Abstract
Objectives
This study postulated that antihypertensive therapy with renin-angiotensin-aldosterone system (RAAS) inhibition may mitigate vascular endothelial growth factor inhibitor (VEGFi)–mediated increases in blood pressure more effectively than other antihypertensive medications in patients receiving VEGFi therapy.
Background
VEGFi therapy is commonly used in the treatment of cancer. One common side effect of VEGFi therapy is elevated blood pressure. Evidence suggests that the RAAS may be involved in VEGFi-mediated increases in blood pressure.
Methods
This retrospective cohort analysis was performed using a de-identified version of the electronic health record at Vanderbilt University Medical Center in Nashville, Tennessee. Subjects with cancer who were exposed to VEGFi therapy were identified, and blood pressure and medication data were extracted. Changes in mean systolic and diastolic blood pressure in response to VEGFi therapy in patients receiving RAAS inhibitor (RAASi) therapy before VEGFi initiation were compared with changes in mean systolic and diastolic blood pressure in patients not receiving RAASi therapy before VEGFi initiation.
Results
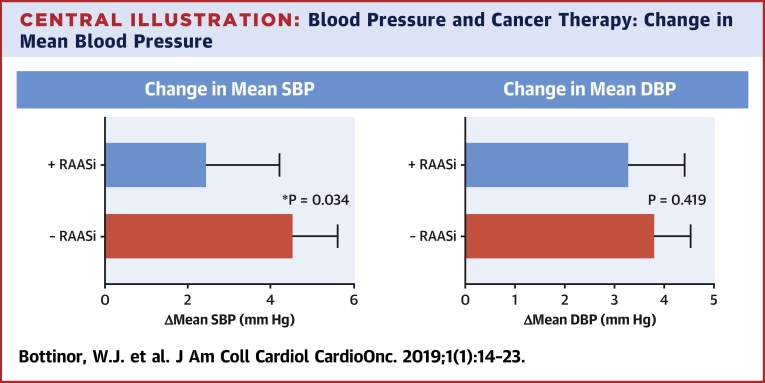
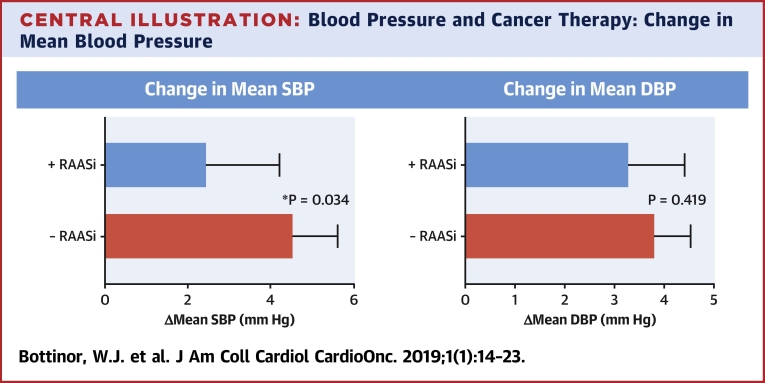
Mean systolic and diastolic blood pressure rose in both groups after VEGFi use; however, patients who had RAASi therapy before VEGFi initiation had a significantly lower increase in systolic blood pressure as compared with patients with no RAASi therapy (2.46 mm Hg [95% confidence interval: 0.7 to 4.2] compared with 4.56 mm Hg [95% confidence interval: 3.5 to 5.6], respectively; p = 0.034).
Conclusions
In a real-world clinical population, RAASi therapy before VEGFi initiation may ameliorate VEGFi-mediated increases in blood pressure. Randomized clinical trials are needed to further our understanding of the role of RAASi therapy in VEGFi-mediated increases in blood pressure.
Key Words: cancer, hypertension, renin-angiotensin-aldosterone, vascular endothelial growth factor, VEGF, VEGF inhibitors
Abbreviations and Acronyms: ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; CI, confidence interval; EHR, electronic health record; ICD, International Classification of Diseases; IQR, interquartile range; RAAS, renin-angiotensin-aldosterone system; RAASi, renin-angiotensin-aldosterone system inhibitor; VEGF, vascular endothelial growth factor; VEGFi, vascular endothelial growth factor inhibitor
Central Illustration
Angiogenesis is modulated through vascular endothelial growth factor (VEGF) receptor signaling. Inhibition of this pathway treats cancer, but it can also lead to deleterious events that limit drug tolerability and treatment success. Increases in blood pressure occur almost universally with VEGF inhibitor (VEGFi) therapy, and hypertension is the most common cardiovascular toxicity of this therapy, occurring in up to 80% of patients (1).
Uncontrolled hypertension increases the risk for serious complications such as renal dysfunction, reversible posterior leukoencephalopathy syndrome, stroke, heart attack, and cardiomyopathy, and it can be a dose-limiting factor for treatment with VEGFi therapy 2, 3. These risks may be further enhanced by treatment strategies that combine VEGFi with other therapies that also carry cardiovascular risks (4). Defining optimal treatment strategies in this unique group of patients is essential.
The pathophysiology and optimal therapy for VEGFi-mediated increases in blood pressure are unclear. Proposed mechanisms include capillary rarefaction, increased vascular stiffness, increased vascular tone secondary to decreased nitric oxide production, increased endothelin-1 activation, and glomerular injury 2, 3, 5, 6. The renin-angiotensin-aldosterone system (RAAS) has also been suggested. Genetic polymorphisms in components of the renin-angiotensin system have been implicated in the efficacy of VEGFi therapy (7). In addition, experimental models suggest that an imbalance between the RAAS and VEGF contributes to VEGFi-mediated hypertension 8, 9.
