Abstract
Current management for spinal cord injury aims to reduce secondary damage and recover sensation and movement. Acute spinal cord injury is often accompanied by spinal cord compartment syndrome. Decompression by durotomy and/or myelotomy attempts to relieve secondary damage by completelyrelieving the compression of the spinal cord, removing the necrotic tissue, decreasing edema, reducing hemorrhage, and improving blood circulation in the spinal cord. However, it is controversial whether durotomy and/or myelotomy after spinal cord injury are beneficial to neurological recovery. This review compares the clinical effects of durotomy with those of myelotomy in the treatment of spinal cord injury. We found that durotomy has been performed more than myelotomy in the clinic, and that durotomy may be safer and more effective than myelotomy. Durotomy performed in humans had positive effects on neurological function in 92.3% of studies in this review, while durotomy in animals had positive effects on neurological function in 83.3% of studies. Myelotomy procedures were effective in 80% of animal studies, but only one clinical study of myelotomy has reported positive results, of motor and sensory improvement, in humans. However, a number of new animal studies have reported that durotomy and myelotomy are ineffective for spinal cord injury. More clinical data, in the form of a randomized controlled study, are needed to understand the effectiveness of durotomy and myelotomy.
Keywords: decompression, durotomy, intraspinal pressure, laminectomy, myelotomy, neurological recovery, spinal cord compartment syndrome, spinal cord injury, spinal cord interstitial pressure
Introduction
Primary trauma to the spinal cord initiates pathological changes that cause secondary damage, including hemorrhage, vasospasm, ischemia, edema, inflammation, and apoptosis (Li and Chandler, 2014). The primary mechanical injury and the secondary injury together make up the pathophysiology of spinal cord injury (SCI) (Fehlings et al., 1989). The mechanisms of axonal injury may differ from those that occur in gray matter (Bengtsson and Siesjo, 1990). Current management of acute SCI is aimed at decreasing secondary injury and promoting neural regeneration. A recent clinical study has reported that surgical interventions are promising for the treatment of SCI (Huang et al., 2018).
Changes in the local autoregulation of blood pressure after injury induce vascular spasm and ischemia, and cause a decrease in perfusion pressure (Tator, 1995; La et al., 2004; Telemacque et al., 2018). Fractures and dislocations place external compression on the spinal cord. Edemas cause a decrease in epidural and subarachnoid space volume and block the epidural veins, arteriole of the cord, and cerebrospinal fluid (CSF) flow, and then induce arachnoid adhesion. The lack of blood supply and CSF infusion then exacerbates the spinal cord edema, causing external and internal compression. These symptoms have been described as “spinal compartment syndrome” (Cao et al., 2015). Surgical decompression aims to relieve this pressure (Rouanet et al., 2017).
Intramedullary spinal cord hemorrhage (hematomyelia) can present in SCI (Leep et al., 2009). It is controversial whether durotomy and/or myelotomy are beneficial to neurological recovery. Decompression by durotomy and myelotomy can be useful for spinal cord hemorrhage; however, while laminectomy can relieve some compression of the cord, it is insufficient for extensive edema (Grant et al., 2015; Telemacque et al., 2018). Swelling and hemorrhage can cause the spinal cord to enlarge, resulting in high interstitial pressure in the spinal cord (Allen, 1911). The cord becomes swollen and the subarachnoid space is occluded in severe cases of SCI.
This review compares the effects of durotomy and myelotomy in the treatment of SCI in humans and animals.
Retrieval Strategy
This article reviewed the clinical evidence regarding surgical therapy in the management of acute traumatic SCI with hemorrhage or edema in humans and animals. This review focused on studies, treatment methods, and the outcomes of these treatments. From January to June 2019, PubMed, Cochrane, Web of Science, Google Scholar, and Epic Browser were used to collect articles published from 1911 to 2019 using the following Medical Subject Headings (MeSH): SCI, decompression of the spinal cord, durotomy, duroplasty, myelotomy, spinal cord compartment syndrome, and neurological recovery. The identified articles were then used to conduct our review.
Durotomy Investigations
Clinical studies
In a study conducted by Perkins and Deane (1988), three patients demonstrated complete neurological recovery and three patients partially recovered following durotomy. Specifically, three of the patients improved to Frankel grade E; one patient recovered gait in the lower limbs; one patient changed from grade A to grade D, and one patient changed from grade A to grade B. All patients showed functional improvement.
Qu et al. (2015) performed durotomy and myelotomy in 21 cases of extensive spinal cord swelling with/without intramedullary hemorrhage. At 2 weeks post-surgery, Magnetic Resonance Imaging (MRI) revealed that the swelling had disappeared. Cases of The American Spinal Injury Association (ASIA) grade A remained as grade A, but the locomotion and sensory levels of the other patients were enhanced after decompression by durotomy.
Qu and Guo (2015) reported that, in acute SCI treatment, laminectomy alone led to residual cord deformation followed by edema, causing subarachnoid occlusion and expansion of the damaged cord, thereby resulting in further cord compression. Therefore, durotomy is necessary to relieve the pressure on the dura and expand the intrathecal space.
Salvok et al. (2015) performed decompression by durotomy in 12 patients who were injured at the level of the thoracic spine, followed by the local administration of cortexin. Positive dynamics were observed in 10 patients, but no neurological dynamics were observed in two patients. The authors emphasized that performing durotomy with the administration of cortexin may help to further improve motor or sensory function after durotomy. The authors have also used cortexin in other studies for the treatment of spinal cord injury (Tsymbaliuk et al., 2014a, b; Salvok et al., 2015).
In a duroplasty study conducted by Cao et al. (2015), there was sensory recovery in 14 cases and motor function recovery in nine cases. In addition, neuropathic pain was relieved completely in 10 cases.
A study of patients after lumbar fusion (Mohamed et al., 2015) revealed that patients who underwent dural incision after lumbar fusion were more likely to develop pseudoarthrosis. The rate for developing pseudoarthrosis in the durotomy group was 35% compared with 14% in the group that did not undergo durotomy. From this study, it was noted that durotomy can have negative effects on patients.
