Keywords: axon, behavior, corticospinal tract, ketogenic diet, ketone supplementation, myelin, neuroprotection, spinal cord injury
Abstract
We have previously shown that induction of ketosis by ketogenic diet (KD) conveyed neuroprotection following spinal cord injury in rodent models, however, clinical translation may be limited by the slow raise of ketone levels when applying KD in the acute post-injury period. Thus we investigated the use of exogenous ketone supplementation (ketone sodium, KS) combined with ketogenic diet as a means rapidly inducing a metabolic state of ketosis following spinal cord injury in adult rats. In uninjured rats, ketone levels increased more rapidly than those in rats with KD alone and peaked at higher levels than we previously demonstrated for the KD in models of spinal cord injury. However, ketone levels in KD + KS treated rats with SCI did not exceed the previously observed levels in rats treated with KD alone. We still demonstrated neuroprotective effects of KD + KS treatment that extend our previous neuroprotective observations with KD only. The results showed increased neuronal and axonal sparing in the dorsal corticospinal tract. Also, better performance of forelimb motor abilities were observed on the Montoya staircase (for testing food pellets reaching) at 4 and 6 weeks post-injury and rearing in a cylinder (for testing forelimb usage) at 6 and 8 weeks post-injury. Taken together, the findings of this study add to the growing body of work demonstrating the potential benefits of inducing ketosis following neurotrauma. Ketone salt combined with a ketogenic diet gavage in rats with acute spinal cord injury can rapidly increase ketone body levels in the blood and promote motor function recovery. This study was approved by the Animal Care Committee of the University of British Columbia (protocol No. A14-350) on August 31, 2015.
Introduction
Despite extensive research efforts, effective strategies for promoting functional improvement after spinal cord injury (SCI) remain elusive (Hug and Weidner, 2012; Singh et al., 2014; Ahuja et al., 2017). Primary mechanical injury to the spinal cord leads to neuron death and axonal tract disruption, as well as extensive glial cell loss. Furthermore, secondary injury mechanisms (e.g., hemorrhage, ischemia, vascular changes, and oxidative damage) propagate damage to initially spared tissue, incurring significant losses of potentially functional neural substrates (Oyinbo, 2011; Hilton et al., 2017). Thus, neuroprotective strategies that focus on mitigating tissue loss from secondary injury by increasing the sparing of neurons, axons, and/or glial cells represent promising approaches for improving the outcome of SCI (Ahuja et al., 2016; Hayta and Elden, 2018).
More recently, diet-based strategies have been investigated for their neuroprotective efficacy following neurotrauma (Davis et al., 2008; Gomez-Pinilla and Gomez, 2011; Jeong et al., 2011; Streijger et al., 2013; Wang et al., 2017; Lu et al., 2018; McDougall et al., 2018). Of these, among the most promising is the ketogenic diet (KD), which has been clinically validated for pediatric intractable epilepsy (Winesett et al., 2015). The high-fat, low-carbohydrate intake of the KD induces a metabolic state that is characterized by fatty acid oxidation and hepatic ketogenesis, which results in elevation of plasma ketone levels. Ketone bodies (i.e., β-hydroxybutyrate (βHB) and acetoacetate) are metabolized by the mitochondria of target tissues to acetyl-coenzyme A, which increases adenosine triphosphate yield via an increased tricarboxylic acid cycle flux and oxidative phosphorylation (Sato et al., 1995; Achanta and Rae, 2017). Ketone bodies are metabolized downstream of pyruvate dehydrogenase (a rate-limiting enzyme of glycolysis), and are considered a more energy-efficient fuel than glucose (Lutas and Yellen, 2013; Magistretti and Allaman, 2015). In vitro studies have shown that ketones reduce neuronal death and prevent changes in neuronal membrane properties that are induced by glutamate excitotoxicity (Maalouf et al., 2007; Koppel and Swerdlow, 2018). Therefore, a reliance on ketone bodies in pathological conditions may benefit for many tissue types, including the injured spinal cord.
Several clinical and pre-clinical studies have found evidence for the KD’s neuroprotective effects on several central nervous system pathologies, including Alzheimer’s disease (Newport et al., 2015), Parkinson’s disease (Shaafi et al., 2016), amyotrophic lateral sclerosis (Zhao et al., 2006), and possibly acute SCI (Yarar-Fisher et al., 2018). Moreover, the putative neuroprotective role of ketones has been demonstrated by improved histological and behavioral outcomes in models of traumatic injury to the brain and spinal cord for both the KD and every-other-day-fasting regimes (Plunet et al., 2008; Jeong et al., 2011; Streijger et al., 2013; Wang et al., 2017; Lu et al., 2018; Ari et al., 2019). Despite this promising evidence, the clinical application of the KD may be limited due to the unpalatable nature associated with the requirement of high fat ingestion (Kesl et al., 2016).
Previous work has found that the ketone levels increase during the first 12–16 hours after KD onset; however, this may miss the critical early period during which ketones are most beneficial (Plunet et al., 2010). Therefore, the application of exogenous ketone supplements as a means of more rapidly increasing ketone levels may provide clearer clinical benefits. Recent studies have confirmed the efficacy of exogenous ketone supplements in inducing a state of ketosis (Clarke et al., 2012; Kesl et al., 2016; Stubbs et al., 2017). The consumption of drinks containing exogenous ketone supplements, such as ketone esters or ketone salts (KSs), has been considered as a possible alternative to the KD (Stubbs et al., 2017). Therefore, in the current study we investigated whether the application of a sodium/potassium βHB mineral salt (ketone calcium sodium, sold under the trade name KetoCaNa) combined with the KD following SCI in rats would more quickly raise blood ketone levels and confer neuroprotective effects.
Materials and Methods
Ethics statement
All procedures performed in this study were approved by the Animal Care Committee of the University of British Columbia (protocol No. A14-350) on August 31, 2015, in accordance with the guidelines established by the Canadian Council for Animal Care. Investigators were blinded to the treatment group of the animals during surgery, as well as for all behavioral and histological assessments.
Animals
Forty-nine young adult male Sprague-Dawley rats (weighing ~350 g, aged 3–4 months; Charles River Breeding Laboratory; Wilmington, MA, USA) were used. Among these, 15 rats were included in the initial experiment (no injury). The remaining 34 rats were included in the second experiment (spinal cord injury). Animals were group-housed (up to 4 animals per cage) in standard rat housing cages in a 12-hour dark/light cycle and had ad libitum access to standard rodent food and water prior to the injury.
