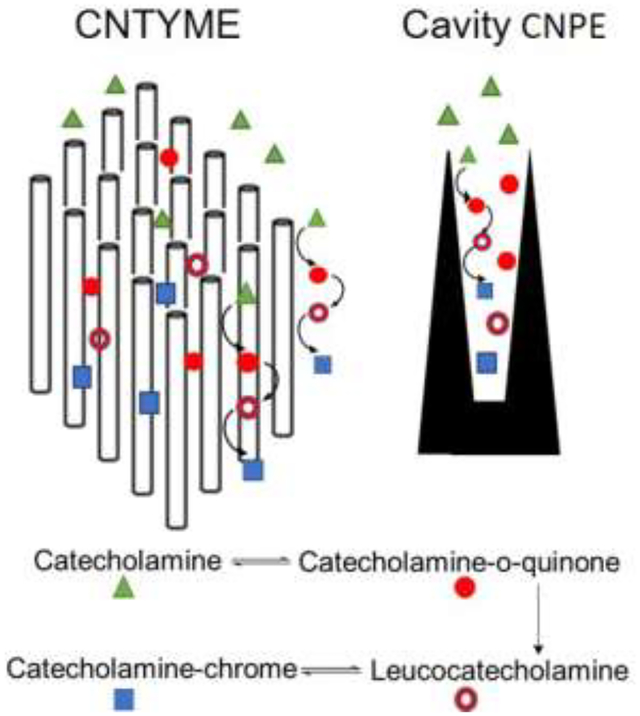
Abstract
Carbon nanotube yarn microelectrodes (CNTYMEs) are an alternative to carbon-fiber microelectrodes (CFMEs) with interesting electrochemical properties because analyte is momentarily trapped in cavities between the CNTs. Here, we compare fast-scan cyclic voltammetry (FSCV) detection of catecholamines, including dopamine, norepinephrine, and epinephrine, at CNTYMEs, CFMEs, as well as cavity carbon nanopipette electrodes (CNPEs). At CFMEs, current decreases dramatically at high FSCV repetition frequencies. At CNTYMEs, current is almost independent of FSCV repetition frequency because the analytes are trapped in the crevices between CNTs, and thus the electrode acts like a thin-layer cell. At CFMEs, small cyclization product peaks are observed due to an intramolecular cyclization reaction to form leucocatecholamine, which is electroactive, and these peaks are largest for the secondary amine epinephrine. At CNTYMEs, more of the leucocatecholamine cyclization product is detected for all catecholamines because of the enhanced trapping effects, particularly at higher repetition rates where the reaction occurs more frequently and more product is accumulated. For epinephrine, the secondary peaks have larger currents than the primary oxidation peaks at 100 Hz, and similar trends are observed with faster scan rates and 500 Hz repetition frequencies. Finally, we examined CNPEs, which also momentarily trap neurotransmitters. Similar to CNTYMEs, at CNPEs, catecholamines have robust cyclization peaks, particularly at high repetition rates. Thus, CNTYMEs and CNPEs have thin layer cell behavior that facilitates high temporal resolution measurements, but catecholamines CVs are complicated by cyclization reactions. However, those additional peaks could be useful in discriminating the analytes, particularly epinephrine and norepinephrine.
Graphical Abstract
Introduction
Fast-scan cyclic voltammetry (FSCV) is used to detect neurotransmitters in vivo because it has a rapid time resolution and provides a unique cyclic voltammogram (CV) that enables analyte identification.[1–4] Carbon-fiber microelectrodes (CFMEs) have strong adsorption for dopamine, enhancing the sensitivity and electron transfer.[5–7] However, FSCV for dopamine at CFMEs is usually limited to 10 Hz repetition frequency because at higher frequencies, the signal decreases dramatically when there is less time at the holding potential for dopamine to be adsorbed on the electrode surface.[8] Recently, carbon nanomaterial electrodes have been developed to enhance dopamine detection.[9,10] For example, a carbon nanotube yarn microelectrode (CNTYME), made from aligned carbon nanotube bundles twisted into a thread,[7,11,12] has highly sensitive dopamine detection with FSCV and a more reversible CV with smaller peak-to-peak separation (ΔEp).[13],[14] Our group demonstrated that at CNTYMEs, dopamine peak current is independent of the FSCV repetition frequency, which enables CNTYMEs to maintain a high sensitivity with improved temporal resolution.[9,15] This frequency independent response is due to surface roughness, as crevices on the scale of the diffusion layer trap dopamine, leading to thin layer cell electrochemical behavior.[16,17] Practically, this occurs at CNT yarns and fibers with micron scale surface roughness,[9,17,18] and the CNTYME performance is improved by increasing the surface roughness using laser activation or an antistatic gun.[19,20] Trapping effects are also observed at other electrodes, including cavity carbon nanopipette microelectrodes (CNPEs),[9,21] where cavity CNPEs have a nano-sized cavity at the tip that also gives rise to thin layer cell electrochemical behavior.[21] However, all the FSCV research on CNTYMEs and cavity CNPEs is focused on dopamine, and other neurotransmitters have not been studied.[13,19]
Dopamine, norepinephrine, and epinephrine are classified as catecholamine neurotransmitters due to their similar chemical structure.[22] Dopamine is the major neurotransmitter associated with reward, and is dysregulated during drug abuse, and also regulates locomotion and is depleted during Parkinson’s disease.[23] Norepinephrine release in the brain influences sleep, attention, and feeding behavior.[24] Epinephrine causes fight or flight responses, such as increasing heart rate and contractility.[25] Catecholamines can be detected electrochemically, but their CVs are largely indistinguishable with normal oxidation limits at carbon electrodes.[26,27] However, Wightman’s group also observed a second anodic peak for epinephrine at around 1.3 V due to the oxidation of the secondary amine group, which can be used to distinguish CVs of epinephrine and norepinephrine.[22,26,28] In 1967, Ralph Adams published oxidation pathways of catecholamines and showed that the o-quinone species produced by oxidation can be cyclized and produce leucocatecholamine products that undergo further redox reactions.[29] These leucocatecholamine oxidation peaks are larger for epinephrine, a secondary amine, but are typically observed only at high concentrations. More recently, Mao’s group reported cyclization of dopamine at a carbon nanotube-based biosensor, which enhances selective dopamine detection in the presence of ascorbic acid and DOPAC (3,4-Dihydroxyphenylacetic acid).[30] In addition, we observed the cyclization products of dopamine in CNPEs with FSCV;[21] however, it is not clear how the cyclization of epinephrine and norepinephrine will be affected at electrodes with a structure that facilitates thin layer cell behavior.
The goal of this study is to compare the FSCV response of dopamine, norepinephrine, and epinephrine at CNTYMEs and CNPEs. Neurotransmitters were measured with FSCV with varied scan rates and repetition frequencies. At CNTYMEs and CNPEs, current is maintained for catecholamines at higher repetition frequencies, in contrast to CFMEs, where current decreases significantly with higher repetition frequencies. In addition, the secondary oxidation peaks that result from the cyclization reactions are larger at CNTYMEs and cavity CNPEs than at CFMEs. Secondary peaks are more prominent at high repetition rates because more product accumulates in a short amount of time. Epinephrine has the largest secondary anodic peak because it has a secondary amine group, which promotes cyclization, while norepinephrine has the smallest cyclization peak. Therefore, the differences in CV shapes at these electrodes might allow discrimination of the analytes. All of the catecholamines are detected with higher temporal resolution at CNTYMEs and CNPEs, but that secondary reactions due to cyclization are more prominent because of the trapping effects.
Experimental section
Electrode Fabrication
CFMEs were fabricated by inserting T-650 carbon fibers into a glass capillary (0.68mm ID X 1.2mm OD) and pulled by a glass puller (model PE-21, Narishige, Tokyo, Japan) to form a cylinder tip. The fiber was cut to 100 μm from the glass tip junction and insulated by Epon Resin 828 (Miller-Stephenson, Danbury, CT) mixed with 14% 1,3-phenylenediamine hardener at 85 °C. After overnight curing at room temperature, the electrodes were placed in the oven at 100°C for two hours and then transferred to the 150°C oven overnight.
Commercial carbon nanotube yarn with 50 μm diameter was purchased from the Nanoworld Lab, Department of Chemical and Environmental Engineering, University of Cincinnati. A glass capillary was pulled by a glass pipette puller and cut to have an opening about 100 μm. A piece of 1.5 cm long CNT yarn was gently inserted into the opening tip in the isopropanol solution to remove impurities on the surface and prevent the change of geometry by external force. When the capillary dried, the electrode open tip was then fully immerged and epoxied with Epon Resin 828. Curing procedure was the same as CFMEs, and electrodes were polished to a 45° angle on a fine diamond abrasive plate (Sutter Instruments model BV-10, Novato, CA). Finally, the electrodes were soaked in isopropanol solution for 10 minutes to remove impurities deposited during the polishing process.
