Abstract
The over-activated microglial cells induce neuroinflammation which has the main role in neurological disorders. The over-activated microglia can disturb neuronal function by releasing inflammatory mediators leading to neuronal dysfunctions and death. Thus, inhibition of over-activated microglia may be an effective therapeutic approach for modulating neuroinflammation. Experimental studies have indicated anti-neuroinflammatory effects of flavonoids such as green tea catechins. The current research was aimed to review the effect of green tea catechins in inhibiting microglial cells, inflammatory cascades, and subsequent neurological diseases.
Keywords: catechins, green tea, microglia, neuroinflammation, neurological disease
Introduction
One of the common beverages with numerous health effects is green tea which mainly used in Asia, North Africa, Europe, and the United States. Green tea has antioxidant, anti-inflammatory anticarcinogenic, and antimicrobial properties (Chatterjee et al., 2012; Crew et al., 2015; Reygaert, 2018). This plant has several pharmacological properties against neurodegenerative diseases, cardiovascular diseases, obesity, and diabetes (Bhardwaj and Khanna, 2013; Huang et al., 2014; Fu et al., 2017; Mancini et al., 2017). Green tea contains several polyphenols including flavonoids. The most common polyphenols of green tea are the flavonoids, and the most significant portion (80–90%) of flavonoids is catechins. The four main catechins in green tea include (–)-epigallocatechin-3-gallate (EGCG), (–)-epigallocatechin (EGC), (–)-epicatechin-3-gallate (ECG), (–)-epicatechin (EC), with an abundance of 60%, 20%, 14%, and 6%, respectively (Reygaert, 2018). Catechins exert antioxidant activity by scavenging free radicals, inducing endogenous antioxidant enzymes activation as well as binding to divalent metals (Higdon and Frei, 2003; Mandel et al., 2005).
Numerous evidence has indicated that catechins have protective effects against neuroinflammation. Catechins can influence the protein kinase B (Akt), mitogen-activated protein kinase (MAPK), AMP-activated protein kinase (AMPK), canonical Wnt pathway (WNT/β-catenin), nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), phosphatidylinositol 3 kinase (PI3K)/Akt, signal transducer and activator of transcription 3 (STAT3), and active protein 1 signaling (Leong et al., 2009; Liu et al., 2012; Oh et al., 2014; Syed Hussein et al., 2015).
Microglia cells in the central nervous system (CNS) play a main role in the immune system and modulating homeostasis of neurons (Galloway et al., 2019). An activated form of microglia protects the CNS from neuronal damage through induction inflammation. Microglia cells induce neuroinflammatory cascades by releasing reactive oxygen species (ROS), reactive nitrogen species (RNS), cytokines (Harry and Kraft, 2008; Sochocka et al., 2017). It has been shown that the over-production of inflammatory cytokines leads to apoptotic damage. Several findings have indicated an association between microglial cells and several neurological disorders including Alzheimer’s disease (AD) and Parkinson’s disease (PD) (Samarghandian et al., 2016b; Bachiller et al., 2018).
Several clinical trial studies have been designed to evaluate drugs against neuroinflammation (Cummings et al., 2019; Dong et al., 2019; Kim et al., 2019). In recent years, strong evidence has shown that catechins are potential therapeutic agents for several diseases such as cardiovascular disease, cancer, infectious diseases, arthritis, atherosclerosis, ischemic stroke (Miura et al., 2001; Suzuki et al., 2004; Babu and Liu, 2008; Yang and Wang, 2016; Reygaert, 2018), and neurological disorders induced by microglia. Catechins across the blood-brain barrier and suppresses microglia activation by decreasing the expression of inducible nitric oxide synthase (iNOS). The inhibition effects of catechins on NO generation and the related molecular signaling pathways as well as its effects in decreasing oxidative stress cause an anti-neuroinflammatory impact on microglia (Sochocka et al., 2017; Bachiller et al., 2018). Additionally, catechins have a neuroprotective impact on neuronal and microglial cells through suppressing the PI3k/Ak, iNOS, cyclooxygenase-2 (COX-2), and heat shock protein 60/heat shock factor-1 expression and stimulating the nuclear factor erythroid 2-related factor 2 (Nrf-2) activation and the antioxidant response element (ARE) (Harry and Kraft, 2008; Sochocka et al., 2017; Bachiller et al., 2018).
Glial cells are mostly found in the CNS. Microglial cells, one type of glial cells, are 10–15% of the total cells in the CNS and distributed in all regions of the adult brain and spinal cord (Dong et al., 2019). Several phenotypes of microglia have been recognized including “classic activation (M1), alternative activation (M2a), alternative type II activation (M2b), and acquired deactivation (M2c)” (Akhmetzyanova et al., 2019). Activated macrophage was classified according to the mannose receptor expression. Enzyme arginase 1, Ym1, a heparin-binding lectin, FIZZ1, CD206, a mannose receptor were recognized as a specific marker for the M2 phenotype (Akhmetzyanova et al., 2019). M1 microglia generates ROS, mediators such as tumor necrosis factor-α (TNF-α), interleukin 1 beta (IL-1β), IL-6, and IL-12, leading to inflammatory damage (Akhmetzyanova et al., 2019). M1 microglia also act as the neurotoxic factor by producing proinflammatory cytokines and inducing the formation of a glial scar (Akhmetzyanova et al., 2019). This phenotype can modulate synaptic pruning for phagocytosis (Akhmetzyanova et al., 2019). The alternative activated form consists of M2a and M2b subgroups. M2a respond to IL-4 and IL-13 leading to an elevation in phagocytic activity, and release of insulin-like growth factor-1, trophic polyamines, and anti-inflammatory cytokines (Akhmetzyanova et al., 2019). M2a can remove cellular debris and induce tissue regeneration. M2b type is stimulated by ligation of immunoglobulin Fc-gamma-receptors leading to an increase in the expression of IL-12, IL-10, and HLA-DR. The formation of deactivation microglia (M2c) is stimulated by the IL-10 or glucocorticoids resulted in an increased expression of transforming growth factor, sphingosine kinase, and CD163, a membrane-bound receptor for haptoglobin/hemoglobin complexes (Akhmetzyanova et al., 2019).
The number of activated microglial cells is low in the normal condition in the CNS. However, these cells rapidly activated in response to a variety of stress signaling molecules (Gerlach et al., 1994; Cummings et al., 2019). Their response covers changes from the integrity of brain structure to modifications in their microenvironment. Microglial cells have receptors for CNS signaling molecules including adenosine triphosphate (Fahn and Cohen, 1992), calcitonin gene-related peptide (Cheng et al., 2015), acetylcholine and noradrenaline (DeMaagd and Philip, 2015). Microglial cells as an innate immunity cell destroy and clear foreign materials via phagocytic and cytotoxic mechanisms. These cells are also antigen-presenting cells and participate in the inflammatory mechanism by releasing mediators and other signaling factors (Riederer et al., 1989).
The majority of secreted molecules by activated microglia have inflammatory activities leading to neurotoxic damage. The most released molecules from microglia are pro-inflammatory mediators including TNF-α, IL-1β as well as free radicals such as superoxide, NO, and fatty acid metabolites (Lee et al., 2000; Sutherland et al., 2005; Kuriyama et al., 2006; Samarghandian et al., 2015). Activated microglia are responsible for harmful events such as neuronal damage and cell death via producing toxic molecules including inflammatory cytokines (Aktas et al., 2004), complement components (Sutherland et al., 2005), chemokines (Koh et al., 2006), and reactive oxygen species (Levites et al., 2001). IL-1, IL-6, TNF-α are mostly released by activated microglia (Haque et al., 2006). Pro-inflammatory cytokines released by activated microglial have the main role in neurological diseases. Neuroinflammation is a complex process can be both harmful and beneficial. Microglial cells may begin neuroinflammation in the brain. Neuroinflammation is amplified by microglia activation due to a prolonged neuroinflammatory response and its destructive effects in CNS.
