Abstract
Context
Once islet autoantibody–positive individuals are identified, predicting which individuals are at highest risk for type 1 diabetes (T1D) is important. A metabolic risk score derived from 2-hour oral glucose tolerance test (OGTT) data, the Diabetes Prevention Trial-Type 1 risk score (DPTRS), can accurately predict T1D. However, 2-hour OGTTs are time-consuming and costly.
Objective
We aimed to determine whether a risk score derived from 1-hour OGTT data can predict T1D as accurately as the DPTRS. Secondarily, we evaluated whether a 1-hour glucose value can be used for diagnostic surveillance.
Methods
The DPTRS was modified to derive a 1-hour OGTT risk score (DPTRS60) using fasting C-peptide, 1-hour glucose and C-peptide, age, and body mass index. Areas under receiver operating curves (ROCAUCs) were used to compare prediction accuracies of DPTRS60 with DPTRS in Diabetes Prevention Trial–Type 1 (DPT-1) (n = 654) and TrialNet Pathway to Prevention (TNPTP) (n = 4610) participants. Negative predictive values (NPV) for T1D diagnosis were derived for 1-hour glucose thresholds.
Results
ROCAUCs for T1D prediction 5 years from baseline were similar between DPTRS60 and DPTRS (DPT-1: 0.805 and 0.794; TNPTP: 0.832 and 0.847, respectively). DPTRS60 predicted T1D significantly better than 2-hour glucose (P < .001 in both cohorts). A 1-hour glucose of less than 180 mg/dL had a similar NPV, positive predictive value, and specificity for T1D development before the next 6-month visit as the standard 2-hour threshold of less than 140 mg/dL (both ≥ 98.5%).
Conclusion
A 1-hour OGTT can predict T1D as accurately as a 2-hour OGTT with minimal risk of missing a T1D diagnosis before the next visit.
Keywords: autoantibody, diagnosis, metabolic risk, oral glucose tolerance test, prediction, type 1 diabetes
The TrialNet Pathway to Prevention (TNPTP) study and its precursor, the Diabetes Prevention Trial—Type 1 Diabetes Study (DPT-1), have screened a large number of relatives of people with type 1 diabetes (T1D) for autoantibodies that commonly precede the diagnosis of the disease. In TNPTP, when participants are identified as having one or more positive islet autoantibodies, they have the opportunity to be monitored for T1D progression risk as well as to enroll in clinical prevention trials. Two-hour oral glucose tolerance tests (OGTTs) are used for monitoring risk and diagnostic surveillance of T1D.
A large number of relatives of people with T1D continue to be identified through the TNPTP study, and programs screening children in the general population for islet autoimmunity have identified thousands of children with early stages of T1D. These general population screening programs are becoming increasingly widespread (1-4). Given the large number of islet autoantibody–positive individuals that will continue to be identified through autoantibody vs antibody screening programs and eligible for diabetes prevention trials, measures that can predict which individuals will progress from autoantibody positivity to clinical T1D as well as who will progress most quickly are important both for prevention trial enrollment and clinical diagnosis surveillance.
In assessments of prediction measures from 2-hour OGTTs, which are used for monitoring T1D risk and diagnosis, the DPT-1 Risk Score (DPTRS) has been shown to accurately predict T1D both in DPT-1 and TNPTP (5, 6). DPTRS is a composite measure based on log fasting C-peptide, age, log body mass index (BMI), and the sum of glucose values and C-peptide values at 30, 60, 90, and 120 minutes. However, 2-hour OGTTs impose significant time and cost burdens on research participants and staff. In this study our primary aim was to determine whether a 1-hour OGTT risk score can reliably predict risk of progression to clinical T1D, and secondarily if a 1-hour glucose threshold value could be identified for diagnostic surveillance. That threshold would indicate whether the 1-hour OGTT should be extended to a 2-hour OGTT so that a T1D diagnosis is not missed.
To assess whether 1-hour OGTTs can accurately predict T1D risk, we developed a risk score, DPTRS60. Similar to DPTRS, DPTRS60 includes log fasting C-peptide, age, and log BMI. However, instead of including the sum of glucose and C-peptide at 30, 60, 90, and 120 minutes, DPTRS60 includes only the 1-hour glucose and 1-hour C-peptide values. We then compared the DPTRS60, DPTRS, and 2-hour glucose both in DPT-1 and TNPTP participants for the prediction of T1D. Next, because a 2-hour OGTT value of greater than 200 mg/dL is needed for a diagnosis of diabetes, we assessed whether a 1-hour glucose threshold could provide sufficient diagnostic surveillance.