VEGF is expressed by renal podocytes and is indispensable for normal development 5, 10, 11. In murine models, RAAS activation leads to a compensatory increase in VEGF expression, and VEGFi therapy results in loss of glomerular endothelial cells and in a malignant hypertensive phenotype with severe glomerulosclerosis (8). In some preclinical studies, the angiotensin-converting enzyme (ACE) inhibitor ramipril ameliorated all of these effects (9). However, other studies have not confirmed this finding (12). The current recommended management strategies for VEGFi-mediated hypertension support the use of ACE inhibitor and angiotensin receptor blocker (ARB) therapy but acknowledge a lack of data specific to the pathophysiology and management of VEGFi-mediated hypertension 2, 3. As such, no formal guidelines exist, and current recommendations are based on expert opinion and general cardiovascular principles rather than on data specific to this group of patients.
We hypothesized that antihypertensive agents that inhibit the RAAS system are more efficacious in modulating VEGFi-mediated blood pressure elevation. Electronic health records (EHRs) have been used in several recent precision medicine projects in both oncology and hypertension 13, 14, 15, 16. To test this hypothesis, we used the EHR to investigate the effect of pre-treatment RAASi therapy on change in mean systolic and mean diastolic blood pressure in patients treated with VEGFis.
Methods
This retrospective cohort analysis was reviewed and approved by the Vanderbilt University (Nashville, Tennessee) Institutional Review Board (IRB #171796). We used Vanderbilt’s Synthetic Derivative database, a version of Vanderbilt’s EHR with Health Insurance Portability and Accountability Act identifiers removed by de-identification software and custom algorithms (17). The Synthetic Derivative contains more than 2.8 million unique subject records from patients seeking care. Records in the database contain clinical data, including basic demographics, clinical care notes, laboratory and diagnostic study results, medication data, and International Classification of Diseases (ICD) and Current Procedural Terminology codes. Methodology for data collection and de-identification within the Synthetic Derivative has been previously described (17).
Data extraction
We developed an algorithm using regular expression and natural language processing to extract structured, quantitative data. Our algorithm identified subjects with records in both the Synthetic Derivative and the Vanderbilt Cancer Registry. This registry was founded in the 1930s and is the institutional repository for data regarding individuals with cancer at Vanderbilt University Medical Center. Information in the database includes the number of patients with newly diagnosed cancer each year, the type of cancer at diagnosis, the state and county of residence, and the health care outcomes for each patient (18). Subjects were included if the VEGFi therapy was mentioned at least twice on 2 separate dates within the record and outpatient blood pressure readings were recorded 6 weeks before and 6 weeks after VEGFi initiation. A complete list of VEGFi therapies included in the algorithm is provided in Supplemental Table 1. Exclusion criteria included subjects less than 18 years of age or pregnant at the time of VEGFi initiation.
Data were extracted through February of 2018. Baseline characteristics over the lifetime of the subject, including sex, age, race, body mass index, and estimated glomerular filtration rate, were collected. Comorbidities, including heart failure, coronary artery disease, hypertension, hyperlipidemia, diabetes, sleep apnea, and nephrectomy, were identified using ICD-9th revision, ICD-10th revision, and Current Procedural Terminology codes (Supplemental Table 2).
A manual chart review of 20 randomly selected, nonoverlapping subjects was performed using a standardized adjudication form (Supplemental Figure 1). On the basis of this review, the algorithm correctly identified all 20 subjects. The accuracy of the algorithm to collect blood pressure data was also assessed. All available outpatient blood pressure recordings during this defined time period were extracted in 100% of the subjects.
To ensure an accurate start date for VEGFi therapy in our subjects, a manual chart review was performed for all 1,013 subjects identified by our algorithm. Subjects whose specific VEGFi prescribed and start dates could be determined, through on clinician notes, prescription records, and infusion records, within a 7-day period and who had blood pressure recordings in the 6 weeks before and after VEGFi initiation were used for all subsequent data collection and analysis.
All outpatient blood pressure readings 6 weeks before and up to 6 weeks following initiation of VEGFi therapy were collected. These blood pressures were obtained according to routine clinical practice in our medical center and included blood pressure readings obtained on separate clinical visits, as well as multiple readings obtained during the same clinical visit. Blood pressure readings that occurred after a change in antihypertensive medications were censored. Nonphysiological data suggestive of entry error were deleted. Our criteria for nonphysiological data were systolic blood pressure ≥300 mm Hg, diastolic blood pressure ≤20 mm Hg, diastolic blood pressure greater than systolic blood pressure, or pulse pressure <5 mm Hg. Our criteria for a plausible range of physiological hemodynamics were based on a population study of mean blood pressure in adults (19). This resulted in the deletion of 11 of 8,442 blood pressure recordings. Using these data, mean systolic and mean diastolic blood pressures were calculated for the 6-week intervals before and after VEGFi initiation. The 6-week time interval was selected on the basis of previous data demonstrating that the hypertensive effect of VEGFi therapy can be detected within the first 24 hours after initiation and plateaus within days 20, 21, 22, 23.
Antihypertensive medication data were extracted by electronic-prescribing tools and MedEx, a previously described and validated natural language processing software program capable of extracting medication information from clinical notes 24, 25. We required the presence of at least 1 of the following identifiers—drug dose, route, frequency or duration—for a MedEx-identified drug to be counted. Drug data were extracted using a complete, previously defined, and validated list of antihypertensive agents (Supplemental Table 3) (16). RAASis were defined as aldosterone antagonists, ACE inhibitors, ARBs, renin inhibitors, and any combination medications containing a drug from these classes. For each antihypertensive medication, our algorithm identified the date of drug initiation so that any changes in antihypertensive regimen during the study period could be identified and accounted for in our statistical analysis.