The activated microglia produce inflammatory mediators and acts as phagocytosis in the acute neuroinflammation condition. Acute neuroinflammation response carries beneficial effects such as reparative function such as engulfing dead cells and debris from the injury. Chronic neuroinflammation is a self-sustaining process after the initial injury characterized by a cycle of maintained microglia activation and persistent release of inflammatory mediators (Rezai-Zadeh et al., 2005; Samarghandian et al., 2016a). Chronic neuroinflammation often has harmful and damaging effects in nervous tissues as well as the protective role (Levites et al., 2002; Rezai-Zadeh et al., 2005). There is numerous evidence for the role of microglia-induced chronic inflammation in the pathogenesis of CNS diseases by producing several neurotoxic molecules (Choi et al., 2001; Levites et al., 2003; Mandel et al., 2003; Singh et al., 2010; Johnson and Johnson, 2015; Unno et al., 2017; Staurengo-Ferrari et al., 2019). The present study was designed to review studies on the anti-inflammatory and inhibitory effects of catechins on microglia cells for the treatment of neurodegenerative diseases. Additionally, the underlying mechanisms of catechins on microglia activation were reviewed.
Search Strategy and Selection Criteria
All available articles about Green tea catechins, microglial activation and neurological disorders were obtained by searching main databases such as Google Scholar, Web of Science, Scopus, MEDLINE and PubMed. The studies were selected by using the following search terms: (epi-gallocatechin-3-gallate OR epigallocatechin OR epicatechin-3-gallate OR epicatechin) AND (microglia) AND (neurological disorders). Available English articles were selected for performing this study.
Green Tea Catechins and Neurological Diseases
Neurodegeneration is a key characteristic of neurological diseases such as AD, PD, and amyotrophic lateral sclerosis. It has been shown that several molecular signaling pathways are responsible for inducing neurodegenerative diseases. Neurodegeneration is characterized by destructing the function of proteins, the ubiquitin-proteasome system, antioxidants system (Riederer et al., 1989; Fahn and Cohen, 1992; Gerlach et al., 1994; Berg et al., 2001). In AD patients most common finding is amyloid or “senile” plaques which comprised of extracellular accumulation of insoluble amyloid-β (Aβ) peptide and intracellular neurofibrillary tangles (Cheng et al., 2015). In PD patients, the characteristic pathology is the loss of substantia nigra (SN) dopaminergic neurons and depigmentation of the SN in midbrain. The histopathological evaluations indicate PD patients have alpha-synuclein, neurofilaments, and ubiquitinated proteins in SN (DeMaagd and Philip, 2015). Numerous evidence has indicated that green tea may be effective against neurological disorders such as AD and PD, which leads to a decrease in the prevalence of cognitive function (Kuriyama et al., 2006). A report of the United States indicated that consuming 2 cups/day or more of tea has been accompanied by a decrease in the risk of PD.
Among the green tea catechins, the effect of EGCG on neurological disorders has been mostly studied. In this context, it has been found that EGCG ameliorated age-related cognitive function and improved cerebral ischemia/reperfusion (Lee et al., 2000; Sutherland et al., 2005). It has been also reported that EGCG attenuated neuroinflammation and neuronal damage in experimental autoimmune encephalomyelitis (Aktas et al., 2004). EGCG ameliorated death signals in the G93A-mutated mice model of amyotrophic lateral sclerosis (Koh et al., 2006). Additionally, it has been found that the administration of green tea extract and EGCG have protective effects in dopaminergic neurons loss in mice (Levites et al., 2001); cognition function in rats (Haque et al., 2006); and also reduced amyloidosis in the cerebellum of transgenic mice model of AD (Rezai-Zadeh et al., 2005). The neuroprotective effects of EGCG have been also indicated by in vitro studies. In this context, the preventive effect of EGCG against neuronal cell death induced by the neurotoxins 6-hydroxydopamine and 1-methyl-4-phenylpyridinium in human neuroblastoma SH-SY5Y cells have been reported (Levites et al., 2002). Additionally, it was found that EGCG protected primary hippocampal neurons (Choi et al., 2001) and rat PC12 cells (Levites et al., 2003; Mandel et al., 2003) against Aβ-induced toxicity.
Molecular targets of catechins in decreasing microglial activation and related neuroinflammation
Catechin can across the blood-brain barrier and directly influence on the activation of microglia (Unno et al., 2017). Strong evidence has indicated the pharmacological properties of catechins such as antioxidant, anti-inflammatory, and neuroprotective activities (Unno et al., 2017). The neuroprotective activity of catechins acts through suppression of apoptosis, TNF-α, COX-2, ROS, RNS, and iNOS (Singh et al., 2010). Oxidative stress and inflammation are able to stimulate the Nrf2-ARE pathway (Staurengo-Ferrari et al., 2019) that are responsible for protection cells by inducing antioxidant genes (Staurengo-Ferrari et al., 2019).
Nrf-2 can stimulate the activation of several antioxidant genes including heme oxygenase 1. It has been indicated that modulation of Nrf-2 and heme oxygenase 1 are the main therapeutic targets for treating neurological disorders (Johnson and Johnson, 2015). Catechins induce Nrf-2 and heme oxygenase 1 expression in microglia eventually decreasing oxidative stress and inflammation. Therefore, catechins are important neuroprotective agents acting through the Nrf-2 pathway activation (Scapagnini et al., 2011). In addition, catechins exert neuroprotective effects via inhibiting TNF-α and other inflammatory mediators. The STAT3 signaling pathway has a main role in the regulation of immunity and inflammation (Yu et al., 2009). The activation of STAT3 is caused by the JAK family of tyrosine-kinases, specifically JAK1 (Yu et al., 2009). STAT3 activation via v-src causes the NF-κB signaling pathway activation which eventually generates inflammatory mediators including IL-6 (Yu et al., 2009). In addition, persistent activation of STAT3 is related to various disorders including cancer and immunodeficiency (Yu et al., 2009).
Catechins regulate the NF-κB pathway activation by inhibiting STAT3 signaling (Fan et al., 2017). The PI3K/Akt signaling pathway has a main role in stimulating microglia. Suppression of the PI3K/Akt pathway with catechins significantly down-regulated the activation of microglia and pro-inflammatory cytokines (Fan et al., 2017). Peroxisome proliferation-activated receptor-γ (PPARγ), a transcriptional factor, modulates the inflammatory cytokines production in microglia (Khan et al., 2019). Activated-PPARγ attaches to the peroxisome proliferators response element (PPRE) leading to suppression of inflammatory cascades (Khan et al., 2019). Catechins induced the activation of the PPARγ pathway which inhibited the NF-κB pathway in an experimental model of AD (Singh et al., 2011). Catechins inhibited the neuroinflammatory signaling by decreasing the active protein 1 activation (Azam et al., 2019). Altogether, catechins decrease the activation of NF-κB (Lee et al., 2009), STAT3 (He et al., 2015), MAPK/JNK (Chen et al., 2013), PI3K/Akt, iNOS as well as the production of Aβ, NO and COX-2 (Nan et al., 2018). Additionally, catechins stimulate the activation of anti-inflammatory molecules including the Nrf-2 and PPARα-γ pathways (Han et al., 2014).
Effects of Catechins on Microglia Functions In vitro studies
Activated microglia produces an excessive amount of proinflammatory cytokines, ROS, and RNS leading to the development of neurodegenerative diseases. Green tea catechins showed various therapeutic effects regarding microglia functions and neurodegenerative diseases which have been found in experimental research. Voltage-gated proton channels are necessary for nicotinamide adenine dinucleotide phosphate oxidase-dependent ROS productions are expressed in microglia. After injuries such as ischemic stroke, proton channel activities play a vital role in brain injury. Jin et al. (2013) examined the effects of EGCG on these proton channel functions in the murine microglial BV2 cells. Results showed EGCG blocked proton flow with an IC50 of 3.7 µM. This inhibition may be the main mechanism for EGCG for decreasing microglial activation and neurotoxic effects. The principal pathological feature of AD, the most common neurodegenerative disease, is Aβ deposition in the brain. Aβ serves as an important stimulator for microglial and induces chronic inflammation leading to neurodegeneration and subsequently neuronal cell death (Khandelwal et al., 2011).
