivir | Remidisvir |
| Chloroquine | Arbidol |
| Silvestrol | Chloroquine |
| Corticosteroids | Tocilizumab |
| Examples of potential treatments for COVID-19 are IDX-184, Sofosbuvir & EIDD-2801 (isopropyl ester of N4-hydroxycytidine) | |
9.2. COVID-19
The global need for a specific anti-COVID-19 drug has led to great efforts to accelerate the search for an effective antiviral agent. The current treatment employed for COVID-19 does not differ greatly from the MERS treatment management approach, e.g. broad-spectrum antivirals are used as first line treatments (Wang et al., 2020a, Wang et al., 2020b). A list of anti-COVID-19 agents that have been used is presented in Table 5.
Potential compounds undergoing further investigation include EIDD-2801 (isopropyl ester of N4-hydroxycytidine), Sofosbuvir and IDX-184. Along with these, Ribavirin and Remidisvir may potentially have a high therapeutic value in treating COVID-19 (Barnard et al., 2006, Lajoie et al., 2017, Vincent et al., 2005). Due to the absence of evidence, the WHO’s interim guidelines (from the 22nd February 2020) do not support the use of systemic corticosteroids for the treatment of viral pneumonia and ARDS in suspected COVID-19 cases. However, the efficacy and associated adverse effects of glucocorticoids in COVID-19 treatment currently need further elucidation (Zhang et al., 2020). Many firms are developing vaccines for the COVID-19 virus. The vaccine (ChAdOx1 nCoV-19) that has been developed by the University of Oxford and backed by the UK government has already been given to the first two candidates in Oxford, England, on the 23rd April 2020. Furthermore, around 1,100 people are expected to be injected with the vaccine as part of the trial (BBC, 2020). The vaccine was prepared by the serum institute in India by removing the harmful properties of the pathogen to decrease its virulence, whilst at the same time keeping it alive. However, more time is needed to obtain enough data to know if the vaccine works. Vaccine trials are already ongoing in China and the USA, and are due to commence in Germany at the end of the current month (Sarvepalli, 2020). For now, most patients are receiving antiviral therapy in addition to ventilation support to manage COVID-19 symptoms (D. Wang et al., 2020a, Wang et al., 2020b).
10. Sequelae
A sequela is the lingering effect formed after the acute phase of an illness or injury has disappeared. There is no time limit on when a sequela can develop. For example, a sequela might appear early in cerebral infarction, or it may be delayed by months or years, due to a previous injury (Petrignani et al., 2018).
10.1. MERS
A cohort study that assessed the health-related quality of life of 78 survivors who required hospitalisation in Saudi Arabia between 2016 and 2017 found that MERS survivors experienced a degree of lung impairment (Batawi et al., 2019). The study reported the presence of impairment in the diffusion capacity of the lungs for carbon monoxide up to 2 years after recovery. In addition, changes in air space were evident through chest radiographs and high-resolution CT scans. MERS survivors also described significant weakening in physical health at approximately 14 months after illness compared to a previously published sample of healthy individuals in Saudi Arabia. As many MERS patients admitted to ICU were given high doses of corticosteroids, the sequelae from this type of therapy could contribute to reduced quality of life in survivors (Bleibtreu et al., 2018). Corticosteroid-induced myopathy, muscle wasting and weakness has also been previously reported among SARS-CoV survivors (Herridge et al., 2003, Ng et al., 2004). Additionally, the study revealed that critically ill MERS survivors had emotional damage which might be due to psychological trauma. Similar findings were reported from South Korea, where 73 survivors who had suffered from severe pneumonia developed significant impairments in pulmonary function compared to those with mild or no pneumonia (Park et al., 2018).
10.2. COVID-19
As of the 20th May, about 1,804,347 cases (37.34%) worldwide had recovered from COVID-19. Researchers believe that those who recover can be left with damaged lungs. However, it is too early to confirm any long-term effects. Investigators in Hong Kong and China analysed more than 152 lung scans of recovered patients and found ground glass opacity in both lungs, in addition to fluid or debris filled sacs in the lungs. It is expected that with these sequelae, the long-term effects are likely to progressively worsen. Another report on the sequelae of COVID-19 indicates that 20–30% of recovered COVID-19 patients suffer a decline in lung capacity due to irreversible lung fibrosis (Cox, 2020). COVID-19 has also caused scarring and stiffening of the lung tissue. Permanent damage to the lungs may progress into severe respiratory issues and ARDS (Wang et al., 2020a, Wang et al., 2020b). Moreover, anxiety, depression and trauma-related symptoms have been associated with HCoV outbreaks (Troyer, Kohn and Hong, 2020). It is believed that older patients may be less able to return to their pre-illness state, whereas the chance of lung tissue recovery is higher for younger patients. Therefore, there is also a need to study the long-term impact of COVID-19 to establish a framework for treatment.