Research Design and Methods
Study population
Participants were autoantibody-positive relatives of people with T1D from DPT-1 (n = 654) and TNPTP (n = 4610), which have been previously described (7-9). TNPTP (and its precursor, DPT-1) screens relatives of people with T1D for the presence of islet autoantibodies and offers close monitoring and/or enrollment into clinical prevention trials. Participants in DPT-1 were all positive for islet cell autoantibody (ICA). TNPTP participants were positive for autoantibodies to insulin, glutamic acid decarboxylase 65-kDa isoform (GAD65), islet antigen 2 (IA-2), zinc transporter 8 (ZnT8), and/or ICA. DPT-1 and TNPTP participants completed 2-hour OGTTs at baseline and at 6- to 12-month intervals thereafter. For the OGTTs, samples were obtained in the fasting state and at 30, 60, 90, and 120 minutes after the ingestion of a 1.75 g/kg glucose dose (maximum, 75 g of carbohydrates). All participants included in the analyses were positive for at least one autoantibody and had completed 2-hour baseline OGTTs. Those with diabetic range baseline OGTTs (fasting glucose ≥ 126 mg/dL or 2-hour glucose ≥ 200 mg/dL) or baseline OGTTs lacking complete glucose and C-peptide data were excluded from the analysis. The diagnosis of T1D was based on standard American Diabetes Association criteria (10). All participants provided written informed consent and assent when applicable, and the study was approved by the ethics boards of all participating institutions.
Laboratory measures
Plasma glucose levels were measured by the glucose oxidase method. C-peptide was measured with the Tosoh assay in TNPTP participants and radioimmunoassay in DPT-1 participants, which have previously been shown to have similar values in split samples (5). Islet autoantibodies were measured from venous blood samples. ICA values were determined with the standard indirect immunofluorescence method using cryo-cut sections of frozen sections of human pancreas at the DPT-1 and TNPTP ICA core laboratory (Gainesville, Florida, USA, and New Orleans, Louisiana, USA). Titers greater than or equal to 10 Juvenile Diabetes Foundation units were considered positive for ICA. The ICA assays had a specificity of 100% with a sensitivity of 74.4% (11). Measurements of islet autoantibodies to insulin, GAD65, IA-2, and ZnT8 were performed in the Clinical Immunology Laboratory at the Barbara Davis Center using radioimmunoassays as previously described (12-14). In the 2018 Islet Autoantibody Standardization Program, sensitivity and specificity were 66% and 99% respectively for insulin autoantibody, 82% and 99% respectively for autoantibodies to GAD65, 62% and 100% respectively for IA-2, and 68% and 100% respectively for ZnT8.
Data analysis
Descriptive analyses were used to summarize characteristics in DPT-1 and TNPTP cohorts. Kaplan-Meier and Cox proportional hazards models were used to calculate hazard ratios (HRs) and log-rank tests to assess differences in characteristics between those who developed T1D and those who did not. Participants at risk of developing T1D who enrolled in prevention trials were censored at the time of entry into the trial. DPTRS was previously developed based on a Cox proportional-hazards model using DPT-1 data and validated in the TNPTP cohort, and is calculated as:
The DPTRS60 was also derived from a Cox proportional-hazards model based on the DPT-1 cohort using 1-hour OGTTs instead of the 2-hour OGTTs used for the standard DPTRS, and is calculated as:
The DPTRS60 model was also externally validated using the TNPTP cohort.
Because our primary end point of interest was time to T1D diagnosis, time-dependent analyses for the area under the receiver operating characteristic curves (ROCAUCs) were used to assess how well DPTRS60, DPTRS, and 2-hour glucose discriminated between participants who developed T1D and those who did not at iterative time points. P values were computed to compare the ROCAUC of markers at each time point using the independent and identically distributed representation of the ROCAUC estimators (15). Higher vs lower risk of T1D development was compared using TNPTP data. Because impaired glucose tolerance (IGT), defined as a 2-hour glucose of 140 mg/dL or greater, corresponded to approximately the 85th percentile of the distribution of all 2-hour glucose measures, we also evaluated the prognostic utility of the 85th percentiles for DPTRS60 and DPTRS and compared the resulting dichotomized risk factors using HRs, 5-year cumulative incidence curves, and time-dependent ROC curves.