Winestone et al. (2017) compared laminectomy, durotomy, and piotomy in the treatment of SCI. The procedure of durotomy reduced intramedullary pressure (IMP) by 75%. When the pia mater was incised, mean cervical IMP was reduced by over 90%, while the mean thoracic IMP decreased by over 100%. Moreover, the effect of pial incision was statistically significant in contrast with the two prior procedures alone.
Telemacque et al. (2018) performed durotomy and duroplasty on patients, which resulted in positive dynamics in six patients. Of the two patients with ASIA grade A, one improved to grade C and one improved to grade D. Two patients with ASIA grade B recovered to grade D, and two patients with ASIA grade C improved to grade D; however, one patient with ASIA grade C showed no change.
Jiaxin et al. (2018) used intradural microsurgery in SCI patients with the aim of improving neurological recovery. Three months after surgery, 52 remained ASIA grade A, 16 recovered to grade B, 13 improved to grade C, and six recovered to grade D.
After performing durotomy with duroplasty, Zhu et al. (2019) revealed that the ASIA grade was improved by one grade in four cases, two grades in 11 cases, and three grades in one case. Before durotomy, patients had spinal cord edema, spinal cord swelling, and tortuous swollen blood vessels. After durotomy, however, microcirculation in the spinal cord was improved and CSF pulsation was restored.
Animal studies
Ianotti et al. (2006) revealed that CSF blockage can be avoided by performing duroplasty. Duroplasty also decreased the magnitude of abrasions and the development of scar tissue and cystic cavities. At 5 weeks post-injury, controls had large cystic cavities, whereas patients who had undergone duroplasty had a reduced amount of cystic cavities.
A study conducted by Smith et al. (2010) investigated the effects of durotomy with duroplasty on the recovery of 72 rats after cervical SCI. The rats that received a dural incision and dural graft had decreased cavitation, inflammation, and scar formation, and better functional recovery compared with the group which received contusion alone.
Zhang et al. (2016) performed durotomy in rats and reported that intrathecal decompression with dural incision resulted in improved neurological function. Microscopic observations revealed that more white matter was conserved in these rats, and they had fewer vacuoles and less axonal destruction.
Saaodun and Papadopoulos (2016) investigated the effect of the dura in spinal cord compression and demonstrated that inserting a graft over the dura controlled the cerebrospinal fluid. Duroplasty caused the abrasion capacity to decrease (P = 0.01) and there were fewer cystic cavities after trauma (P = 0.001). Duroplasty also decreased the percolation of Ectodermal Dysplasia 1 (ED1-positive macrophages around the injured area and secondary damage was reduced.
Jalan et al. (2017) performed durotomy and duroplasty in 64 rats and reported no improvement in movement; catwalk gait analysis demonstrated a decline in coordination among the limbs in these rats. Moreover, after dural incision, the pain threshold was lowered. In the group that received the dural graft, there were larger lesions, increased white matter, and more compression after graft placement.
Camlar et al. (2019) conducted a study to analyze the effect of durotomy on neurological function. Tarlov test scores were not significantly different among the groups; duroplasty did not give positive results.
Clinical studies of durotomy investigations involving intraspinal pressure monitoring
Phang et al. (2015) reported that the increase in intradural space at the trauma area was 50% in the durotomy group compared with 20% in the laminectomy group (P < 0.05). They also demonstrated a more adequate decompression, with 78% having cerebrospinal fluid visible in MRI compared with 0% (P < 0.01). In the durotomy group, the mean intraspinal pressure (ISP) was lower, the mean spinal cord perfusion pressure was greater, and the mean spinal vascular pressure reactivity index was lower (0.04 compared with 0.14, P < 0.01). This study concluded that dural incision enhanced the radiological as well as physiological parameters more effectively than lamina removal alone.
Saadoun et al. (2017) demonstrated that any attempts at decreasing ISP or elevating spinal cord perfusion pressure after traumatic SCI can boost neural regeneration. During the observation period of 9–12 months, ISP and spinal cord perfusion pressure (P < 0.05) were both associated with progress in ASIA grade. The greater the ISP and the lower the spinal cord perfusion pressure, the greater the chance of developing ischemia, which causes secondary damage.
Tykocki et al. (2017) stated that incision and repair of the dura reduces ISP and ischemia. Previous studies have shown that, if the spinal cord is decompressed within 1 day of injury, the possibility of neural recovery is higher at 6 months after surgery (Saadoun and Papadopoulos, 2016; Tykocki, 2017). Their studies revealed that both the dura and the pia contribute greatly to the increase in ISP during the acute phase, while only the dura contributes to ISP increase during the sub-acute phase.
Myelotomy Investigations
Animal studies
A study in dogs (Allen, 1911) revealed that, after SCI, hemorrhage occurred, which inhibited conductive function or destroyed the spinal cord permanently. After myelotomy, the dogs recovered, showing slight awkwardness in the hind limb that did not prevent running or jumping. Because movement was restored, this myelotomy procedure obtained good results.
Rivlin and Tator (1979) conducted two separate experiments, each including 20 rats. The controls received durotomy while the experiment group received myelotomy. When myelotomy was performed, the clinical results were more consistent and there was a significant improvement in recovery compared with the durotomy group (P < 0.01).
Hu et al. (2015) conducted experiments in rats using aquaporin-4 (AQP4) and aquaporin-9 (AQP9) to examine the effects of myelotomy on AQP4, AQP9, and swelling after SCI. After myelotomy, less swelling of the cord was observed within 4 and 6 days. Myelotomy was also associated with downregulation of AQP4 and AQP9 expression, as well as enhanced movement.
In a meta-analysis, Qin et al. (2018) collected six clinical trials in rats in which myelotomy reduced edema, promoted movement, and decreased the possibility of secondary injuries. At 1 week after acute SCI, Basso, Beattie, and Bresnahan scale scores in the moderate injury subgroup were higher in myelotomy groups than in contusion control groups (P < 0.001), which suggests a protective effect of myelotomy. Moreover, at 2 weeks after injury, the Basso, Beattie, and Bresnahan scale scores in the severe and moderate injury subgroups were higher in the myelotomy groups than in the contusion groups (P < 0.001), which also suggests a protective effect of myelotomy.