SCI model establishment
Animals were subjected to a C5 cervical hemi-contusion SCI, as described previously (Lee et al., 2012). Briefly, a unilateral C5 laminectomy was performed to expose the cervical spinal cord, followed by affixing of the dorsal processes of C4–6 with a custom designed clamp rigidly fixed at a 22.5° angle (with respect to the horizon). The exposed spinal cord was then subjected to a hemi-contusion injury using the Infinite Horizons impactor device (Precision Systems & Instrumentation, Lexington, KY, USA), which was set to deliver a moderately severe injury (force: 150 kdyne; impact velocity: 100 m/s). The injury was performed on the animal’s preferred side as determined by pre-injury behavioral screening with the Montoya staircase pellet reaching task.
KD + KS intervention
To induce a metabolic state of ketosis, animals in the KD + KS treatment group were fed with KD (F5848; paste; Bio-Serv; Frenchtown, NJ, USA) supplemented with ketone salt (calcium/sodium βHB mineral salt; 1000–1500 mg βHB; sold under the trade name KetoCaNa by KetoSports, Urbana, IL, USA; intragastric administration) starting at 3 hours post-injury and delivered at 12-hour intervals for 4 days post-injury. Note that KD was continued for the entire expriment (Additional Figure 1 (339KB, pdf) ). The calcium/sodium βHB mineral salt used contains a high proportion of βHB (61.5% w/w), and is sold as a supplement for high-demand physical activities (Cox et al., 2016). Control animals were provided ad libitum access to standard rodent food and water, and received an equal volume of vehicle (water; intragastric administration) at the same dosing intervals (Additional Figure 1 (339KB, pdf) ). For the feasibility study, non-injured animals were randomly assigned to a control group, KD group, or KD + KS group (n = 5 per group). Ketone levels in blood samples obtained through tail vein puncture were measured in the morning (for baseline) and at 1 or 4 hours after oral gavage using a βHB concentration monitor (Abbott Laboratories; Lake Bluff, IL, USA).
Behavioral assessment
Behavioral assessments were performed at 2, 4, 6, and 8 weeks post-injury to assess functional improvement (compared to pre-injury baselines). As described previously (Montoya et al., 1991; Nikkhah et al., 1998), the Montoya staircase reaching task was used to assess forepaw grasping ability. Briefly, animals were fasted for 21 hours (to increase motivation) prior to placement in the staircase apparatus for 15 minutes. The number of pellets eaten using the ipsilateral and contralateral paw was recorded and used as a proxy of forelimb grasping ability. Pellets were color-coded according to the level of the staircase to distinguish between misplaced and remaining pellets. The reaching score (%) was calculated as the number of pellets eaten divided by the total number of pellets. As described previously (Schallert et al., 2000; Hilton et al., 2013; Streijger et al., 2013), cylinder rearing was used to assess forelimb usage. Briefly, animals were placed in a Plexiglas® cylinder (Bristol, PA, USA) for 15 minutes. During rearing motion (i.e., vertical exploration of the cylinder with the forepaws), use of the ipsilateral and contralateral paw was recorded. The percent of ipsilateral and dual limb use was calculated based on the number of times the ipsilateral limb was used for both initial and subsequent rearing events over a total of 20 independent rears.
Tissue processing
At the experimental endpoint, animals were sacrificed by an overdose of chloral hydrate (100 mg/kg, intraperitoneal injection) and were transcardially perfused with PBS, followed by 4% paraformaldehyde fixative solution. Once fixed, cervical spinal cord tissue (~C2–8) was harvested, post-fixed in 4% paraformaldehyde overnight, then cryoprotected in subsequent 12%, 18%, and 24% sucrose solutions. Tissue samples were then frozen over dry ice in Tissue-Tek® optimal cutting temperature embedding medium (Sakura Finetek USA Inc., Torrance, CA, USA) and stored at –80°C until further processing. Spinal cord cross-sections were cut using a cryostat at a 20-µm thickness.
Immunohistochemistry
Spinal cord tissue sections were stained using established immunohistochemical methods. Briefly, delipidation was initially performed on thawed spinal cord sections via a step-wise series of ethanol solutions of increasing and decreasing concentrations (50, 70, 90, 100, 90, 70, and 50% for 2 minutes each). Slides were then incubated with 10% normal donkey serum (NDS) (in 0.01 M PBS with 0.1% Triton X-100) for 30 minutes, followed by overnight application (~14–16 hours) of primary antibodies at room temperature. Primary antibodies used in the current study included guinea pig anti-NeuN for neuron labeling (1:500; Cat #ABN90P; Millipore, Badford, MA, USA), rabbit anti-glial fibrillary acidic protein for astrocyte labeling (1:1000; Cat #AB5541; Chemicon, Badford, MA, USA), mouse anti-βIII tubulin for tubulin labeling (1:1000; Cat #T8660; Sigma, St. Louis, MO, USA), mouse anti-SMI-312 for neurofilament labeling (1:1000; Cat #SMI-31R; Biolegend, San Diego, CA, USA), and chicken anti-myelin basic protein (MBP) for myelin sheath labeling (1:200; Cat #MBP; AvesLabs, Tigard, OR, USA). Following washing, secondary antibodies raised in donkey and conjugated to Alexa fluorophores (405, 488, 594, and/or 647 nm) (all in 1:200; Jackson ImmunoResearch, West Grove, PA, USA) were applied to the tissue sections for 2 hours at room temperature. For the white matter sparing analysis, spinal cord sections were also stained with eriochrome cyanine , following established protocols.