Next, we calculated the sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) for the standard 2-hour glucose threshold of 140 mg/dL or greater and potential 1-hour glucose thresholds 155, 160, 170, 180, and 190 mg/dL. Given the interest to potentially use 1-hour OGTTs to monitor at-risk participants, we evaluated potential 1-hour glucose thresholds that would necessitate completing a full 2-hour OGTT to minimize the number of participants diagnosed with T1D prior to their biannual follow-up appointment.
All analyses were performed using SAS 9.4 (SAS Institute) or R 3.3.2 using the packages timeROC (21) and survival. P values are 2-sided, and significance was declared as P less than .05.
Results
Baseline characteristics of DPT-1 and TNPTP cohorts are shown in Table 1. DPT-1 participants (n = 654) had a median age of 11.2 years and median BMI percentile of 64.7%; 55.5% were male. The median age and BMI percentile of the TNPTP (n = 4610) cohort was 12.3 years and 68.8%; 48.3% were male. The mean follow-up time was 3.1 years ± 1.7 years for DPT-1 participants and 2.6 years ± 2.6 years for TNPTP participants.
Table 1.
Baseline characteristics of the Diabetes Prevention Trial–Type 1 cohort (n = 654) and the TrialNet Pathway to Prevention cohort (n = 4610)
| Baseline characteristics | DPT-1 (n = 654) | TNPTP (n = 4610) |
|---|---|---|
| Age, y | ||
| Median (range) | 11.2 (3.0-46.0) | 12.3 (1.3-49.5) |
| Mean (SD) | 14.0 (9.6) | 17.3 (12.9) |
| HR (95% CI) | 0.95 (0.94-0.97) | 0.95 (0.94-0.96) |
| Log-rank P | < .001 | < .001 |
| BMI percentile | ||
| Median (range) | 64.7 (0.0-100.0) | 68.8 (0.0-100.0) |
| Mean (SD) | 59.8 (28.8) | 63.2 (28.9) |
| Missing (n) | 0 | 26 |
| HR (95% CI) | 1.00 (0.99-1.01) | 1.00 (0.99-1.00) |
| Log-rank P | .13 | .79 |
| Sex, male | ||
| No. (%) | 363 (55.5) | 2223 (48.3) |
| Not reported (n) | 0 | 8 |
| HR (95% CI) (ref = female) | 1.04 (0.81-1.35) | 1.23 (1.04-1.45) |
| Log-rank P | .76 | .17 |
| Follow-up time, y | ||
| Median (range) | 3.0 (0.0-7.2) | 1.8 (0.0-12.96) |
| Mean (SD) | 3.1 (1.8) | 2.6 (2.6) |
| Type 1 diabetes events | ||
| No. (%) | 238 (36.4) | 541 (11.7) |
Abbreviations: BMI, body mass index; DPT-1, Diabetes Prevention Trial–Type 1; HR, hazard ratio; ref, reference; TNPTP, TrialNet Pathway to Prevention.
Comparisons between 1-hour OGTTs and 2-hour OGTTs for assessing prediction accuracy and diagnostic surveillance in autoantibody-positive cohorts are shown below.