11. Prevention
Saudi Arabia has implemented rapid and effective infection control and isolation measures to prevent the spread of MERS and COVID-19. These measures are focused on 4 main aspects: confirmed cases, close contact handling, community prevention and protection of healthcare workers.
11.1. MERS
11.1.1. Confirmed cases
Approximately 2.519 laboratory confirmed cases of infection with MERS-CoV were registered for the period from June 2012 to January 2020, according to the WHO. Around 866 of these cases were associated with deaths (WHO, 2020b). Although transmission of the disease is not fast, SMOH advises taking all possible measures and that suspected cases should be quarantined prior to having a laboratory test result, which takes between 12 and 24 h. This is crucial to combat the spread of MERS to a new family member or a healthcare worker (Altamimi et al., 2020). Mild symptomatic cases that result in a positive PCR may be dealt with by isolation at home, whereas severe to moderate cases should be isolated in hospital until they recover, and then discharged for isolation at home for an extended period. Both mild and severe cases are re-tested after 7 days, and the test is subsequently repeated every 3 days until a negative result is obtained (Garbati et al., 2016). The WHO have stated that the ongoing efforts to combat MERS transmission have been successful in reducing the prevalence of MERS-CoV (WHO, 2020b).
11.1.2. Close contact handling
All close contacts of MERS patients were required to report to selected medical centres by the Department of Health for close contact handling. Home quarantine measures were suggested for those in close contact with a suspected case in a bid to reduce the possibility of MERS-CoV transmission.
11.1.3. Community prevention
Patients with both suspected and confirmed cases of MERS‐CoV are advised to visit one of the specialised testing centres to detect community transmission. In addition, public education is provided regarding infection prevention and control and updated regularly via social media. This includes the use of face masks, hand hygiene, disinfection of surfaces for fomites, avoiding direct contact with infected people, covering the mouth with a tissue when coughing or sneezing and other simple hygiene measures (Garbati et al., 2016). The authorities have also warned of the dangers of consuming raw or undercooked animal products, including milk and meat, as they may have a high risk of MERS-CoV infection. The advice is not to have direct contact with camel excretions and subsequently touch mucous membranes, as this can be a key source of infection. Three specialised centres have been set up in the Kingdom to deal with these cases (22.51).
11.1.4. Protection of healthcare workers
Healthcare workers are told to use personal protective equipment (PPE), i.e. N95 masks, face shield or goggles, gloves and isolation gowns as strict infection control measures to prevent droplet and contact transmission. It is not always possible to identify patients with MERS-CoV infection early. This is due to the fact that, like other respiratory infections, the early symptoms of MERS-CoV are non-specific. Therefore, SMOH emphasises that healthcare workers should always apply standard precautions consistently with all patients. Additionally, patients with confirmed cases are advised to wear a mask to avoid further spread of the virus via droplets. When providing care to patients with symptoms of acute respiratory infection, contact precautions and eye protection should be used. In line with the WHO, SMOH encourages all healthcare units to continue surveillance for new MERS cases and to carefully review any unusual patterns.
11.2. COVID-19
11.2.1. Confirmed cases
During early March 2020, the Saudi Center for Disease Prevention and Control (Wegaya) indicated the need for urgent isolation and quarantine during the COVID-19 outbreak in the country. It is advised that suspected COVID-19 patients should be placed in an area separated from other patients. Extra precautions should promptly be implemented when dealing with patients to avoid getting ill, as the disease is able to transmit rapidly from human to human. All confirmed cases are kept in hospital for isolation and treatment until fully recovered. Suspected cases are also required to quarantine at home until a negative result is confirmed via PCR testing. To find high risk cases on admission to hospital, from March 2020, the patient’s travel history is collected from the immigration department and incorporated into the hospital authority computer system (Clinical Management System). Temperature checking is maintained as a routine check for arrivals into Saudi Arabia along with 14 days of quarantine. Additionally, respiratory specimens have been collected from all travellers and sent for viral testing at regional SMOH laboratories since the 15th March 2020 (Al-Tawfiq and Memish, 2020).