For the tissue sparing analysis, eriochrome cyanine staining was visualized using a Zeiss Axiophot2 microscope (Carl Zeiss; Oberkochen, Germany) with a 10× objective on cross-sections ranging from 2.4 mm rostral and caudal to the lesion epicenter (i.e., the location at which there was the least amount of spared white matter) at intervals of 0.4 mm. Captured images were then analyzed by manually tracing and measuring the area of white and gray matter using SigmaScan Pro version 5.0 (Systat Software Inc., Chicago, IL, USA). Briefly, the spared white matter (determined independently by trained volunteer students) of the injured spinal cord were normalized to the contralateral side of the same section to obtain the index of spared myelin area. Immunofluorescence of antigen-bound secondary antibodies was visualized using a Zeiss Axio Observer Z1 confocal microscope (Carl Zeiss) (axon/myelinated axons: Plan-Apochromat 63× oil NA 1.4; gray matter neurons: Plan-Apochromat 40× oil NA 1.4) equipped with a Yokogawa X-1 spinning disk (Yokogawa; Tokyo, Japan). To ensure consistency, brightness and contrast were kept constant for all images for any given analysis. For the assessment of neuronal sparing (Chen et al., 2016), two boxes were placed in the ventral (200 μm × 200 μm) and dorsal (200 μm × 200 μm) horn, respectively (Additional Figure 2A (138KB, pdf) ). The density of axons and myelinated axons were assessed in three boxes (50 μm × 50 μm) placed in the dorsal corticospinal tract, lateral white matter next to the dorsal horn, and the ventral white matter medial from the ventral nerve root (Additional Figure 2B (138KB, pdf) ). To account for variability between animals, the numbers of spared neurons (NeuN+), axons (SMI-312+, β-III tubulin+), and myelinated axons (axons surrounded by MBP+ sheaths) were normalized to the contralateral side of the same cross-section (presented as an index/percentages) (Additional Figure 2 (88.8KB, pdf) ).
Statistical analysis
All data are presented as the mean ± standard error (SEM). Between-group differences were assessed using a two-way analysis of variance followed by Tukey’s post hoc test or by two-tailed unpaired Student’s t-tests. For all statistical analyses, P < 0.05 was considered to be statistically significant. GraphPad Prism Version 6.0 (GraphPad Software, San Diego, CA, USA) was used for all analyses.
Results
Exogenous KS supplementation raises blood ketone levels above those of KD in uninjured rats
To test if KS combined with the KD would increase ketone levels to a greater degree than the KD alone, we performed an initial study in uninjured rats. Baseline βHB levels were not different between the control, KD, and KD + KS groups (Figure 1). At 1 hour after the first gavage of KS, there were no differences in blood ketone levels between KD and KD + KS groups. However, 25 hours after starting the KD, both the KD and KD + KS groups had higher ketone levels than the control group, and the KD + KS group had significantly higher levels than the KD group (P < 0.05; Figure 1). We observed similar differences at 49 hours after starting the KD (P < 0.05). Given that the KD + KS group had significantly higher ketone levels than both the control and KD groups, we investigated whether KD + KS treatment would further increase ketone levels after SCI.
Figure 1.
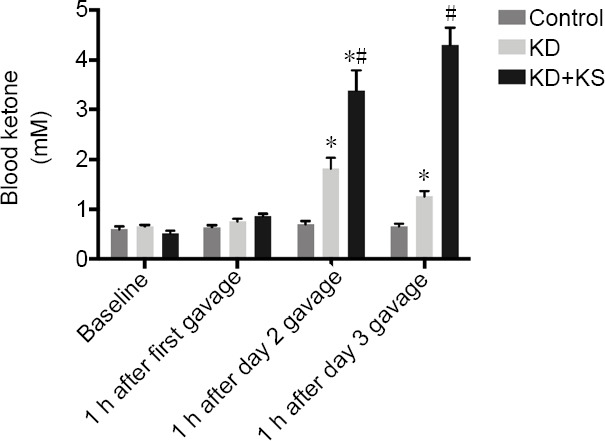
The effect of ketone salt combined with the ketogenic diet on blood ketone levels in uninjured rats.
Concentrations of β-hydroxybutyrate at baseline and 2 days following gavage in the different groups. One hour after the first gavage is also one hour after starting the KD; 1 hour after the day 2 gavage is 25 hours after starting the KD; and 1 hour after the day 3 gavage is 49 hours after starting the KD. Data are expressed as the mean ± SEM (n = 5 per group). *P < 0.05, vs. control group; #P < 0.05, vs. KD group (two-way analysis of variance and Tukey’s multiple comparisons test).
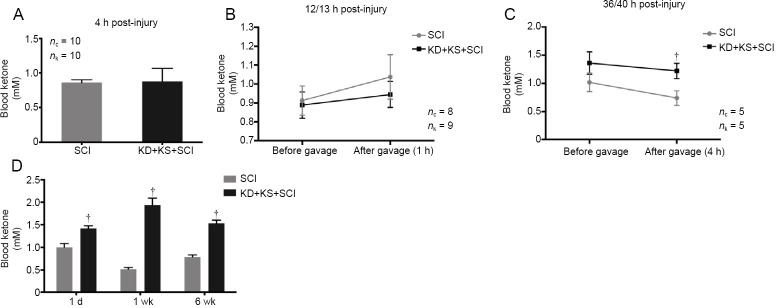
KS supplementation does not elicit a sustained further increase in ketone levels above those in KD-fed rats after SCI
Blood βHB levels measured 1 hour after the initial gavage (4 hours post-injury) failed to reveal a significant increase above control levels (P > 0.05). Similarly, no significant difference was observed 13 hours post-injury, with blood βHB levels increasing slightly in both groups (P > 0.05; Figure 2B). βHB levels measured at 36 hours post-injury revealed non-significantly elevated levels of βHB in the KD + KS group prior to gavage (P = 0.23; two-tailed Student’s t-test) and a significant difference at 4 hours post-gavage (40 hours post-injury) (P < 0.05; two-tailed Student’s t-test; Figure 2A–C). As expected, the KD + KS treatment induced a long-lasting state of ketosis, with blood βHB increasing above control levels as early as 1 day post-injury, peaking at 1 week post-injury, and remaining elevated for at least 6 weeks post-injury (Figure 2D). However, in the acute phase after SCI, supplementation with KS via an oral gavage (in combination with KD) did not appear to elicit a prolonged further increase in ketone levels; the levels were comparable to those seen after KD only in the uninjured rats (Figure 1) and to those observed in our previous results in animals that received only a KD after SCI (Streijger et al., 2013). This could be attributed to an increased ketone body metabolism after injury. We therefore tested whether the KD + KS regimen had neuroprotective effects after SCI.
Figure 2.
The effect of ketone salt combined with the ketogenic diet on blood ketone levels in spinal cord injury rats.