Cheng-Chung Wei et al. (2016) investigated the application of EGCG for indicating the effects of green tea catechins in microglial activation following Aβ. Results showed that EGCG markedly decreased the expression of TNFα, IL-1β, IL-6, and iNOS synthase in the EOC 13.31 microglia cell. EGCG also inhibited ROS-induced NF-κB activation following Aβ exposure. The study showed that EGCG was neuroprotective against Aβ-induced neuroinflammatory response of microglia and inhibited neurotoxicity. In another experiment by Kim et al. (2009), EGCG suppressed the expression of iNOS, NO and peroxynitrite generation against Aβ-induced ROS/NOS in BV2 microglia. Zhong et al. (2019) found that EGCG inhibited the activation of canonical and non-canonical inflammasome through the Toll-like receptor 4 (TLR4)/NF-κB pathway in microglia cell exposed to Aβ in vitro and in APP/PS1 mice. In rat primary microglia and hippocampus of APP/PS1 mice, EGCG reduced inflammation and neurotoxicity of microglia by decreasing the stimulation of canonical NLRP3 and non-canonical caspase-11-dependent inflammasome through activating of TLR4/NF-κB pathway. Neuronal injury or neuroinflammation can induce NO production via microglial activation. The reaction between NO and cysteine thiols leading to S-nitroso-proteins formation. Qu et al. (2014) indicated that EGCG inhibits S-nitrosylation of proteins in microglial cells exposed to lipopolysaccharide (LPS) via activating Nrf-2. Mazzio et al. (2016) screened natural products including EGCG for antibacterial and anti-inflammatory agents in activated macrophages and microglial cells. Results showed that EGCG expresses anti-inflammatory effects at IC50 11.3 (μg/mL) and toxicity at LC50 24.8 (μg/mL) in the LPS activated BV-2 cells. Brain-derived neurotrophic factor, one of the major neurotrophic factors have been shown to modulate homeostasis of neuroinflammation. EGCG and minocycline caused the up-regulation of brain-derived neurotrophic factor to prevent the expression of COX-2 and pro-inflammatory cytokines (Lai et al., 2018). Additionally, EGCG and minocycline increased the up-regulation of Brain-derived neurotrophic factor expression in microglia via inducing of the AMPK signaling. EGCG prevented LPS-induced the release of NO and TNF-α from microglial via down-regulation of iNOS and TNF-α expression. EGCG protected against microglial activation-caused neuronal damage in the human dopaminergic SH-SY5Y cell line and in primary rat mesencephalic cells. 67 kDa laminin receptor (67LR) is an important component in the activation and migration macrophage (Li et al., 2004).
Ren et al. (2014) investigated the impact of EGCG on cell migration and 67LR in the LPS-activated macrophagic RAW264.7 cells. EGCG ameliorated the LPS-induced cell migration dose-dependently. EGCG also caused membrane translocation of 67LR from the membrane surface towards the cytoplasm in a dose-dependent manner. The harmful neuronal effects of microglia activation occur following cerebral ischemia/reperfusion injuries of the brain. Catechin markedly blocked the hypoxia/reperfusion-induced reduction in cell viability and elevation in oxidative stress and apoptosis in microglia. Catechin also up-regulated the phosphorylation of Akt and mammalian target of rapamycin and blocked the hypoxia/reperfusion-caused autophagy in microglia. The findings indicated catechin could reverse hypoxia/reperfusion-induced autophagy-activated microglia apoptosis/death through the ROS-regulated Akt/mammalian target of rapamycin signaling pathway (Chen et al., 2014). The research finding indicated the effects of green tea extract and EGCG on memory function following cerebral ischemia in in vitro and in vivo ischemic brain injury (Wu et al., 2012). EGCG had an anti-inflammatory impact in the BV-2 microglial cells. EGCG also prevented LPS-induced the generation of NO and decreased the expression of COX2 and iNOS in the BV-2 cells. Green tea extract and its active polyphenol EGCG attenuated learning and memory impairment in a cerebral ischemia animal model and is thought to be due to the reduction of oxidative stress and neuroinflammation. Researchers showed treatment with EGCG markedly elevated the proliferation of subventricular zone neural progenitor cells and the migration of subventricular zone neuroblasts as well as functional improvement, perhaps via M2 phenotype induction in microglia in the ischemic animal model (Zhang et al., 2017). It was found that AKT signaling involved in EGCG-caused proliferation and neuronal differentiation in neural progenitor cells. This study showed that EGCG has protective effects in spontaneous recovery after ischemic stroke (Zhang et al., 2017).
EC may generate its neuroprotective effects through activating the Nrf-2 pathway. In this regard, Leonardo et al. (2013) indicated that EC treatment decreased forelimb motor coordination dysfunction in the wild type mice. The protection was accompanied by a decrease in brain damage and microglial activation. ECCG showed neuroprotective effects by decreasing infrasound-induced neuronal destruction by preventing microglia-mediated inflammation through the NF-κB pathway (Cai et al., 2014). Huang et al. (2005) showed (+)-catechin isolated from Green tea protected murine microglia from ROS-caused DNA damage and cell cycle arrest via inhibiting tertbutylhydroperoxide-induced translocation of NF-κB. Huang et al. (2006) indicated that (+)-catechin significantly inhibited DNA fragmentation and apoptosis of microglia cells via increasing the expression of poly ADP ribose polymerase and activating the antioxidant enzymes which were exposed to tertbutylhydroperoxide. Hussein et al. (2015) indicated that (+)-catechin ameliorated LPS-induced iNOS and COX-2 and prevented the production of microglial NO, ROS as well as suppressed the TNF-α and IL-6 production in the BV-2 cells. Catechin also inhibited Akt activation in the LPS-caused inflammation in BV-2 microglial cells. (+)-Catechin showed an anti-inflammatory impact on the BV-2 cells by decreasing the production of NF-κB via p38 MAPK, Akt, ERK, and AMPK pathways. A summary of the in vitro effect of green tea catechins on microglial cells has been shown in Additional Table 1.
Table 1.
In vitro effects of green tea catechins
| Authors | Species and cell types | Agent | Dose/Route | In vitro effects |
|---|---|---|---|---|
| Jin et al., 2013 | Murine microglial BV2 cells | EGCG | IC50 of 3.7 μM | Suppressed microglial activation by inhibiting the proton channel |
| Cheng-Chung Wei et al., 2016 | EOC 13.31 mouse immortalized microglia activated via Aβ | EGCG | 5, 10, 20 μM | Suppressed expression of TNF-α, IL-1β, IL-6, and iNOS in microglial cells; Protected against microglia-induced cytotoxicity via inhibiting MAPK-dependent release of TNF-α |
| Kim et al., 2009 | BV2 microglial cells; exposed to Aβ | EGCG | 2, 5, 10 μM | Suppressed increased expression of iNOS, nitric oxide and peroxynitrite in BV2 microglia cells |
| Zhong et al., 2019 | Mouse microglial cell line BV2; rat primary microglial cells; treated with LPS (1 μg/mL) for 1 h and Aβ1 (10 μM) | EGCG | 10 μM | Recovered neurotoxicity from microglial conditioned media; Reduced microglial expressions of caspase-1 p20, NLRP3, and caspase-11 p26 |
| Qu et al., 2014 | BV-2 cells SNOC; exposed to 100 ng/mL LPS for 20 h; In vitro: (10, 20, 40, 80, or 200 μM) for 30 min; ex vivo: 20 μM SNOC | EGCG | 10 μM | Attenuated S-nitrosylation of proteins after microglial cells activation via modulation of Nrf-2-mediated oxidative stress response |