Solutions and Electrochemistry
Dopamine hydrochloride, norepinephrine hydrochloride and epinephrine hydrochloride were purchased from Sigma-Aldrich (St. Louis, MO). Analytes were dissolved in 0.1 M HClO4 to make 10 mM stock solutions and were diluted daily to 1 μM in PBS pH 7.4 buffer (131.25 mM NaCl, 3.00 mM KCl, 10 mM NaH2PO4, 1.2 mM MgCl2, 2.0 mM Na2SO4, and 1.2 mM CaCl2).
The buffer and analytes were supplied to the flow cell using a dual syringe pump and were injected through a six-port switching valve (Valco Instruments Co. Inc. Houston, TX).[31] Electrodes were filled with 1 M KCl and inserted into an electrode holder with a silver wire to connect to the headstage of the Dagan ChemClamp (Dagan, Minnesota). The applied triangular waveform was from −0.4 V to 1.3 V versus a Ag/AgCl reference electrode. Scan rate was 400 V/s with frequencies ranging from 10 Hz to 100 Hz, or 2000 V/s for frequencies from 5 Hz to 500 Hz. When multiple frequencies were tested, they were tested from low to high frequency and all three analytes were tested before changing to a higher frequency to prevent electro-activation of carbon electrode surface. FSCV data were acquired with HDCV software, developed at University of North Carolina, Chapel Hill. All CVs are averaged from 5 CVs across 0.5 s in time, and background subtracted by averaging 10 background CVs (1 s). For flow injection analysis data, CVs were taken at the end of a 5 s injection of analyte, to best highlight the secondary peaks which grow in over time.
Surface Characterization
For scanning electrochemical microscopy (SEM), CFMEs and CNTYMEs were taped on a flat aluminum stage. The polished surface of the CNT yarn disk electrode was placed upward to guarantee the image capture. SEM was performed on a ZEISS Merlin High-Resolution Scanning electron microscope (Oberkochen, Germany), provided by Center for Nanophase Materials Science (CNMS), Oak Ridge National Laboratory.
Results and Discussion
Surface characteristics
First, we compared the structural and electrochemical properties of CFMEs and CNTYMEs to detect catecholamines. Figure 1 shows the surfaces of CFMEs and CNTYMEs characterized by SEM. The T650 carbon fiber, 7 μm in diameter, has a relatively smooth surface, but also has striations (Fig. 1A). Polished disk electrodes are used for the CNTYME, and Figure 1B shows the center region, which has a forest structure formed by vertically aligned CNTs. The CNTYME has a rough surface, with many tufts of CNTs with crevices in between. RZ is a roughness factor which represents the distance from the top of the highest peak to the lowest valley, and is about 0.95 μm for the CNTYME, similar to previous work.[20]
Figure 1.
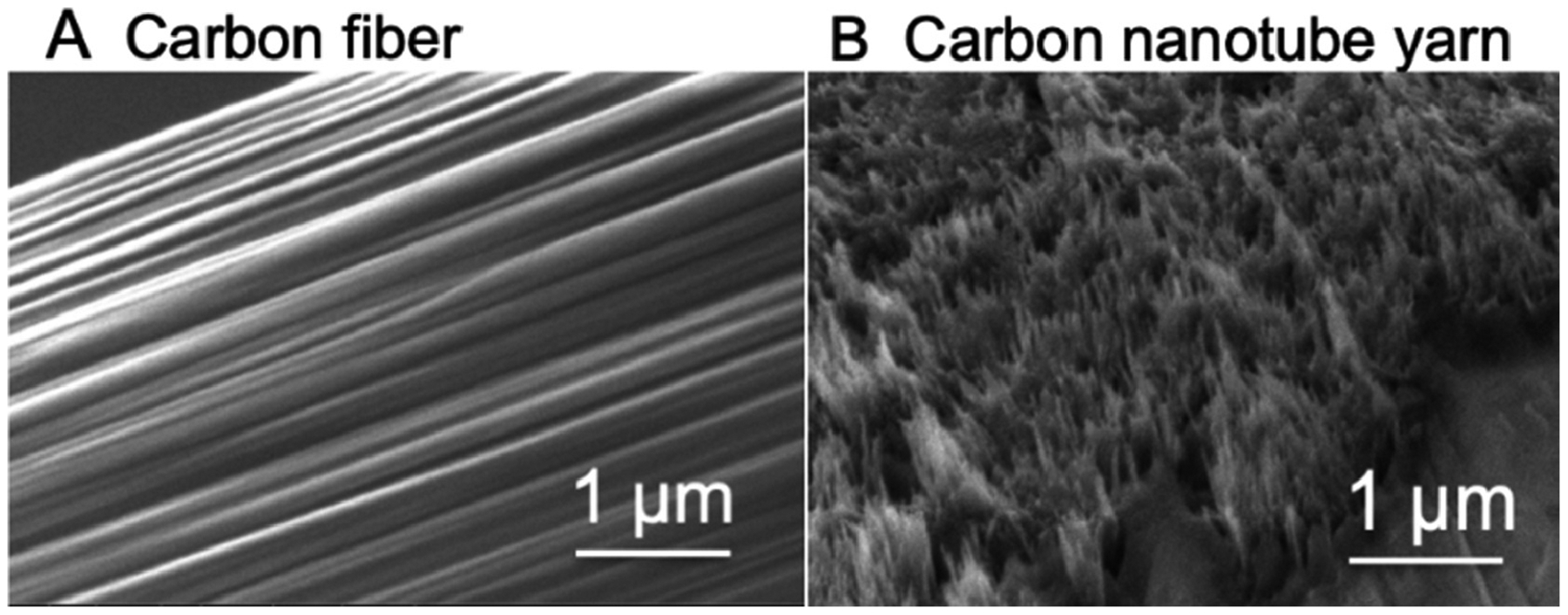
SEM images. (A) T650 carbon fiber surface, (B) CNTYME surface.
Oxidation current decays with higher repetition frequencies at CFMEs
To study the effect of repetition frequency on FSCV measurements, cyclic voltammograms of 1 μM catecholamines were measured at CFMEs at frequencies from 10 Hz to 100 Hz. Figure 2A shows a cyclic voltammogram of 1 μM dopamine, which has an oxidation peak for dopamine at 0.6 V and a reduction peak on the backwards scan around −0.2 V.[32] At 10 Hz, the reduction peak current (14 ± 2 nA) is less than the oxidation peak current (31 ± 3 nA), because dopamine-ortho-quinone (DOQ) has a larger desorption rate constant and desorbs more readily from the CFME.[8] At 100 Hz, oxidation currents are much smaller, less than 10 nA, but the CVs are more symmetrical and the oxidation currents (4.4 ± 0.5 nA) are more similar to the reduction currents (3.2 ± 0.3 nA). Similar CV features were also observed for norepinephrine and epinephrine (Fig. 2B and 2C). The peak currents at 10 Hz are larger than those at 100 Hz, and at 10 Hz, the oxidation peak currents at 0.6 V are larger than reduction peak currents at −0.2 V. Dopamine has the highest peak current among the three catecholamine analytes, likely due to stronger adsorption.
Figure 2.
