Central nervous system (CNS) injuries and neurodegenerative diseases show a broad spectrum of common pathophysiological processes, including oxidative stress, neuroinflammation, excitotoxicity, demyelination and neurotransmission dysfunctions. Over the past decades, valuable experimental investigations have helped to clarify the role and timing of these multiple molecular and cellular mechanisms in each of these particular disorders, which usually overlap and critically contribute to long-term disability. However, up to now, no definite cures or effective disease-modifying therapies are available for any of these conditions. This has led to an active search of novel therapeutic approaches, including the repositioning of existing drugs for new indications, as a valid approach to promptly move candidate molecules to clinical trials.
Progesterone, a steroid with a crucial role in the reproductive function in mammals, stands as one of these promising repositioning molecules to modulate the complex array of cellular and molecular events observed in several of these central nervous system diseases (Stein and Sayeed, 2019). Indeed, a great number of preclinical studies have provided solid basis for supporting a protective effect of progesterone in stroke, traumatic brain injury, spinal cord trauma, central and peripheral neuropathies, multiple sclerosis, and Alzheimer’s and Parkinson’s disease (González et al., 2019, 2020).
Notwithstanding this remarkable number of studies exploring the beneficial effects of progesterone in CNS disorders, few of them offer a deeper look at the different receptors and complex signaling cascades involved. This perspective aims at expanding our view on the variety of receptors and signaling pathways that might be involved in progesterone-mediated actions in the nervous system as part of a rational strategy to promote a successful translation of steroid-based therapies for the treatment of neurological diseases.
Unveiling the variety of receptors engaged by progesterone: Progesterone, acting as a neurosteroid/neuroactive steroid, exhibits a significant amount of neuroprotective, anti-inflammatory and pro-myelinating actions in experimental models neurodegeneration and/or nervous system injuries (González et al., 2019, 2020). The central nervous system presents a wide diversity of progesterone receptors in both neurons and glial cells, including the “classical” nuclear receptor (PR). Acting as a ligand-activated transcription factor, PR binds to steroid-response elements within the promoter region of target genes regulating their expression. PR-A and PR-B, the two major PR isoforms, are transcribed from two distinct promoter regions of a single gene and exhibit distinct transcriptional activity, PR-B being a stronger transcriptional activator than PR-A. Further, extranuclear PR activation may trigger Src/Ras/MAPK and or PI3K/Akt pathways, eventually modulating the transcription of genes even in the absence of progesterone-response elements.
By using PR knockout mice (PRKO)-lacking both PR-A and PR-B isoforms- our laboratory and others have shed light on the crucial role of this “classical” receptor in mediating the beneficial actions of progesterone in the nervous system. In fact, PR seems to be the main mediator of the remyelinating effects of progesterone, since the enhancement in the expression of the myelin basic protein induced by the steroid in organotypic cerebellar slices is lost in PRKO mice (Ghoumari et al., 2003). In addition, the lack of PR also impeded the increase in the density of oligodendrocyte precursor cells and the prevention of the apoptotic death of these cells in the injured spinal cord of these mice after progesterone administration, in clear contrast to the observed actions of the steroid in wild-type littermates (Labombarda et al., 2015).
Progesterone also requires a functional PR receptor for displaying several of its neuroprotective actions (Gonzalez et al., 2020). Indeed, progesterone was unable to prevent the loss of motoneuron and cell death in organotypic slices of the spinal cord from PRKO mice subjected to contusion injury. More recently, the expression of PR has been shown to be necessary for reducing brain damage and motor impairment after stroke (Zhu et al., 2019) and for the induction of brain-derived neurotrophic factor, a potential mediator of the neuroprotective effects of progesterone (Jodhka et al., 2009).
Further, the role of PR in neuroinflammatory conditions was also proven in PRKO mice subjected to spinal cord injury, in which progesterone administration did not decrease reactive gliosis and failed to reduce the injury-induced expression of pro-inflammatory cytokines (Labombarda et al., 2015). Although PR has been detected in culture glial cells, including oligodendrocytes and astrocytes, its expression has not been found in surveillance microglial cells. Certainly, much remains to be explored regarding the role of PR in the modulation of glial responses, in particular during in vivo neuroinflammatory challenges and demyelinating disorders.