| Mazzio et al., 2016 | BV-2 microglia cells; exposed to LPS 1 μg/mL | EGCG | IC50 11.3 (μg/mL) | Anti-inflammatory effects at IC50 11.3 (μg/mL) and Toxicity at LC50 24.8 (μg/mL) in activated BV-2 microglia cells |
| Lai et al., 2018 | BV-2 microglial cell; exposed to Brain-derived neurotrophic factor | EGCG | 25, 50, 100 μM | upregulated Brain-derived neurotrophic factor expression in microglia through AMPK signaling |
| Li et al., 2004 | Microglia from Spraque-Dawley rat brains at postnatal days 1-2; exposed to LPS 500 ng/mL | EGCG | 1, 10, 100 μM; 30 min before LPS | Inhibited LPS-activated microglial secretion of nitric oxide and TNF-α through the downregulation of inducible nitric oxide synthase and TNF-α expression |
| Ren et al., 2014 | Macrophagic RAW264.7 cells; exposed to LPS | EGCG | 1-25 μM | Inhibited microglia activation and migration via internalization of 67LR |
| Wu et al., 2012 | Murine microglial BV-2 cell; exposed to 0.5 μg/mL LPS | EGCG | 2, 10, 25 μM; 1 h before intervention | anti-inflammatory effects in BV-2 microglia cells; inhibited nitric oxide production and reduced cyclooxygenase-2 and inducible nitric oxide synthase expression in BV-2 cells |
| Zhang et al., 2017 | Neural progenitor cells from ipsilateral SVZ, 14 days post-ischemic/reperfusion injured C57BL/6 mice | EGCG | 10, 20, 40 μM | Functional recovery of SVZ neuroblasts through M2 phenotype induction in microglia |
| Leonardo et al., 2013 | Mouse cortical neurons isolated from 0-to 1-day-old wild type and Nrf2-/- pups | EC | Pretreatment at 50, 100 μM; EC was washed out 6 h prior to oxygen-glucose deprivation | Reduced microglial activation/recruitment in wild type mice by 56.4 ± 13.0% |
| Huang et al., 2005 | murine microglia N9 cells; exposed to tBHP | (+)-Catechin | 0.06, 0.13, 0.25, 0.5, 1, 2 μM | Protected microglia cells from DNA lesion by the blockage of NF-κB Activation |
| Huang et al., 2006 | murine microglia cell line (N9); exposed to tert-butylhydroperoxide | (+)-Catechin | 0.063, 0.125, 0.25, 0.5, 1 μM | Neuroprotective against microglia cell injury via enhancing activities of CAT and SOD; Inhibited activation of caspase-3; increased the expression of PARP to repair DNA lesion |
| Syed Hussein et al., 2015 | BV-2 microglial cells; exposed to LPS | (+)-Catechin | 3.125, 6.25, 12.5, 25, 50, 100 μM; pretreatment 2 h before LPS | Inhibited microglial NO and ROS production; inhibited microglia cells inflammation via blocking activation of Akt |
67LR: 67 kDa laminin receptor; Aβ: Amyloid β; Akt: protein kinase B; AMPK: AMP-activated protein kinase; CAT: catalase; EC: (-)-epicatechin; EGCG: (-)-epigallocatechin-3-gallate; IL: interleukin; iNOS: inducible nitric oxide synthase; LPS: lipopolysaccharide; MAPK: mitogen-activated protein kinase; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3: NOD-like receptors family pyrin domain containing 3; NO: nitric oxide; Nrf-2: nuclear factor erythroid 2-related factor 2; PARP: poly ADP ribose polymerase; ROS: reactive oxygen species; SNOC: S-nitrosocysteine; SOD: superoxide dismutase; SVZ: subventricular zone; tBHP: tertbutylhydroperoxide; TNF-α: tumor necrosis factor alpha.
In vivo studies
In vivo studies investigated the impact of green tea catechins on microglia function. EGCG showed protective effects against spinal cord gliosis by modulating the expression of Ras homologue gene family member A, fatty acid synthase and TNF-α in thermal hyperalgesia mice model (Ren et al., 2014). EGCG treatment suppressed the expression of Ras homologue gene family member A, fatty acid synthase, and TNF-α proteins. It also reduced the reactivity of astroglia and microglia in the spinal cord. Sharman et al. (2019) assessed the effects of curcumin, curcumin + EGCG, docosahexaenoic acid and α-lipoic acid (ALA) + curcumin, or a combination of EGCG, docosahexaenoic acid and α-lipoic acid in male Tg2576 transgenic mice. The combination of flavonoids decreased microglial activation. Obesity disturbs energy metabolism leading to CNS dysfunction. In a mouse model with a high-fat diet impact of EGCG on brown adipose tissue thermogenesis and neuroinflammation was studied by Zahou et al. (2015).
EGCG markedly blocked the high-fat diet-induced obesity by increasing BAT thermogenesis as well as decreased the overactivation of microglia and inflammation in hypothalamus by modulating the NF-kB and STAT3 signaling pathways. In another study regarding the pathological processes of AD, Li et al. (2006) tested EGCG effect on cerebral amyloidosis in the transgenic mice model of AD. EGCG reduced the immune-reactivity of microglial cells in the cortex and hippocampus of mice. EGCG (100 mg/kg, intraperitoneal injection) decreased brain water content and vascular permeability in the rat model of traumatic brain injury (Zhang et al., 2015). EGCG decreased the mRNA expression of IL-1β and TNF-α induced by traumatic brain injury. EGCG inhibited microglia activation by decreasing CD68 mRNA expression. Seong et al. (2016) assessed the effect of EGCG on neurogenesis in the dentate gyrus after neuroinflammation induced by LPS. Due to microglia recruitment which induced by LPS, the pro-inflammatory cytokine production was decreased by EGCG treatment via regulating the TLR4/NF-κB pathway. Neuroprotective effects of EGCG in a transgenic mice model of amyotrophic lateral sclerosis was investigated by Xu et al. (2006).
EGCG-treated transgenic mice showed an increase in the number of motor neurons, and a decrease in the activation of microglial, NF-κB, iNOS as well as a caspase-3 cleavage in the spinal cords. It is found that inflammation and neuronal loss with cognitive deficits are related to human immunodeficiency virus-associated dementia. Rrapo et al. (2009) showed that EGCG reduced astrogliosis, glial fibrillary acidic protein expression, activated microglia, and neuronal loss through apoptosis in a human immunodeficiency virus-1 transgenic mice model. Ren et al. (2018) investigated the anti-inflammatory impact of green tea extract against the LPS-induced retinal inflammation in rats. Green tea extract decreased the number of activated microglia cells dose-dependently. Green tea extract has also suppressed the activation of astrocytes and Müller glia in the retina. EC significantly ameliorated the expression of TNF-α, iNOS, NF-κB and total nitrite levels in the brain of rats exposed to doxorubicin.
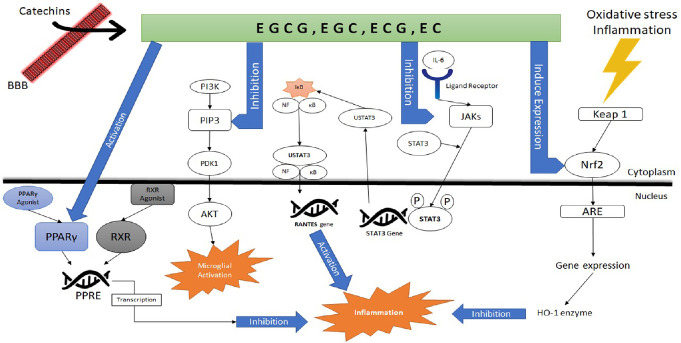
The findings showed that EC against neuroinflammation during antineoplastic therapy by inhibiting TNF-α, NF-κB, and iNOS release from activated microglia (Mohamed et al., 2011). Leonardo et al. (2013) studied the effects of EC on anatomical and functional changes in the brain of ischemic male C57BL/6 mice. Results showed that Iba1 marker of microglia/macrophage increased in the ipsilateral hemispheres of mice 7 days after intervention whereas pretreatment with 15 mg/kg EC blocked this effect. Mice treated with 15 mg/kg also showed a trend toward reduction in infarct volume relative to vehicle controls. Zeng et al. (2014) investigated the effect of EC (40 mg/kg per day, for 6 months) in APP/PS1 transgenic mice. Results showed that long-term feeding of EC decreased total Aβ in the brain. In addition, EC decreased microgliosis and astrocytosis in the brain of mice with AD. The main effects of green tea catechins on microglial cells in animal models are summarized in Additional Table 2. The catechins effects on CNS signaling pathways is shown in Figure 1.
Table 2.