We defined our primary outcomes as a difference in the change of mean systolic blood pressure and mean diastolic blood pressure between subjects using a RAASi before VEGFi initiation and those not using a RAASi before VEGFi initiation. Because the existing published reports are most robust for ACE inhibitor and ARB use, we also assessed the difference in the change of mean systolic and diastolic blood pressure for ACE inhibitor and ARB use alone instead of all RAASis. In addition, we examined the effect of using a RAASi alone without any other antihypertensive medications on the difference in the change of mean systolic and diastolic blood pressure compared with subjects using no antihypertensive medication before VEGFi initiation.
Statistical analysis
Data are expressed as mean ± SD for continuous variables and as absolute value and proportion for categorical variables, unless stated otherwise. Between-group comparisons were made using Student’s t-test or the chi square test as appropriate. Because the number of potential confounders was not large in comparison with the effective sample size, we used direct covariate adjustment to maintain power and sample size and to ensure a representative sample group 26, 27, 28. Linear regression modeling was used to identify the effect of pre-specified covariates known to influence blood pressure. Variables were selected a priori on the basis of clinical knowledge, content expertise, and current published reports. All pre-specified variables were included in the final model. In our analytic approach, nonlinear terms (spline) were used for all continuous variables initially and were removed because of nonsignificance. Variables included baseline mean systolic and diastolic blood pressure, age, sex, race, body mass index, glomerular filtration rate, history of heart failure, coronary artery disease, hypertension, hyperlipidemia, diabetes, sleep apnea, specific VEGFi prescribed, and the presence of antihypertensive medication changes during the 6 weeks after VEGFi initiation. The variable specific VEGFi prescribed was treated as a categorical variable according to the frequency of drug prescription within our study group. This resulted in 3 categories: bevacizumab, sorafenib, and all other VEGFis prescribed. An alpha level of <0.05 was defined as statically significant. Statistical analysis was performed using IBM SPSS Statistics version 25 (IBM Corp., Armonk, New York) and reconfirmed using R Statistical Software (R Foundation, Vienna, Austria).
Results
Demographic and clinical characteristics
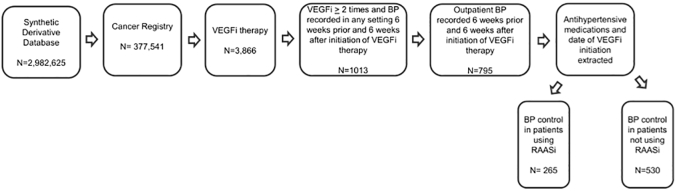
We identified 377,541 unique subjects with a diagnosis of cancer. Of these subjects, 3,867 included at least 1 mention of a VEGFi in the initial review of the subject record. A total of 1,013 unique subjects met inclusion criteria and did not meet any of the exclusion criteria. By manual chart review, the date of VEGFi initiation could be determined within a 7-day window for 844 individuals. We were then able to collect outpatient blood pressure readings during both the 6 weeks before and the 6 weeks after initiation of VEGFi therapy for 795 of these individuals, thus representing our final group of patients. Of these subjects, 265 were using RAASis before initiation of VEGFi (Figure 1). The median number of days between first mention of RAASi in the EHR and VEGFi initiation was 458 days (SD = 1,033 days).
Figure 1.
Flow Diagram for Cohort Selection
The database investigated was the Synthetic Derivative (a de-identified version of Vanderbilt University’s electronic health record). Subjects within the Synthetic Derivative with a history of cancer were identified on the basis of their presence in the Vanderbilt Cancer Registry. From these subjects, 3,866 had a mention of a vascular endothelial growth factor inhibitor (VEGFi). Within this group, we required a mention of a vascular endothelial growth factor inhibitor ≥2 times and blood pressure (BP) recorded in any setting 6 weeks before and 6 weeks after initiation of vascular endothelial growth factor inhibitor therapy. These criteria narrowed our cohort to 1,013 subjects. We then narrowed this selection to our final cohort by including only those subjects with outpatient blood pressure recordings. On the basis of these selection criteria we identified 265 patients using renin-angiotensin-aldosterone system inhibitor (RAASi) therapy before initiation of vascular endothelial growth factor inhibitor and 530 patients not using renin-angiotensin-aldosterone system inhibitor therapy before vascular endothelial growth factor inhibitor initiation.
Regardless of RAASi use before VEGFi start, bevacizumab and sorafenib were the most commonly prescribed VEGFis (Table 1). The most common cancer types were genitourinary, gastrointestinal, neurological, and lung or thoracic (Table 1). In unadjusted analysis, subjects using a RAASi before VEGFi therapy were older (68 years vs. 61 years of age) and more likely to be male (63% vs. 46%) (p < 0.001 for both comparisons). Subjects using a RAASi were more likely to be white (92% vs. 88%) compared with subjects not using a RAASi (p = 0.048). RAASi use was significantly associated with higher body mass index and lower estimated glomerular filtration rates. There was also a significant association between RAASi use and an increase in cardiovascular comorbidities, including a history of hyperlipidemia (74% vs. 54%), diabetes (46% vs. 25%), hypertension (92% vs. 56%), and obstructive sleep apnea (17% vs. 8%), a decrease in heart failure (62% vs. 83%) (p < 0.001 for all comparisons), and an increase in history of coronary disease (21% vs. 14%) (p = 0.012).
Table 1.