(A) β-Hydroxybutyrate concentration 4 hours post-injury in the KD + KS + SCI (black; n = 10) and SCI groups (gray; n = 10). (B) Blood ketone levels 12 hours post-injury in the KD + KS + SCI (black; n = 9) and SCI groups (gray; n = 8) prior to gavage and 1 hour after gavage (13 hours post-injury). (C) Blood ketone levels 36 hours post-injury in the KD + KS + SCI (black; n = 5) and SCI groups (gray; n = 5) prior to gavage and 4 hours post-gavage (40 hours post-injury). (D) Blood ketone levels in the KD + KS + SCI (black; n = 15) and SCI groups (gray; n = 17) at 1 day (measured 4 hours after the gavage), 1 week, and 6 weeks post injury. KS was only given for 4 days after SCI. Data are expressed as the mean ± SEM (n = 5 per group). †P < 0.05, vs. SCI group (two-tailed unpaired Student’s t-tests).
KD + KS improves forelimb motor performance in SCI rats
Animals in the KD + KS + SCI group displayed a significantly improved performance on the Montoya staircase test (considered an effective assessment of forelimb extension and grasping skills) as compared to SCI rats at 4 weeks (P = 0.002; two-tailed Student’s t-test) and 6 weeks post injury (P = 0.048; two-tailed Student’s t-test), which is indicative of an improved forelimb dexterity (Figure 3A). Furthermore, usage preference of the affected forelimb (as measured by the cylinder rearing test) was also significantly increased in the KD + KS + SCI group versus SCI rats at 6 weeks (P = 0.02; two-tailed Student’s t-test) and 8 weeks post injury (P = 0.01; two-tailed Student’s t-test) as compared to SCI rats (Figure 3B).
Figure 3.
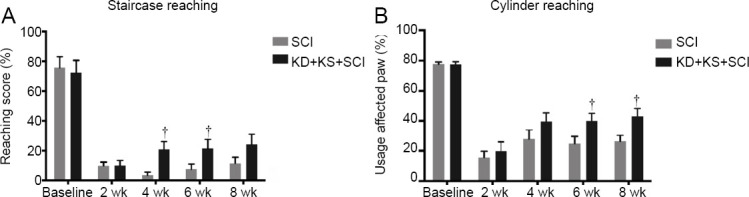
The effect of ketone salt combined with the ketogenic diet on the functional recovery of the affected forepaw in spinal cord injury rats.
(A, B) Performance on the Montoya staircase (A) and cylinder rearing tests (B) in the KD + KS + SCI (black; n = 15) and SCI (gray; n = 17) groups at baseline, 2, 4, 6, and 8 weeks post injury. Data are expressed as the mean ± SEM (n = 5 per group). †P < 0.05, vs. SCI group (two-tailed Student’s t-tests).
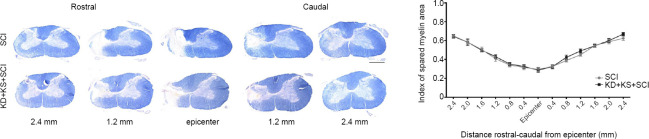
No effect of KD + KS on white matter sparing in SCI rats
Despite previous reports of enhanced tissue sparing in KD models (Streijger et al., 2013), we did not observe a difference in the spared white matter area, as visualized using eriochrome cyanine staining (P > 0.05; two-tailed Student’s t-test; Figure 4). However, this method fails to discriminate intact versus degenerated myelin, which is not rapidly cleared and remains for some time in the injured spinal cord.
Figure 4.
The effect of ketone salt combined with the ketogenic diet on white matter sparing in spinal cord injury rats 8 weeks post injury.
Representative images of eriochrome cyanine-stained spinal cord sections (A) and quantification of spared white matter at various distances rostral-caudal from the lesion epicentre (B) 8 weeks post-injury for the KD + KS + SCI (black; n = 15) and SCI groups (gray; n = 17). The spared myelin area was not larger in the KD + KS + SCI group than in the control group. No significant differences were found. Bar scale: 500 μm. Data are expressed as the mean ± SEM (n = 5 per group), and were analyzed using two-tailed unpaired Student’s t-tests.
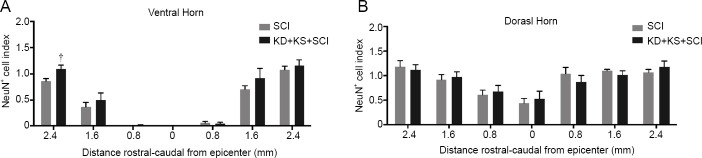
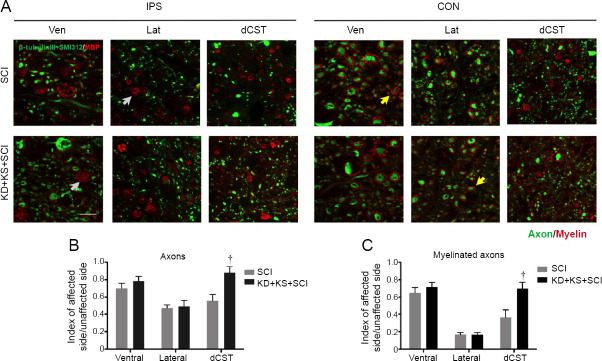
KD + KS increases neuronal and axonal survival in the spinal cord of SCI rats
Increased neuronal sparing, as revealed by the number of NeuN+ cells, was observed at 2.4 mm rostral to the lesion epicenter in the KD + KS + SCI group as compared to SCI rats (P = 0.01; two-tailed Student’s t-test; Figure 5). No significant between-group differences were found in the dorsal horn. As expected, many axonal tracts ipsilateral to the injury displayed signs of degeneration (i.e., small cavitation and myelin debris), which were largely absent from the contralateral side (Figure 6A). Assessment of axonal number revealed a significantly higher axonal density in the dorsal corticospinal tract of the KD + KS + SCI group compared to the SCI group (P = 0.004; two-tailed Student’s t-test; Figure 6B), which indicates an axonal sparing effect. Quantification of myelinated axon density revealed a similar increase in the KD + KS + SCI group (P = 0.007; two-tailed Student’s t-test; Figure 6C). The specific skilled voluntary movements assessed by the Montoya staircase and cylinder reaching task are influenced by corticospinal projections and motor neurons in the ventral horn (Hilton et al., 2016); therefore, our histological and behavioral results are consistent with a focal sparing of these populations.
Figure 5.
The effect of ketone salt combined with the ketogenic diet on neuronal sparing in the spinal cord of spinal cord injury rats.