11.2.2. Close contact handling
Suspected cases and close contacts are required to self-isolate for 14 days at home or in their accommodation and monitor their daily body temperature, signs and symptoms (Al-Tawfiq and Memish, 2020, Yezli and Khan, 2020).
11.2.3. Community prevention
In accordance with the recommendations of the health authorities, Saudi has applied proactive preventive measures to reduce the spread of COVID-19 within the community. For example, on the 26th of February 2020, Saudi Arabia temporarily suspended entry to individuals seeking to perform the Umrah pilgrimage to Mecca or visit the prophet’s Mosque in Medina. Furthermore, this restriction applied to all tourists travelling from countries with an extensive spread of COVID-19. Subsequently, on the 8th of March, all schools and universities were locked down. All public events, prayers, holy sites for Muslims, public museums, public libraries, sports centres and venues were either closed or cancelled to avoid clusters of infections. Visitors or those returning to the Saudi international airports were taken to stay in a quarantine centre (5-star hotel) for a period of two weeks. This was applied on the 9th of March 2020. Following this, on the 15th of March 2020, Saudi Arabia prohibited entry to all travellers, so all local and international airports have been locked down to date. Likewise, public services and cross-border transportation have also been suspended. Saudi Arabia imposed a dusk to dawn curfew on the 23rd of March 2020, meaning that people were only allowed to leave their homes between 6 am and 7 pm. After the number of confirmed deaths had increased to 38, the duration of these daily curfews was extended to 24 h for 5 cities and 4 governorates on the 7th of April. Similar measures were imposed on several more cities later in the same month (78.79). Recently, curfews have been eased across the country, but 24-hour lockdowns are still in place in Mecca and neighbouring areas. Outside Mecca and the lockdown areas, curfews will be eased between 9 am and 5 pm until the 22nd May. Authorities now plan to enforce a nationwide 24-hour curfew during the 5-day Muslim festival of Eid Al-Fitr, starting on the 23rd of May.
11.2.4. Protection of healthcare workers
Healthcare workers exposed to a COVID-19 case are required to respect international guidelines approved by SMOH and the WHO to limit the spread of COVID-19. All healthcare workers are advised to use personal protective equipment (PPE), especially those who have direct exposure to patients with suspected or confirmed COVID-19. Every person in a public hospital is required to wear a surgical mask to prevent cross contamination (Yezli and Khan, 2020).
12. Hajj & Umrah in the time of COVID-19
Saudi Arabia is a place of pilgrimage and therefore is an important religious gathering place for Muslims. One of the largest religious gatherings is the Hajj and Umrah, which is likely to provide serious challenges in terms of outbreak containment. Hajj is an annual Islamic ritual attended by over two million Muslims from more than 188 countries, so during the outbreak there is a risk of mass exposure and spread all over the world (Atique and Itumalla, 2020). This could facilitate the spread of COVID-19 to multiple countries, including those that have been less affected so far, whilst also re-spreading to countries that have recovered or are recovering from the outbreak. Muslims also travel to Saudi for Umrah (mini-pilgrimage) which requires similar considerations to those mentioned above. As a result, it is strongly advised that the prevailing situation with regard to COVID-19 should be observed and evaluated by the Ministry of Hajj and Umrah in Saudi Arabia, along with religious scholars, to make appropriate decisions about the next Hajj which is fast approaching (July 2020). Potential measures with regard to this are vital for further mitigation of the pandemic (Farooq et al., 2020). To successfully limit the extent of the COVID-19 outbreak, the following points are recommended:
-
1.
Above all, prevent or reduce the transmission from the natural host. This requires more knowledge of the natural ecology of the virus. Such transmission risks should be considered when new contact between bats and humans takes place, or when human settlements extend into bat habitats. Also, social science can be employed to better convey the message to the general public regarding interactions with specific animals, and when to avoid these (based on disease ecology knowledge) (Lu et al., 2020b).
-
2.