Meyer et al. (2018) performed myelotomy in a number of rats and reported a rise in foot-stepping angle FSA (P < 0.001) 7 days after surgery, as well as a continued rise after 2 weeks (P < 0.001). However, there were no statistically significant differences between the two groups (P > 0.05). Thus, this study did not report positive results for decompression by myelotomy.
Clinical study
Koyanagi et al. (1989) recorded patients treated by myelotomy. Imaging revealed edema in all cases. Surgery was performed within 5 and 21 hours after trauma, and there were no complications after surgery. Within 1.5 years, movement in the upper limbs was enhanced and sensation impairment was decreased. In this study, myelotomy was successful for treating cervical SCI accompanied by edema. The authors noted that early myelotomy is effective in preventing secondary injury.
Discussion
Surgeons sometimes perform laminectomy followed by duroplasty to help relieve pressure on the spinal cord. Some studies have found that laminectomy alone is sufficient, others found laminectomy followed by duroplasty to be more efficient, and others found myelotomy to be the most effective. From Figure 1, it is apparent that CSF pulsation of the spinal cord does not recover with decompression by laminectomy after SCI. However, after further decompression by durotomy, the spinal cord is fully released and CSF pulsation is recovered; next, a duroplasty is performed to increase the space under the dura mater. In contrast, during myelotomy, the spinal cord is incised and necrotic tissue can be removed. Myelotomy is used mainly in patients with severe SCI.
Figure 1.
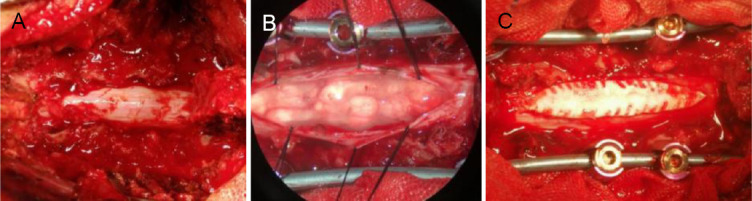
Recovery of CSF pulsation after laminectomy.
(A) There was no recovery of CSF pulsation after laminectomy. (B) Laminectomy was followed by durotomy and CSF pulsations were recovered. (C) Duroplasty was performed after durotomy to enlarge the dural space. CSF: Cerebrospinal fluid. Pictures of typical cases are from previous clinical operations by our research team.
Decompression alleviates the pressure from edema and hemorrhage, preventing blood or oxygen shortages. There is evidence that osseous decompression may not be enough to improve spinal cord perfusion pressure, and that durotomy or even piotomy with removal of the necrotic tissue is necessary (Newton et al., 2017). Decompression inhibits primary and secondary injuries. It has been demonstrated that, if the spinal cord is decompressed within 1 day after injury, improved sensory and locomotor functions are apparent within 6 months after surgery (La et al., 2004; Fehlings et al., 2012).
Many studies have focused on neurological recovery after SCI (Huang and Sharma, 2013), because neural regeneration is essential in patients recovering from SCI. Low blood pressure after SCI causes ischemia, but surgical decompression can reduce this condition. Durotomy with duroplasty and myelotomy help to relieve the secondary injury, which is a major factor in determining patient outcome after damage. Durotomy and myelotomy have been performed worldwide, revealing that edema can be controlled when necrosis is cleared. It has been observed that durotomy with myelotomy facilitates the expulsion of necrotic material when performed in the 4 days following trauma (Layer et al., 2017). Myelotomy has also been reported to improve neurological recovery in rats, and in some instances mannitol has been used with myelotomy to treat rats after SCI (Iwasaki et al., 1981; Yang et al., 2013).
Durotomy aims at decreasing the CSF pressure that is formed after compartment syndrome, thus improving blood distribution (Werndle et al., 2014; Zhang et al., 2016; Jiaxin et al., 2018). After duroplasty, mean arterial pressure should be maintained above 85 mmHg within the first week to improve spinal cord perfusion and achieve favorable results (Saoudun et al., 2008; Anderson et al., 2010; Awwad et al., 2014). To manage compartment-like syndrome after SCI, duroplasty is necessary to reduce ISP and improve spinal cord perfusion pressure. The swelling of the spinal cord reaches its maximum at around 3 days post-injury (Maikos et al., 2008; Grassner et al., 2017).
It is recommended that decompression be performed at the level of the dura, because ischemia produced by intradural pressure causes permanent damage (Perkins and Deane, 1988). In one study, laminectomy lowered the intramedullary pressure by 15–25%. When compared with that of durotomy, 15–25% is a smaller percentage; while durotomy with piotomy produced an 89–100% reduction (Winestone et al., 2017). Durotomy decreases intracranial pressure and also limits perifocal edema and ischemia in the injured area (Werndle et al., 2014). It is best if decompressive surgery is performed within 24 hours under stable vital signs (Krengel et al., 1993; Duh et al., 1994; Botel et al., 1997; Campagnolo et al., 1997; Guest et al., 2002; Fehlings and Arvin, 2009; Fehlings et al., 2017a, b). The use of high-dose intravenous methylprednisolone after durotomy for SCI has been controversial because of randomized trials with positive results (Hadley and Walters, 2013). There is a 28% overall conversion rate after conventional osseous decompression surgery (Bourassa et al., 2016).
Multilevel full-scale decompression and stabilization can improve blood supply and CSF circulation in SCI as well as reduce pathological changes (Bowsell et al., 2012; Aganesov, 2013; Chen et al., 2014). Previous studies have demonstrated that durotomy is an effective method for reducing secondary injury (Ida and Tachibana, 1995; Smith, 2009; Chavanne, 2011). Surgery is considered to be largely responsible for decompression and for facilitating neurological restoration (Wilson et al., 2017; Bergmeister et al., 2018; Ter et al., 2018; Bandeira et al., 2019). Studies in Central Europe have indicated that neurological recovery is superior if surgical decompression occurs within the first 8 hours post-injury (Jug et al., 2015; Grassner et al., 2016).
Table 1 shows a summary of the studies that were used, including the type of decompression, author and year, brief description of the study, and the results obtained.
Table 1.