Less information is available on the role of PR isoforms expression after nervous system injury or during disease. Since both isoforms show distinct transcriptional properties, any disruption of their balanced expression may impact on the regulation of particular and/or common arrays of target genes. Interestingly, the unequal expression of these two isoforms between the brain and retina after traumatic brain injury might account for the differential anti-inflammatory actions of progesterone within these tissues (Allen et al., 2016). To add complexity, the regulation of the expression of PR A/B isoforms may not only depend on DNA methylation and the chromatin basal state of their respective promoters but also on the participation small non-coding RNAs termed microRNAs (miRNAs) that mediate gene silencing at the post-transcriptional level.
Another exciting perspective has been opened after the identification of a set of novel proteins, including synaptic proteins and regulators of metabolism, that form complexes with PR in a ligand- and isoform-specific manner. Through the use of combined mass spectrometry and reverse phase protein arrays, Acharya et al. (2017) provided clear evidences that several female mouse hypothalamic proteins form complexes with PR-A, PR-B, or both in a ligand-dependent manner, including synapsin-I and synapsin-II, suggesting that both isoforms function in synaptic plasticity (Acharya et al., 2017). In support of this notion, progesterone increased synaptic density in primary cortical neurons, suggesting a function for progesterone in synapse formation in cortical neurons. Further, differential interactions of signaling molecules with PR may be important for isoform-specific rapid effects of PR in brain. Indeed, they also found that MAPK1 kinase associated with PR-B, but not PR-A, and detected hormone-dependent interactions between Src kinase with PR-A, but not PR-B. Moreover, both PR isoforms were associated with a key regulator of energy homeostasis, FoxO1, pointing to a novel role for PR in energy metabolism. These relevant data open up new challenges to unravel in the context of CNS disorders.
Although most studies have mainly focused their attention on the genomic actions of the well-characterized “classical” PR (Gonzalez et al., 2020), additional receptors and different intracellular targets may explain the multiple actions of progesterone. Indeed, membrane progesterone receptors (mPRs) and membrane progesterone binding protein (PGRMC1) have been also described in the CNS. Without being related to nuclear steroid receptors, mPRs and PGRMC1 and 2 can set up fast cell-surface actions in the nervous system, appearing as potential players to be considered when designing steroid-based therapies.
The membrane-bound mPRs are associated with G protein that, depending on the receptor subtype involved, activate intracellular pathways that may lead to increase or decrease AMPc levels. The expression of mPR receptor subtypes in specific cellular and CNS locations validate their role in mediating the protective actions of progesterone during inflammation and altered ion and water homeostasis in the injured CNS. However, the cellular distribution and the concrete function of different mPR isoforms in progesterone signaling in health and disease have not been fully depicted and clearly deserve further research.
Far from being clarify, PGRMC1 intracellular signaling pathways and functions remain intriguing. PGMR1 has been described as an adaptor protein for mPRs (Thomas et al., 2014) facilitating mPR signaling and as alternative partner to mediate progesterone actions on mitochondria (Gonzalez et al., 2020). More recently, Wu et al. (2019) also found that progesterone significantly improves glucose metabolism both in cellular and transgenic models of Alzheimer’s disease, through a mechanism involving the activation of PGRMC1, offering a new target to explore during the administration of this steroid in pathological conditions.
Notably, progesterone and several of its related metabolites are modulators of certain neurotransmitter systems and channels, such as γ-aminobutyric acid (GABA) and N-methyl-D-aspartate receptors, transient receptor potential (TRP) channels and sigma-1 receptor (Sigma-1R), adding intricacy to steroids actions in the nervous system. In line with this notion, a recent report (Morales-Lazaro et al., 2019), provided valuable insights regarding the molecular interplay between the Sigma-1R, steroids, and ion channels, shedding light on novel aspects of progesterone in pain modulation. The Sigma-1R is a protein mainly localized to the endoplasmic reticulum where it functions as a chaperone and binds progesterone, a major endogenous antagonist of this receptor. Interestingly, Sigma-1R and TRP vanilloid channels (TRPV1), with pivotal roles in painful signal transduction, are associated in the endoplasmic reticulum, improving TRPV1 stability and resulting in suitable TRPV1 expression in the plasma membrane, where the channel transmits noxious signals. To be noted, they found that antagonism of Sigma-1R by progesterone results in the down-regulation of TRPV1 expression in the plasma membrane of sensory neurons and, consequently, a decrease in nociceptive responses to harmful stimulation. This observations were achieved both in male rats treated with a synthetic antagonist of Sigma-1R and in pregnant rats, which display high levels of circulating progesterone and exhibited attenuated pain-induced behavior through the disruption of the Sigma-1R/TRPV1 complex. Therefore, elevated levels of progesterone, such as those found during pregnancy, could confer protection against painful situations and open new roads to explore possible progesterone interactions with Sigma-1R in pathological pain conditions.