(A, B) NeuN+ cell quantification in the ventral (A) and dorsal horn (B) in the KD + KS + SCI (gray; n = 11) and SCI groups (black; n = 14) at various distances rostral-caudal from the lesion epicenter 8 weeks post-injury. NeuN+ cell counts (index) were normalized to the contralateral side. Data are expressed as the mean ± SEM (n = 5 per group). †P < 0.05, vs. SCI group (two-tailed unpaired Student’s t-tests).
Figure 6.
The effect of ketone salt combined with the ketogenic diet on axonal sparing in spinal cord of spinal cord injury rats.
(A) Representative images of spinal cord tissue 2.5 mm caudal to the epicenter immunostained for axons (β-tubulin III and SMI-312; green) and myelin (MBP; red) 8 weeks post-injury, both ipsilateral (IPS) and contralateral (CON) to injury. Yellow arrows indicate examples of myelinated axons. Gray arrows indicate examples of putative myelin debris. Bar scale: 20 μm. (B, C) Quantification of axons (B) and myelinated axons (C) in the KD + KS + SCI (black; n = 12) and SCI groups (gray; n = 12) 8 weeks post-injury in the dorsal corticospinal tract (dCST), lateral white matter (Lat), and ventral white matter (Ven). Numbers were normalized to the contralateral side (index). Data are expressed as the mean ± SEM (n = 5 per group). †P < 0.05, vs. SCI group (two-tailed unpaired Student’s t-tests). MBP: Myelin basic protein.
Discussion
In the current study, we first demonstrated that exogenous ketone supplementation raises blood ketone levels more than the KD alone in uninjured animals. However, after SCI, exogenous KS supplementation did not have a pronounced prolonged effect on ketone levels, which is indicative of a fast usage of ketone bodies after injury. Nonetheless, we successfully induced higher ketone levels as early as 1 day after injury with KD + KS treatment. Importantly, we observed improved functional recovery of the forelimb, neuronal survival in the ventral horn, as well as axonal sparing in the dorsal corticospinal tract. This tract plays a role in skilled forelimb movement (Wang et al., 2017), and, collectively, our results highlight the therapeutic potential of a combined dosing regimen of KS and KD during the first 4 days following SCI.
Several potential mechanisms have been proposed to explain the neuroprotective effects of promoting a state of ketosis (through the KD, medium-chain triglyceride diet, or fasting) (Maalouf et al., 2009; Levy et al., 2012; Gano et al., 2014). Increased levels of ketones in the blood are known to enhance metabolic and mitochondrial functions. For example, mitochondrial breakdown of βHB alters the NAD+/NADH and ubiquinon/ubiquinol ratios and increases the redox span between complex I and complex II of the electron transport chain, which reduces production of reactive oxygen species (Gano et al., 2014). Alternatively, KD has also been proposed to mitigate excitotoxic damage to neurons (Levy et al., 2012; Lutas and Yellen, 2013; Wang et al., 2018). Indeed, both acetoacetate and βHB have been shown to reduce spontaneous neuronal firing in brain slice cultures through regulation of KATP channels (Ma et al., 2007) and through inhibition of the vesicular glutamate uptake transporter by acetoacetate (Juge et al., 2010). Recently, Wang and colleagues demonstrated that KD activates autophagic pathways and reduces brain injury during PTZ-kindled seizures. This effect of KD is likely exerted via a reduction of mitochondrial cytochrome C release (Wang et al., 2018). Additionally, ketones may directly regulate intracellular molecular pathways. Using a focal ischemia model, Puchowicz and coworkers reported 3-fold increases in hypoxia inducible factor-1α and Bcl-2 (anti-apoptotic) protein levels in diet-induced ketotic animals (Puchowicz et al., 2008). Lu and colleagues (Lu et al., 2018) found that KD attenuates oxidative stress and inflammation after SCI by activating Nrf2 and suppressing the nuclear factor-κB signaling pathways. Finally, the anti-oxidative stress effects of ketones might occur through histone deacetylase inhibition, as demonstrated in a kidney oxidative stress injury (Shimazu et al., 2013).
In previous work, we found that the neuroprotective effects of KD partially depend on monocarboxylate transporters, whereby monocarboxylate transporter inhibition via α-cyano-4-hydroxycinnamate attenuated KD-induced tissue sparing following SCI (Streijger et al., 2013). Furthermore, Rahman and colleagues reported that the hydrox-yl-carboxylic acid receptor 2 (HCA2) is required for the βHB-mediated neuroprotection in a stroke model (as the effect was lost in HCA2–/– animals) (Rahman et al., 2014). This receptor is expressed on microglial cells and a variety of monocytes (Offermanns and Schwaninger, 2015). Activation of HCA2 receptors has been found to occur with an eriochrome cyanine of 0.7 mM βHB, which is easily attained in nutritional ketosis, and this induces a protective monocyte phenotype involving prostaglandin D2 production and inhibition of nuclear factor-κB (Rahman et al., 2014). In addition, there is evidence for a regulatory role of βHB on inflammatory cells through suppression of the NOD-, LRR-, and pyrin domain-containing protein 3 inflammasome, which is a multiprotein complex that stimulates pro-inflammatory cytokine production (Walsh et al., 2014; Youm et al., 2015). This suppression was recently demonstrated to occur at concentrations of several mM and is independent of glycolytic inhibition, tricarboxylic acid cycle flux, uncoupling protein-2 (UCP2), and HCA2 activation (Achanta and Rae, 2017).
Previous studies have demonstrated the potential for the KD to be used as a treatment for neurodegenerative diseases (e.g., Alzheimer disease and Parkinson’s disease) (Gano et al., 2014; Pilla, 2018); however, the unpalatability of the diet remains a major hurdle for clinical translation. Therefore, development of ketone supplements that are capable of inducing sustained therapeutic ketosis without the need to adhere to a strict high-fat, low-carbohydrate diet would be of significant interest. However, the high salt concentration of KS formulations could limit the amounts tolerated. This drawback of ketone sodium could be overcome by using other ketone supplements, such as 1,3-butanediol and 1,3-butanediol acetoacetate diesters (Newport et al., 2015; Kesl et al., 2016; Murray et al., 2016; Stubbs et al., 2017). Kesl et al. (2016) reported that chronic administration of these supplements could induce a state of “nutritional” ketosis, even under a normal non-KD diet. Furthermore, some studies have shown that blood ketone levels are increased by oral ketone ester ((R)-3-hydroxybutyl and (R)-3-hydroxybutyrate) administration, which was associated with enhanced physical and cognitive performance (Murray et al., 2016; Stubbs et al., 2017). The current study showed that exogenous ketone supplementation raised blood ketone levels above those induced by the KD alone in uninjured animals. However, the ketone level elevation was not completely repeatable in SCI rats, which is indicative of a fast metabolism of ketone bodies after injury. Similarly, clinical observations have revealed hypermetabolism in the acute stage in patients with central nervous system trauma. One of the main metabolic changes in response to injury is the development of insulin resistance, and free fatty acids are considered as the primary sources of energy (Şimşek et al., 2014). Therefore, KS administration via an oral gavage might not elicit a prolonged, further increase of blood ketone levels.