Prediction accuracy
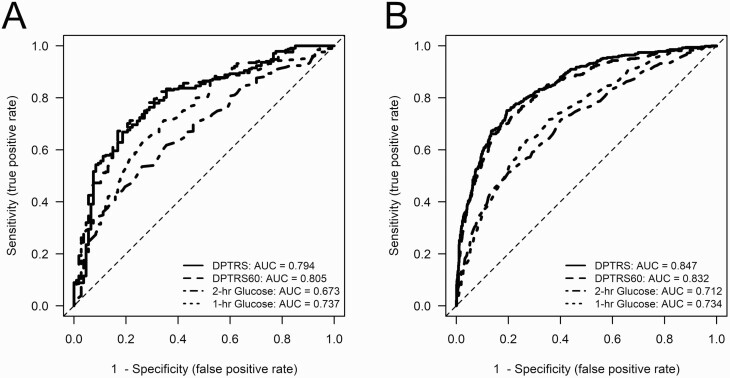
Comparisons of receiver operating characteristic curves. Time-dependent ROC curves were calculated to indicate the prediction accuracy of T1D risk for DPTRS60, DPTRS, and 2-hour glucose in the DPT-1 and TNPTP cohorts. In DPT-1, the estimated ROCAUCs (95% CI) associated with DPTRS60 and DPTRS were very similar at 1 year (0.84 [0.79-0.9) and 0.86 [0.79-0.81], respectively), 2 years (0.79 [0.74-0.84) and 0.81 [0.76-0.86], respectively), 3 years (0.79 [0.74-0.83] and 0.80 [0.75-0.84], respectively), and at 5 years (0.81 [0.760.86] and 0.79 [0.740.85], respectively). The differences for the ROCAUCs were not clinically significant at any of those iterative time points. However, ROCAUCs of the 2-hour glucose were substantially lower at 3 years (0.69 [0.64-0.74]) and at 5 years (0.67 [0.61-0.73]) than both the DPTRS60 and DPTRS ROCAUCs (P < .001 for all comparisons). Figure 1 shows that ROCs for T1D prediction 5 years from baseline are similar between DPTRS60 and DPTRS and more accurate than 2-hour and 1-hour glucose both in the DPT-1 and TNPTP cohorts.
Figure 1.
Receiver operating characteristic curves for type 1 diabetes prediction 5 years from baseline were similar between DPTRS60 and DPTRS and more accurate than 2-hour and 1-hour glucose both in A, DPT-1 and B, TNPTP cohorts. AUC, area under the curve; DPTRS60, 1-hour oral glucose tolerance test risk score; DPTRS, Diabetes Prevention Trial-Type 1 risk score; TNPTP, TrialNet Pathway to Prevention.
The same pattern was also evident in the TNPTP participants (Fig. 1). The ROCAUCs (95% CI) for DPTRS60 and DPTRS were again quite similar at 1 year (0.87 [0.84-0.88] and 0.90 [0.87-0.93], respectively), 2 years (0.86 [0.84-0.88] and 0.87 [0.85-0.90], respectively), 3 years (0.86 [0.84-0.88] and 0.87 [0.85-0.89], respectively), and at 5 years (0.83 [0.81-0.86] and 0.85 [0.82-0.87], respectively). The number of participants was much larger in the TNPTP cohort, resulting in statistically significant P values for comparisons at 3 years (P < .01) and at 5 years (P < .001). However, these differences would not be considered clinically significant. The 2-hour glucose ROCAUCs were again lower at 3 years (0.75 [0.72-0.78]) and at 5 years (0.71 [0.68-0.74]) than those for DPTRS60 and DPTRS (P < .001 for all comparisons).
Discrimination of higher-risk vs lower-risk individuals.
TNPTP data were used to demonstrate how DPTRS60, derived from 1-hour values, can lead to better T1D risk discrimination than IGT (the current standard), and similar risk discrimination as DPTRS, which requires a 2-hour OGTT. The 85th percentiles for DPTRS60 and DPTRS were 4.93 and 7.26, respectively. HRs were calculated for dichotomized risk indicators based on DPTRS60 values of less than 4.93 vs 4.93 or greater, DPTRS values of less than 7.26 vs 7.26 or greater, and 2-hour glucose of 140 mg/dL or greater vs less than 140 mg/dL. The HR for DPTRS60 of 4.93 or greater (vs < 4.93; HR = 9.09 [7.66-10.78]; P < .0001) was similar to the HR for DPTRS of 7.26 or greater (vs < 7.26; HR = 10.45 [8.80-12.41]; P < .001) and higher than the HR for 2-hour glucose of 140 mg/dL or greater (vs < 140; HR = 4.69 [3.95-5.57]; P < .001).