Clinical Characteristics and Blood Pressure Response
| RAASi at Baseline (n = 265) | No RAASi at Baseline (n = 530) | p Value | |
|---|---|---|---|
| Mean age, yrs | 68 ± 10.52 | 61 ± 13.59 | <0.001 |
| Male | 166 (63) | 242 (46) | <0.001 |
| Race | 0.048 | ||
| White | 243 (92) | 467 (88) | |
| Black | 18 (7) | 36 (7) | |
| Other or unknown | 4 (2) | 27 (5) | |
| BMI (kg/m2) | 30 ± 6.91 | 27 ± 6.11 | <0.001 |
| GFR (ml/min) | 78 ± 29.87 | 95 ± 36.81 | <0.001 |
| HLD | 204 (74) | 287 (54) | <0.001 |
| DM | 122 (46) | 132 (25) | <0.001 |
| HTN | 243 (92) | 294 (56) | <0.001 |
| OSA | 46 (17) | 41 (8) | <0.001 |
| CAD | 55 (21) | 73 (14) | 0.012 |
| CHF | 163 (62) | 440 (83) | <0.001 |
| Number of baseline antihypertensives | 3 ± 1.46 | 1 ± 0.91 | <0.001 |
| Antihypertensive class | — | ||
| Beta-blocker | 105 (40) | 106 (20) | |
| RAASi | 265 (33) | 0 | |
| CCB | 99 (37) | 89 (17) | |
| Other diuretics | 265 (33) | 35 (7) | |
| Loop | 120 (35) | 23 (3) | |
| Cancer type | <0.001 | ||
| Genitourinary | 95 (36) | 91 (17) | |
| Eye | 1 (<1) | 1 (<1) | |
| Musculoskeletal (including soft tissue and peripheral nerves) | 7 (3) | 29 (6) | |
| Head and neck | 2 (1) | 3 (1%) | |
| Gastrointestinal | 50 (19) | 130 (25) | |
| Gynecological | 11 (4) | 28 (5) | |
| Endocrine | 4 (2) | 9 (2) | |
| Lung or thoracic | 35 (13) | 58 (11) | |
| Neurological | 24 (9) | 85 (16) | |
| Unknown primary | 1 (<1) | 3 (1) | |
| Hematologic | 11 (4) | 37 (7) | |
| Dermatologic | 12 (5) | 10 (2) | |
| Breast | 12 (5) | 46 (9) | |
| VEGFi | — | ||
| Bevacizumab | 134 (51) | 325 (61) | |
| Sorafenib | 50 (19) | 79 (15) | |
| Axitinib | 5 (2) | 4 (1) | |
| Pazopanib | 30 (11) | 51 (10) | |
| Lenvatinib | 0 | 3 (0.5) | |
| Sunitinib | 37 (25) | 45 (8) | |
| Ramucirumab | 8 (3) | 18 (3) | |
| Vandetanib | 0 | 3 (0.5) | |
| Regorafenib | 1 (0.3) | 1 (0.1) | |
| Aflibercept | 0 | 1 (0.1) | |
| Baseline SBP | 128 ± 13.59 | 123 ± 13.29 | <0.001 |
| Baseline DBP | 76 ± 10.23 | 75 ± 8.92 | 0.500 |
| Response SBP | 131 ± 15.16 | 128 ± 15.31 | 0.006 |
| Response DBP | 79 ± 10.07 | 79 ± 9.66 | 0.948 |
Values are mean ± SD or n (%).
BMI = body mass index; CAD = coronary artery disease; CCB = calcium-channel blocker; CHF = congestive heart failure; DBP = diastolic blood pressure; DM = diabetes mellitus; GFR = glomerular filtration rate; HLD = hyperlipidemia; HTN = hypertension; OSA = obstructive sleep apnea; RAASi = renin-angiotensin-aldosterone system inhibitor; SBP = systolic blood pressure; VEGFi = vascular endothelial growth factor inhibitor.
Subjects using a RAASi before VEGFi were most commonly using an ACE inhibitor or ARB, with only 15 subjects not using an ACE inhibitor or an ARB. On average, subjects had 5 outpatient blood pressure readings in the 6 weeks before VEGFi initiation and 3 outpatient blood pressure readings in the 6 weeks after VEGFi initiation. At baseline, the mean systolic blood pressure for subjects using RAASi was higher than for subjects not using RAASi (p = 0.001), with similar mean diastolic blood pressures. A comparison of the subject groups is displayed in Table 1.
Outcomes
Although mean blood pressure increased in both groups during the 6 weeks after VEGFi therapy, the increase in mean systolic blood pressure was significantly lower in subjects using a RAASi before a VEGFi (2.46 mm Hg [95% confidence interval (CI): 0.7 to 4.2] vs. 4.56 mm Hg [95% CI: 3.5 to 5.6]; p = 0.034) (Central Illustration). This observation was consistent when only ACE inhibitor or ARB use rather than any RAASi was evaluated (2.27 mm Hg [95% CI: 0.43 to 4.1] vs. 4.56 mm Hg [95% CI: 3.5 to 5.6]; p = 0.024). Although the absolute increase in mean diastolic blood pressure after VEGFi initiation was larger in subjects not using RAASis, this difference did not meet our threshold for statistical significance (3.30 mm Hg [95% CI: 2.2 to 4.4] vs. 3.82 mm Hg [95% CI: 3.1 to 4.5]; p = 0.419) (Central Illustration). This finding was also consistent among subjects using only ACE inhibitor or ARB therapy rather than any RAASi (3.15 mm Hg [95% CI: 2.0 to 4.3] vs. 3.82 mm Hg [95% CI: 3.1 to 4.5]; p = 0.314).
Central Illustration.
Blood Pressure and Cancer Therapy: Change in Mean Blood Pressure
Using Student’s t-test, the change in mean systolic blood pressure in subjects with baseline renin-angiotensin-aldosterone system inhibitor use was 2.46 mm Hg (95% confidence interval [CI]: 0.7 to 4.2), and without renin-angiotensin-aldosterone system inhibitor use it was 4.56 mm Hg (95% CI: 3.5 to 5.6; p = 0.034). The change in mean diastolic blood pressure in subjects with baseline renin-angiotensin-aldosterone system inhibitor use was 3.30 mm Hg (95% CI: 2.2 to 4.4), and without renin-angiotensin-aldosterone system inhibitor use it was 3.82 mm Hg (95% CI: 3.1 to 4.5; p = 0.419). The rise in blood pressure was less in those patients who had renin-angiotensin-aldosterone system inhibitor therapy at baseline.