Consistent with findings of a neuroprotective effect of ketosis, we observed an increased sparing of ventral horn neurons and dorsal corticospinal tract axons in the KD + KS group. Importantly, these histological results were consistent with the behavioral improvements observed, as the dorsal corticospinal tract is required for skilled forelimb behaviors, which we tested using the Montoya staircase test. However, we did not observe a significant effect on white matter sparing. This may be due to the non-specific nature of eriochrome cyanine staining (which is a general lipophilic dye), which means that the difference between intact myelin sheaths and myelin debris cannot be effectively discerned. This interpretation is supported by our observation of an increased number of myelinated axons in the dorsal corticospinal tract in the KD + KS group.
Several limitations of this study should be noted. First, we only demonstrated the protective effects of the combined strategy, and did not include a comparison with KD or KS alone. Second, the molecular mechanisms underlying the protective effect observed were not investigated. Third, new exogenous ketone supplementation, such as ketone ester, could be taken into account in the current research, as KS has limited effects on blood ketone levels.
Taken together, our results demonstrate that a combination of KD and KS supplement initiated soon after SCI provides neuroprotection and enhances behavioral recovery. This work adds to research demonstrating the efficacy of inducing ketosis to promote neuroprotection.
Additional files:
Additional Figure 1 (339KB, pdf) : Experiment design (upper) and time line (lower).
Experiment design (upper) and time line (lower).
G: gavage.
Additional Figure 2 (138KB, pdf) : Schema of how NeuN+ cells index (A) and axon index (B) analysis was performed.
Schema of how NeuN+ cells index (A) and axon index (B) analysis was performed.
The frames are the region of interests to show where the boxes were placed. The axon index and the myelinated axon index shared the same analysis box. Scale bars: 500 µm. dCST: dorsal corticospinal tract; GFAP: glial fibrillary acidic protein.
Additional file 1: Open peer review report 1 (88.8KB, pdf) .
Acknowledgments
The authors thank Kaneeka Khosla and Tingting Ma (both are students from University of British Colombia, Canada) for help with the data analysis.
Footnotes
Conflicts of interest: The authors declare that there is no conflict of interests.
Financial support: This study was supported by the Craig Neilsen Foundation (to WT), and the Canadian Institutes for Health Research (to WT). BTT was supported by the China Scholarship Council (No. 201508500102) and a visiting trainee stipend from the Blusson Integrated Cure Partnership. WT holds the John and Penny Ryan British Columbia Leadership Chair in Spinal Cord Research funded in part by the Rick Hansen Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Institutional review board statement: The study was approved by the Animal Care Committee of the University of British Columbia (protocol No. A14-350) on August 31, 2015.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement: Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewers: Syoichi Tashiro, Keio University School of Medicine, Japan; Norihiro Nishida, Yamaguchi University, Japan.
Funding: This study was supported by the Craig Neilsen Foundation (to WT), and the Canadian Institutes for Health Research (to WT). BTT was supported by the China Scholarship Council (No. 201508500102) and a visiting trainee stipend from the Blusson Integrated Cure Partnership. WT holds the John and Penny Ryan British Columbia Leadership Chair in Spinal Cord Research funded in part by the Rick Hansen Foundation.
Chinese Library Classification No. R459.9; R741; R1155.1
P-Reviewer: Tashiro S, Nishida N; C-Editor: Zhao M; S-Editors: Yu J, Li CH; L-Editors: Cason N, Yu J, Song LP; T-Editor: Jia Y
References
- 1.Achanta LB, Rae CD. ß-Hydroxybutyrate in the brain: one molecule, Multiple Mechanisms. Neurochem Res. 2017;42:35–49. doi: 10.1007/s11064-016-2099-2. [DOI] [PubMed] [Google Scholar]
- 2.Ahuja CS, Martin AR, Fehlings M. Recent advances in managing a spinal cord injury secondary to trauma. F1000Res. 2016;5 doi: 10.12688/f1000research.7586.1. F1000 Faculty Rev-1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahuja CS, Wilson JR, Nori S, Kotter MRN, Druschel C, Curt A, Fehlings MG. Traumatic spinal cord injury. Nat Rev Dis Primers. 2017;3:17018. doi: 10.1038/nrdp.2017.18. [DOI] [PubMed] [Google Scholar]
- 4.Ari C, Koutnik AP, DeBlasi J, Landon C, Rogers CQ, Vallas J, Bharwani S, Puchowicz M, Bederman I, Diamond DM, Kindy MS, Dean JB, D Agostino DP. Delaying latency to hyperbaric oxygen-induced CNS oxygen toxicity seizures by combinations of exogenous ketone supplements. Physiol Rep. 2019;7:e13961. doi: 10.14814/phy2.13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen K, Liu J, Assinck P, Bhatnagar T, Streijger F, Zhu Q, Dvorak MF, Kwon BK, Tetzlaff W, Oxland TR. Differential histopathological and behavioral outcomes eight weeks after rat spinal cord injury by contusion, dislocation and distraction mechanisms. J Neurotrauma. 2016;33:1667–1684. doi: 10.1089/neu.2015.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarke K, Tchabanenko K, Pawlosky R, Carter E, Todd King M, Musa-Veloso K, Ho M, Roberts A, Robertson J, Vanitallie TB, Veech RL. Kinetics, safety and tolerability of (R)-3-hydroxybutyl (R)-3-hydroxybutyrate in healthy adult subjects. Regul Toxicol Pharmacol. 2012;63:401–408. doi: 10.1016/j.yrtph.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cox PJ, Kirk T, Ashmore T, Willerton K, Evans R, Smith A, Murray AJ, Stubbs B, West J, McLure SW, King MT, Dodd MS, Holloway C, Neubauer S, Drawer S, Veech RL, Griffin JL, Clarke K. Nutritional ketosis alters fuel preference and thereby endurance performance in athletes. Cell Metab. 2016;24:256–268. doi: 10.1016/j.cmet.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 8.Davis LM, Pauly JR, Readnower RD, Rho JM, Sullivan PG. Fasting is neuroprotective following traumatic brain injury. J Neurosci Res. 2008;86:1812–1822. doi: 10.1002/jnr.21628. [DOI] [PubMed] [Google Scholar]