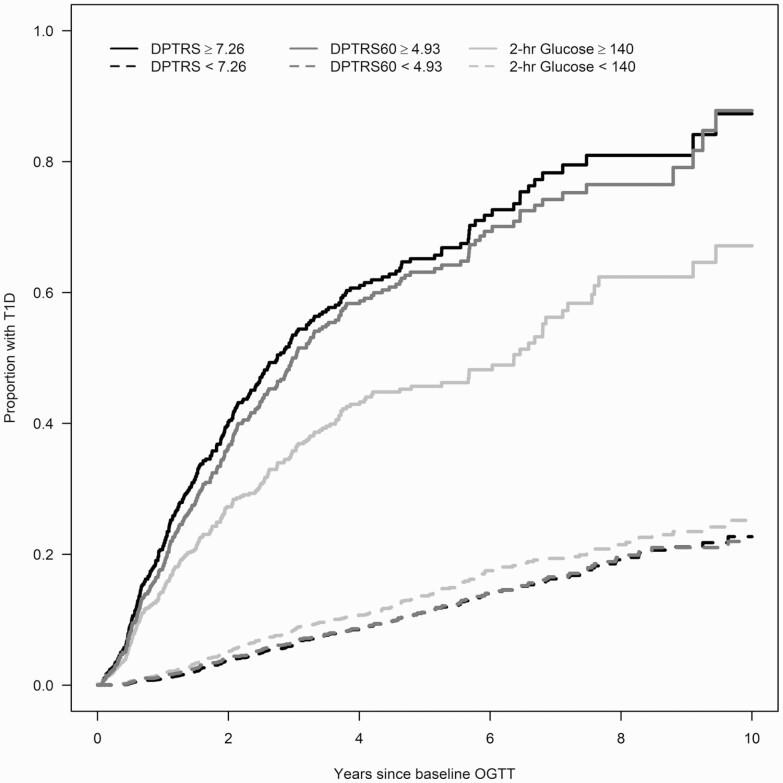
Figure 2 shows the cumulative incidence curves for T1D for the standard 2-hour glucose threshold of 140 mg/dL (IGT) and 85th percentile thresholds for DPTRS and DPTRS60. There was greater differential between the estimated 5-year cumulative incidence of T1D for participants below vs above the 85th percentile for DPTRS60 compared to IGT (DPTRS60: 0.11 [0.10-0.13] and 0.63 [0.58-0.68]; IGT: 0.14 [0.12-0.15] and 0.46 [0.41-0.51]). The DPTRS estimates were similar to those of DPTRS60 (0.11 [0.10-0.13] and 0.65 [0.60-0.70]). In a time-dependent ROC curve analysis using the 85th percentile thresholds, the ROCAUCs (95% CI) of DPTRS60 (0.72 [0.69-0.75]) and DPTRS (0.72 [0.70-0.75]) at 5 years were significantly higher (P < .001 for both) than the ROCAUC of the 2-hour glucose (0.65 [0.63, 0.68]). The estimated ROCAUC curves did not differ significantly between DPTRS60 and DPTRS (P = .507).
Figure 2.
Cumulative incidence curves for standard impaired glucose tolerance (2-hour glucose ≥ 140 mg/dL) and corresponding 85th percentiles DPTRS60 ≥ 4.93 and DPTRS ≥ 7.26 in TrialNet Pathway to Prevention participants. DPTRS60, 1-hour oral glucose tolerance test risk score; DPTRS, Diabetes Prevention Trial-Type 1 risk score; OGTT, oral glucose tolerance test; T1D, type 1 diabetes.
Diagnostic surveillance
Because fasting values are rarely 126 mg/dL or greater (the standard diagnostic fasting threshold), a 2-hour glucose value of 200 mg/dL or greater is the standard threshold used for the diagnosis of T1D. Thus, by current standards 1-hour OGTTs cannot be used for diagnostic purposes. The analyses that follow were designed to examine strategies that would mitigate a missed diagnosis when 1-hour OGTTs are used in place of standard 2-hour OGTTs.
Sensitivity, specificity, and predictive values of 1-hour glucose thresholds
For the standard 2-hour glucose threshold of 140 mg/dL or greater and potential 1-hour glucose thresholds 155, 160, 170, 180, and 190 mg/dL, sensitivity ranged from 24.9% to 79.1%, specificity ranged from 66.5% to 90.1%, PPV ranged from 3.8% to 7.9%, and NPV was high, ranging from 98.5% to 99.5%. The NPV for 1-hour glucose values is important because the higher the NPV, the more likely it is that a person would not be diagnosed with T1D prior to their next biannual follow-up visit. Table 2 shows potential glucose thresholds for the surveillance of T1D diagnosis by NPV at 6 months and number of 2-hour OGTTs that would need to be extended to 2 hours. A 1-hour glucose of 180 mg/dL or greater was most consistently similar to the current standard of 2-hour glucose of 140 mg/dL or greater in terms of NPV and number of 2-hour OGTTs needed in the DPT-1 and TNPTP cohorts. Many of the other 1-hour glucose thresholds also had high NPVs but were less similar to the 2-hour glucose of 140 mg/dL or greater NPV and more divergent, especially in terms of specificity and PPV.