Prevent or reduce the risk of transmission from the intermediate host. In principle, this risk could be avoided by completely separating the primary host from the intermediate host (e.g. bats from camels). This will depend on which animal or animals are proven to constitute the intermediate host or hosts for COVID-19. However, in practice this may prove to be difficult, since live animal markets appear to play an important role in this process. These could be a starting point for measures to be employed with a true One Health approach (Lu, Milinovich and Hu, 2016).
-
3.
Reduce transmission between humans. This is a crucial measure to prevent further development of the outbreak, and is currently the primary measure employed, for good reason (Bleibtreu et al., 2019b).
13. Conclusion
The MERS epidemic began in 2012 and outbreaks have subsequently been seen worldwide. Although MERS-CoV continues to persist in the Middle East 7 years later, MERS has not become pandemic. In contrast, COVID-19 emerged in December 2019 and spread rapidly throughout the whole world within a few weeks (~ten weeks). On the 11th of March 2020, COVID-19 was declared a pandemic by the WHO (WHO, 2020a). The latest data available on causes, transmissions, symptoms, treatments and preventive measures of MERS and COVID-19 have been reviewed. However, more information on the biology, epidemiological features, diagnosis and treatments of various HCoVs are urgently required to further advance the risk assessment and response. This will ultimately have benefits for the control and prevention of the current COVID-19 pandemic. RT-PCR testing, as a diagnostic tool, is used to confirm infection with MERS or COVID-19. However, this may sometimes provide a false negative result. To date, there are no specific treatment regimens or vaccines for HCoVs, and the only antiviral therapy currently employed is broad spectrum. However, a number of antiviral drugs targeting specific HCoVs, along with vaccines, are under development (Jiang et al., 2020, Yu et al., 2020). The WHO has shipped millions of items of personal protective equipment to 105 countries, as well as lab supplies to more than 127 countries. This, however, requires the joint efforts of the public, politicians and scientists. Social distancing, personal hygiene and protection are the most significant and important measures for controlling transmission. This includes wearing a mask, washing hands, decreasing social contact, avoiding crowds and working from home, as well as following the instructions provided by local health authorities and the WHO (Bleibtreu et al., 2019a).
Fear plays a role in economic and social consequences, and this is also a feature of the SARS-CoV outbreak. The human, economic and social crisis created by the COVID-19 pandemic may also increase global unemployment, inequality, exclusion, discrimination and general unrest in the medium and long term, if not properly addressed. In the meantime, therefore, educating the population and strengthening public confidence will be critical.
Ethical approval
Ethical approval is not required.
Declaration of Competing Interest
The authors declare that there no conflict of interests. This work has not been published previously and the manuscript is not under consideration for publication elsewhere. The submission is approved by all authors.
Acknowledgment
The authors would like to thank the Deanship of scientific research at Najran University (NU) for supporting and funding the study grant No. NU/MID/18/002.
Footnotes
Peer review under responsibility of King Saud University.
References
- Al-Tawfiq, J.A., Memish, Z.A., 2020. ‘COVID-19 in the Eastern Mediterranean Region and Saudi Arabia: prevention and therapeutic strategies. International Journal of Antimicrobial Agents. Elsevier B.V., (March). doi: 10.1016/j.ijantimicag.2020.105968. [DOI] [PMC free article] [PubMed]
- Aleanizy F.S. Outbreak of Middle East respiratory syndrome coronavirus in Saudi Arabia: a retrospective study. BMC Infect. Dis. 2017;17(1):1–7. doi: 10.1186/s12879-016-2137-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altamimi A. Demographic variations of MERS-CoV infection among suspected and confirmed cases: an epidemiological analysis of laboratory-based data from Riyadh regional laboratory. Biomed Res. Int. 2020;2020 doi: 10.1155/2020/9629747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atique, S., Itumalla, R., 2020 ‘Hajj in the Time of COVID-19’, Infection, Disease & Health, (March), pp. 4–6. doi: 10.1016/j.idh.2020.04.001. [DOI] [PMC free article] [PubMed]
- Azhar E.I. Evidence for camel-to-human transmission of MERS coronavirus. N. Engl. J. Med. 2014;370(26):2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- Azhar E.I. The Middle East Respiratory Syndrome (MERS) Infect. Dis. Clin. North Am. 2019;33(4):891–905. doi: 10.1016/j.idc.2019.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard D.L. Evaluation of immunomodulators, interferons and known in vitro SARS-CoV inhibitors for inhibition of SARS-CoV replication in BALB/c mice. Antivir. Chem. Chemother. 2006;17(5):275–284. doi: 10.1177/095632020601700505. [DOI] [PubMed] [Google Scholar]
- Batawi S. ‘Quality of life reported by survivors after hospitalization for Middle East respiratory syndrome (MERS)’. Health Qual. Life Outcomes Health Qual. Life Outcomes. 2019;17(1):1–7. doi: 10.1186/s12955-019-1165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BBC, 2020. How India will play a major role in a virus vaccine, BBC. Available at: https://www.bbc.com/news/world-asia-india-52363791 (Accessed: 28 April 2020).