In vivo effects of green tea catechins
| Authors | Species | Agent | Dose/Route | In vivo effects |
|---|---|---|---|---|
| Sharman et al., 2019 | Tg2576 transgenic mice | EGCG + docosahexaenoic acid + α-lipoic acid | EGCG; 50 mg/kg body weight (around 0.0357% = 357 ppm) | Combination treatment decreased microglial activation and number of microglia around plaques in the cortex and hippocampus |
| Zhu et al., 2018 | Obese C57BL/6J mice with the high-fat diet | EGCG | 1% EGCG in diet | Attenuated microglia overactivation by regulating the NF-κB and STAT3 signaling pathways |
| Li et al., 2006 | transgenic mouse model of AD | EGCG | 20 mg/kg per day, in drinking water for 3 months | Decreased CD45, a marker of microglial activation, by 18% in the cortex and 28% in the hippocampus |
| Zhang et al., 2015 | Sprague-Dawley rats; TBI model | EGCG | 100 mg/kg intraperitoneally | Inhibited microglia activation evidenced by decreased CD68 mRNA expression in the brain |
| Seong et al., 2016 | C57BL/6 mice; LPS 0.1 mg/kg injected to the brain | EGCG | 0.5 mg/kg; intraperitoneally; 3 times | Attenuated pro-inflammatory cytokine production by microglial cells through modulating the TLR4-NF-κB pathway |
| Xu et al., 2006 | SOD1-G93A transgenic mice and wild-type mice model of ALS | EGCG | 10 mg/kg, orally | Diminished microglial activation |
| Rrapo et al., 2009 | HIV-1 Tat transgenic mouse | EGCG | 300 mg/kg per day | Mild reduction in activated microglia |
| Ren et al., 2018 | Adult Sprague-Dawley rats; injected with LPS (1 mg/kg) | Green tea extract: EGCG 70.53%, EGC 4.61%, EC 3.88% and GC 0.64% | 550 mg/kg or 275 mg/kg suspension in 0.5 mL distilled water; intragastrical at 2, 8, 26, and 32 h post LPS injection | Suppressed the number of activated microglia cells in a dose-dependent manner; suppressed activation of astrocytes and Müller glia in the retina |
| Mohamed et al., 2011 | Adult Wistar rats; intraperitoneal injections of doxorubicin (13 mg/kg) | EC | 10 mg/kg per day; orally; 2 weeks before intervention | Increased TNF-α due to microglia activation |
| Leonardo et al., 2013 | Male C57BL/6 mice; permanent distal middle cerebral artery occlusion | EC | Pretreatment 90 minutes prior to intervention with 15 mg/kg | Blocked microglia/macrophage marker Iba1 which was increased in the ipsilateral hemispheres of mice 7 d after intervention |
| Zeng et al., 2014 | APP/PS1 transgenic mice | EC and curcumin for 9 months | EC 40 mg/kg per day and curcumin 47 mg/kg per day in diet | Reduced Microgliosis in the brain of AD mice |
| Zhong et al., 2019 | APP/PS1 mice | EGCG | 2 mg/kg per day; Intragastrical | Reduced microglial inflammation and neurotoxicity by suppressing the activation of canonical NLRP3 and non-canonical caspase-11-dependent inflammasome via TLR4/NF-κB pathway in hippocampus |
| Wu et al., 2012 | Sprague-Dawley rats; Focal brain ischemia model ischemia model | Green tea extract and EGCG | Green tea extract (30, 100, and 300 mg/kg) and EGCG (10 mg/kg) | Inhibited NO production, iNOS protein and COX-2 expression in BV-2 microglia cells |
| Leonardo et al., 2013 | wildtype and Nrf-2 C57BL/6 knockout mice; focal brain ischemia model | EC | 50 or 100 μM by gavage 90 min before intervention wildtype mice by 56.4 ± 13.0% | Reduced microglia activation/recruitment in |
AD: Alzheimer’s disease; COX-2: cyclooxygenase-2; EC: (-)-epicatechin; EGC: (-)-epigallocatechin; EGCG: (-)-epigallocatechin-3-gallate; iNOS: inducible nitric oxide synthase; LPS: lipopolysaccharide; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3: NOD-like receptors family pyrin domain containing 3; NO: nitric oxide; Nrf-2: nuclear factor erythroid 2-related factor 2; TBI: traumatic brain injury; TLR4: Toll-like receptor 4; TNF-α: tumor necrosis factor alpha.
Figure 1.
Catechins effect on oxidative stress, inflammation and microglia activation signaling pathways in central nervous system.
Akt: Protein kinase B; AMPK: AMP-activated protein kinase; ARE: antioxidant response element; EC: epicatechin; ECG: epicatechin-3-gallate; EGC: epigallocatechin; EGCG: epigallocatechin-3-gallate; HO-1: heme oxygenase-1; MAPK: mitogen-activated protein kinase; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; Nrf-2: nuclear factor erythroid 2-related factor 2; PI3K: phosphatidylinositol 3 kinase; PPARγ: peroxisome proliferation-activated receptor-γ; PPRE: peroxisome proliferators response element; RXR: retinoid X receptor; STAT3: signal transducer activator of transcription 3.
Conclusions and Remarks
Microglia, as a primary immune cell in the CNS, has an important contribution in the homeostasis of the CNS against pathogens and injuries through generating inflammatory cytokines. However, over activation of microglia under pathological conditions causes excessive production of inflammatory cytokines via microglia leading to neuronal inflammation, injury, and death. Furthermore, one of the initial steps in neurodegenerative diseases is inflammation. Green tea catechins have beneficial effects against numerous diseases. Catechins inhibit/reduce the inflammatory mediators production via inhibiting the activation of microglia. Catechins suppress the levels of TNF-α, IL-1β, IL-6 via modulating NF-κB, Nrf-2 and TLR4/NF-κB pathways. Catechins have inhibitory effects on other intracellular parts such as voltage-gated proton channels which have a role in activating microglia. Taken together, green tea catechins are promising therapeutic agents in inhibiting inflammatory and apoptotic mediators in microglia and subsequently neuroprotective effects in neurodegeneration diseases.
Although several studies indicated involved mechanisms of neuroprotective effects of green tea catechins by modulating activated microglia, it was not indicated effective mechanisms to shift microglia toward a neuroprotective phenotype following catechins administration. It was suggested that more preclinical studies should be designed to identify the suitable pathways that can be modulated by catechins in order to stimulate neuroregeneration for clinical approaches. It was suggested that focuses on the possible effect of catechins on the receptor-mediated, microRNA and cell cycle of microglia for modulating its activity as the future prospective study.
Additional files:
Additional Table 1: In vitro effects of green tea catechins.
Additional Table 2: In vivo effects of green tea catechins.
Footnotes
Conflicts of interest: The authors declare no conflicts of interest.
Financial support: None.
Copyright license agreement: The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check: Checked twice by iThenticate.
Peer review: Externally peer reviewed.