Table 2.
Potential glucose thresholds for surveillance of type 1 diabetes diagnosis by negative predictive value at 6 months and number of 2-hour oral glucose tolerance tests that would need to be completed
| OGTT glucose threshold, mg/dL | n | Sensitivity, % | Specificity, % | Negative predictive value at 6 mo, % | Participants requiring 2-h OGTT, % | |
|---|---|---|---|---|---|---|
| DPT-1 (n = 654) | 2-h ≥ 140 | 206 | 74.80 | 69.29 | 98.9 | 15.1 |
| 1-h ≥ 155 | 188 | 74.80 | 72.03 | 99.3 | 31.5 | |
| 1-h ≥ 160 | 130 | 41.40 | 80.55 | 99.3 | 28.7 | |
| 1-h ≥ 170 | 97 | 33.13 | 85.53 | 98.6 | 19.9 | |
| 1-h ≥ 180 | 63 | 24.86 | 90.84 | 98.5 | 14.8 | |
| 1-h ≥ 190 | 789 | 71.19 | 81.92 | 98.5 | 9.6 | |
| TNPTP (n = 4610) | 2-h ≥ 140 | 1548 | 79.12 | 66.51 | 99.4 | 17.1 |
| 1-h ≥ 155 | 1338 | 75.85 | 70.96 | 99.5 | 33.6 | |
| 1-h ≥ 160 | 1011 | 74.21 | 78.36 | 99.4 | 29.0 | |
| 1-h ≥ 170 | 717 | 67.83 | 84.62 | 99.4 | 21.9 | |
| 1-h ≥ 180 | 500 | 52.15 | 89.52 | 99.4 | 15.6 | |
| 1-h ≥ 190 | 99 | 49.83 | 85.69 | 99.1 | 10.8 |
Abbreviations: DPT-1, Diabetes Prevention Trial–Type 1; OGTT, oral glucose tolerance test; TNPTP, TrialNet Pathway to Prevention.
Conclusions
This study provides several new findings indicating that metabolic data derived from the first hour of an OGTT can reliably be used for prediction and diagnostic surveillance of T1D in islet autoantibody–positive individuals. Composite C-peptide and glucose measures such as DPTRS are superior to glucose measures alone as predictors of and specific markers for T1D (16). Importantly, DPTRS60 had similar accuracy to DPTRS for predicting T1D in an at-risk population. Of note, DPTRS generally had slightly higher ROC curves than DPTRS60 in TNPTP. Although the large numbers of individuals in TNPTP provide power to discern a statistical significance, it is doubtful that the small differences between DPTRS and DPTRS60 would have an impact with regard to prediction and diagnostic surveillance.
In addition, DPTRS60 was appreciably more accurate for predicting T1D risk than the 2-hour glucose, even though the latter is the standard measure used for T1D diagnosis. The development of DPTRS60 was preceded by DPTRS and Index60 (5, 6). DPTRS stemmed from the need to improve the accuracy for prediction of T1D. Although Index60 is in itself a stronger predictor of T1D than glucose measures, it was developed to serve as a prediagnostic or a peridiagnostic end point rather than as predictor of T1D (17). DPTRS60 can thus be viewed as an amalgam of DPTRS and Index60, since DPTRS60 is based on 1-hour C-peptide and glucose values as is Index60, but also takes into account age and BMI, as does DPTRS. The improved prediction of composite C-peptide and glucose measures supports their use for exploring the natural history of T1D, for predicting T1D, and as potential clinical trial diagnostic end points.
Individuals identified as autoantibody positive through TNPTP have 2-hour OGTTs at regular intervals for diagnostic surveillance. Thus, for practical utility a secondary aim was necessary to assess whether a 1-hour OGTT glucose threshold could be identified below which there would be little likelihood of being diagnosed with clinical T1D before the next follow-up visit. A 1-hour glucose threshold of 180 mg/dL appears to provide such diagnostic surveillance. Individuals undergoing OGTTs would be tested with a point-of-care glucose measurement at 1 hour. If the measurement is less than 180 mg/dL, the OGTT would not be extended to 2 hours. However, a 2-hour OGTT would be performed for those participants above that threshold to assess whether T1D is present.