- Bermingham A. Severe respiratory illness caused by a novel coronavirus, in a patient transferred to the United Kingdom from the Middle East, September 2012. Eurosurveillance. 2012;17(40):1–5. doi: 10.2807/ese.17.40.20290-en. [DOI] [PubMed] [Google Scholar]
- Bleibtreu A. Clinical management of respiratory syndrome in patients hospitalized for suspected Middle East respiratory syndrome coronavirus infection in the Paris area from 2013 to 2016. BMC Infect. Dis. 2018;18(1):1–9. doi: 10.1186/s12879-018-3223-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleibtreu, A. et al., 2019a. ‘Focus on Middle East respiratory syndrome coronavirus (MERS-CoV)’, Medecine et Maladies Infectieuses. Elsevier Masson SAS. doi: 10.1016/j.medmal.2019.10.004. [DOI] [PMC free article] [PubMed]
- Bleibtreu A. ‘Focus on Middle East respiratory syndrome coronavirus (MERS-CoV)’, Medecine et Maladies Infectieuses. Elsevier Masson SAS. 2019;50(3):243–251. doi: 10.1016/j.medmal.2019.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoch I.I. Pneumonia of unknown aetiology in Wuhan, China: potential for international spread via commercial air travel. J. Travel Med. 2020;27(2):1–3. doi: 10.1093/jtm/taaa008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2019a. Centers of Disease Control & Prevention, National Center for Immunization and Respiratory Diseases (NCIRD), Division of Viral Diseases. Available at: https://www.cdc.gov/coronavirus/mers/index.htm (Accessed: 20 April 2019).
- CDC, 2019b. Disease Burden of Influenza https, Disease Control and Prevention, National Center for Immunization and Respiratory Diseases (NCIRD). Available at: https://www.cdc.gov/flu/about/burden/index.html.
- CDC, 2020. Human Coronavirus Types, Center for Disease Control and Prevention. Available at: https://www.cdc.gov/coronavirus/types.html (Accessed: 21 April 2020).
- Chen J. Pathogenicity and transmissibility of 2019-nCoV—a quick overview and comparison with other emerging viruses. Microbes Infect.. Elsevier Ltd. 2020;22(2):69–71. doi: 10.1016/j.micinf.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conzade R. Reported direct and indirect contact with dromedary camels among laboratory-confirmed MERS-CoV cases. Viruses. 2018;10(8):1–10. doi: 10.3390/v10080425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corman V.M. Antibodies against MERS coronavirus in dromedary camels, Kenya, 1992–2013. Emerg. Infect. Dis. 2014;20(8):1319–1322. doi: 10.3201/eid2008.140596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox, D., 2020. Some patients who survive COVID-19 may suffer lasting lung damage Science News, Science News. Available at: https://www.sciencenews.org/article/coronavirus-covid-19-some-patients-may-suffer-lasting-lung-damage (Accessed: 29 April 2020).