C-Editors: Zhao M, Li JY; T-Editor: Jia Y
References
- 1.Akhmetzyanova ER, Kletenkov KS, Mukhamedshina YO, Rizvanov A. Different approaches to modulation of microglia phenotypes after spinal cord injury. Front Syst Neurosci. 2019;13:37. doi: 10.3389/fnsys.2019.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aktas O, Prozorovski T, Smorodchenko A, Savaskan NE, Lauster R, Kloetzel PM, Infante-Duarte C, Brocke S, Zipp F. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J Immunol. 2004;173:5794–800. doi: 10.4049/jimmunol.173.9.5794. [DOI] [PubMed] [Google Scholar]
- 3.Azam S, Jakaria M, Kim IS, Kim J, Haque ME, Choi DK. Regulation of Toll-like receptor (TLR) signaling pathway by polyphenols in the treatment of age-linked neurodegenerative diseases: focus on TLR4 signaling. Front Immunol. 2019;10:1000. doi: 10.3389/fimmu.2019.01000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babu PV, Liu D. Green tea catechins and cardiovascular health: an update. Curr Med Chem. 2008;15:1840–1850. doi: 10.2174/092986708785132979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bachiller S, Jiménez-Ferrer I, Paulus A, Yang Y, Swanberg M, Deierborg T, Boza-Serrano A. Microglia in neurological diseases: a road map to brain-disease dependent-inflammatory response. Front Cell Neurosci. 2018;12:488. doi: 10.3389/fncel.2018.00488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berg D, Gerlach M, Youdim MB, Double KL, Zecca L, Riederer P, Becker G. Brain iron pathways and their relevance to Parkinson’s disease. J Neurochem. 2001;79:225–236. doi: 10.1046/j.1471-4159.2001.00608.x. [DOI] [PubMed] [Google Scholar]
- 7.Bhardwaj P, Khanna D. Green tea catechins: defensive role in cardiovascular disorders. Chin J Nat Med. 2013;11:345–353. doi: 10.1016/S1875-5364(13)60051-5. [DOI] [PubMed] [Google Scholar]
- 8.Cai J, Jing D, Shi M, Liu Y, Lin T, Xie Z, Zhu Y, Zhao H, Shi X, Du F, Zhao G. Epigallocatechin gallate (EGCG) attenuates infrasound-induced neuronal impairment by inhibiting microglia-mediated inflammation. J Nutr Biochem. 2014;25:716–725. doi: 10.1016/j.jnutbio.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Chatterjee P, Chandra S, Dey P, Bhattacharya S. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative in vitro study. J Adv Pharm Technol Res. 2012;3:136–138. doi: 10.4103/2231-4040.97298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen CM, Wu CT, Yang TH, Chang YA, Sheu ML, Liu SH. Green tea catechin prevents hypoxia/reperfusion-evoked oxidative stress-regulated autophagy-activated apoptosis and cell death in microglial cells. J Agric Food Chem. 2016;64:4078–4085. doi: 10.1021/acs.jafc.6b01513. [DOI] [PubMed] [Google Scholar]
- 11.Chen E, Xu D, Lan X, Jia B, Sun L, Zheng JC, Peng H. A novel role of the STAT3 pathway in brain inflammation-induced human neural progenitor cell differentiation. Curr Mol Med. 2013;13:1474–1484. doi: 10.2174/15665240113139990076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng X, Zhang L, Lian YJ. Molecular targets in Alzheimer’s disease: from pathogenesis to therapeutics. Biomed Res Int. 2015;2015:760758. doi: 10.1155/2015/760758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng-Chung Wei J, Huang HC, Chen WJ, Huang CN, Peng CH, Lin CL. Epigallocatechin gallate attenuates amyloid β-induced inflammation and neurotoxicity in EOC 13, 31 microglia. Eur J Pharmacol. 2016;770:16–24. doi: 10.1016/j.ejphar.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 14.Choi YT, Jung CH, Lee SR, Bae JH, Baek WK, Suh MH, Park J, Park CW, Suh SI. The green tea polyphenol (-)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001;70:603–614. doi: 10.1016/s0024-3205(01)01438-2. [DOI] [PubMed] [Google Scholar]
- 15.Crew KD, Ho KA, Brown P, Greenlee H, Bevers TB, Arun B, Sneige N, Hudis C, McArthur HL, Chang J, Rimawi M, Cornelison TL, Cardelli J, Santella RM, Wang A, Lippman SM, Hershman DL. Effects of a green tea extract, Polyphenon E, on systemic biomarkers of growth factor signalling in women with hormone receptor-negative breast cancer. J Hum Nutr Diet. 2015;28:272–282. doi: 10.1111/jhn.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cummings J, Lee G, Ritter A, Sabbagh M, Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement (N Y) 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeMaagd G, Philip A. Parkinson’s disease and its management: part 1: disease entity risk, factors pathophysiology, clinical presentation, and diagnosis. P T. 2015;40:504–532. [PMC free article] [PubMed] [Google Scholar]
- 18.Dong Y, Li X, Cheng J, Hou L. Drug development for Alzheimer’s disease: microglia induced neuroinflammation as a target. Int J Mol Sci. 2019;20:558. doi: 10.3390/ijms20030558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fahn S, Cohen G. The oxidant stress hypothesis in Parkinson’s disease: evidence supporting it. Ann Neurol. 1992;32:804–12. doi: 10.1002/ana.410320616. [DOI] [PubMed] [Google Scholar]
- 20.Fan FY, Sang LX, Jiang M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules. 2017;22:484. doi: 10.3390/molecules22030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu QY, Li QS, Lin XM, Qiao RY, Yang R, Li XM, Dong ZB, Xiang LP, Zheng XQ, Lu JL, Yuan CB, Ye JH, Liang YR. Antidiabetic effects of tea. Molecules. 2017 doi: 10.3390/molecules22050849. doi: 103390/molecules22050849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galloway DA, Phillips AEM, Owen DRJ, Moore CS. Phagocytosis in the brain: homeostasis and disease. Front Immunol. 2019;10:790. doi: 10.3389/fimmu.2019.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerlach M, Ben-Shachar D, Riederer P, Youdim MB. Altered brain metabolism of iron as a cause of neurodegenerative diseases. J Neurochem. 1994;63:793–807. doi: 10.1046/j.1471-4159.1994.63030793.x. [DOI] [PubMed] [Google Scholar]
- 24.Han J, Wang M, Jing X, Shi H, Ren M, Lou H. (−)-Epigallocatechin gallate protects against cerebral ischemia-induced oxidative stress via Nrf2/ARE signaling. Neurochem Res. 2014;39:1292–1299. doi: 10.1007/s11064-014-1311-5. [DOI] [PubMed] [Google Scholar]
- 25.Haque AM, Hashimoto M, Katakura M, Tanabe Y, Hara Y, Shido O. Long-term administration of green tea catechins improves spatial cognition learning ability in rats. J Nutr. 2006;136:1043–1047. doi: 10.1093/jn/136.4.1043. [DOI] [PubMed] [Google Scholar]
- 26.Harry GJ, Kraft AD. Neuroinflammation and microglia: considerations and approaches for neurotoxicity assessment. Expert Opin Drug Metab Toxicol. 2008;4:1265–1277. doi: 10.1517/17425255.4.10.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He Q, Bao L, Zimering J, Zan K, Zhang Z, Shi H, Zu J, Yang X, Hua F, Ye X, Cui G. The protective role of (−)-epigallocatechin-3-gallate in thrombin-induced neuronal cell apoptosis and JNK-MAPK activation. Neuroreport. 2015;26:416–423. doi: 10.1097/WNR.0000000000000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 29.Huang J, Wang Y, Xie Z, Zhou Y, Zhang Y, Wan X. The anti-obesity effects of green tea in human intervention and basic molecular studies. Eur J Clin Nutr. 2014;68:1075–1087. doi: 10.1038/ejcn.2014.143. [DOI] [PubMed] [Google Scholar]
- 30.Huang Q, Wu LJ, Tashiro S, Gao HY, Onodera S, Ikejima T. (+)-Catechin, an ingredient of green tea, protects murine microglia from oxidative stress-induced DNA damage and cell cycle arrest. J Pharmacol Sci. 2005;98:16–24. doi: 10.1254/jphs.fpj04053x. [DOI] [PubMed] [Google Scholar]
- 31.Huang Q, Wu LJ, Tashiro S, Onodera S, Ikejima T. Elevated levels of DNA repair enzymes and antioxidative enzymes by (+)-catechin in murine microglia cells after oxidative stress. J Asian Nat Prod Res. 2006;8:61–71. doi: 10.1080/10286020500209087. [DOI] [PubMed] [Google Scholar]