Summary of the animal and human studies of decompression by durotomy and myelotomy
| Method of decompression | Authors | Description of study | Conclusion |
|---|---|---|---|
| Durotomy (animal studies) | Ianotti et al. (2006) | Duroplasty was performed on 12 rats to investigate the effect on scar formation, abrasion size, and blockage of CSF. | Duroplasty reduced abrasion size, scar formation and cystic cavities. |
| Smith et al. (2010) | Durotomy with duroplasty was performed in 72 rats to analyze the effects on functional recovery. | Functional recovery was better in rats treated with durotomy and duroplasty. | |
| Zhang et al. (2016) | Investigated the difference between intrathecal and epidural decompression in 84 rats. | Dural incision facilitated neural regeneration since there were less vacuoles and axon destruction. | |
| Saadoun et al. (2017) | Investigated the effect of a dural graft placement on cerebrospinal fluid. | Secondary damage was receded; the dural graft enhanced recovery. | |
| Jalan et al. (2017) | Investigated the effects of laminectomy, durotomy and duroplasty on 64 rats. | No improvement in movement, pain threshold was lowered. | |
| Camlar et al. (2019) | Investigated the effect of duroplasty on neurological function of 28 rats. | There was no significant difference among the groups. Duroplasty did not show positive results. | |
| Durotomy (clinical studies) | Perkins and Deane (1988) | Performed durotomy for the prevention of secondary injury of 6 patients. | All patients showed improvement in function. One patient had a gait. |
| Qu et al. (2015) | Performed durotomy in 21 patients with cord swelling with or without hemorrhage. | Two patients remained grade A after durotomy; however, other patients had enhancement in motor and sensory levels. | |
| Qu and Guo (2015) | Explained that laminectomy alone was inadequate for decompression. | Durotomy is necessary to relieve pressure and to expand the intrathecal space. | |
| Salvok et al. (2015) | Performed decompression by durotomy in 12 patients at the level of the thoracic spine with administration of cortexin. | Ten out of twelve patients showed positive dynamics with an improvement in ASIA grade. | |
| Cao et al. (2015) | Investigated the effect of durotomy on motor and sensory functions in 18 patients. | Muscle strength was increased; neuropathic pain was relieved; and some cases had recovery of sensation. | |
| Mohamed et al. (2015) | Investigated the effect by durotomy in 17 patients with SCI. | Patients who underwent durotomy were more likely to develop pseudoarthrosis. | |
| Phang et al. (2015) | Investigated the effect of laminectomy and duroplasty in 21 patients with SCI. | Dural incision enhanced the radiological parameters in a more efficacious manner than removal of lamina alone. | |
| Saudun et al. (2017) | Investigated the effect of durotomy with measuring ISP in 45 patients. | A mean ISP of less than 10 mmHg and mean spinal cord perfusion pressure of more than 90 mmHg are more likely to have better neural regeneration and function. | |
| Tykocki et al. (2017) | Investigated the effect of durotomy with the measurement of ISP. | Decompression by dural incision reduces ISP. | |
| Winestone et al. (2017) | Compared laminectomy, durotomy and piotomy in 16 cadavers. | Durotomy was effective in decreasing mean cervical IMP and piotomy was most effective. | |
| Jiaxin et al. (2018) | Performed microsurgery for improving neurological recovery in 87 patients. | Some patients remained ASIA grade A, while others improved in their ASIA grade. | |
| Telemacque et al. (2018) | Investigated the effect of durotomy at the level of the cervical spine in 7 patients with SCI. | Six out of seven patients had improvement in ASIA grade. | |
| Zhu et al. (2019) | Investigated the effects of durotomy and duroplasty in 16 patients with SCI. | There was improvement in microcirculation of the spinal cord, and restoration of cerebrospinal fluid pulsation. | |
| Myelotomy (animal studies) | Allen (1911) | Performed myelotomy in 4 dogs to analyze the effect on locomotion after SCI. | Movement was restored after SCI. |
| Rivlin et al. (1979) | Investigated the effect of myelotomy for SCI in 40 rats. | Myelotomy achieved significant improvement in spinal cord function compared with durotomy. | |
| Hu et al. (2015) | Investigated the effect of myelotomy on 20 rats with SCI. | Myelotomy improved locomotor function and reduced edema in rats with SCI. | |
| Meyer et al. (2018) | Investigated the effect of myelotomy on 20 rats with SCI. | Myelotomy had no benefit on SCI reduced BBB scores. | |
| Qin et al. (2018) | Meta-analysis of six randomized controlled trials | Myelotomy promoted locomotor recovery in rats with SCI, especially in those with moderate injury. | |
| Myelotomy (clinical studies) | Koyanagi et al. (1989) | Report of four cases with SCI. | Early myelotomy was effective in preventing secondary injury. |
BBB: Basso, Beattie, and Bresnahan; CSF: cerebrospinal fluid; IMP: intramedullary pressure; ISP: intraspinal pressure; SCI: spinal cord injury.
Studies conducted by different authors have been reported here and demonstrate that durotomy plus duroplasty and/or myelotomy is a potential procedure for the treatment of SCI involving edema, hemorrhage, necrosis, or spinal cord compartment syndrome. The procedures of durotomy and myelotomy have been conducted by different surgeons, and have been reported to have both positive and negative outcomes.
Some patients had improvement in movement or neurological function after decompression by durotomy or myelotomy. However, in other cases, the results of the experimental groups were not different from those of the control groups. In studies without a positive effect, either the locomotion or sensation of the patients had not improved, or pain sensitivity had increased after the surgery. The effectiveness of the decompression is probably dependent on the severity of the patient’s injury, the physiology of the patient, the surgeon’s experience in decompression, and the conditions or facility in which the treatment takes place.