- Drosten C. Transmission of MERS-coronavirus in household contacts. N. Engl. J. Med. 2014;371(9):828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- Farooq H.Z. (2020) ‘Middle East respiratory syndrome coronavirus (MERS-CoV) — Surveillance and testing in North England from 2012 to 2019’. Int. Soc. Infect. Diseases. 2018;93:237–244. doi: 10.1016/j.ijid.2020.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbati M.A. A comparative study of clinical presentation and risk factors for adverse outcome in patients hospitalised with acute respiratory disease due to mers coronavirus or other causes. PLoS ONE. 2016;11(11):1–12. doi: 10.1371/journal.pone.0165978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemida M.G. Seroepidemiology of middle east respiratory syndrome (MERS) coronavirus in Saudi Arabia (1993) and Australia (2014) and characterisation of assay specificity. Eurosurveillance. 2014;19(23):1–6. doi: 10.2807/1560-7917.ES2014.19.23.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herridge M.S. One-year outcomes in survivors of the acute respiratory distress syndrome. N. Engl. J. Med. 2003;348(8):683–693. doi: 10.1056/NEJMoa022450. [DOI] [PubMed] [Google Scholar]
- Hijawi B. Novel coronavirus infections in Jordan, April 2012: Epidemiological findings from a retrospective investigation. Eastern Mediterranean Health Journal. 2013;19(SUPPL.1):12–18. doi: 10.26719/2013.19.supp1.s12. [DOI] [PubMed] [Google Scholar]
- Hui, D. S. (2017) ‘Epidemic and Emerging Coronaviruses (Severe Acute Respiratory Syndrome and Middle East Respiratory Syndrome)’, Clinics in Chest Medicine. Elsevier Inc, 38(1), pp. 71–86. 10.1016/j.ccm.2016.11.007. [DOI] [PMC free article] [PubMed]
- Hui D.S. Middle East respiratory syndrome coronavirus: risk factors and determinants of primary, household, and nosocomial transmission. Lancet. Infect. Dis. Elsevier Ltd. 2018;18(8):e217–e227. doi: 10.1016/S1473-3099(18)30127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji W. Cross-species transmission of the newly identified coronavirus 2019-nCoV. J. Med. Virol. 2020;92(4):433–440. doi: 10.1002/jmv.25682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S., Du L., Shi Z. An emerging coronavirus causing pneumonia outbreak in Wuhan, China: calling for developing therapeutic and prophylactic strategies. Emerging Microbes Infect. 2020;9(1):275–277. doi: 10.1080/22221751.2020.1723441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killerby M.E. Middle east respiratory syndrome coronavirus transmission. Emerg. Infect. Dis. 2020 doi: 10.3201/eid2602.190697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W. Risk factors for transmission of Middle East respiratory syndrome coronavirus infection during the 2015 outbreak in South Korea. Clin. Infect. Dis. 2017;64(5):551–557. doi: 10.1093/cid/ciw768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korea Centers for Disease Control and Prevention, 2015. ‘Middle East Respiratory Syndrome Coronavirus Outbreak in the Republic of Korea, 2015’, Osong Public Health and Research Perspectives. Elsevier Korea LLC, 6(4), pp. 269–278. doi: 10.1016/j.phrp.2015.08.006. [DOI] [PMC free article] [PubMed]
- Lai C.C. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int. J. Antimicrob. Agents. Elsevier B.V. 2020;55(3) doi: 10.1016/j.ijantimicag.2020.105924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie J., Mwangi L., Fowke K.R. Preventing HIV infection without targeting the virus: How reducing HIV target cells at the genital tract is a new approach to HIV prevention. AIDS Res.Therapy. BioMed Cent. 2017;14(1):1–5. doi: 10.1186/s12981-017-0166-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam, T.T.-Y. et al., 2020. ‘Identification of 2019-nCoV related coronaviruses in Malayan pangolins in southern China’, bioRxiv, p. 2020.02.13.945485. doi: 10.1101/2020.02.13.945485.
- Lu H., Stratton C.W., Tang Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020:401–402. doi: 10.1002/jmv.25678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J., Milinovich G.J., Hu W. A brief historical overview of emerging infectious disease response in China and the need for a One Health approach in future responses. One Health. 2016;99(102) doi: 10.1016/j.onehlt.2016.07.001. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7127800/pdf/main.pdf Available at: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. The Lancet. Elsevier Ltd. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKimm-Breschkin J.L. (2018) ‘Prevention and treatment of respiratory viral infections: presentations on antivirals, traditional therapies and host-directed interventions at the 5th ISIRV Antiviral Group conference’. Antiviral Res. 2017;149:118–142. doi: 10.1016/j.antiviral.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical News Today (2020) MERS-CoV: Symptoms, causes, risk factors, and treatment, Medicalnewstoday.com. Available at: https://www.medicalnewstoday.com/articles/262538#prevention (Accessed: 27 April 2020).