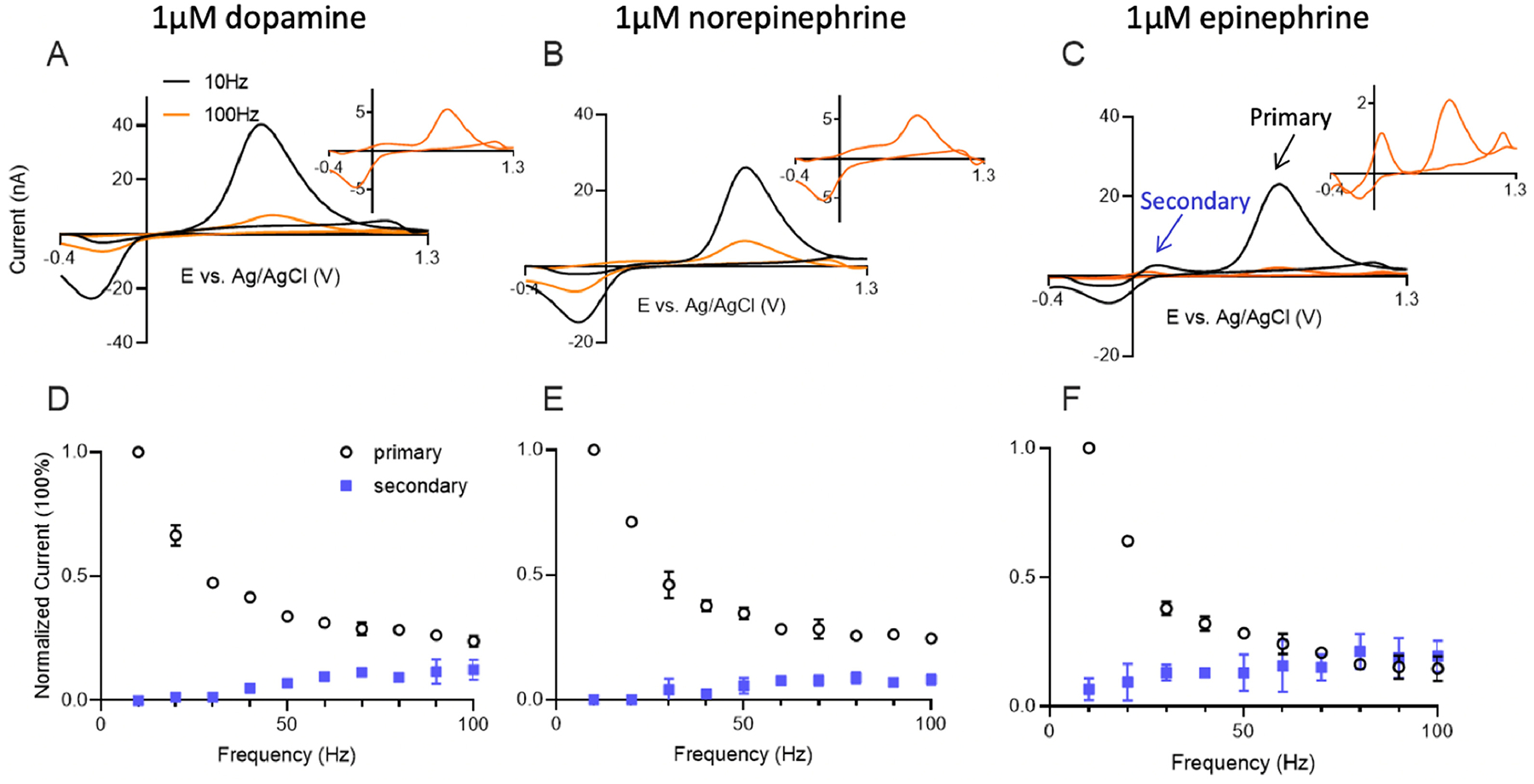
CFME-based detection of catecholamines at different FSCV frequencies. Cyclic voltammograms of (A) dopamine, (B) norepinephrine and (C) epinephrine at 10 Hz (black) and 100 Hz (orange) on CFME with FSCV. The dopamine waveform (−0.4V to 1.3V and back at 400V/s) was applied. Insets are CVs collected at 100 Hz signals. Normalized current (normalized to 10 Hz signal) of the primary and secondary oxidation peaks of (D) dopamine, (E) norepinephrine, and (F) epinephrine at various frequencies. n = 4 electrodes, error bars SEM.
Although the CVs of the three catecholamines have similar oxidation and reduction potentials, there are slight differences. For epinephrine, a secondary redox couple peak due to the intramolecular cyclization reaction is also present, with an oxidation peak at 0.15 V (Fig. 2C). Scheme 1 shows redox pathways and cyclization of catecholamines. The overall anodic pathway of catecholamines is an ECE (Electrochemical-Chemical-Electrochemical) mechanism.[26,33] The catecholamine is oxidized via an electrochemical reaction to generate a corresponding ortho-quinone molecule, which undergoes intramolecular cyclization to produce leucocatecholamine via a Michael addition reaction. This chemical process is irreversible, as leucocatecholamines cannot revert back to the original species once Michael addition occurs.[30] In the following electrochemical process, leucocatecholamine is further oxidized to an aminochrome; thus, a reversible oxidation couple with an oxidation peak at 0.15 V is observed in the cyclic voltammogram.[21,30,34] Epinephrine is the most favorable to undergo the cyclization side reaction, because the methyl group on the secondary amine of epinephrine acts as an electron donor making the nitrogen more nucleophilic, and making it easier to attack the ring structure of the quinone. Secondary peaks are therefore larger for epinephrine.[28] Epinephrine also has another oxidation peak that occurs at 1.3 V on back scan, which is due to the oxidation of the amine group.[28] As a secondary amine, the methylated amine group on epinephrine is more easily oxidized than the primary amines on dopamine and norepinephrine. Researchers have separated epinephrine from norepinephrine based on the peak at 1.3 V, which is much larger on the voltammogram of epinephrine.[22]
Scheme 1.
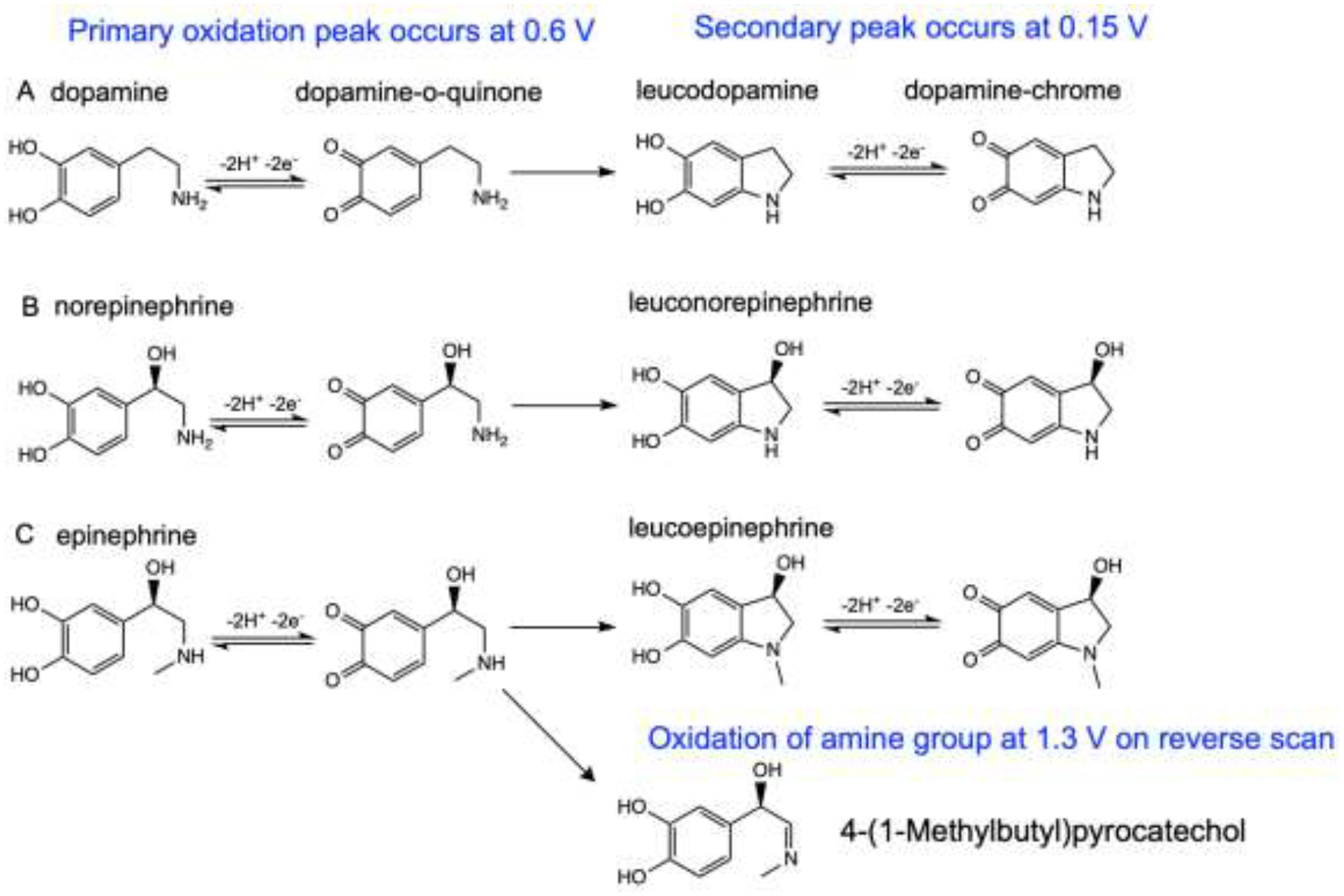
Oxidation reaction schemes of (A) dopamine, (B) norepinephrine and (C) epinephrine.
Figure 2D–F plots the oxidation current vs repetition frequency at CFMEs. For dopamine (Fig. 2D), there is a dramatic decay of the primary oxidation current as FSCV repetition frequency increases. The current at 100 Hz is only 20% of that at 10 Hz. Norepinephrine and epinephrine (Fig. 2E, 2F) have similar trends as dopamine, with primary oxidation currents that decay with increasing frequency. With higher repetition frequency, all three analytes also have an increasing secondary peak current from the cyclization product. The decrease in primary oxidation current for epinephrine is larger than for norepinephrine and dopamine, but its secondary peak current increases the most. More epinephrine-o-quinone molecules are consumed in the chemical process and secondary peaks grow, but the primary peak decreases because less epinephrine-o-quinone is reduced back to epinephrine and detected on the next scan. Cyclization is observed more at 100 Hz than 10 Hz because the number of electrochemical cycles is increased; thus, if a given percentage of o-quinone species will cyclize on each scan, there is more of that cyclization product being accumulated with ten times more scans per second. Overall, the primary oxidation current for all the catecholamines at CFMEs decreases with increasing frequency, limiting the temporal response. Note that the overall amount of cyclization observed at CFMEs is low due to the diffusion of o-quinones.
