Abstract
Accurately predicting the onset and course of a disease in an individual is a major unmet challenge in medicine due to the complex and dynamic nature of disease progression. Continuous data from wearable technologies and biomarker data with a fine time resolution provide a unique opportunity to learn more about disease evolution and to usher in a new era of personalized and real-time medicine. Herein, we propose the potential of real-time, continuously measured physiological data as a noninvasive biomarker approach for detecting disease transitions, using allogeneic hematopoietic stem cell transplant (HCT) patient care as an example. Additionally, we review a recent computational technique, the landscape dynamic network biomarker method, that uses biomarker data to identify transition states in disease progression and explore how to use it with both biomarker and physiological data for earlier detection of graft-versus-host disease specifically. Throughout, we argue that increased collaboration across multiple fields is essential to realizing the full potential of wearable and biomarker data in a new paradigm of personalized and real-time medicine.
Introduction
Accurately predicting the onset and subsequent course of a disease (including the effects of treatment) in an individual patient is a major unmet challenge in medicine and a significant barrier to realizing the full potential of personalized medicine. Various biomarkers and physiological data have been sought to address the prediction problem, including germline genetic polymorphisms [1-6], proteins and DNA/RNA markers measured in blood or diseased tissue [7-18], clinical and demographic features used to develop risk scores [19-22] and physiological measurements from wearable technologies [23-32]. Although such prediction approaches have been successful at a population-level (e.g., blood lipid profiles and cardiovascular risk), their ability to accurately predict occurrence and timing of disease events in individuals has been limited.
To predict the occurrence and timing of disease events, we must continue to learn more about pre-diagnosis and even pre-symptom disease states. For most diseases, symptoms prompt diagnosis and medical attention, so relatively little is known about the transition from a healthy to disease state. Moreover, tools to study these shifts have historically been limited. Emerging data, however, indicates that detection of disease at its earliest, pre-symptom stages can lead to preventive and therapeutic interventions with substantial positive clinical impact. For example, detection and intervention of "pre-diabetes" in asymptomatic individuals can prevent progression to diabetes and its end-organ complications [30,33,34]. Likewise, early awareness of precancerous cervical changes through Pap smears, followed by localized treatment, dramatically reduced mortality from cervical cancer [35]. One can imagine that early detection of other evolving events, such as drug toxicities, emerging infections, blood clots, and more, could profoundly improve patient outcomes. However, the predominant “reactive” approach of modern medicine continues to overshadow early disease detection in many settings.
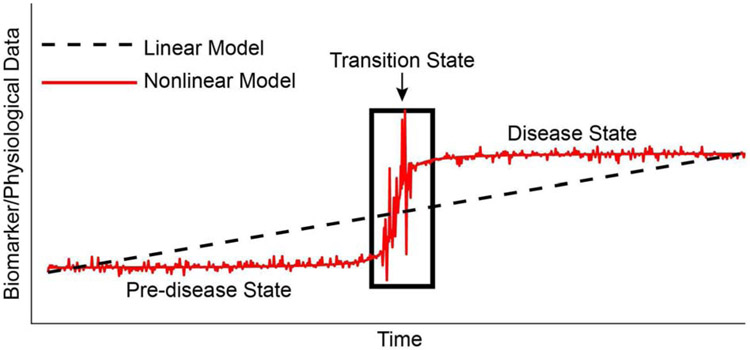
One reason traditional biomarker approaches have not succeeded for individual prediction and early detection is because they are incongruent with the fact that disease processes, and the human hosts in which they occur, represent complex dynamical systems (see [36] for a comprehensive introduction). The complex dynamical system perspective has profound implications for thinking about prediction and early detection of disease [7,8,10,14,37]. First, it suggests that instead of viewing prediction as a one-time, fixed assertion, it would be useful to revise the concept to one of "dynamic prediction" or "dynamic forecasting", in which predictions are changing over time based on new data about the disease process in an individual. Second, it suggests that early detection needs to be done in "real-time", because complex dynamical processes such as disease evolution tend to proceed nonlinearly, with prolonged periods of relative stability interspersed with shorter periods of rapid evolution (see Figure 1). The periods of rapid evolution represent transition states, or “tipping points”, where signals begin to exceed background noise, yet the disease process is not well-established and can be interrupted more easily with an intervention [14,15,37]. It is not surprising then that the current biomarker approaches in clinical medicine, which are typically based on a "snapshot" model with measurements at a single or few points in time, fail to make accurate predictions and detect disease in a timely fashion in individuals.