Progesterone may also exert important effects involving its conversion to allopregnanolone that does not bind PR and acts through non-classical pathways. For instance, the anesthetic and antiseizure activity of progesterone mainly occurs through its conversion to the neurosteroid allopregnanolone, a potent endogenous positive modulator of the GABAA receptor complex, the principal mediator of the fast inhibitory transmission within the CNS. However, allopregnanolone binding is not limited to GABAA receptors but also can bind to mPRs and to pregnane X receptor that might mediate several of the neuroprotective effects of this steroid. In line with these beneficial roles of allopregnanolone, Mensah-Nyagan et al. developed a series of novel synthetic analogs of allopregnanolone, as part of a crucial process to enhance the efficacy of steroid-based therapies (for a review, Gonzalez et al., 2019). In vitro testing has revealed that these compounds, acting as mitochondrial neuromodulators and neuroprotective drugs, show notable advantages over the original molecule to counteract mitochondrial bioenergetic deficits and improve neuronal cell survival under oxidative stress, widening the options to be translated from the laboratory to the bedside.
Conclusions and future directions: Based upon an active research over the past decades, it is now clear that targeting a unique mechanism or signaling pathway to overcome the complex network of processes that take place during CNS trauma or neurodegenerative disease represents an inadequate approach. This has led to the search of novel strategies such as repositioning of drugs with several mechanisms of action, as is the case of progesterone. It is now clear that an array of receptors, including neurotransmitter receptors, may be acting in an integrated and complex fashion to promote the neuroprotective progesterone-dependent actions in the nervous system (Figure 1).
Figure 1.
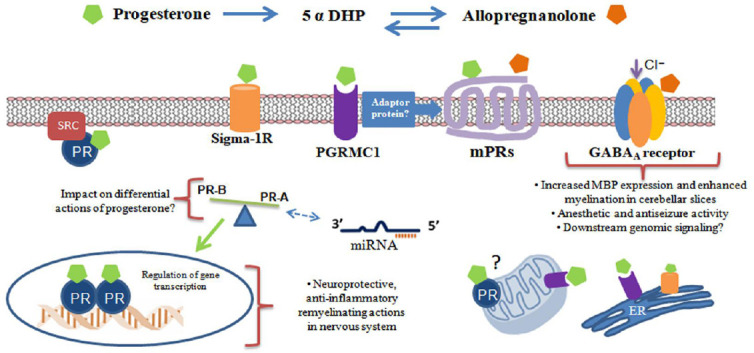
Multiple receptors and signaling partners are involved in progesterone and allopregnanolone actions in central nervous system.
Progesterone can bind to an array of progesterone receptors including the classic nuclear receptor (PR), progesterone membrane receptors (mPR) and the membrane-associated protein progesterone receptor-membrane component 1 (PGRMC1). A balance between PR-A and PR-B isoforms may influence progesterone effects. In addition, small-non coding RNAs (miRNAs) control the level of PR expression and in turn operate as mediators of progesterone signaling. The functions of mitochondrial PR and PGRMC1 remain to be explored. Progesterone also acts as a competitive antagonist of Sigma 1R associated to the endoplasmic reticulum. Finally, the conversion to allopregnanolone, that does not bind to PR but may act as a potent positive allosteric modulator of γ-aminobutyric acid (GABA)A receptor, extends the variety of progesterone-associated mechanisms. DHP: Dihexadecyl phosphate; ER: endoplasmic reticulum.
However, and despite the wealth of experimental evidence supporting the beneficial effects of progesterone, this steroid, like many drugs that were successful in pre-clinical trials, did not work as expected in clinical trials. Certainly, many lessons have been learnt from clinical trials that forced to revise many aspects of their design. Moreover, refining our knowledge on progesterone’s mechanisms of action for the different pathological should be mandatory to provide solid support for translational success.
Indeed, novel aspects of progesterone signaling are emerging that introduce unexpected factors to take into account when designing progesterone-based therapies. This complex scenario includes the interaction of PR with synaptic proteins, the bioconversion of progesterone to reduced metabolites, and the differential expression of mPRs, PGRMC1 and PR isoforms in each pathological condition. All these challenges release the way to novel research in the field and open future perspectives on how to fill the gap between human and animal studies to develop effective steroid-based therapies for the treatment of CNS trauma, neuropathic pain and neurodegenerative diseases.
I apologize to all authors whose work could not be cited here due to paper length limitation. I would like to thank Dr. H. Coirini for his help with the final version of the figure included in this perspective.