CNTYMEs maintain high oxidation currents at higher repetition frequencies
Next, CNTYMEs were tested with 1 μM catecholamines at different FSCV repetition frequencies. Figure 3 shows the CVs of dopamine (Figure 3A), norepinephrine (Figure 3B), and epinephrine (Figure 3C) at 10 Hz and 100 Hz. The primary oxidation peak current of dopamine collected at CNTYMEs is 5.2 ± 0.2 nA at 10 Hz, which is smaller than that of CFMEs, because CNTYMEs are disk electrode that have a smaller surface area than the cylindrical CFMEs. The anodic current is 3.9 ± 0.2 nA, which is more reversible than CFMEs. At 100 Hz, the peak current for dopamine is only slightly decreased at CNYMEs and the secondary oxidation peak at 0.15 V is clearly observed. Norepinephrine has a similar CV shape as dopamine at different frequencies, but the secondary oxidation peak is harder to observe. Epinephrine, however, has multiple oxidation peaks and at 100 Hz, the primary peak is smaller than the secondary peaks at 0.15 V and 1.3 V. Compared to CFMEs, the reduction peaks for all three analytes are larger, particularly at high frequencies, indicating more reversibility. Thus, observation of secondary peaks is enhanced at CNTYMEs.
Figure 3.
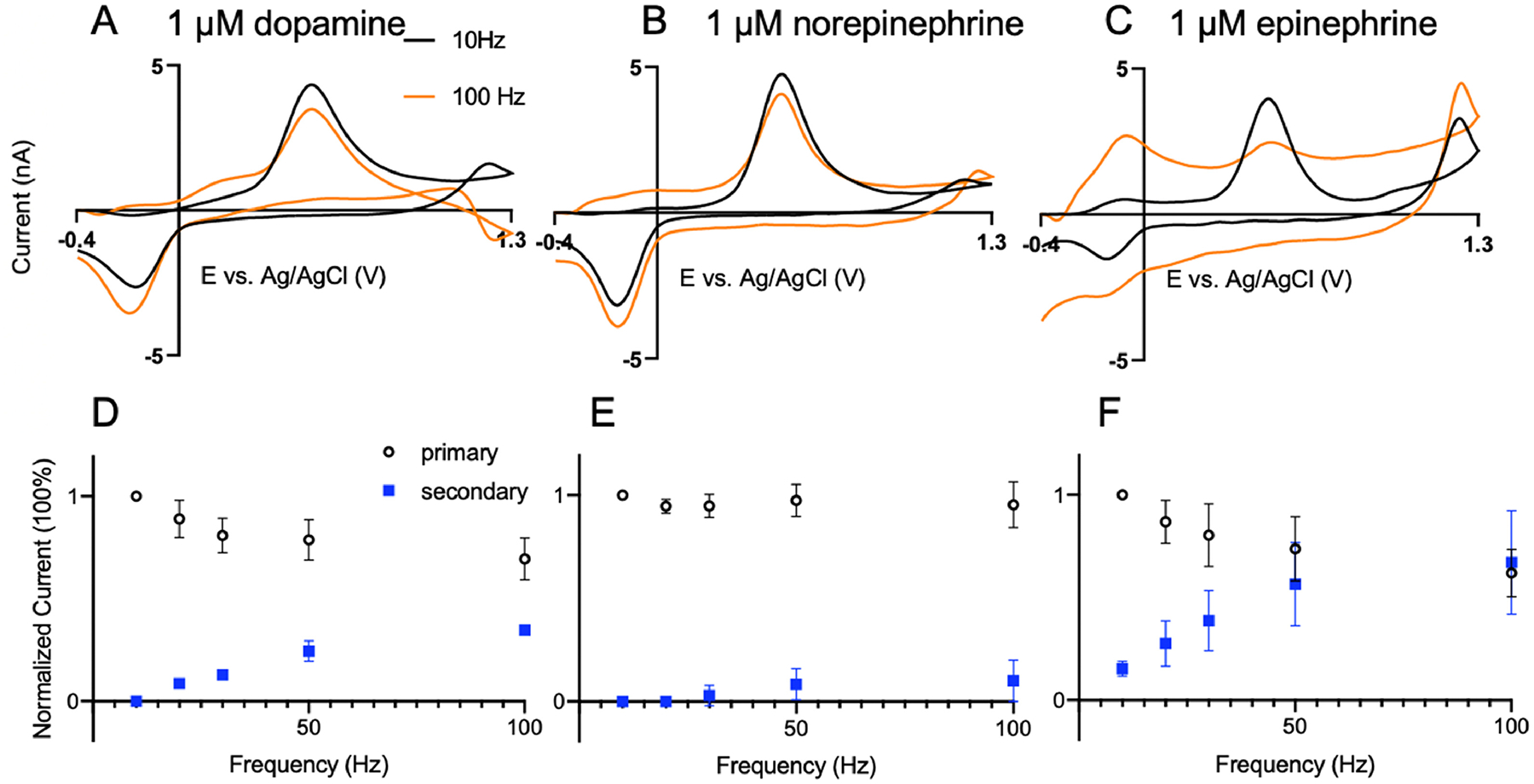
CNTYMEs for catecholamine detection. CVs at 10 Hz (black) and 100 Hz (orange) for (A) 1 μM dopamine, (B) 1 μM norepinephrine, and (C) 1 μM epinephrine. Normalized current vs repetition frequency for (D) dopamine, (E) norepinephrine, and (F) epinephrine. Both the primary oxidation peak (black) and the secondary oxidation peak (blue) from the cyclization reaction are plotted. n = 4
To understand the effects of repetition frequency on current, normalized oxidative currents for the primary and secondary reactions were plotted vs frequency (Fig. 3D, E, F). For dopamine and norepinephrine, the primary peak (0.6 V) decreases only slightly, while the secondary (0.15 V) oxidation peak increases at higher frequencies. Norepinephrine has little secondary peak and thus the current for the primary oxidation is nearly independent of repetition frequency. However, for epinephrine, the primary current drops more at high frequencies because there are more secondary reactions occurring. The primary peak drops as the epinephrine is consumed by the chemical reaction and the secondary peak rises due to reversible detection of the leucoepinephrine redox couple. At higher frequencies, there are more electrochemical scans and so more leucoepinephrine is accumulated, since the cyclization reaction is an irreversible process.
Enhanced secondary peaks at CNTYMEs
To compare CNTYMEs and CFMEs, Figure 4 shows the normalized CVs for dopamine, norepinephrine, and epinephrine at 100 Hz. The primary oxidation current was normalized to account for difference in surface area between CNTYME and CFME. CNTYMEs have larger secondary peaks for all three analytes than CFMEs. For epinephrine, secondary peaks for CNTYMEs are larger than primary peaks for CNTYMEs, but not for CFMEs. Secondary peaks are larger at CNTYMEs because the o-quinone and cyclization products become trapped in the crevices between CNTs and therefore are more likely to be detected. In a 5 second injection of analyte in a flow cell, CV shapes change at different time points. Figure S1 shows the CVs early after exposure to the analyte (Fig. S1A) and late in the 5 s exposure to the analyte at CNTYMEs. At the later time point (4 s into the injection) the CVs have higher secondary peak currents because more cyclized molecules accumulated near the electrode surface over time. Epinephrine has the highest secondary peak current increase because it forms more cyclization products, while the norepinephrine CV does not change much over time because it is not as likely to cyclize. The peak-to-peak separation (ΔEp) is smaller at CNTYMEs for the primary oxidation, due to faster electron transfer kinetics at CNTs,[7] and the secondary peak potentials also shift to more negative potentials due to catalytic effects. The increasing oxidation peak at 1.3 V at CNTYMEs may also result from the electrocatalytic effect because the reaction can occur at lower potentials, so there is more time for the reaction to occur with the given potential limits.[7]
Figure 4.
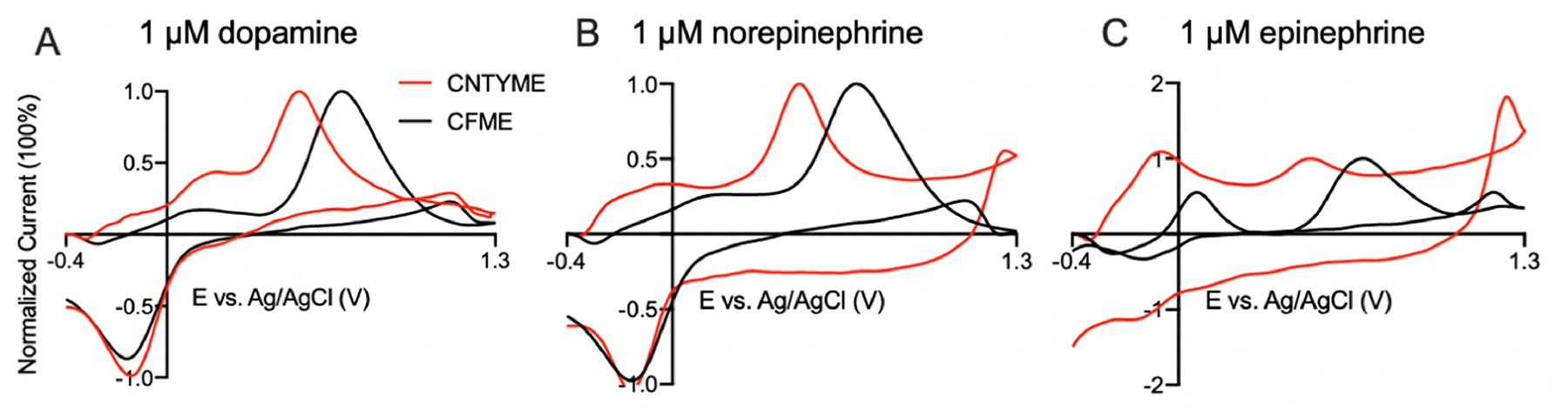
Comparison of CVs for catecholamines at CNTYMEs and CFMEs at 100 Hz. (A) dopamine, (B) norepinephrine, and (C) epinephrine.