Figure 1: Schematic of the nonlinear evolution of disease.
The black line represents the linear model of disease whereby disease progression happens uniformly and in a constant manner to the disease state. The red curve represents the nonlinear evolution of disease whereby a stable pre-disease state undergoes a rapid transition (transition state) to a disease state. Intervention at the transition state may result in better outcomes.
Wearable sensor technologies (i.e., consumer-grade wrist-worn fitness trackers, as well medical-grade wearable devices; see Figure 2) provide a unique opportunity to transform the one-time, fixed assertion view of prediction to a real-time and personalized approach to early detection of disease evolution. First, wearable devices are practical because they are passive, continuous monitors that typically require little attention from the subject or clinician. Second, they can be implemented in inpatient or remote settings, allowing for continuous, noninvasive monitoring of patients in the comfort of their own homes. Finally, wearables provide objective data measurements of many physiological parameters that may be correlated with surrogate endpoints, and thereby, supply a platform for real-time feedback to the patient and clinician [38].
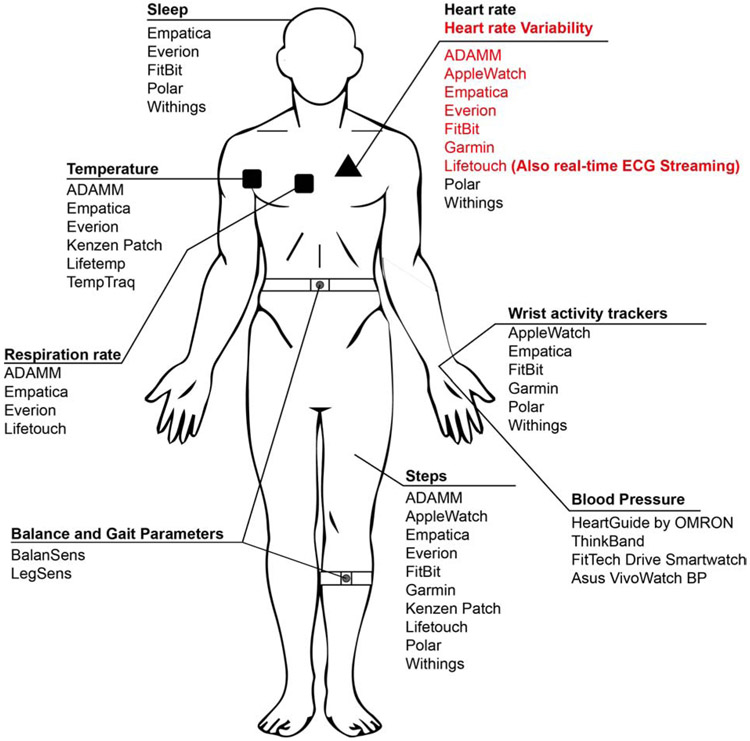
Figure 2: Examples of current wearable technologies, based on the data they collect.
Various physiological data streams available from some currently available wearable technologies. The list presented is not meant to be exhaustive, and there may be other important wearable technologies on the market.
The advantages of wearable technologies can address the challenge of studying human hosts as complex dynamical systems in a unique way. As stated above, disease evolution typically starts with prolonged periods of relative stability. Wearable technologies, with continuous and real-time data collection, capture these prolonged periods of relative stability in a personalized way. Furthermore, the personalization gives context to the shorter periods of rapid evolution that signify disease tipping points. In addition, data collection with the fine-time resolution offered by wearables allows for the detection of the shorter periods of instability that may not be seen with traditional clinical methods. Finally, wearables provide a platform for the integration of many technologies that can inform clinicians immediately of changes in physiology. Real-time notifications and feedback avoid the need for patients to self-report changes, as patientreported data can often be significantly delayed [39].
With the current advantages of wearable technologies, it is surprising that, in most clinical applications, wearables are commonly used only for physical activity tracking [40]. To realize the full potential of wearables devices to advance medicine, research efforts must expand to employ a growing number of other continuous physiological measurements, as the tools for such measurements continue to improve--e.g., heart and respiration rate, heart rate variability, continuous ECG and EEG monitoring, body temperature, blood pressure, the release of certain biochemicals, and others (see Figure 2 for examples). The specific data streams that are most informative can be expected to vary based on the specific disease type. Moreover, coupling physiological data streams with more dynamically collected molecular biomarker data (e.g., blood biomarkers, gene expression profiles) will be important, in order to relate sensor data to underlying disease mechanisms. Herein, we propose the potential of real-time, continuously measured physiological data as a noninvasive biomarker approach for detecting disease transitions, using allogeneic hematopoietic stem cell transplant (HCT) patient care as an example. Furthermore, we discuss a recently described computational method using biomarker data, which we argue could be coupled with physiological data from wearables to earlier and more accurately identify disease tipping points.