Decompression by durotomy has been used widely in humans and has shown promising results in enhancing neurological function. In the studies mentioned in this review, the majority of durotomy cases showed positive dynamics; there was marked recovery of locomotion as well as sensory recovery. Positive effects of neurological function by durotomy were seen in 92.3% of human studies and 66.7% of animal studies. In some of these studies, there was an improvement in ASIA grade after durotomy, while others reported reduced abrasion size, reduced cystic cavities, and less scar formation. Moreover, myelotomy had positive effects in 80% of animal cases, where it can be effective for removing necrotic tissue. Myelotomy use is aimed at removing necrotic tissue, decreasing edema, and reducing hemorrhage in the spinal cord. Positive results were reported in the one human study presented here; there was an improvement in upper limb movement, a decrease in sensation impairment, and reduced secondary damage after SCI. Durotomy and myelotomy are able to reduce ISP to a greater extent than laminectomy, which can better improve blood circulation of the spinal cord.
However, durotomy and myelotomy are still often believed to be dangerous, and as a result, some surgeons prefer not to perform these procedures. Myelotomy, in particular, destroys the blood-brain barrier of the spinal cord, leading to aggravated inflammation in the injured spinal cord, which may cause negative outcomes. For this reason, durotomy may be a safer and more effective procedure, with more successful results and less injury than myelotomy.
Durotomy or duroplasty in most studies showed a positive effect in both clinical SCI patients and animal experiments, although some studies reported negative outcomes. The ASIA scale and ISP can be used to objectively reflect the validity of these surgical methods. In addition, some animal experiments have a lack of histological evidence and may even support more aggressive myelotomy. However, it should be noted that there are not enough clinical data, and a randomized controlled study is needed to determine the effectiveness of durotomy and myelotomy. When adequate data are obtained, researchers should then confirm whether neurological function of the damaged cord can be improved by durotomy and duroplasty using ISP monitoring accompanied by spinal cord perfusion pressure optimization.
Acknowledgments
We would like to thank the authors and all who contributed to the ideas of this review for editing the content and revising the manuscript.
Footnotes
Conflicts of interest: The authors declare that there are no conflicts of interest associated with this manuscript.
Financial support: This work was financially supported by the National Key Research and Development Program of China, No. 2016YFC1100100 (to XDG).
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Funding: This work was financially supported by the National Key Research and Development Program of China, No. 2016YFC1100100 (to XDG).
C-Editor: Zhao M; S-Editors: Wang J, Li CH; L-Editors: Gardner B, Maxwell R, Qiu Y, Song LP; T-Editor: Jia Y
References
- 1.Aganesov AG. The future and the past of surgery for the complicated spine trauma. Khirurgiia (Mosk) 2013;1:5–12. [PubMed] [Google Scholar]
- 2.Allen A. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column. JAMA. 1911;LVII:878–880. [Google Scholar]
- 3.Awwad W, Bassi M, Shrier I, Al-Ahaideb A, Steele RJ, Jarzem PF. Mitigating spinal cord interstitial injuries: the effect of durotomy in decreasing cord interstitial pressure in vitro. Eur J Orthop Surg Traumatol. 2014;24:261–267. doi: 10.1007/s00590-013-1409-5. [DOI] [PubMed] [Google Scholar]
- 4.Bandeira F, Yusoff NZ, Yam GH, Mehta JS. Corneal re-innervation following refractive surgery treatments. Neural Regen Res. 2019;14:557–565. doi: 10.4103/1673-5374.247421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bengtsson F, Siesjo BK. Cell damage in cerebral ischemia: physiological, biochemical and structural aspects. In: Schurrr A, Rigor BM, editors. Cerebral ischemia and resuscitation. Boca Raton, FL: CRC; 1990. pp. 215–233. [Google Scholar]
- 6.Bergmeister KD, Daeschler SC, Rhodius P, Schoenle P, Böcker A, Kneser U, Harhaus L. Promoting axonal regeneration following nerve surgery: a perspective on ultrasound treatment for nerve injuries. Neural Regen Res. 2018;13:1530–1533. doi: 10.4103/1673-5374.237113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boswell S, Sather M, Kebriaei M, Lydiatt K, Bowdino B, Tomes D, Treves J, Hellbusch L. Combined open decompressive laminectomy and verteboplasty for treatment of thoracolumbar fractures retrospective view of 41 cases. Clin Neurol Neurosurg. 2012;114:902–906. doi: 10.1016/j.clineuro.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 8.Bötel U, Gläser E, Niedeggen A. The surgical treatment of acute spinal paralysed patients. Spinal Cord. 1997;35:420–428. doi: 10.1038/sj.sc.3100407. [DOI] [PubMed] [Google Scholar]
- 9.Bourassa-Moreau É, Mac-Thiong JM, Li A, Ehrmann Feldman D, Gagnon DH, Thompson C, Parent S. Do patients with complete spinal cord injury benefit from early surgical decompression? Analysis of neurological improvement in a prospective cohort study. J Neurotrauma. 2016;33:301–306. doi: 10.1089/neu.2015.3957. [DOI] [PubMed] [Google Scholar]
- 10.Camlar M, Turk C, Buhur A, Oltullu F, Oren M, Senoglu M, Ozer F. Does decompressive duraplasty have a neuroprotective effect on spinal trauma: an experimental study. World Neurosurg. 2019;126:E288–294. doi: 10.1016/j.wneu.2019.02.043. [DOI] [PubMed] [Google Scholar]