Using a scan rate of 400 V/s allows a maximum frequency of ~115 Hz. To understand the behavior at higher repetition frequencies, higher scan rates were employed. With a scan rate of 2000 V/s, the scan time is reduced to 1.7 ms, which allows a maximum repetition frequency of about 500 Hz. Figure 5 compares a 2000 V/s scan rate with 5 Hz or 500 Hz repetition frequencies for CFMEs and CNTYMEs. The anodic currents were normalized to the primary oxidative current to compare the relative heights of the secondary peaks. Cyclic voltammograms at 2000 V/s have a larger ΔEp, because peaks shift with scan rate in FSCV due to sluggish electron transfer.[35] The primary anodic peak occurs around 1.0 V at CFMEs and 0.8 V at CNTYMEs, and secondary peak occurs at 0.5 V, whereas reduction peaks are not observed. Overall, there are dramatically larger secondary peaks for dopamine, norepinephrine, and epinephrine at 500 Hz at CNTYMEs and those secondary peaks are larger than at CFMEs. Epinephrine, the catecholamine with the most cyclization reaction, has the largest secondary peak, which predominates over the primary peak.
Figure 5.
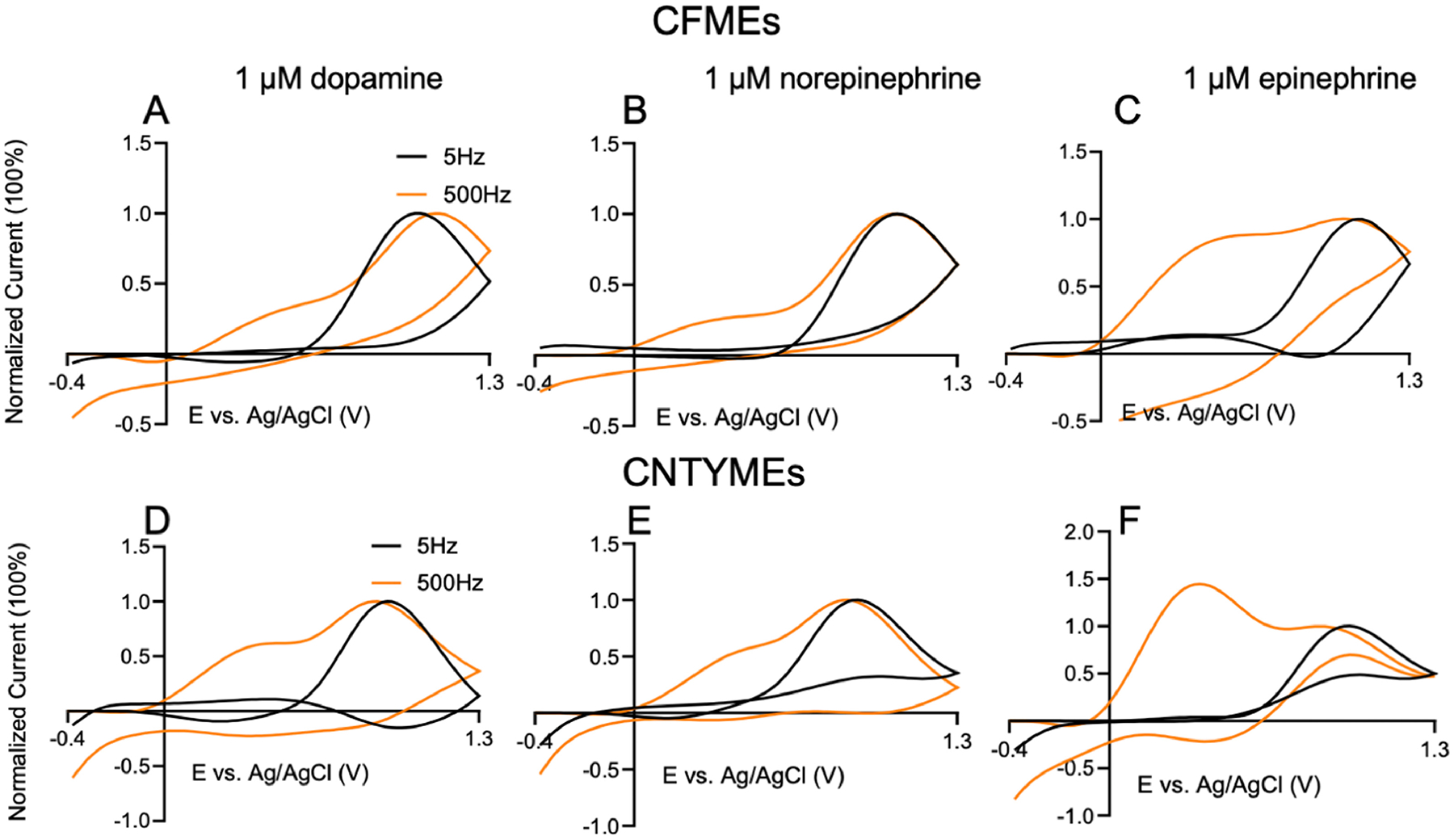
Cyclic voltammogram with 2000V/s scan rate and 5 Hz (black) and 500 Hz (orange) repetition frequencies. At CFMEs: (A) dopamine, (B) norepinephrine, and (C) epinephrine. At CNTYMEs: (D) dopamine, (E) norepinephrine, (F) epinephrine. CVs normalized to make primary oxidation current 100 %.
Ratios of primary to secondary anodic current were further analyzed for the three catecholamines. At 500 Hz (2000 V/s, Fig. 6), the secondary to primary peak ratio for dopamine is approximately 0.6 at CNTYMEs, while it is 0.4 for norepinephrine, and 1.2 for epinephrine. A ratio larger than 1 means the secondary peak current is larger the primary current. There is a significant main effect of analyte (Two-way ANOVA with Tukey’s multiple comparison test, p < 0.001, n = 4 electrodes) and electrode type (p = 0.0244) on the current ratios (Figure 6). The secondary peak current ratios at CNTYMEs are significantly larger than those at CFMEs for all three analytes (p < 0.1). At both CFMEs and CNTYMEs, epinephrine has larger ratio of secondary peak currents than dopamine or norepinephrine, because it easily cyclizes. CNTYMEs have a rougher surface that traps the analyte and enhances the secondary oxidation peaks.
Figure 6.