- 32.Jin S, Park M, Song JH. (−)-Epigallocatechin-3-gallate inhibits voltage-gated proton currents in BV2 microglial cells. Eur J Pharmacol. 2013;698:154–160. doi: 10.1016/j.ejphar.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 33.Johnson DA, Johnson JA. Nrf2—a therapeutic target for the treatment of neurodegenerative diseases. Free Radic Biol Med. 2015;88:253–267. doi: 10.1016/j.freeradbiomed.2015.07.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khan MA, Alam Q, Haque A, Ashafaq M, Khan MJ, Ashraf GM, Ahmad M. Current progress on peroxisome proliferator-activated receptor gamma agonist as an emerging therapeutic approach for the treatment of Alzheimer’s disease: an update. Curr Neuropharmacol. 2019;17:232–246. doi: 10.2174/1570159X16666180828100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khandelwal PJ, Herman AM, Moussa CE. Inflammation in the early stages of neurodegenerative pathology. J Neuroimmunol. 2011;238:1–11. doi: 10.1016/j.jneuroim.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim CY, Lee C, Park GH, Jang JH. Neuroprotective effect of epigallocatechin-3-gallate against β-amyloid-induced oxidative and nitrosative cell death via augmentation of antioxidant defense capacity. Arch Pharm Res. 2009;32:869–881. doi: 10.1007/s12272-009-1609-z. [DOI] [PubMed] [Google Scholar]
- 37.Kim SH, Noh MY, Kim HJ, Oh KW, Park J, Lee S, Moon Y, Kim YE, Bae JS, Jin HK K-ARPI. A therapeutic strategy for Alzheimer’s disease focused on immune-inflammatory modulation. Dement Neurocogn Disord. 2019;18:33–46. doi: 10.12779/dnd.2019.18.2.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koh SH, Lee SM, Kim HY, Lee KY, Lee YJ, Kim HT, Kim J, Kim MH, Hwang MS, Song C, Yang KW, Lee KW, Kim SH, Kim OH. The effect of epigallocatechin gallate on suppressing disease progression of ALS model mice. Neurosci Lett. 2006;395:103–107. doi: 10.1016/j.neulet.2005.10.056. [DOI] [PubMed] [Google Scholar]
- 39.Kuriyama S, Hozawa A, Ohmori K, Shimazu T, Matsui T, Ebihara S, Awata S, Nagatomi R, Arai H, Tsuji I. Green tea consumption and cognitive function: a cross-sectional study from the Tsurugaya Project 1. Am J Clin Nutr. 2006;83:355–361. doi: 10.1093/ajcn/83.2.355. [DOI] [PubMed] [Google Scholar]
- 40.Lai SW, Chen JH, Lin HY, Liu YS, Tsai CF, Chang PC, Lu DY, Lin C. Regulatory effects of neuroinflammatory responses through brain-derived neurotrophic factor signaling in microglial cells. Mol Neurobiol. 2018;55:7487–7499. doi: 10.1007/s12035-018-0933-z. [DOI] [PubMed] [Google Scholar]
- 41.Lee JW, Lee YK, Ban JO, Ha TY, Yun YP, Han SB, Oh KW, Hong JT. Green tea (-)-epigallocatechin-3-gallate inhibits β-amyloid-induced cognitive dysfunction through modification of secretase activity via inhibition of ERK and NF-κ B pathways in mice. J Nutr. 2009;139:1987–1993. doi: 10.3945/jn.109.109785. [DOI] [PubMed] [Google Scholar]
- 42.Lee S, Suh S, Kim S. Protective effects of the green tea polyphenol (-)-epigallocatechin gallate against hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci Lett. 2000;287:191–194. doi: 10.1016/s0304-3940(00)01159-9. [DOI] [PubMed] [Google Scholar]
- 43.Leonardo CC, Agrawal M, Singh N, Moore JR, Biswal S, Doré S. Oral administration of the flavanol (-)-epicatechin bolsters endogenous protection against focal ischemia through the Nrf2 cytoprotective pathway. Eur J Neurosci. 2013;38:3659–3668. doi: 10.1111/ejn.12362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leong H, Mathur PS, Greene GL. Green tea catechins inhibit angiogenesis through suppression of STAT3 activation. Breast Cancer Res Treat. 2009;117:505–515. doi: 10.1007/s10549-008-0196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levites Y, Amit T, Mandel S, Youdim MB. Neuroprotection and neurorescue against Abeta toxicity and PKC-dependent release of nonamyloidogenic soluble precursor protein by green tea polyphenol (-)-epigallocatechin-3-gallate. FASEB J. 2003;17:952–954. doi: 10.1096/fj.02-0881fje. [DOI] [PubMed] [Google Scholar]
- 46.Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem. 2002;277:30574–30580. doi: 10.1074/jbc.M202832200. [DOI] [PubMed] [Google Scholar]
- 47.Levites Y, Weinreb O, Maor G, Youdim MB, Mandel S. Green tea polyphenol (-)-epigallocatechin-3-gallate prevents. N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-induced dopaminergic neurodegeneration. J Neurochem. 2001;78:1073–1082. doi: 10.1046/j.1471-4159.2001.00490.x. [DOI] [PubMed] [Google Scholar]
- 48.Li Q, Gordon M, Tan J, Morgan D. Oral administration of green tea epigallocatechin-3-gallate (EGCG) reduces amyloid beta deposition in transgenic mouse model of Alzheimer’s disease. Exp Neurol. 2006;198:576. [Google Scholar]
- 49.Li R, Huang YG, Fang D, Le WD. (−)‐Epigallocatechin gallate inhibits lipopolysaccharide‐induced microglial activation and protects against inflammation‐mediated dopaminergic neuronal injury. J Neurosci Res. 2004;78:723–731. doi: 10.1002/jnr.20315. [DOI] [PubMed] [Google Scholar]
- 50.Liu D, Li P, Song S, Liu Y, Wang Q, Chang Y, Wu Y, Chen J, Zhao W, Zhang L, Wei W. Pro-apoptotic effect of epigallo-catechin-3-gallate on B lymphocytes through regulating BAFF/PI3K/Akt/mTOR signaling in rats with collagen-induced arthritis. Eur J Pharmacol. 2012;690:214–225. doi: 10.1016/j.ejphar.2012.06.026. [DOI] [PubMed] [Google Scholar]
- 51.Mancini E, Beglinger C, Drewe J, Zanchi D, Lang UE, Borgwardt S. Green tea effects on cognition, mood and human brain function: A systematic review. Phytomedicine. 2017;34:26–37. doi: 10.1016/j.phymed.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 52.Mandel S, Reznichenko L, Amit T, Youdim MB. Green tea polyphenol (-)-epigallocatechin-3-gallate protects rat PC12 cells from apoptosis induced by serum withdrawal independent of P13-Akt pathway. Neurotox Res. 2003;5:419–424. doi: 10.1007/BF03033171. [DOI] [PubMed] [Google Scholar]
- 53.Mandel SA, Avramovich-Tirosh Y, Reznichenko L, Zheng H, Weinreb O, Amit T, Youdim MB. Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress and PKC signaling pathway. Neurosignals. 2005;14:46–60. doi: 10.1159/000085385. [DOI] [PubMed] [Google Scholar]
- 54.Mazzio EA, Li N, Bauer D, Mendonca P, Taka E, Darb M, Thomas L, Williams H, Soliman KF. Natural product HTP screening for antibacterial (E. coli 0157: H7) and anti-inflammatory agents in (LPS from E. coli O111: B4) activated macrophages and microglial cells; focus on sepsis. BMC Complement Altern Med. 2016;16:467. doi: 10.1186/s12906-016-1429-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miura Y, Chiba T, Tomita I, Koizumi H, Miura S, Umegaki K, Hara Y, Ikeda M, Tomita T. Tea catechins prevent the development of atherosclerosis in apoprotein E-deficient mice. J Nutr. 2001;131:27–32. doi: 10.1093/jn/131.1.27. [DOI] [PubMed] [Google Scholar]
- 56.Mohamed RH, Karam RA, Amer MG. Epicatechin attenuates doxorubicin-induced brain toxicity: critical role of TNF-α, iNOS and NF-κB. Brain Res Bull. 2011;86:22–28. doi: 10.1016/j.brainresbull.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 57.Nan W, Zhonghang X, Keyan C, Tongtong L, Wanshu G, Zhongxin X. Epigallocatechin-3-gallate reduces neuronal apoptosis in rats after middle cerebral artery occlusion injury via pi3k/akt/enos signaling pathway. Biomed Res Int. 2018;2018:6473580. doi: 10.1155/2018/6473580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Oh S, Gwak J, Park S, Yang CS. Green tea polyphenol EGCG suppresses Wnt/beta-catenin signaling by promoting GSK-3beta- and PP2A-independent beta-catenin phosphorylation/degradation. Biofactors. 2014;40:586–595. doi: 10.1002/biof.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qu Z, Meng F, Zhou H, Li J, Wang Q, Wei F, Cheng J, Greenlief CM, Lubahn DB, Sun GY, Liu S, Gu Z. NitroDIGE analysis reveals inhibition of protein S-nitrosylation by epigallocatechin gallates in lipopolysaccharide-stimulated microglial cells. J Neuroinflammation. 2014;11:17. doi: 10.1186/1742-2094-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ren JL, Yu QX, Liang WC, Leung PY, Ng TK, Chu WK, Pang CP, Chan SO. Green tea extract attenuates LPS-induced retinal inflammation in rats. Sci Rep. 2018;8:429. doi: 10.1038/s41598-017-18888-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ren X, Guo X, Chen L, Guo M, Peng N, Li R. Attenuated migration by green tea extract (−)-epigallocatechin gallate (EGCG): involvement of 67 kDa laminin receptor internalization in macrophagic cells. Food Funct. 2014;5:1915–1919. doi: 10.1039/c4fo00143e. [DOI] [PubMed] [Google Scholar]