In addition to its use for diagnostic surveillance, the comparability of the 180 mg/dL glucose 1-hour threshold to the 140 mg/dL 2-hour glucose threshold suggests that it could also be used as another criterion for IGT. Thus, 1-hour glucose measurements could also serve other purposes that are provided by 2-hour measurements. IGT is currently used both as an entry criterion and as an end point in prevention trials, and for staging the progression of T1D from stage 1 to stage 2 (18).
The use of 1-hour OGTTs would result in more convenience and less time expenditure for research participants. Because fewer school hours and work hours would be missed, the willingness of autoantibody-positive individuals to participate would likely increase. One-hour OGTT measurements for prediction and surveillance would also result in lower research costs. Although it is difficult to accurately estimate the actual cost savings, decreasing the time for needed research or clinical staff support and the number of laboratory tests should lessen costs considerably. Reducing the duration of 2-hour OGTTs to 1-hour OGTTs will become especially important as increased numbers of children at risk for T1D are identified, T1D prevention trials increase, and the number of participants thereby undergoing OGTTs for screening and further monitoring will inevitably increase. It is important to note that although we chose the 180 mg/dL 1-hour glucose threshold for demonstrating how an alternative monitoring approach might be used to include 1-hour OGTTs in T1D studies, it is still possible that other 1-hour thresholds would better fit the objectives of some studies. The basis for using 180 mg/dL was this threshold’s performance overall in the analyses, especially its concordance with the standard 140 mg/dL 2-hour glucose threshold.
In conclusion, this study provided several new findings: 1) DPTRS60, a risk score based on data obtained within 1-hour of a glucose challenge, had similar accuracy to DPTRS (a validated risk score based on data obtained within 2 hours of a glucose challenge) for predicting T1D in an at-risk population; 2) DPTRS60 was appreciably more accurate for predicting T1D risk than the 2-hour glucose, even though the latter is the standard measure used; and 3) a 1-hour glucose threshold of 180 mg/dL or greater has a similar NPV and specificity to a 2-hour glucose of 140 mg/dL or greater, and therefore could be safely used for diagnostic surveillance of T1D. Importantly, these findings were validated in 2 independent cohorts of autoantibody-positive individuals at risk for T1D. Our findings show that for purposes of prediction and surveillance, the duration of OGTTs can be reduced from 2 hours to 1 hour in the majority of individuals at risk for T1D who participate in research with minimal to no loss of accuracy and clinical oversight.
Acknowledgments
Financial Support: The Type 1 Diabetes TrialNet Pathway to Prevention Study Group is a clinical trials network funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), the National Institute of Allergy and Infectious Diseases, and The Eunice Kennedy Shriver National Institute of Child Health and Human Development (through the cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085465, U01 DK085453, U01 DK085461, U01 DK085463, U01 DK085466, U01 DK085499, U01 DK085504, U01 DK085505, U01 DK085509, U01 DK103180, U01-DK103153, U01-DK085476, and U01-DK103266), and the Juvenile Diabetes Research Foundation International (JDRF). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF.
Author Contributions: K.S. wrote the manuscript. J.S. wrote the manuscript and researched the data. M.W. researched the data. S.G. researched the data. H.I. reviewed/edited the manuscript. H.E. reviewed/edited the manuscript. A.S. contributed to the discussion and reviewed/edited the manuscript.
Glossary
Abbreviations
- BMI
body mass index
- DPT-1
Diabetes Prevention Trial—Type 1 Diabetes Study
- DPTRS
DPT-1 Risk Score
- GAD65
glutamic decarboxylase 65-kDa isoform
- HR
hazard ratio
- IA-2
islet antigen 2
- ICA
islet cell autoantibody
- IGT
impaired glucose tolerance
- OGTT
oral glucose tolerance test
- ROCAUC
area under the receiver operating characteristic curve
- T1D
type 1 diabetes
- TNPTP
TrialNet Pathway to Prevention
- ZnT8
zinc transporter 8
Additional Information
Disclosure Statement: The authors have nothing to disclose.
Data Availability
TrialNet data can be requested from the NIDDK public repository. The data sets generated and analyzed during the present study will be made available by request from the NIDDK Central Repository at https://repository.niddk.nih.gov/studies/trialnet.