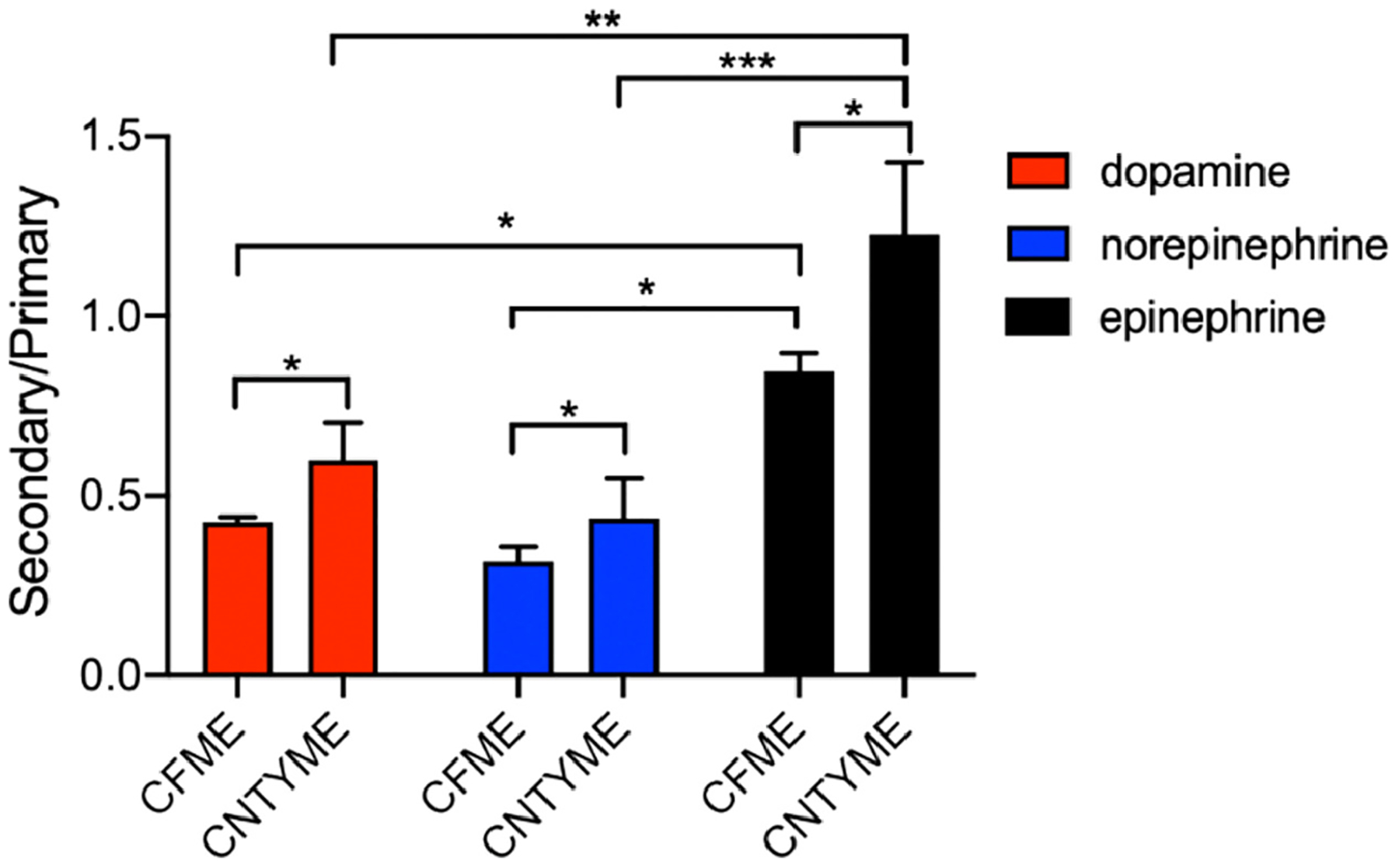
Ratios of secondary to primary anodic peak currents for catecholamines at CNTYMEs and CFMEs. Peak currents were obtained at 500 Hz, with a scan rate of 2000 V/s (Two-way ANOVA with Tukey’s multiple comparison test, error bars are SEM with n = 4). * p<0.05, **p < 0.01, ***p<0.001
Cavity carbon nanopipette microelectrodes are also frequency independent with more secondary peaks
To prove that the enhancement of secondary peaks is due to trapping effects, we tested another type of electrode with a geometry that is known to momentarily trap analytes. Cavity carbon-nanopipette microelectrodes (CNPEs) have tip diameters of 200–400 nm and a cavity that is 500 nm long. Their electrochemical properties have been tested for dopamine,[21,36–38] and FSCV detection of dopamine is frequency independent due to the trapping effect within the cavity. Here, we explored the current response of dopamine, norepinephrine, and epinephrine at different frequencies at cavity CNPEs. The surface area of cavity CNPEs is much smaller than CFMEs and CNTYMEs[36] so 10 μM dopamine, norepinephrine, and epinephrine were tested.
The CVs in Figure 7 indicate peak currents decrease slightly with increasing repetition frequency for dopamine and norepinephrine, but not as at CFMEs. Dopamine (Fig. 7A) and norepinephrine (Figure 7B) have smaller drops in current from 10 to 100 Hz, whereas epinephrine has the largest current drop, but also the largest secondary peaks. Interestingly, epinephrine has a well-defined secondary oxidation couple, but the peak at 1.3 V is not as large as for CNTYMEs. This indicates that the 1.3 V peak is likely enhanced at CNTYMEs due to electrocatalytic effects and not trapping effects. The trends for dopamine (Fig. 7D) and norepinephrine (Fig.7E) primary and secondary currents at CNPEs are similar to CNTYMEs. The primary currents do not decay as much as CFMEs with increasing frequency and the secondary peak increases with increasing frequency. However, the primary current of epinephrine (Fig. 7F) decays a lot as the secondary anodic peak increases. The secondary oxidation peak current dominates the primary one at 100 Hz. With increasing frequencies, more epinephrine-o-quinone is made and more irreversibly cyclizes to leucoepinephrine, which is detected in future scans. Overall, the catecholamine neurotransmitter electrochemistry is similar at both CNTYMEs and cavity CNPEs. They both have thin layer cell behavior and trapping effects that lead to frequency independent current response and enhanced secondary oxidation peaks.
Figure 7.
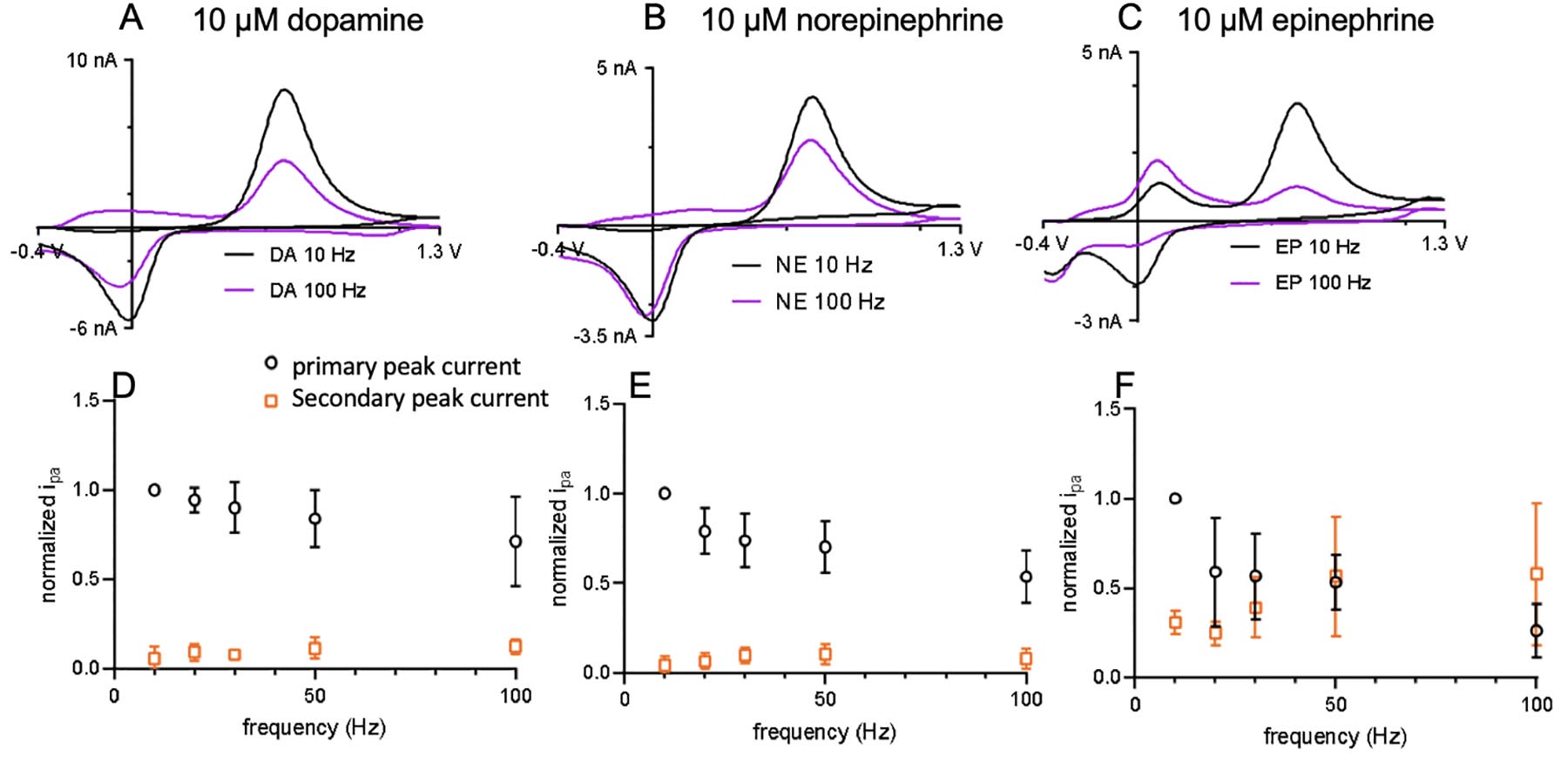
Cavity carbon nanopipette microelectrode detection of catecholamines at different FSCV frequencies. Cyclic voltammograms of 10 μM (A) dopamine, (B) norepinephrine, and (C) epinephrine at 10 Hz and 100 Hz. Normalized current vs frequency for CNPEs (D) dopamine, (E) norepinephrine, (F) epinephrine.
Discussion
Electrochemical performance for catecholamines is different at CNTYMEs than for CFMEs. The CVs at CNTYMEs have a smaller peak-to-peak difference, more reversible CV shapes, and more frequency independent peak current responses. Secondary oxidation peaks are more readily apparent at CNTYMEs, especially at higher repetition frequencies, a result that is similar to catecholamine oxidation at cavity CNPEs. These results lead to the hypothesis that CNTYMEs act as a thin-layer electrode and that secondary peaks are enhanced by trapping effects.