Multivariable linear regression modeling was used to account for baseline differences between cases with RAASi exposure and those without. RAASi exposure before VEGFi was significantly associated with a decrease in mean systolic and mean diastolic blood pressure (95% CI: −4.94 to −0.60; p = 0.012; and 95% CI: −2.74 to −0.05; p = 0.042, respectively). Further, a prior history of hypertension and escalation of antihypertensive therapy after VEGFi initiation were significantly associated with increased mean systolic and diastolic blood pressures after VEGFi initiation. An increase in mean systolic blood pressure tended to be associated with age, whereas a decrease in mean diastolic blood pressure tended to be associated with female sex. Variables accounted for the in the model, 95% CIs and p values, are displayed in Table 2.
Table 2.
Linear Regression Modeling Demonstrating the Effect of Baseline RAASi on Change in Mean Blood Pressure After VEGFi Initiation
| Change Between Groups in Mean SBP β (95% CI) | Change Between Groups in Mean SBP Significance (p Value) | Change Between Groups in Mean DBP β (95% CI) | Change Between Groups in Mean DBP Significance (p Value) | |
|---|---|---|---|---|
| RAASi | −2.71 (−4.83 to −0.60) | 0.012 | −1.40 (−2.74 to -0.05) | 0.042 |
| Age | 2.31 (1.04 to 3.58) | <0.001 | −0.69 (−1.48 to 0.10) | 0.088 |
| Sex (male vs. female) | 0.57 (−1.18 to 2.33) | 0.522 | −1.53 (−2.65 to −0.40) | 0.008 |
| Race | 0.152 | 0.616 | ||
| Black vs. white | 3.35 (−0.12 to 6.82) | 1.10 (−1.11 to 3.31) | ||
| Other vs. white | −0.77 (−5.22 to 3.68) | −0.103 (−2.93 to 2.73) | ||
| BMI | 1.12 (−0.07 to 2.31) | 0.066 | 0.38 (−0.37 to 1.13) | 0.318 |
| GFR | −0.38 (−1.49 to 0.74) | 0.509 | −0.61 (−1.32 to 0.10) | 0.093 |
| HTN | 2.91 (0.79 to 5.03) | 0.007 | 2.57 (1.21 to 3.92) | <0.001 |
| DM | 1.65 (−0.30 to 3.59) | 0.097 | −0.76 (−1.99 to 0.48) | 0.228 |
| CHF | −1.39 (−4.94 to 2.16) | 0.442 | −1.77 (−4.03 to 0.50) | 0.126 |
| OSA | 1.46 (−1.45 to 4.38) | 0.324 | 0.10 (−1.75 to 1.95) | 0.915 |
| CAD | 0.96 (−1.50 to 3.42) | 0.444 | −0.03 (−1.54 to 1.59) | 0.972 |
| Baseline mean SBP | −7.57 (−8.81 to −6.33) | <0.001 | ||
| Baseline mean DBP | −5.48 (−6.26 to −4.71) | <0.001 | ||
| Medication change | 2.90 (0.60 to 5.19) | 0.014 | 1.86 (0.41 to 3.31) | 0.012 |
| VEGFi | 0.452 | 0.001 | ||
| Sorafenib vs. bevacizumab | 0.023 (−2.24 to 2.70) | −0.28 (−1.85 to 1.29) | ||
| Other vs. bevacizumab | 1.34 (−0.77 to 3.44) | 2.37 (1.03 to 3.72) | ||
| Number of baseline antihypertensive agents | −1.18 (−2.95 to 0.59) | 0.190 | −0.69 (−1.81 to 0.44) | 0.230 |
CI = confidence interval; other abbreviations as in Table 1.
We also examined the effect of RAASi monotherapy. Although this analysis did not reach statistical significance, the results are summarized here. In our subgroup analysis of 368 subjects, 311 were not using any antihypertensive therapy, and 57 were using only a RAASi before VEGFi initiation. In this model, mean systolic and diastolic blood pressure did increase in both groups; however, the increase in mean systolic blood pressure was 2.53 mm Hg (95% CI: −1.2 to 6.3) in subjects using RAASi before VEGFi compared with 4.52 mm Hg (95% CI: 3.2 to 5.8) in those who were not (p = 0.256). The increase in mean diastolic blood pressure after VEGFi initiation was 3.84 mm Hg (95% CI: 1.3 to 6.4) in subjects using RAASi before VEGFi compared with 4.14 mm Hg (95% CI: 3.3 to 5.0) in those who were not (p = 0.799). In a regression model accounting for covariates, RAASi monotherapy tended to be associated with a decrease in mean systolic blood pressure (−2.31mm Hg; 95% CI: −5.81 to 1.2; p = 0.196) and diastolic blood pressure (−1.08 mm Hg; 95% CI: −3.37 to 1.2; p = 0.352) (Supplemental Table 4).
Discussion
Our findings suggest that RAASi use before VEGFi initiation may ameliorate VEGFi-mediated increases in blood pressure in a real-world clinical setting. These findings may support the hypothesis that an imbalance between RAAS and VEGF could potentially play a role in VEGFi-mediated elevations in blood pressure, a hypothesis that is supported by previous studies, although not definitely proven 8, 9.