- 11.Campagnolo D, Esquieres R, Kopacz K. Effect of timing of stabilizationon length of stay and medical complications following spinal cord injury. J Spinal Cord Med. 1997;20:331–334. doi: 10.1080/10790268.1997.11719484. [DOI] [PubMed] [Google Scholar]
- 12.Cao XJ, Feng SQ, Fu CF, Gao K, Guo JS, Guo XD, He XJ, Huang ZW. Repair protection and regeneration of spinal cord injury. Neural Regen Res. 2015;10:1953–1975. doi: 10.4103/1673-5374.172314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chavanne A, Pettigrew DB, Holtz JR, Dollin N, Kuntz C. Spinal cord intramedullary pressure in cervical kyphotic deformity: a cadaveric study. Spine (Phila Pa 1976) 2011;36:1619–1626. doi: 10.1097/BRS.0b013e3181fc17b0. [DOI] [PubMed] [Google Scholar]
- 14.Chen T, Long L, Cao G, Cai Y, Liao W. Treatment of thoracolumbar burst fractures by posterior laminectomy decompression and bone grafting via injured vertebrae. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2014;28:1236–1240. [PubMed] [Google Scholar]
- 15.Duh M, Shepard M, Wilberger J, Bracken M. The effectiveness of surgery on the treatment of acute spinal cord injury and its relation to pharmacological treatment. Neurosurgery. 1994;35:240–248. doi: 10.1227/00006123-199408000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Fehlings MG, Arvin B. The timing of surgery in patients with central spinal cord injury. J Neurosurg Spine. 2009;10:1–2. doi: 10.3171/2008.10.SPI0822. [DOI] [PubMed] [Google Scholar]
- 17.Fehlings MG, Tator CH, Linden RD. The relationships among the severity of spinal cord injury, motor and somatosensory evoked potentials and spinal cord blood flow. Electroencephalogr Clin Neurophysiol. 1989;741:241–259. doi: 10.1016/0168-5597(89)90055-5. [DOI] [PubMed] [Google Scholar]
- 18.Fehlings MG, Tetreault LA, Wilson JR, Aarabi B, Anderson P, Arnold PM, Brodke DS, Burns AS, Chiba K, Dettori JR, Furlan JC, Hawryluk G, Holly LT, Howley S, Jeji T, Kalsi-Ryan S, Kotter M, Kurpad S, Marino RJ, Martin AR, et al. A clinical practice guideline for the management of patients with acute spinal cord injury and central cord syndrome: recommendations on the timing (≤ 24 hours versus > 24 hours) of decompressive surgery. Global Spine J. 2017a;7:195S–202S. doi: 10.1177/2192568217706367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fehlings MG, Tetreault LA, Wilson JR, Kwon BK, Burns AS, Martin AR, Hawryluk G, Harrop JS. A clinical practice guideline for the management of acute spinal cord injury: introduction, rationale and scope. Global Spine J. 2017b;7:84S–94S. doi: 10.1177/2192568217703387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fehlings MG, Vaccaro A, Wilson JR, Singh A, Cadotte D, Harrop JS, Aarabi B, Shaffrey C, Dvorak M, Fisher C, Arnold P, Massicotte EM, Lewis S, Rampersaud R. Early versus delayed decompression for traumatic cervical spinal cord injury: results of the surgical timing in acute spinal cord injury study (STASCIS) PLoS One. 2012;7:540–542. doi: 10.1371/journal.pone.0032037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant RA, Quon JL, Abbed KM. Management of acute traumatic spinal cord injury. Curr Treat Options Neurol. 2015;17:334. doi: 10.1007/s11940-014-0334-1. [DOI] [PubMed] [Google Scholar]
- 22.Grassner L, Grillhösl A, Griessenauer CJ, Thomé C, Bühren V, Strowitzki M, Winkler PA. Spinal meninges and their role in spinal cord injury: a neuroanatomical review. J Neurotrauma. 2017;35:403–410. doi: 10.1089/neu.2017.5215. [DOI] [PubMed] [Google Scholar]
- 23.Grassner L, Wutte C, Klein B, Mach O, Riesner S, Panzer S, Vogel M, Bühren V, Strowitzki M, Vastmans J, Maier D. Early decompression (< 8 h) after traumatic cervical spinal cord injury improves functional outcome as assessed by spinal cord independence measure after one year. J Neurotrauma. 2016;33:1658–1666. doi: 10.1089/neu.2015.4325. [DOI] [PubMed] [Google Scholar]
- 24.Guest J, Eleraky MA, Apostolides PJ, Dickman CA, Sonntag VK. Traumatic central cord syndrome: results of surgical management. J Neurosurg. 2002;97:25–32. doi: 10.3171/spi.2002.97.1.0025. [DOI] [PubMed] [Google Scholar]
- 25.Hadley MN, Walters BC. Introduction to the guidelines for the management of acute cervical spine and spinal cord injuries. Neurosurg. 2013;72:5–16. doi: 10.1227/NEU.0b013e3182773549. [DOI] [PubMed] [Google Scholar]
- 26.Hardwell DM, Gibson JL, Fessler RD, Pettigrew DB, Kuntz C. Pia matter significantly contributes to spinal cord intraparenchymal pressure in a simulated, model of edema. Spine. 2016;41:E524–529. doi: 10.1097/BRS.0000000000001306. [DOI] [PubMed] [Google Scholar]
- 27.Hu AM, Li JJ, Sun W, Yang DG, Yang ML, Du LL, Gu R, Gao F, Li J, Chu HY, Zhang X, Gao LJ. Myelotomy reduces spinal cord edema and inhibits aquaporin-4 and aquaporin-9 expression in rats with spinal cord. Spinal Cord. 2015;53:98–102. doi: 10.1038/sc.2014.209. [DOI] [PubMed] [Google Scholar]
- 28.Huang H, Hari SS, Chen L, Ali O, Ziad M, Al Z, Saberi H, Muresanu DF, He XJ. Review of clinical neurorestorative strategies for spinal cord injury: exploring history and latest progresses. J Nurs Res. 2018;6:171–178. [Google Scholar]
- 29.Huang H, Sharma H. Neurorestoratology, one of the most promising new disciplines at the forefront of neuroscience and medicine. J. Neurorestoratol. 2013;1:37–41. [Google Scholar]