- Memish Z.A. Middle East respiratory syndrome coronavirus in Bats, Saudi Arabia. Emerg. Infect. Dis. 2013;19(11):1819–1823. doi: 10.3201/eid1911.131172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel E. Absence of Middle East respiratory syndrome coronavirus in camelids, Kazakhstan, 2015. Emerg. Infect. Dis. 2016;22(3):555–557. doi: 10.3201/eid2203.151284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguel, E. et al., 2017. ‘Risk factors for MERS coronavirus infection in dromedary camels in Burkina Faso, Ethiopia, and Morocco, 2015’, Eurosurveillance, 22(13). doi: 10.2807/1560-7917.ES.2017.22.13.30498. [DOI] [PMC free article] [PubMed]
- Mohd, H.A. et al., 2016. ‘Predictors of MERS-CoV infection: A large case control study of patients presenting with ILI at a MERS-CoV referral hospital in Saudi Arabia’. Travel Med. Infect. Disease. Elsevier Ltd, 14(5), pp. 464–470. doi: 10.1016/j.tmaid.2016.09.008. [DOI] [PMC free article] [PubMed]
- Müller M.A. Mers coronavirus neutralizing antibodies in camels, eastern Africa, 1983–1997. Emerg. Infect. Dis. 2014;20(12):2093–2095. doi: 10.3201/eid2012.141026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M.A. Presence of Middle East respiratory syndrome coronavirus antibodies in Saudi Arabia: a nationwide, cross-sectional, serological study. Lancet. Infect. Dis. 2015;15(5):559–564. doi: 10.1016/S1473-3099(15)70090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C.K. Six month radiological and physiological outcomes in severe acute respiratory syndrome (SARS) survivors. Thorax. 2004;59(10):889–891. doi: 10.1136/thx.2004.023762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W.B. Correlation between pneumonia severity and pulmonary complications in Middle East respiratory syndrome. J. Korean Med. Sci. 2018;33(24):1–5. doi: 10.3346/jkms.2018.33.e169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrignani M. (2018) ‘Chronic sequelae and severe complications of norovirus infection: a systematic review of literature’. J. Clin. Virol. 2017;105:1–10. doi: 10.1016/j.jcv.2018.05.004. [DOI] [PubMed] [Google Scholar]
- Petrosillo, N. et al., 2020. ‘COVID-19, SARS and MERS: are they closely related?’, Clin. Microbiol. Infect. Elsevier Ltd, (xxxx). doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed]
- Reusken C. Middle East respiratory syndrome coronavirus neutralising serum antibodies in dromedary camels: a comparative serological study. Lancet. Infect. Dis. 2013;13(10):859–866. doi: 10.1016/S1473-3099(13)70164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reusken C. Specific serology for emerging human coronaviruses by protein microarray. Eurosurveillance. 2013;18(14):2–7. doi: 10.2807/1560-7917.es2013.18.14.20441. [DOI] [PubMed] [Google Scholar]
- Reusken C.B. Middle east respiratory syndrome coronavirus (MERS-CoV) RNA and neutralising antibodies in milk collected according to local customs from dromedary camels, Qatar, April 2014. Eurosurveillance. 2014;19(23):1–5. doi: 10.2807/1560-7917.ES2014.19.23.20829. [DOI] [PubMed] [Google Scholar]
- Sabir J.S.M. Co-circulation of three camel coronavirus species and recombination of MERS-CoVs in Saudi Arabia. Science. 2016;351(6268):81–84. doi: 10.1126/science.aac8608. [DOI] [PubMed] [Google Scholar]
- Sarvepalli D. ‘Coronavirus disease 2019: a comprehensive review of etiology. Pathogen. Diagn. Ongoing Clinical Trials’, Cureus. 2020;12(5) doi: 10.7759/cureus.8076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shereen M.A. COVID-19 infection: origin, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shim E. ‘Transmission potential and severity of COVID-19 in South Korea’. Int. Soc. Infect. Dis. 2020;93:339–344. doi: 10.1016/j.ijid.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses. 2019;11(1) doi: 10.3390/v11010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi S. Inclusion of MERS-spike protein ELISA in algorithm to determine serologic evidence of MERS-CoV infection. J. Med. Virol. 2018;90(2):367–371. doi: 10.1002/jmv.24948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav. Immun. 2020 doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent M.J. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virology Journal. 2005;2:1–10. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA – J. Am. Med. Assoc. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y. Temporal changes of CT findings in 90 patients with COVID-19 pneumonia: a longitudinal study. Rdiol. 2020 doi: 10.14358/PERS.80.2.000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegaya, 2020. Diseas Interactive Dashboard-Daily updates, Saudi Center for Disease Prevention and Control. Available at: https://covid19.cdc.gov.sa/daily-updates/ (Accessed: 23 April 2020).