To examine the role of the RAAS in VEGFi-mediated blood pressure elevations, we compared the difference in the change of mean blood pressure in subjects using a RAASi alone or as part of a multidrug antihypertensive regimen with subjects without RAASis and with or without the use of other antihypertensive drug classes. Even though subjects using RAASis were older and had higher baseline mean blood pressures, both of which are known risk factors for VEGFi-mediated increases in blood pressure, subjects using RAASis had a smaller increase in mean blood pressure than subjects not using this drug class (29).
We attempted to account for baseline differences between our 2 groups by using a linear regression model in which all clinical characteristics with significant differences between both groups were included in the model. Even after accounting for these differences, a significantly smaller increase in mean systolic and diastolic blood pressure was observed for patients using a RAASi before VEGFi therapy.
We aimed to clarify the role of RAASi use alone further by comparing changes in mean blood pressure in subjects using only a RAASi before VEGFi initiation with those using no antihypertensive medications. Our regression model for this small subgroup analysis again demonstrated the ability of RAASi therapy to modulate VEGFi-mediated increases in blood pressure, although this was not statistically significant.
The potential role of the RAAS system in VEGFi-mediated hypertension is an area of scientific uncertainty, and other potential mechanisms have been identified, including endothelin-1 activation (30). Our findings, however, are supported by several studies in murine models. In models overexpressing renin, renal VEGF expression is increased (8). When VEGFi is used in this model, loss of glomerular endothelial cells, glomerulosclerosis, and malignant hypertension are more severe compared with control models (8). Belcik et al. (9) demonstrated increased afterload, elevated plasma angiotensin II, left ventricular remodeling, and glomerular disease in mice treated with VEGFi. In this model, coadministration of ramipril almost entirely prevented these adverse effects. In addition, Nagasawa et al. (31) demonstrated a 50% reduction in blood pressure rise in rats treated with oral captopril and sorafenib versus sorafenib alone. These studies suggest that VEGFi may lead to a state of relative RAAS overactivation, thereby creating an iatrogenic imbalance between RAAS and VEGF.
Other basic models have not as clearly demonstrated a link between RAAS and VEGFi-mediated hypertension. There is some evidence to suggest a decrease in renin levels with VEGFi exposure. However, in these studies aldosterone levels did not appear to decline and may have increased 32, 33. Therefore, it is possible that RAAS is involved in VEGFi-mediated hypertension but that aldosterone rather than renin is a more prominent factor. We were not able to evaluate renin and aldosterone levels in our cohort because of the limited availability of these data.
Additional conflicting evidence for the role of RAASis in VEGFi-mediated hypertension includes the observation of superior blood pressure control in models using calcium-channel blockade or endothelin receptor antagonism rather than ACE inhibitors 12, 32. Although this study seemingly contradicts the findings of Nagasawa et al., methodological differences between these studies, such as subcutaneous rather than oral administration of an ACE inhibitor, may account for these results (12). Interestingly, decreased proteinuria and renal injury were observed in this model with ACE inhibitor therapy, and the investigators suggested that renal injury, although perhaps not the primary factor, may further contribute to VEGFi-mediated hypertension. The hypothesis of potential renal involvement is supported by clinical studies describing glomerular disease consistent with thrombotic microangiopathy in patients treated with bevacizumab (5). In a corollary animal model, renal disease preceded all cases of VEGFi-mediated hypertension (5). Given that ACE inhibitors and ARBs are used for the treatment of proteinuria, this observation may support the use of these agents in VEGFi-mediated increases in blood pressure.
Three prospective human studies investigated VEGFi-mediated hypertension. One study examined changes in levels of VEGF, catecholamines, endothelin-1, urotensin II, renin, and aldosterone (34). In this study renin and aldosterone levels did not change after sorafenib initiation; however, vascular stiffness did increase. These results were confirmed by Catino et al. (35), who demonstrated increases in arterial stiffness, resistance, and pulsatile load in the setting of sunitinib use. Although the absence of an increase in renin and aldosterone may indicate that the RAAS system is not involved in VEGFi-mediated hypertension, this finding may not fully eliminate the possibility of a relative imbalance between VEGF and RAAS such that the absence of a compensatory decrease in renin and aldosterone after VEGFi administration could possibly play a role in VEGFi-mediated hypertension. In particular, given the role of RAAS in vascular stiffness, it is possible that RAASi may be more effective in patients using sorafenib or sunitinib (36). Although our study did not address levels of these vasoactive mediators, it did help to address the hypothesis of a potential relative imbalance between RAAS and VEGF by investigating the effects of RAASi therapy, a hypothesis that currently remains unproven.
The third study investigated the role of prophylactic calcium-channel blockade in patients receiving the VEGFi cediranib. Although prophylactic treatment with calcium-channel blockers did not reduce the incidence of mild or moderate hypertension, the incidence of severe hypertension was lower in patients receiving prophylaxis with calcium-channel blockers. Notably, before developing severe hypertension, 44% of patients who were randomized to no antihypertensive prophylaxis had been started on antihypertensive therapy because of blood pressure increases. This may influence interpretation of these results given the number of patients in the no–antihypertensive therapy group who were started on antihypertensive therapy before developing severe hypertension (23). Our study did not investigate the use of calcium-channel blockade specifically, but more than one-third of subjects were using RAASis in the setting of calcium-channel blockade.
Strengths of our study include our subject size, use of a previously validated database, and medication extraction programming. Our study was able to replicate previous work that has shown that age and existing hypertension are risk factors for VEGFi-mediated increases in blood pressure (29). In addition, our results suggest a benefit to RAASis, a class of agents supported by current management recommendations for VEGFi-mediated elevations in blood pressure.