Thin layer cell electrodes were first developed in the 1960s and have two flat working electrodes separated by a thin electrolyte space, where the gap is smaller than the diffusion layer thickness.[33,39] Mass transfer can be neglected in a thin-layer cell, and the analyte concentration on the surface is maintained due to lack of depletion. Cyclic voltammograms in a thin-layer cell have different properties: the peak current is proportional to scan rate (rather than the square root of scan rate with diffusion control) and the voltammogram is perfectly symmetrical and has a ΔEp of 0 for a reversible redox couple.[33]
CNTYMEs consist of vertically aligned CNTs, which can be treated as separate working electrodes, with cavities in between them that act as the narrow gaps in a thin-layer cell. If the depth of the cavity is larger than the diffusion layer, molecules will be trapped in the cavity and will remain close to the CNT for the timescale of the experiment. For one dimensional diffusion, the diffusion layer thickness is calculated by
The calculated diffusion length is 0.9 μm, based on a diffusion coefficient of 2×10−6 cm2 s−1 [40] and time for DOQ to exist of 4.25 ms with 400 V/s scan rate (the time is based on when oxidation of DA occurs but DOQ is not yet reduced back to DA). Thus, the diffusion layer thickness is approximately the same as the surface roughness of CNTYMEs, which was measured to be 0.95 μm by laser scanning confocal microscopy. Note however, the trapping effect will be larger in two or three dimensions, because the cavities are deep and narrow, and catecholamine will hit “walls” as it diffuses. Thus, the CNTYME has a structure that makes it equivalent to thousands of small thin layer cells. Consistent with this theory, the CVs obtained from CNTYMEs exhibit a smaller ΔEp and a larger cathodic current compared with CFMEs. The same result is observed at cavity CNPEs, which also have a thin-layer like structure. Unlike CNTYMEs, CNPEs have one, submicron-sized cavity, instead of surface roughness, but molecules are still trapped inside, increasing the local concentration of the analytes.
The thin layer structure leads to the frequency independent current response of catecholamines at CNTYMEs. The kinetics of dopamine are adsorption controlled at carbon fibers,[8] but the theory and behavior for adsorption control is derived from thin-layer electrochemistry because it assumes there is no diffusion.[33] At carbon electrodes, if dopamine remained adsorbed to the electrode while it was oxidized to DOQ and the reduced back to dopamine, the current response would be frequency independent. However, the rate for DOQ desorption is 10-fold higher than for dopamine at CFMEs, and much of the DOQ falls off and diffuses away from the electrode surface before it is reduced back to dopamine.[41] Time at the holding potential is necessary to re-adsorb dopamine, and that time is controlled by the repetition frequency.[8,42] At CNTYMEs or cavity CNPEs, molecules that enter the cavities can’t diffuse out on the FSCV time scale. Thus, even if DOQ desorbs from the electrode, it would still be trapped near the surface and be able to undergo redox reaction back to dopamine. If no side reactions occur, the reaction would be perfectly reversible and repetition frequency independent at CNTYMEs. The response of norepinephrine is the most ideal, because it had the lowest rate of side reaction.
The thin layer structure of CNTYMEs also contributes to detecting more secondary oxidation peaks. For a CFME, if DOQ desorbed and cyclized, it might diffuse away from the surface and the cyclization product would not be detected; indeed, leucodopamine is typically only observed with high concentrations, in the mM range.[40] Here, cyclization products were observed at low concentrations more frequently for all the catecholamines at CNTYMEs and CNPEs because leucocatecholamines are restricted near the surface. However, the downside to observing cyclization peaks is that the primary oxidation peak decreases with repetition frequency, because some of the original catecholamine is not recycled in the redox reaction, but is “consumed” in the side chemical reaction. Therefore, for molecules that undergo extensive cyclization, the primary current decays with frequency at CNTYMEs, but for a different reason than the decay observed at CFMEs. Still, CNTYMEs maintained higher currents for high frequencies than CFMEs. The upside to observing more cyclization products is that it could be a method to distinguish different molecules. Norepinephrine and epinephrine are more easily discriminated at CNTYMEs, especially at 10 Hz FSCV frequency, because side peaks are amplified for epinephrine. Differentiating dopamine and norepinephrine is still challenging, but using more advanced data analysis techniques, such as machine learning or principal components analysis,[43,44] it may be possible to discriminate the shapes because there are differences in the secondary peaks in the CVs.
Our work also shows cyclization peak currents are larger with increasing frequencies for all three catecholamines. When applying the dopamine waveform at 10 Hz, the voltage ramp where redox reactions occur is only 8.5% of the time, but at 100 Hz, the voltage ramp takes 85% of the time. With ten times number of reactions happening in a given time at 100 Hz, the product concentrations also increase. Leucocatecholamines are further oxidized to aminochromes and this reaction is reversible, so the same product can be detected again on future scans. Higher cyclization peak currents are observed at both CFMEs and CNTYMEs at high frequencies. Moreover, with the thin-layer structure on CNTYMEs, the accumulated leucocatecholamines are trapped in the restricted area near the electrode where they are detected. Thus, CNTYMEs have enhanced secondary peaks due to both trapping effect and frequency effects.
Conclusions
CNTYMEs are a good alternative to CFMEs in detection of neurotransmitters due to the fast electron transfer kinetics, good electrochemical properties, and high temporal resolution. Both CNTYMEs and cavity CNPEs have trapping effects caused by thin-layer structure: CNTYMEs have micron-sized surface roughness, whereas cavity-CNPEs have nano-cavities on the tip. Overall, this paper shows that CNTYMEs and cavity CNPEs have surface structures that give rise to thin layer cell electrochemistry on the time scale of FSCV. The trapping effects cause the primary current to be largely independent of FSCV frequency, because any o-quinone that desorbs is trapped near the electrode surface and can be detected again. However, catecholamines can also cyclize and detection of these products is amplified by trapping effects at CNTYMEs and cavity CNPEs, especially at higher frequencies. Epinephrine has the highest rate of cyclization and thus detection of secondary peaks is more evident at CNTYMEs and at high repetition frequencies. Thus, this study shows that thin layer cell electrodes are useful in fundamental understanding of ECE reactions and may be useful for discriminating different compounds with similar structure based on secondary products.
Supplementary Material
Acknowledgement
This study is supported by National Institution of Health (NIH) grant R01 EB026497 and NSF grant CHE-1763337 (M.V.M.). A portion of this research was conducted at Nanoscale Material Characterization Facility, University of Virginia (NMCF, UVA) and Center for Nanophase Materials Science, Oak Ridge National Laboratory (CNMS, ORNL), which is a DOE Office of Science User Facility under user agreement CNMS 2019-034.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- [1].Jaquins-Gerstl A, Michael AC, A review of the effects of FSCV and microdialysis measurements on dopamine release in the surrounding tissue, Analyst 140 (2015) 3696–3708. 10.1039/c4an02065k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Roberts JG, Sombers LA, Fast-Scan Cyclic Voltammetry: Chemical Sensing in the Brain and Beyond, Anal. Chem 90 (2018) 490–504. 10.1021/acs.analchem.7b04732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Nguyen MD, Venton BJ, Fast-scan Cyclic Voltammetry for the Characterization of Rapid Adenosine Release, Comput. Struct. Biotechnol. J 13 (2015) 47–54. 10.1016/j.csbj.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ganesana M, Lee ST, Wang Y, Venton BJ, Analytical Techniques in Neuroscience: Recent Advances in Imaging, Separation, and Electrochemical Methods., Anal. Chem 89 (2017) 314–341. 10.1021/acs.analchem.6b04278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Huffman ML, Venton BJ, Carbon-fiber microelectrodes for in vivo applications, Analyst 134 (2009) 18–24. 10.1039/b807563h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yang C, Denno ME, Pyakurel P, Venton BJ, Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review, Anal. Chim. Acta 887 (2015) 17–37. 10.1016/j.aca.2015.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].McCreery RL, Advanced carbon electrode materials for molecular electrochemistry, Chem. Rev 108 (2008) 2646–2687. 10.1021/cr068076m. [DOI] [PubMed] [Google Scholar]