- 62.Reygaert WC. Green tea catechins: their use in treating and preventing infectious diseases. Biomed Res Int. 2018;2018:9105261. doi: 10.1155/2018/9105261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rezai-Zadeh K, Shytle D, Sun N, Mori T, Hou H, Jeanniton D, Ehrhart J, Townsend K, Zeng J, Morgan D, Hardy J, Town T, Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Riederer P, Sofic E, Rausch WD, Schmidt B, Reynolds GP, Jellinger K, Youdim MB. Transition metals, ferritin, glutathione and ascorbic acid in parkinsonian brains. J Neurochem. 1989;52:515–520. doi: 10.1111/j.1471-4159.1989.tb09150.x. [DOI] [PubMed] [Google Scholar]
- 65.Rrapo E, Zhu Y, Tian J, Hou H, Smith A, Fernandez F, Tan J, Giunta B. Green Tea-EGCG reduces GFAP associated neuronal loss in HIV-1 Tat transgenic mice. Am J Transl Res. 2009;1:72–79. [PMC free article] [PubMed] [Google Scholar]
- 66.Samarghandian S, Azimi-Nezhad M, Borji A, Hasanzadeh M, Jabbari F, Farkhondeh T, Samini M. Inhibitory and cytotoxic activities of chrysin on human breast adenocarcinoma cells by induction of apoptosis. Pharmacogn Mag. 2016a;12:S436–S440. doi: 10.4103/0973-1296.191453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Samarghandian S, Azimi-Nezhad M, Farkhondeh T. Crocin attenuate Tumor Necrosis Factor-alpha (TNF-α) and interleukin-6 (IL-6) in streptozotocin-induced diabetic rat aorta. Cytokine. 2016b;88:20–28. doi: 10.1016/j.cyto.2016.08.002. [DOI] [PubMed] [Google Scholar]
- 68.Samarghandian S, Azimi-Nezhad M, Shabestari MM, Azad FJ, Farkhondeh T, Bafandeh F. Effect of chronic exposure to cadmium on serum lipid, lipoprotein and oxidative stress indices in male rats. Interdiscip Toxicol. 2015;8:151–154. doi: 10.1515/intox-2015-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scapagnini G, Sonya V, Nader AG, Calogero C, Zella D, Fabio G. Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol. 2011;44:192–201. doi: 10.1007/s12035-011-8181-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seong KJ, Lee HG, Kook MS, Ko HM, Jung JY, Kim WJ. Epigallocatechin-3-gallate rescues LPS-impaired adult hippocampal neurogenesis through suppressing the TLR4-NF-κB signaling pathway in mice. Korean J Physiol Pharmacol. 2016;20:41–51. doi: 10.4196/kjpp.2016.20.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharman MJ, Gyengesi E, Liang H, Chatterjee P, Karl T, Li QX, Wenk MR, Halliwell B, Martins RN, Münch G. Assessment of diets containing curcumin, epigallocatechin-3-gallate, docosahexaenoic acid and α-lipoic acid on amyloid load and inflammation in a male transgenic mouse model of Alzheimer’s disease: Are combinations more effective. Neurobiol Dis. 2019;124:505–519. doi: 10.1016/j.nbd.2018.11.026. [DOI] [PubMed] [Google Scholar]
- 72.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh R, Akhtar N, Haqqi TM. Green tea polyphenol epigallocatechi3-gallate: Inflammation and arthritis. Life Sci. 2010;86:907–918. doi: 10.1016/j.lfs.2010.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sochocka M, Diniz BS, Leszek J. Inflammatory response in the CNS: friend or foe. Mol Neurobiol. 2017;54:8071–8089. doi: 10.1007/s12035-016-0297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Staurengo-Ferrari L, Badaro-Garcia S, Hohmann MSN, Manchope MF, Zaninelli TH, Casagrande R, Verri WA., Jr Contribution of Nrf2 modulation to the mechanism of action of analgesic and anti-inflammatory drugs in pre-clinical and clinical stages. Front Pharmacol. 2019;9:1536. doi: 10.3389/fphar.2018.01536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sutherland BA, Shaw OM, Clarkson AN, Jackson DN, Sammut IA, Appleton I. Neuroprotective effects of (-)-epigallocatechin gallate following hypoxia-ischemia-induced brain damage: novel mechanisms of action. FASEB J. 2005;19:258–260. doi: 10.1096/fj.04-2806fje. [DOI] [PubMed] [Google Scholar]
- 77.Suzuki M, Tabuchi M, Ikeda M, Umegaki K, Tomita T. Protective effects of green tea catechins on cerebral ischemic damage. Med Sci Monit. 2004;10:166–174. [PubMed] [Google Scholar]
- 78.Syed Hussein SS, Kamarudin MN, Kadir HA. (+)-Catechin attenuates NF-kappaB activation through regulation of Akt, MAPK and AMPK signaling pathways in LPS-induced BV-2 microglial cells. Am J Chin Med. 2015;43:927–952. doi: 10.1142/S0192415X15500548. [DOI] [PubMed] [Google Scholar]
- 79.Unno K, Pervin M, Nakagawa A, Iguchi K, Hara A, Takagaki A, Nanjo F, Minami A, Nakamura Y. Blood-brain barrier permeability of green tea catechin metabolites and their neuritogenic activity in human neuroblastoma SH‐SY5Y cells. Mol Nutr Food Res. 2017;61:1700294. doi: 10.1002/mnfr.201700294. [DOI] [PubMed] [Google Scholar]
- 80.Wu KJ, Hsieh MT, Wu CR, Wood WG, Chen YF. Green tea extract ameliorates learning and memory deficits in ischemic rats via its active component polyphenol epigallocatechin-3-gallate by modulation of oxidative stress and neuroinflammation. Evid Based Complement Alternat Med. 2012;2012:163106. doi: 10.1155/2012/163106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu Z, Chen S, Li X, Luo G, Li L, Le W. Neuroprotective effects of (-)-epigallocatechin-3-gallate in a transgenic mouse model of amyotrophic lateral sclerosis. Neurochem Res. 2006;31:1263–1269. doi: 10.1007/s11064-006-9166-z. [DOI] [PubMed] [Google Scholar]
- 82.Yang CS, Wang H. Cancer preventive activities of tea catechins. Molecules. 2016 doi: 10.3390/molecules21121679. doi: 103390/molecules21121679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu H, Pardoll D, Jove R. STATs in cancer inflammation and immunity: a leading role for STAT3. Nat Rev Cancer. 2009;9:798–809. doi: 10.1038/nrc2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zeng YQ, Wang YJ, Zhou XF. Effects of (−)epicatechin on the pathology of APP/PS1 transgenic mice. Front Neurol. 2014;5:69. doi: 10.3389/fneur.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang B, Wang B, Cao S, Wang Y. Epigallocatechin-3-gallate (EGCG) attenuates traumatic brain injury by inhibition of edema formation and oxidative stress. Korean J Physiol Pharmacol. 2015;19:491–497. doi: 10.4196/kjpp.2015.19.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang JC, Xu H, Yuan Y, Chen JY, Zhang YJ, Lin Y, Yuan SY. Delayed treatment with green tea polyphenol EGCG promotes neurogenesis after ischemic stroke in adult mice. Mol Neurobiol. 2017;54:3652–3664. doi: 10.1007/s12035-016-9924-0. [DOI] [PubMed] [Google Scholar]
- 87.Zhong X, Liu M, Yao W, Du K, He M, Jin X, Jiao L, Ma G, Wei B, Wei M. Epigallocatechin-3-gallate attenuates microglial inflammation and neurotoxicity by suppressing the activation of canonical and noncanonical inflammasome via TLR4/NF-κB pathway. Mol Nutr Food Res. 2019;63:e1801230. doi: 10.1002/mnfr.201801230. [DOI] [PubMed] [Google Scholar]
- 88.Zhou J, Mao L, Xu P, Wang Y. Effects of (−)-epigallocatechin gallate (EGCG) on energy expenditure and microglia-mediated hypothalamic inflammation in mice ded a high-fat diet. Nutrients. 2018 doi: 10.3390/nu10111681. doi: 103390/nu10111681. [DOI] [PMC free article] [PubMed] [Google Scholar]