Study limitations
Limitations of our study are predominantly related to the retrospective nature of the study’s design, as well as the inherent limitations of EHR use. Among such limitations are the inability to determine the actual VEGFi start date and the requirement of a 7-day window. In addition, blood pressures were obtained clinically and not by a single uniform protocol, as in clinical trials. Although this is certainly a limitation, these outcomes better reflect the data available to practitioners managing patients using VEGFis in daily practice. In addition, we cannot exclude the possibility that regression to the mean influenced our results, given the direction of association between mean baseline blood pressures and our outcome. However, because of a rise in blood pressure in both groups, we believe that regression to the mean would not fully explain our results, and this association could also be explained by differences in clinical practice patterns such as more intensified blood pressure management for subjects with higher blood pressure readings at baseline. Further, although we were able to account for the addition of antihypertensive agents after VEGFi initiation, we were not able to account for changes in antihypertensive doses, discontinuation of a medication, or medication compliance. Finally, in subjects using more than 1 antihypertensive drug class, we could discern the effect of RAASi therapy on mean blood pressure, but not the effect of each antihypertensive drug class. Similarly, we were not powered to examine the effects of specific VEGFis. Despite these limitations, our study provides real-world clinical data regarding antihypertensive therapy in patients receiving VEGFi therapy and may support the hypothesis that RAAS contributes to VEGFi-mediated elevations in blood pressure.
Conclusions
Our results may support the hypothesis that an iatrogenic imbalance between RAAS and VEGF could contribute to VEGFi-mediated elevations in blood pressure. In addition, RAASi use before VEGFi therapy may better protect patients from VEGFi-mediated increases in blood pressure. Future prospective clinical trials and direct mechanistic studies are warranted to confirm the validity of this hypothesis and determine whether RAAS blockade should be initiated prophylactically in patients receiving VEGFi therapy.
Perspectives.
COMPETENCY IN MEDICAL KNOWLEDGE: VEGFis commonly result in blood pressure increases. However, the underlying pathophysiology and optimal approach to hypertension treatment are not clearly defined. The RAAS may play a role in VEGFi-mediated hypertension, and RAASi therapy may be efficacious in ameliorating these increases in blood pressure.
TRANSLATIONAL OUTLOOK: Further prospective studies are needed to understand fully the potential prophylactic benefit of RAASi therapy before VEGFi use.
Footnotes
Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institute of Health under Award Number UL1 TR000445. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Dr. Brittain has reported membership on the advisory board of Bayer. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Appendix
For supplemental tables and a figure, please see the online version of this paper.
Appendix
References
- 1.Li W., Croce K., Steensma D.P., McDermott D.F., Ben-Yehuda O., Moslehi J. Vascular and metabolic implications of novel targeted cancer therapies: focus on kinase inhibitors. J Am Coll Cardiol. 2015;66:1160–1178. doi: 10.1016/j.jacc.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 2.Maitland M.L., Bakris G.L., Black H.R. Initial assessment, surveillance, and management of blood pressure in patients receiving vascular endothelial growth factor signaling pathway inhibitors. J Natl Cancer Inst. 2010;102:596–604. doi: 10.1093/jnci/djq091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ederhy S., Izzedine H., Massard C. Cardiac side effects of molecular targeted therapies: towards a better dialogue between oncologists and cardiologists. Crit Rev Oncol Hematol. 2011;80:369–379. doi: 10.1016/j.critrevonc.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Cella D., Grunwald V., Escudier B. Patient-reported outcomes of patients with advanced renal cell carincoma treated with nivolumab plus ipilimumab versus sunitinib (CheckMate 214): a randomised, phase 3 trial. Lancet Oncol. 2019;20:297–310. doi: 10.1016/S1470-2045(18)30778-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eremina V., Jefferson J.A., Kowalewska J. VEGF inhibition and renal thrombotic microangiopathy. N Engl J Med. 2008;358:1129–1136. doi: 10.1056/NEJMoa0707330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lankhorst S., Kappers M.H., van Esch J.H., Danser A.H., van den Meiracker A.H. Hypertension during vascular endothelial growth factor inhibition: focus on nitric oxide, endothelin-1, and oxidative stress. Antioxid Redox Signal. 2014;20:135–145. doi: 10.1089/ars.2013.5244. [DOI] [PubMed] [Google Scholar]
- 7.Moreno-Munoz D., de la Haba-Rodriguez J.R., Conde F. Genetic variants in the renin-angiotensin system predict response to bevacizumab in cancer patients. Eur J Clin Invest. 2015;45:1325–1332. doi: 10.1111/eci.12557. [DOI] [PubMed] [Google Scholar]
- 8.Advani A., Kelly D.J., Advani S.L. Role of VEGF in maintaining renal structure and function under normotensive and hypertensive conditions. Proc Natl Acad Sci U S A. 2007;104:14448–14453. doi: 10.1073/pnas.0703577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Belcik J.T., Qi Y., Kaufmann B.A. Cardiovascular and systemic microvascular effects of anti-vascular endothelial growth factor therapy for cancer. J Am Coll Cardiol. 2012;60:618–625. doi: 10.1016/j.jacc.2012.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrara N., Carver-Moore K., Chen H. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 11.Shalaby F., Rossant J., Yamaguchi T.P. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- 12.Lankhorst S., Kappers M.H., van Esch J.H. Treatment of hypertension and renal injury induced by the angiogenesis inhibitor sunitinib: preclinical study. Hypertension. 2014;64:1282–1289. doi: 10.1161/HYPERTENSIONAHA.114.04187. [DOI] [PubMed] [Google Scholar]