- World Health Organization, 2019. Laboratory testing for 2019 novel coronavirus (2019-nCoV) in suspected human cases, WHO.
- WHO, 2020a. Coronavirus disease (COVID-19) outbreak Who.int. (2020). Coronavirus disease (COVID-19) outbreak. [online] Available at: https://www.who.int/westernpacific/emergencies/covid-19 [Accessed 1 May 2020]. WHO. Available at: https://www.who.int/westernpacific/emergencies/covid-19 (Accessed: 1 May 2020).
- WHO, 2020b. Epidemic and pandemic diseases., WHO EMRO | MERS situation update. Available at: http://www.emro.who.int/pandemic-epidemic-diseases/mers-cov/mers-situation-update-january-2020.html (Accessed: 20 May 2020).
- WHO, 2020c. WHO Coronavirus Disease (COVID-19) Dashboard Covid19., WHO Coronavirus Disease (COVID-19) Dashboard. Available at: https://covid19.who.int/?gclid=CjwKCAjwqpP2BRBTEiwAfpiD-4yF0-v4xvX60WLMnDZv-woOoIJzwWUphBe3dWacFXBVlwmGI6 (Accessed: 20 May 2020).
- Wrapp D. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367(6483):1260–1263. doi: 10.1126/science.aax0902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. ‘Characteristics of and Important Lessons from the Coronavirus Disease 2019 (COVID-19) Outbreak in China: Summary of a Report of 72314 Cases from the Chinese Center for Disease Control and Prevention’. J. Am. Med. Assoc. 2020;323(13) doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Xie M., Chen Q. ‘Insight into 2019 novel coronavirus — an updated intrim review and lessons from SARS-CoV and MERS-CoV’. Int. Soc. Infect. Diseases. 2020;94:119–124. doi: 10.1016/j.ijid.2020.03.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie, X. et al., 2020 ‘Chest CT for Typical 2019-nCoV Pneumonia: Relationship to Negative RT-PCR Testing’, Radiology, p. 200343. doi: 10.1148/radiol.2020200343. [DOI] [PMC free article] [PubMed]
- Yezli, S., Khan, A., 2020. ‘COVID-19 social distancing in the Kingdom of Saudi Arabia: Bold measures in the face of political, economic, social and religious challenges’, Travel Med. Infect. Disease. Elsevier, (March), p. 101692. doi: https://doi.org/10.1016/j.tmaid.2020.101692. [DOI] [PMC free article] [PubMed]
- Yi Y. COVID-19: what has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020;16(10):1753–1766. doi: 10.7150/ijbs.45134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo J.H. The fight against the 2019-nCoV outbreak: an arduous march has just begun. J. Korean Med. Sci. 2020;35(4):2019–2021. doi: 10.3346/jkms.2020.35.e56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F. Measures for diagnosing and treating infections by a novel coronavirus responsible for a pneumonia outbreak originating in Wuhan, China. Microbes Infect. 2020;22(2):74–79. doi: 10.1016/j.micinf.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki A.M. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. N. Engl. J. Med. 2012;367(19):1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- Zhang W. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The experience of clinical immunologists from China. Clin. Immunol. 2020;214(March) doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Zowalaty M.E., Järhult J.D. From SARS to COVID-19: A previously unknown SARS- related coronavirus (SARS-CoV-2) of pandemic potential infecting humans – Call for a One Health approach. One Health. 2020;9(February) doi: 10.1016/j.onehlt.2020.100124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A. ‘Coronaviruses-drug discovery and therapeutic options’. Nat. Publish. Group. 2016;15(5):327–347. doi: 10.1038/nrd.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zumla A., Hui D.S., Perlman S. Middle East respiratory syndrome. The Lancet. 2015;386(9997):995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]