- 9.Gano LB, Patel M, Rho JM. Ketogenic diets, mitochondria and neurological diseases. J Lipid Res. 2014;55:2211–2228. doi: 10.1194/jlr.R048975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gomez-Pinilla F, Gomez AG. The influence of dietary factors in central nervous system plasticity and injury recovery. PM R. 2011;3:S111–S116. doi: 10.1016/j.pmrj.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayta E, Elden H. Acute spinal cord injury: A review of pathophysiology and potential of non-steroidal anti-inflammatory drugs for pharmacological intervention. J Chem Neuroanat. 2018;87:25–31. doi: 10.1016/j.jchemneu.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 12.Hilton BJ, Moulson AJ, Tetzlaff W. Neuroprotection and secondary damage following spinal cord injury: concepts and methods. Neurosci Lett. 2017;652:3–10. doi: 10.1016/j.neulet.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Hilton BJ, Assinck P, Duncan GJ, Lu D, Lo S, Tetzlaff W. Dorsolateral funiculus lesioning of the mouse cervical spinal cord at C4 but not at C6 results in sustained forelimb motor deficits. J Neurotrauma. 2013;30:1070–1083. doi: 10.1089/neu.2012.2734. [DOI] [PubMed] [Google Scholar]
- 14.Hilton BJ, Anenberg E, Harrison TC, Boyd JD, Murphy TH, Tetzlaff W. Re-establishment of cortical motor output maps and spontaneous functional recovery via spared dorsolaterally projecting corticospinal neurons after dorsal column spinal cord injury in adult mice. J Neurosci. 2016;36:4080–4092. doi: 10.1523/JNEUROSCI.3386-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hug A, Weidner N. From bench to beside to cure spinal cord injury: lost in translation. Int Rev Neurobiol. 2012;106:173–196. doi: 10.1016/B978-0-12-407178-0.00008-9. [DOI] [PubMed] [Google Scholar]
- 16.Jeong MA, Plunet W, Streijger F, Lee JHT, Plemel JR, Park S, Lam CK, Liu J, Tetzlaff W. Intermittent fasting improves functional recovery after rat thoracic contusion spinal cord injury. J Neurotrauma. 2011;28:479–492. doi: 10.1089/neu.2010.1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kesl SL, Poff AM, Ward NP, Fiorelli TN, Ari C, Van Putten AJ, Sherwood JW, Arnold P, D’Agostino DP. Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride and lipoprotein levels in Sprague-Dawley rats. Nutr Metab (Lond) 2016;13:9–9. doi: 10.1186/s12986-016-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koppel SJ, Swerdlow RH. Neuroketotherapeutics: A modern review of a century-old therapy. Neurochem Int. 2018;117:114–125. doi: 10.1016/j.neuint.2017.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee JHT, Streijger F, Tigchelaar S, Maloon M, Liu J, Tetzlaff W, Kwon BK. A contusive model of unilateral cervical spinal cord injury using the infinite horizon impactor. J Vis Exp. 2012:3313. doi: 10.3791/3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Levy RG, Cooper PN, Giri P. Ketogenic diet and other dietary treatments for epilepsy. Cochrane Database Syst Rev. 2012:CD001903. doi: 10.1002/14651858.CD001903.pub2. [DOI] [PubMed] [Google Scholar]
- 22.Lu Y, Yang YY, Zhou MW, Liu N, Xing HY, Liu XX, Li F. Ketogenic diet attenuates oxidative stress and inflammation after spinal cord injury by activating Nrf2 and suppressing the NF-κB signaling pathways. Neurosci Lett. 2018;683:13–18. doi: 10.1016/j.neulet.2018.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Lutas A, Yellen G. The ketogenic diet: metabolic influences on brain excitability and epilepsy. Trends Neurosci. 2013;36:32–40. doi: 10.1016/j.tins.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening K(ATP) channels. J Neurosci. 2007;27:3618–3625. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maalouf M, Rho JM, Mattson MP. The neuroprotective properties of calorie restriction, the ketogenic diet and ketone bodies. Brain Res Rev. 2009;59:293–315. doi: 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maalouf M, Sullivan PG, Davis L, Kim DY, Rho JM. Ketones inhibit mitochondrial production of reactive oxygen species production following glutamate excitotoxicity by increasing NADH oxidation. Neuroscience. 2007;145:256–264. doi: 10.1016/j.neuroscience.2006.11.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magistretti PJ, Allaman I. A cellular perspective on brain energy metabolism and functional imaging. Neuron. 2015;86:883–901. doi: 10.1016/j.neuron.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 28.McDougall A, Bayley M, Munce SE. The ketogenic diet as a treatment for traumatic brain injury: a scoping review. Brain Inj. 2018;32:416–422. doi: 10.1080/02699052.2018.1429025. [DOI] [PubMed] [Google Scholar]
- 29.Montoya CP, Campbell-Hope LJ, Pemberton KD, Dunnett SB. The “staircase test”: a measure of independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991;36:219–228. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- 30.Murray AJ, Knight NS, Cole MA, Cochlin LE, Carter E, Tchabanenko K, Pichulik T, Gulston MK, Atherton HJ, Schroeder MA, Deacon RMJ, Kashiwaya Y, King MT, Pawlosky R, Rawlins JNP, Tyler DJ, Griffin JL, Robertson J, Veech RL, Clarke K. Novel ketone diet enhances physical and cognitive performance. FASEB J. 2016;30:4021–4032. doi: 10.1096/fj.201600773R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Newport MT, VanItallie TB, Kashiwaya Y, King MT, Veech RL. A new way to produce hyperketonemia: use of ketone ester in a case of Alzheimer’s disease. Alzheimers Dement. 2015;11:99–103. doi: 10.1016/j.jalz.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nikkhah G, Rosenthal C, Hedrich HJ, Samii M. Differences in acquisition and full performance in skilled forelimb use as measured by the ‘staircase test’ in five rat strains. Behav Brain Res. 1998;92:85–95. doi: 10.1016/s0166-4328(97)00128-9. [DOI] [PubMed] [Google Scholar]