This work was partly supported by grants from Consejo Nacional de Investigaciones Científicas y Tecnológicas (CONICET, PIP 112-201501-00266), Agencia Nacional de Promoción Científica y Técnica (PICT 2018-02152), Fundación René Barón and Fundación Williams.
Footnotes
Copyright license agreement: The Copyright License Agreement has been signed by the author before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
Open peer reviewer: María Miranda, Universidad Cardenal Herrera-CEU, Spain.
P-Reviewer: Miranda M; C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Acharya KD, Nettles SA, Sellers KJ, Im DD, Harling M, Pattanayak C, Vardar-Ulu D, Lichti CF, Huang S, Edwards DP, Srivastava DP, Denner L, Tetel MJ. The progestin receptor interactome in the female mouse hypothalamus: interactions with synaptic proteins are isoform specific and ligand dependent. eNeuro. 2017 doi: 10.1523/ENEURO.0272-17.2017. doi: 101523/ENEURO0272-172017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen RS, Sayeed I, Oumarbaeva Y, Morrison KC, Choi PH, Pardue MT, Stein DG. Progesterone treatment shows greater protection in brain vs. retina in a rat model of middle cerebral artery occlusion: Progesterone receptor levels may play an important role. Restor Neurol Neurosci. 2016;34:947–963. doi: 10.3233/RNN-160672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghoumari AM, Ibanez C, El-Etr M, Leclerc P, Eychenne B, O’Malley BW, Baulieu EE, Schumacher M. Progesterone and its metabolites increase myelin basic protein expression in organotypic slice cultures of rat cerebellum. J Neurochem. 2003;86:848–859. doi: 10.1046/j.1471-4159.2003.01881.x. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez SL, Coronel MF, Raggio MC, Labombarda F. Progesterone receptor-mediated actions and the treatment of central nervous system disorders: An up-date of the known and the challenge of the unknown. Steroids. 2020;153:108525. doi: 10.1016/j.steroids.2019.108525. [DOI] [PubMed] [Google Scholar]
- 5.González SL, Meyer L, Raggio MC, Taleb O, Coronel MF, Patte-Mensah C, Mensah- Nyagan AG. Allopregnanolone and progesterone in experimental neuropathic pain: former and new insights with a translational perspective. Cell Mol Neurobiol. 2019;39:523–537. doi: 10.1007/s10571-018-0618-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jodhka PK, Kaur P, Underwood W, Lydon JP, Singh M. The differences in neuroprotective efficacy of progesterone and medroxyprogesterone acetate correlate with their effects on brain-derived neurotrophic factor expression. Endocrinology. 2009;150:3162–3168. doi: 10.1210/en.2008-1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Labombarda F, Jure I, Gonzalez S, Lima A, Roig P, Guennoun R, Schumacher M, De Nicola AF. A functional progesterone receptor is required for immunomodulation, reduction of reactive gliosis and survival of oligodendrocyte precursors in the injured spinal cord. J Steroid Biochem Mol Biol. 2015;154:274–284. doi: 10.1016/j.jsbmb.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Morales-Lazaro SL, Gonzalez-Ramirez R, Rosenbaum T. Molecular interplay between the Sigma-1 receptor, steroids and ion channels. Front Pharmacol. 2019;10:419. doi: 10.3389/fphar.2019.00419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein DG, Sayeed I. Repurposing and repositioning neurosteroids in the treatment of traumatic brain injury: A report from the trenches. Neuropharmacology. 2019;147:66–73. doi: 10.1016/j.neuropharm.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 10.Thomas P, Pang Y, Dong J. Enhancement of cell surface expression and receptor functions of membrane progestin receptor α (mPRα) by progesterone receptor membrane component 1 (PGRMC1): evidence for a role of PGRMC1 as an adaptor protein for steroid receptors. Endocrinology. 2014;155:1107–1119. doi: 10.1210/en.2013-1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H, Wu ZG, Shi WJ, Gao H, Wu HH, Bian F, Jia PP, Hou YN. Effects of progesterone on glucose uptake in neurons of Alzheimer’s disease animals and cell models. Life Sci. 2019;238:116979. doi: 10.1016/j.lfs.2019.116979. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Frechou M, Schumacher M, Guennoun R. Cerebroprotection by progesterone following ischemic stroke: Multiple effects and role of the neural progesterone receptors. J Steroid Biochem Mol Biol. 2019;185:90–102. doi: 10.1016/j.jsbmb.2018.07.014. [DOI] [PubMed] [Google Scholar]