- 30.Iannotti C, Zhang YP, Shields LB, Han Y, Burke DA, Xu XM, Shields CB. Dural repair reduces connective tissue scar invasion and cystic cavity formation after acute spinal cord laceration injury in adult rats. J Neurotrama. 2006;23:853–865. doi: 10.1089/neu.2006.23.853. [DOI] [PubMed] [Google Scholar]
- 31.Iida H, Tachibana S. Spinal cord intramedullary pressure: Direct cord traction test. Neurol Med Chir (Tokyo) 1995;35:75–77. doi: 10.2176/nmc.35.75. [DOI] [PubMed] [Google Scholar]
- 32.Iwasaki Y, Ito T, Isu T, Tsuru M. Effect of combined treatment of mannitol and myelotomy on experimental spinal cord injury (author’s transl) Neurol Med Chir (Tokyo) 1981;21:917–921. doi: 10.2176/nmc.21.917. [DOI] [PubMed] [Google Scholar]
- 33.Jalan D, Saini N, Zaidi M, Pallottie A, Elkabes S, Heary RF. Effects of early surgical decompression on functional and histological outcomes after severe experimental thoracic spinal cord injury. J Neurosurg Spine. 2017;26:62–75. doi: 10.3171/2016.6.SPINE16343. [DOI] [PubMed] [Google Scholar]
- 34.Jiaxin X, Xunnding D, Yu F, Ning C, Xin Z, Fang F, Shemin Z, Yaping F. Early intradural microsurgery improves neurological recovery of acute spinal cord injury: a study of 87 cases. J Neurorestoratol. 2018;6:152–157. [Google Scholar]
- 35.Jug M, Kejžar N, Vesel M, Al Mawed S, Dobravec M, Herman S, Bajrović FF. Neurological recovery after traumatic cervical spinal cord injury is superior if surgical decompression and instrumented fusion are performed within 8 hours versus 8 to 24 hours after injury: a single center experience. J Neurotrauma. 2015;32:1385–1392. doi: 10.1089/neu.2014.3767. [DOI] [PubMed] [Google Scholar]
- 36.Koyanagi I, Iwasaki Y, Isu T, Akino M, Abe H, Mitsumori K, Nakagawa T, Sakuragi M, Saitoh H, Nomura M. Myelotomy for acute cervical cord injury: report of four cases. Neurol Med Chir (Tokyo) 1989;29:302–306. doi: 10.2176/nmc.29.302. [DOI] [PubMed] [Google Scholar]
- 37.Krengel W, Anderson P, Henley M. Early stabilization and decompression for incomplete paraplegia due to a thoracic-level spinal cord injury. Spine. 1993;18:2080–2087. doi: 10.1097/00007632-199310001-00027. [DOI] [PubMed] [Google Scholar]
- 38.La RG, Conti A, Cardali S, Cacciola F, Tomasello F. Does early decompression improve neurological outcome of spinal cord injured patients? Appraisal of the literature using a meta-analytical approach. Spinal Cord. 2004;42:503–512. doi: 10.1038/sj.sc.3101627. [DOI] [PubMed] [Google Scholar]
- 39.Layer RT, Ulich TR, Coric D, Arnold PM, Guest JD, Heary RH, Hsieh PC, Jenkins L, Kim KD, Lee KS, Masuoka LK, Neff KM, Ray WZ, Theodore N, Fehlings MG. New clinical-pathological classification of intraspinal injury following traumatic acute complete thoracic spinal cord injury: postdurotomy/myelotomy observations. Clin Neurosurg. 2017;64:105–109. doi: 10.1093/neuros/nyx204. [DOI] [PubMed] [Google Scholar]
- 40.Leep HN, Wijdicks EF. Intramedullary spinal cord hemorrhage (hematomyelia) Rev Neurol Dis. 2009;6:E54–61. [PubMed] [Google Scholar]
- 41.Li Y, Chandler L, Walker, Yi Ping Zhang, Christopher B, Shields, Xiao-Ming Xu. Surgical decompression in acute spinal cord injury: a review of clinical evidence, animal model studies, and potential future directions of investigation. Front Biol. 2014;9:127–136. doi: 10.1007/s11515-014-1297-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maikos JT, Qian Z, Metaxas D, Shreiber DI. Finite element analysis of spinal cord injury in the rat. J Neurotrauma. 2008;25:795–816. doi: 10.1089/neu.2007.0423. [DOI] [PubMed] [Google Scholar]
- 43.Meyer C, Bendella H, Rink S, Gensch R, Seitz R, Stein G, Manthou M, Papamitsou T, Nakamura M, Bouillon B, Galea M, Batchelor P, Dunlop S, Angelov D. The effect of myelotomy following low thoracic spinal cord compression injury in rats. Exp Neurol. 2018;306:10–21. doi: 10.1016/j.expneurol.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 44.Mohamed B, Rafael G, Nicholas R, AbtMohamed B, Macki Z, Timothy FW. Durotomy is associated with pseudoarthrosis following lumbar fusion. J Clin Neurosci. 2015;22:544–548. doi: 10.1016/j.jocn.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 45.Newton C, Laureen D, Michael G. Spinal cord edema after spinal cord injury: from pathogenesis to management. Brain Edema. 2017:261–275. [Google Scholar]
- 46.Perkins PG, Deane RH. Long term follow up of six patients with acute spinal cord injury following dural decompression. Injury. 1988;19:397–401. doi: 10.1016/0020-1383(88)90132-5. [DOI] [PubMed] [Google Scholar]
- 47.Phang I, Werndle MC, Saadoun S, Varsos G, Czosnyka M, Zoumprouli A, Papadopoulos MC. Expansion duroplasty improves intraspinal pressure, spinal cord perfusion, pressure and vascular pressure reactivity index in patients with traumatic spinal cord injury. J Neurotrauma. 2015;32:865–874. doi: 10.1089/neu.2014.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qin C, Zhang WH, Yang DG, Yang ML, Du LJ, Li JJ. Myelotomy promotes locomotor recovery in rats subjected to spinal cord injury: a meta-analysis of six randomized controlled trials. Neural Regen Res. 2018;13:1096–1106. doi: 10.4103/1673-5374.233454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qu Y, Guo X. Durotomy and dural grafting to treat lower cervical spine injuries with extensive spinal cord edema. Neural Regen Res. 2015;10:12. [Google Scholar]
- 50.Qu Y, Luo Z, Guo X, Cui W, Sun T T, Huang Z, Wu Y, Shao Z. The durotomy or myelotomy for the spinal cord extensive swelling with/without intramedullary hemorrhage. Zhonghua Guke Zazhi. 2015;35:707–713. [Google Scholar]