References
- 1. Raab J, Haupt F, Scholz M, et al. ; Fr1da Study Group . Capillary blood islet autoantibody screening for identifying pre-type 1 diabetes in the general population: design and initial results of the Fr1da Study. BMJ Open. 2016;6(5):e011144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gesualdo PD, Bautista KA, Waugh KC, et al. Feasibility of screening for T1D and celiac disease in a pediatric clinic setting. Pediatr Diabetes. 2016;17(6):441-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simmons KM, Youngkin E, Alkanani A, et al. Screening children for type 1 diabetes-associated antibodies at community health fairs. Pediatr Diabetes. 2019;20(7):909-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ziegler AG, Kick K, Bonifacio E, et al. ; Fr1da Study Group . Yield of a public health screening of children for islet autoantibodies in Bavaria, Germany. JAMA. 2020;323(4):339-351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sosenko JM, Skyler JS, Mahon J, et al. ; Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups . Use of the Diabetes Prevention Trial–Type 1 Risk Score (DPTRS) for improving the accuracy of the risk classification of type 1 diabetes. Diabetes Care. 2014;37(4):979-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sosenko JM, Skyler JS, Palmer JP; Diabetes Type 1 TrialNet and Diabetes Prevention Trial–Type 1 Study Groups . The development, validation, and utility of the Diabetes Prevention Trial–Type 1 Risk Score (DPTRS). Curr Diab Rep. 2015;15(8):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Diabetes Prevention Trial–Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685-1691. [DOI] [PubMed] [Google Scholar]
- 8. Skyler JS, Krischer JP, Wolfsdorf J, et al. Effects of oral insulin in relatives of patients with type 1 diabetes: the Diabetes Prevention Trial–Type 1. Diabetes Care. 2005;28(5):1068-1076. [DOI] [PubMed] [Google Scholar]
- 9. Sosenko JM, Skyler JS, Krischer JP, et al. ; Diabetes Prevention Trial–Type 1 Study Group . Glucose excursions between states of glycemia with progression to type 1 diabetes in the Diabetes Prevention Trial–Type 1 (DPT-1). Diabetes. 2010;59(10):2386-2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care. 2019;42(Suppl 1):S13-S28. [DOI] [PubMed] [Google Scholar]
- 11. Greenbaum CJ, Cuthbertson D, Krischer JP; Disease Prevention Trial of Type I Diabetes Study Group . Type I diabetes manifested solely by 2-h oral glucose tolerance test criteria. Diabetes. 2001;50(2):470-476. [DOI] [PubMed] [Google Scholar]
- 12. Yu L, Robles DT, Abiru N, et al. Early expression of antiinsulin autoantibodies of humans and the NOD mouse: evidence for early determination of subsequent diabetes. Proc Natl Acad Sci U S A. 2000;97(4):1701-1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wenzlau JM, Juhl K, Yu L, et al. The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci U S A. 2007;104(43):17040-17045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bonifacio E, Yu L, Williams AK, et al. Harmonization of glutamic acid decarboxylase and islet antigen-2 autoantibody assays for National Institute of Diabetes and Digestive and Kidney Diseases consortia. J Clin Endocrinol Metab. 2010;95(7):3360-3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Blanche P, Dartigues JF, Jacqmin-Gadda H. Estimating and comparing time-dependent areas under receiver operating characteristic curves for censored event times with competing risks. Stat Med. 2013;32(30):5381-5397. [DOI] [PubMed] [Google Scholar]
- 16. Sosenko JM, Palmer JP, Rafkin-Mervis L, et al. Glucose and C-peptide changes in the perionset period of type 1 diabetes in the Diabetes Prevention Trial–Type 1. Diabetes Care. 2008;31(11):2188-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nathan BM, Boulware D, Geyer S, et al. ; Type 1 Diabetes TrialNet and Diabetes Prevention Trial–Type 1 Study Groups . Dysglycemia and Index60 as prediagnostic end points for type 1 diabetes prevention trials. Diabetes Care. 2017;40(11):1494-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Insel RA, Dunne JL, Atkinson MA, et al. Staging presymptomatic type 1 diabetes: a scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015;38(10):1964-1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
TrialNet data can be requested from the NIDDK public repository. The data sets generated and analyzed during the present study will be made available by request from the NIDDK Central Repository at https://repository.niddk.nih.gov/studies/trialnet.