- 13.Afghahi A., Mathur M., Thompson C.A. Use of gene expression profiling and chemotherapy in early-stage breast cancer: a study of linked electronic medical records, cancer registry data, and genomic data across two health care systems. J Oncol Pract. 2016;12:e697–e709. doi: 10.1200/JOP.2015.009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Afghahi A., Purington N., Han S.S. Higher absolute lymphocyte counts predict lower mortality from early-stage triple-negative breast cancer. Clin Cancer Res. 2018;24:2851–2858. doi: 10.1158/1078-0432.CCR-17-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dumitrescu L., Ritchie M.D., Denny J.C. Genome-wide study of resistant hypertension identified from electronic health records. PloS One. 2017;12 doi: 10.1371/journal.pone.0171745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shuey M.M., Gandelman J.S., Chung C.P. Characteristics and treatment of African-American and European-American patients with resistant hypertension identified using the electronic health record in an academic health centre: a case-control study. BMJ Open. 2018;8 doi: 10.1136/bmjopen-2018-021640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roden D.M., Pulley J.M., Basford M.A. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–369. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dagny S. Warner named medical director of Cancer Registry. VUMC Reporter. February 11, 2016 [Google Scholar]
- 19.Wright J.D., Hughes J.P., Ostchega Y., Yoon S.S., Nwankwo T. Mean systolic and diastolic blood pressure in adults aged 18 and over in the United States, 2001-2008. Natl Health Stat Report. 2011:1–22. 44. [PubMed] [Google Scholar]
- 20.Maitland M.L., Kasza K.E., Karrison T. Ambulatory monitoring detects sorafenib-induced blood pressure elevations on the first day of treatment. Clin Cancer Res. 2009;15:6250–6257. doi: 10.1158/1078-0432.CCR-09-0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson E.S., Matulonis U.A., Ivy P. Rapid development of hypertension and proteinuria with cediranib, an oral vascular endothelial growth factor receptor inhibitor. Clin J Am Soc Nephrol. 2010;5:477–483. doi: 10.2215/CJN.08111109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azizi M., Chedid A., Oudard S. Home blood-pressure monitoring in patients receiving sunitinib. N Engl J Med. 2008;358:95–97. doi: 10.1056/NEJMc072330. [DOI] [PubMed] [Google Scholar]
- 23.Langenberg M.H., van Herpen C.M., De Bono J. Effective strategies for management of hypertension after vascular endothelial growth factor signaling inhibition therapy: results from a phase II randomized, factorial, double-blind study of Cediranib in patients with advanced solid tumors. J Clin Oncol. 2009;27:6152–6159. doi: 10.1200/JCO.2009.22.2273. [DOI] [PubMed] [Google Scholar]
- 24.Xu H., Stenner S.P., Doan S., Johnson K.B., Waitman L.R., Denny J.C. MedEx: a medication information extraction system for clinical narratives. J Am Med Inform Assoc. 2010;17:19–24. doi: 10.1197/jamia.M3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H., Jiang M., Oetjens M. Facilitating pharmacogenetic studies using electronic health records and natural-language processing: a case study of warfarin. J Am Med Inform Assoc. 2011;18:387–391. doi: 10.1136/amiajnl-2011-000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brazauskas R., Logan B.R. Observational studies: matching or regression? Biol Blood Marrow Transplant. 2016;22:557–563. doi: 10.1016/j.bbmt.2015.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rose S., Laan M.J. Why match? Investigating matched case-control study designs with causal effect estimation. Int J Biostat. 2009;5 doi: 10.2202/1557-4679.1127. Article 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenland S., Morgenstern H. Matching and efficiency in cohort studies. Am J Epidemiol. 1990;131:151–159. doi: 10.1093/oxfordjournals.aje.a115469. [DOI] [PubMed] [Google Scholar]
- 29.Hamnvik O.P., Choueiri T.K., Turchin A. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer. 2015;121:311–319. doi: 10.1002/cncr.28972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lankhorst S., Kappers M.H., van Esch J.H., Danser A.H., van den Meiracker A.H. Mechanism of hypertension and proteinuria during angiogenesis inhibition: evolving role of endothelin-1. J Hyperens. 2013;31:444–454. doi: 10.1097/HJH.0b013e32835c1d1b. [DOI] [PubMed] [Google Scholar]
- 31.Nagasawa T., Hye Khan M.A., Imig J.D. Captopril attenuates hypertension and renal injury induced by the vascular endothelial growth factor inhibitor sorafenib. Clin Exp Pharmacol Physiol. 2012;39:454–461. doi: 10.1111/j.1440-1681.2012.05699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kappers M.H., van Esch J.H., Sluiter W., Sleijfer S., Danser A.H., van den Meiracker A.H. Hypertension induced by the tyrosine kinase inhibitor sunitinib is associated with increased circulating endothelin-1 levels. Hypertension. 2010;56:675–681. doi: 10.1161/HYPERTENSIONAHA.109.149690. [DOI] [PubMed] [Google Scholar]
- 33.Thijs A., van Herpen C., van der Graaf W.T.A., Rongen G.A. EACPT-0028 - early rise in aldosteron is correlated with increase in blood pressure in sunitinib treated patients with metastatic renal cell carcinoma. Clin Ther. 2014;36:e11. [Google Scholar]
- 34.Veronese M.L., Mosenkis A., Flaherty K.T. Mechanisms of hypertension associated with BAY 43-9006. J Clin Oncol. 2006;24:1363–1369. doi: 10.1200/JCO.2005.02.0503. [DOI] [PubMed] [Google Scholar]
- 35.Catino A.B., Hubbard R.A., Chirinos J.A. Longitudinal assessment of vascular function with sunitinib in patients with metastatic renal cell carcinoma. Circ Heart Fail. 2018;11 doi: 10.1161/CIRCHEARTFAILURE.117.004408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jia G., Aroor A.R., Hill M.A., Sowers J.R. Role of renin-angiotensin-aldosterone system activation in promoting cardiovascular fibrosis and stiffness. Hypertension. 2018;72:537–548. doi: 10.1161/HYPERTENSIONAHA.118.11065. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.