- 33.Offermanns S, Schwaninger M. Nutritional or pharmacological activation of HCA(2) ameliorates neuroinflammation. Trends Mol Med. 2015;21:245–255. doi: 10.1016/j.molmed.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp. 2011;71:281–299. doi: 10.55782/ane-2011-1848. [DOI] [PubMed] [Google Scholar]
- 35.Pilla R. Clinical applications of ketogenic diet-induced ketosis in neurodegenerative and metabolism-related pathologies. Cancer. 2018;15:16. [Google Scholar]
- 36.Plunet WT, Lam CK, Lee JHT, Liu J, Tetzlaff W. Prophylactic dietary restriction may promote functional recovery and increase lifespan after spinal cord injury. Ann N Y Acad Sci. 2010;1198(Suppl 1):E1–E11. doi: 10.1111/j.1749-6632.2010.05564.x. [DOI] [PubMed] [Google Scholar]
- 37.Plunet WT, Streijger F, Lam CK, Lee JHT, Liu J, Tetzlaff W. Dietary restriction started after spinal cord injury improves functional recovery. Exp Neurol. 2008;213:28–35. doi: 10.1016/j.expneurol.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 38.Puchowicz MA, Zechel JL, Valerio J, Emancipator DS, Xu K, Pundik S, LaManna JC, Lust WD. Neuroprotection in diet-induced ketotic rat brain after focal ischemia. J Cereb Blood Flow Metab. 2008;28:1907–1916. doi: 10.1038/jcbfm.2008.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rahman M, Muhammad S, Khan MA, Chen H, Ridder DA, Müller-Fielitz H, Pokorná B, Vollbrandt T, Stölting I, Nadrowitz R, Okun JG, Offermanns S, Schwaninger M. The β-hydroxybutyrate receptor HCA2 activates a neuroprotective subset of macrophages. Nat Commun. 2014;5:3944. doi: 10.1038/ncomms4944. [DOI] [PubMed] [Google Scholar]
- 40.Sato K, Kashiwaya Y, Keon CA, Tsuchiya N, King MT, Radda GK, Chance B, Clarke K, Veech RL. Insulin, ketone bodies and mitochondrial energy transduction. FASEB J. 1995;9:651–658. doi: 10.1096/fasebj.9.8.7768357. [DOI] [PubMed] [Google Scholar]
- 41.Schallert T, Fleming SM, Leasure JL, Tillerson JL, Bland ST. CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical, ablation, parkinsonism and spinal cord injury. Neuropharmacology. 2000;39:777–787. doi: 10.1016/s0028-3908(00)00005-8. [DOI] [PubMed] [Google Scholar]
- 42.Shaafi S, Najmi S, Aliasgharpour H, Mahmoudi J, Sadigh-Etemad S, Farhoudi M, Baniasadi N. The efficacy of the ketogenic diet on motor functions in Parkinson’s disease: A rat model. Iran J Neurol. 2016;15:63–69. [PMC free article] [PubMed] [Google Scholar]
- 43.Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, Grueter CA, Lim H, Saunders LR, Stevens RD, Newgard CB, Farese RV, Jr, de Cabo R, Ulrich S, Akassoglou K, Verdin E. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Science. 2013;339:211–214. doi: 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Şimşek T, Şimşek HU, Cantürk NZ. Response to trauma and metabolic changes: posttraumatic metabolism. Ulus Cerrahi Derg. 2014;30:153–159. doi: 10.5152/UCD.2014.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014;6:309–331. doi: 10.2147/CLEP.S68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Streijger F, Plunet WT, Lee JHT, Liu J, Lam CK, Park S, Hilton BJ, Fransen BL, Matheson KAJ, Assinck P, Kwon BK, Tetzlaff W. Ketogenic diet improves forelimb motor function after spinal cord injury in rodents. PLoS One. 2013;8:e78765. doi: 10.1371/journal.pone.0078765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, Magor-Elliott S, Hiyama S, Stirling M, Clarke K. On the metabolism of exogenous ketones in humans. Front Physiol. 2017;8:848. doi: 10.3389/fphys.2017.00848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walsh JG, Muruve DA, Power C. Inflammasomes in the CNS. Nat Rev Neurosci. 2014;15:84–97. doi: 10.1038/nrn3638. [DOI] [PubMed] [Google Scholar]
- 49.Wang BH, Hou Q, Lu YQ, Jia MM, Qiu T, Wang XH, Zhang ZX, Jiang Y. Ketogenic diet attenuates neuronal injury via autophagy and mitochondrial pathways in pentylenetetrazol-kindled seizures. Brain Res. 2018;1678:106–115. doi: 10.1016/j.brainres.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 50.Wang X, Liu Y, Li X, Zhang Z, Yang H, Zhang Y, Williams PR, Alwahab NSA, Kapur K, Yu B, Zhang Y, Chen M, Ding H, Gerfen CR, Wang KH, He Z. Deconstruction of corticospinal circuits for goal-directed motor skills. Cell. 2017;171:440–455e14. doi: 10.1016/j.cell.2017.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Winesett SP, Bessone SK, Kossoff EHW. The ketogenic diet in pharmacoresistant childhood epilepsy. Expert Rev Neurother. 2015;15:621–628. doi: 10.1586/14737175.2015.1044982. [DOI] [PubMed] [Google Scholar]
- 52.Yarar-Fisher C, Kulkarni A, Li J, Farley P, Renfro C, Aslam H, Bosarge P, Wilson L, Barnes S. Evaluation of a ketogenic diet for improvement of neurological recovery in individuals with acute spinal cord injury: a pilot, randomized safety and feasibility trial. Spinal Cord Ser Cases. 2018;4:88. doi: 10.1038/s41394-018-0121-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, D’Agostino D, Planavsky N, Lupfer C, Kanneganti TD, Kang S, Horvath TL, Fahmy TM, Crawford PA, Biragyn A, Alnemri E, Dixit VD. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21:263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, Humala N, Thiyagarajan M, Wang J, Pasinetti GM. A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis. BMC Neurosci. 2006;7:29. doi: 10.1186/1471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experiment design (upper) and time line (lower).
G: gavage.
Schema of how NeuN+ cells index (A) and axon index (B) analysis was performed.
The frames are the region of interests to show where the boxes were placed. The axon index and the myelinated axon index shared the same analysis box. Scale bars: 500 µm. dCST: dorsal corticospinal tract; GFAP: glial fibrillary acidic protein.