- 51.Rivlin AS, Tator CH. Effect of vasodilators and myelotomy on recovery after acute spinal cord injury in rats. J Neurosurg. 1979;50:349–352. doi: 10.3171/jns.1979.50.3.0349. [DOI] [PubMed] [Google Scholar]
- 52.Rouanet C, Reges D, Rocha E, Gagliardi V, Silva GS. Traumatic Spinal cord injury:current concepts and treatment updates. Arq Neuropsiquiatr. 2017;75:387–396. doi: 10.1590/0004-282X20170048. [DOI] [PubMed] [Google Scholar]
- 53.Saadoun S, Chen S, Papadopoulos MC. Intraspinal pressure and spinal cord perfusion pressure predict neurological outcome after traumatic spinal cord injury. J Neurol Neurosurg Psychiatry. 2017;88:452–453. doi: 10.1136/jnnp-2016-314600. [DOI] [PubMed] [Google Scholar]
- 54.Saadoun S, Papadopoulos MC. Spinal cord injury:Is monitoring from the injury site the future. Crit Care Med. 2016;20:308. doi: 10.1186/s13054-016-1490-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saadoun S, Werndle MC, Lopez HL, Papadopoulos MC. The dura causes spinal cord compression after spinal cord injury. Br J Neurosurg. 2016;5:582–584. doi: 10.3109/02688697.2016.1173191. [DOI] [PubMed] [Google Scholar]
- 56.Salvok M, Tysmbaliuk V, Dzyak L. The method of multilevel decompression of thoracic spine with the durotomy and the local administration of cortexin in the setting in the intradural space in the patients with spinal cord injury. Int J Neurohabilitation. 2015;2:173. [Google Scholar]
- 57.Saoudun S, Bell BA, Verkman AS, Papadopoulos MC. Greatly improved neurological outcome after spinal cord compression injury in AQP4- deficient mice. Brain. 2008;131:1087–1098. doi: 10.1093/brain/awn014. [DOI] [PubMed] [Google Scholar]
- 58.Smith JS, Anderson R, Pham T, Bhatia N, Steward O, Gupta R. Role of early surgical decompression of the intradural space after surgical spinal cord injury in an animal model. J Bone Joint Surg Am. 2010;92:1206–1214. doi: 10.2106/JBJS.I.00740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tator CH. Update on pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995;5:407–413. doi: 10.1111/j.1750-3639.1995.tb00619.x. [DOI] [PubMed] [Google Scholar]
- 60.Telemacque D, Zhu FZ, Chen KF, Chen Li, Ren ZW, Yao S, Qu YZ, Guo XD. Method of Decompression by durotomy and duroplasty for cervical spinal cord injury in patients without fracture or dislocation. J Neurorestoratol. 2018:158–164. [Google Scholar]
- 61.Ter Wengel PV, De Witt Hamer PC, Pauptit JC, van der Gaag NA, Oner FC, Vandertop WP. Early surgical decompression improves neurological outcome after complete traumatic cervical spinal cord injury: a meta analysis. J Neurotrauma. 2018;36:835–844. doi: 10.1089/neu.2018.5974. [DOI] [PubMed] [Google Scholar]
- 62.Tsymbaliuk VI, Dzyak LA, Salkov NN, Rodnsky AG, Demchenko E. Antioxidant activity of cortexin and electro stimulationin dynamics of restoration of mobility in rats after spinal cord injury. J Exp Clin Physiol Biochem. 2014a;4:5–12. [Google Scholar]
- 63.Tsymbaliuk VI, Dzyak LA, Salkov NN, Rodnsky AG, Tkachenko S. Experimental study of effectiveness of local application of elecctrostimulation, cortexin and methylprednisolone in acute spinal cord injury. J Med Perspect. 2014b;19:51–56. [Google Scholar]
- 64.Tykocki T, Poniatowski Ł, Czy M, Koziara M, Wynne-Jones G. Intraspinal pressure monitoring and extensive duroplasty in the acute phase of traumatic spinal cord injury: a systemic review. World Neurosurg. 2017;105:145–152. doi: 10.1016/j.wneu.2017.05.138. [DOI] [PubMed] [Google Scholar]
- 65.Werndle MC, Saadoun S, Phang I, Czosnyka M, Varsos GV, Czosnyka ZH, Smielewski P, Jamous A, Bell BA, Zoumprouli A, Papadopoulos MC. Monitoring of spinal cord perfusion pressure in acute spinal cord injury: Initial findings of the injured spinal cord pressure evaluation study. Crit Care Med. 2014;42:646–655. doi: 10.1097/CCM.0000000000000028. [DOI] [PubMed] [Google Scholar]
- 66.Wilson JR, Tetreault LA, Kwon BK, Arnold PM, Mroz TE, Shaffrey C, Harrop JS, Chapman JR, Casha S, Skelly AC, Holmer HK, Brodt ED, Fehlings MG. Timing of decompression in patients with acute spinal cord injury: a systematic review. Global Spine J. 2017;7:95S–115S. doi: 10.1177/2192568217701716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Winestone JS, Farley CW, Curt BA, Chavanne A, Dollin N, Pettigrew DBx, Kuntz C. Laminectomy, durotomy and piotomy effects on spinal cord intramedullary pressure in severe cervical and thoracic kyphotic deformity: a cadaveric study. J Neurosurg Psychiatr. 2017;88:452–453. doi: 10.3171/2011.10.SPINE11377. [DOI] [PubMed] [Google Scholar]
- 68.Yang D, Li J, Gu R, Hu A, Yang M, Du LY, Zhang X, Sun W, Gao F, Wu Y, He J, Feng, Chu H. Myelotomy suppresses autophagic marker LC3-II expression and elevates mTORC1 expression and improves neurological function recovery in rats with spinal cord injury. Neurol Asia. 2013;18:401–407. [Google Scholar]
- 69.Zhang J, Wang H, Zhang CJ, Li WG. Intrathecal decompression versus epidural decompression in the treatment of severe spinal cord injury in rat model: a randomized, controlled preclinical research. J Orthop Surg Res. 2016;11:34. doi: 10.1186/s13018-016-0369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhu F, Yao S, Ren Z, Telemacque D, Qu Y, Chen K, Yang F, Zeng L, Guo X. Early durotomy with duroplasty for severe adult spinal cord injury without radiographic abnormality: a novel concept and method of surgical decompression. Eur Spine J. 2019;28:2275–2282. doi: 10.1007/s00586-019-06091-1. [DOI] [PubMed] [Google Scholar]