- [8].Bath BD, Michael DJ, Trafton BJ, Joseph JD, Runnels PL, Wightman RM, Subsecond adsorption and desorption of dopamine at carbon-fiber microelectrodes, Anal. Chem 72 (2000) 5994–6002. 10.1021/ac000849y. [DOI] [PubMed] [Google Scholar]
- [9].Yang C, Trikantzopoulos E, Jacobs CB, Venton BJ, Evaluation of carbon nanotube fiber microelectrodes for neurotransmitter detection: Correlation of electrochemical performance and surface properties, Anal. Chim. Acta 965 (2017) 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cao Q, Hensley DK, V Lavrik N, Venton BJ, Carbon nanospikes have better electrochemical properties than carbon nanotubes due to greater surface roughness and defect sites, Carbon N. Y 155 (2019) 250–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Li W, Jayasinghe C, Shanov V, Schulz M, Spinning Carbon Nanotube Nanothread under a Scanning Electron Microscope, Materials (Basel) 4 (2011) 1519–1527. 10.3390/ma4091519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Jayasinghe C, Chakrabarti S, Schulz MJ, Shanov V, Spinning yarn from long carbon nanotube arrays, J. Mater. Res 26 (2011) 645–651. 10.1557/jmr.2010.91. [DOI] [Google Scholar]
- [13].Schmidt AC, Wang X, Zhu Y, Sombers LA, Carbon nanotube yarn electrodes for enhanced detection of neurotransmitter dynamics in live brain tissue, ACS Nano 7 (2013) 7864–7873. 10.1021/nn402857u. [DOI] [PubMed] [Google Scholar]
- [14].Jacobs CB, Ivanov IN, Nguyen MD, Zestos AG, Venton BJ, High temporal resolution measurements of dopamine with carbon nanotube yarn microelectrodes, Anal. Chem 86 (2014) 5721–5727. 10.1021/ac404050t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yang C, Venton BJ, High performance, low cost carbon nanotube yarn based 3D printed electrodes compatible with a conventional screen printed electrode system, 2017 IEEE Int. Symp. Med. Meas. Appl (2017) 100–105. 10.1109/MeMeA.2017.7985857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Akinoglu EM, Kätelhön E, Pampel J, Ban Z, Antonietti M, Compton RG, Giersig M, Nanoscopic carbon electrodes: Structure, electrical properties and application for electrochemistry, Carbon 130 (2018) 768–774. 10.1016/j.carbon.2018.01.064. [DOI] [Google Scholar]
- [17].Cao Q, Puthongkham P, Venton BJ, Review: New insights into optimizing chemical and 3D surface structures of carbon electrodes for neurotransmitter detection, Anal. Methods 11 (2019) 247–261. 10.1039/c8ay02472c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zestos AG, Venton BJ, Communication—Carbon Nanotube Fiber Microelectrodes for High Temporal Measurements of Dopamine, J. Electrochem. Soc 165 (2018) G3071–G3073. 10.1149/2.0111812jes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang C, Trikantzopoulos E, Nguyen MD, Jacobs CB, Wang Y, Mahjouri-Samani M, Ivanov IN, Venton BJ, Laser treated carbon nanotube yarn microelectrodes for rapid and sensitive detection of dopamine in vivo, ACS Sensors 1 (2016) 508–515. 10.1021/acssensors.6b00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang C, Wang Y, Jacobs CB, Ivanov IN, Venton BJ, O2 plasma etching and antistatic gun surface modifications for CNT yarn microelectrode improve sensitivity and antifouling properties, Anal. Chem 89 (2017) 5605–5611. 10.1021/acs.analchem.7b00785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang C, Hu K, Wang D, Zubi Y, Lee ST, Puthongkham P, Mirkin MV, Venton BJ, Cavity Carbon-Nanopipette Electrodes for Dopamine Detection, Anal. Chem 91 (2019) 4618–4624. 10.1021/acs.analchem.8b05885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ciolkowski EL, Cooper BR, Jankowski JA, Jorgenson JW, Wightman RM, Direct Observation of Epinephrine and Norepinephrine Cosecretion from Individual Adrenal Medullary Chromaffin Cells, J. Am. Chem. Soc 114 (1992) 2815–2821. 10.1021/ja00034a009. [DOI] [Google Scholar]
- [23].Venton BJ, Wightman RM, Psychoanalytical electrochemistry: Dopamine and behavior, Anal. Chem 75 (2003) 414 A–421 A. [Google Scholar]
- [24].Meredith ME, May JM, Regulation of embryonic neurotransmitter and tyrosine hydroxylase protein levels by ascorbic acid, Brain Res 1539 (2013) 7–14. 10.1016/j.brainres.2013.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Wong DL, Epinephrine biosynthesis: hormonal and neural control during stress., Cell. Mol. Neurobiol 26 (2006) 891–900. 10.1007/s10571-006-9056-6. [DOI] [PubMed] [Google Scholar]
- [26].Adams RN, Anodic Oxidation Pathways of Aromatic Hydrocarbons and Amines, Acc. Chem. Res 2 (1969) 175–180. 10.1021/ar50018a003. [DOI] [Google Scholar]
- [27].Baur JE, Kristensen EW, May LJ, Wiedemann DJ, Wightman RM, Fast-scan voltammetry of biogenic amines., Anal. Chem 60 (1988) 1268–1272. [DOI] [PubMed] [Google Scholar]
- [28].Pihel K, Schroeder TJ, Wightman RM, Rapid and selective cyclic voltammetric measurements of epinephrine and norepinephrine as a method to measure secretion from single bovine adrenal medullary cells, Anal. Chem 66 (1994) 4532–4537. 10.1021/ac00096a021. [DOI] [Google Scholar]
- [29].Hawley MD, Tatawawadi SV, Piekarski S, Adams RN, Electrochemical Studies of the Oxidation Pathways of Catecholamines, J. Am. Chem. Soc 89 (1967) 447–450. 10.1021/ja00978a051. [DOI] [PubMed] [Google Scholar]
- [30].Xiang L, Lin Y, Yu P, Su L, Mao L, Laccase-catalyzed oxidation and intramolecular cyclization of dopamine: A new method for selective determination of dopamine with laccase/carbon nanotube-based electrochemical biosensors, Electrochim. Acta 52 (2007) 4144–4152. 10.1016/j.electacta.2006.11.040. [DOI] [Google Scholar]
- [31].Strand AM, Venton BJ, Flame etching enhances the sensitivity of carbon-fiber microelectrodes, Anal. Chem 80 (2008) 3708–3715. 10.1021/ac8001275. [DOI] [PubMed] [Google Scholar]
- [32].Venton BJ, Cao Q, Fundamentals of fast-scan cyclic voltammetry for dopamine detection, Analyst 145 (2020) 1158–1168. 10.1039/C9AN01586H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bard AJ, Faulkner LR, Electrochemical methods: fundamentals and applications, 2nd ed., John Wiley and Sons, New York, 2001. [Google Scholar]
- [34].Borovansky J, Edge R, Land EJ, Navaratnam S, Pavel S, Ramsden CA, Riley PA, Smit NPM, Mechanistic studies of melanogenesis: The influence of N-substitution on dopamine quinone cyclization, Pigment Cell Res 19 (2006) 170–178. 10.1111/j.1600-0749.2006.00295.x. [DOI] [PubMed] [Google Scholar]
- [35].Keithley RB, Takmakov P, Bucher ES, Belle AM, Owesson-White CA, Park J, Wightman RM, Higher sensitivity dopamine measurements with faster-scan cyclic voltammetry, Anal. Chem 83 (2011) 3563–3571. 10.1021/ac200143v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Hu K, Wang D, Zhou M, Bae JH, Yu Y, Xin H, Mirkin MV, Ultrasensitive Detection of Dopamine with Carbon Nanopipets, Anal. Chem 91 (2019) 12935–12941. 10.1021/acs.analchem.9b02994. [DOI] [PubMed] [Google Scholar]
- [37].Wang Y, Wang D, Mirkin MV, Resistive-pulse and rectification sensing with glass and carbon nanopipettes, Proc. R. Soc. A Math. Phys. Eng. Sci 473 (2017) 20160931 10.1098/rspa.2016.0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Hu K, Wang Y, Cai H, V Mirkin M, Gao Y, Friedman G, Gogotsi Y, Open carbon nanopipettes as resistive-pulse sensors, rectification sensors, and electrochemical nanoprobes, Anal. Chem 86 (2014) 8897–8901. 10.1021/ac5022908. [DOI] [PubMed] [Google Scholar]
- [39].Davis JM, Fan FRF, Bard AJ, Currents in thin layer electrochemical cells with spherical and conical electrodes, J. Electroanal. Chem 238 (1987) 9–31. 10.1016/0022-0728(87)85163-X. [DOI] [Google Scholar]
- [40].Trouillon R, Lin Y, Mellander LJ, Keighron JD, Ewing AG, Evaluating the diffusion coefficient of dopamine at the cell surface during amperometric detection: disk vs ring microelectrodes, Anal. Chem 85 (2013) 6421–6428. 10.1021/ac400965d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Fowler JC, Changes in extracellular adenosine levels and population spike amplitude during graded hypoxia in the rat hippocampal slice., Naunyn. Schmiedebergs. Arch. Pharmacol 347 (1993) 73–8. [DOI] [PubMed] [Google Scholar]
- [42].Kawagoe KT, Zimmerman JB, Wightman RM, Principles of voltammetry and microelectrode surface states, J. Neurosci. Methods 48 (1993) 225–240. 10.1016/0165-0270(93)90094-8. [DOI] [PubMed] [Google Scholar]
- [43].Puthongkham P, Venton BJ, Recent advances in fast-scan cyclic voltammetry, Analyst 145 (2020) 1087–1102. 10.1039/C9AN01925A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Johnson JA, Rodeberg NT, Wightman RM, Failure of Standard Training Sets in the Analysis of Fast-Scan Cyclic Voltammetry Data, ACS Chem. Neurosci 7 (2016) 349–359. 10.1021/acschemneuro.5b00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