Acute GVHD in HCT patients: A model system to investigate dynamic prediction and real-time detection approaches
Allogeneic HCT, commonly used for treating leukemia and other blood disorders, involves ablation of a patient's endogenous blood-forming stem cells, followed by infusion of hematopoietic stem cells from a donor [41,42]. As standard practice, patients undergoing transplant are followed very closely for the first 100 days, with the first month spent in-hospital and the subsequent 2 months involving frequent clinic visits up to twice a week. The first 100 days after transplant are especially critical as patients are at high risk (up to 40%) for developing acute graft-versus-host disease (aGVHD), the most common cause of morbidity and mortality aside from leukemia (or other blood disorder) itself in HCT patients [41].
Acute GVHD is caused by activation and proliferation of donor T-cells, leading to an attack on host tissues, most significantly the lining of the gut, resulting in massive epithelial cell apoptosis [43]. Acute GVHD is treated with high doses of immunosuppressive drugs, but can be difficult to control, especially when it has become well-established [43]. Therefore, there is a crucial need to develop approaches for early detection of aGVHD to enable earlier, more effective intervention, and thereby reduce mortality. Although blood-based biomarkers have been developed, they have had limited clinical utility to date [44,45]. We propose that wearable sensor technologies provide a noninvasive means to continuously monitor HCT patients and potentially identify when patients are nearing the tipping point of severe aGVHD.
Recently, we showed that, in a mouse model of aGVHD, continuous monitoring of body temperature revealed signals associated with aGVHD in the first-week post-transplant [28]. These signals corresponded to time frames that were prior to what is currently possible with any other reliable, noninvasive means [28]. Moreover, the work used unsupervised machine learning algorithm approaches to identify animals subsequently developing aGVHD [28], providing evidence that continuously measured physiological data contains information that could predict aGVHD. Incorporating more sophisticated mathematical and computational techniques that include biological interpretation and confounding factors may help to translate this approach to detect human aGVHD in human HCT. Simply with a low-cost, noninvasive, and passive approach of continuously monitoring body temperature, there is potential to detect aGVHD at an early stage and intervene quickly to reduce disease severity. In order to translate such findings to improved personalized, real-time clinical care, more multidisciplinary collaborations are needed to enable physiological data readily available from current wearable devices to be analyzed for more accurate and prompt prediction of disease-state transition and ultimately be incorporated into clinical research and patient care workflows.
In the case of early aGVHD detection then, continuous monitoring of body temperature in the mouse model showed promise for detecting the disease-state transition earlier than with traditional methods [28]. Integration of additional physiological data types such as heart and respiration rate, blood pressure, heart rate variability, and others may aid in even earlier and more robust prediction of disease onset. Furthermore, since high-time resolution (daily) blood biomarker sampling is possible in HCT patients because they are hospitalized and have central venous access catheters, there is a unique opportunity to investigate how coupling physiological data with daily blood-based biomarker measurements and genetic data may increase the effectiveness of early disease detection methods. Additionally, not only should future research focus on how to couple the two data streams, but also to ask whether passive, noninvasive physiological data are suitable surrogates for biospecimen-based biomarker data. Ultimately, this may remove the need for invasive data collection methods. However, to harness all of the multi-parameter data streams, robust and validated mathematical and computational techniques are needed.
The Landscape-Dynamic Network Biomarker Method: A technique to detect disease tipping points
One such computational approach, the landscape Dynamic Network Biomarker (l-DNB) method, was recently published by Liu et al [14]. The assumptions of l-DNB account for the nonlinearity of disease onset by assuming a period of stability in physiological and biological parameters with a transition state and eventual disease state. The goal of the l-DNB theory is to identify the transition state by looking at changes in the data across time. The advantage of the landscape-DNB method over previous DNB methods is that l-DNB quantifies the tipping point without any clustering algorithms or other heuristic procedures [14].
To detect the tipping point, the l-DNB method computes a global DNB score, IDNB, at each time point, based on the landscape of local DNB scores, IS. There are three major steps to compute the global DNB score: (1) construction of a single sample network (SSN), (2) calculation of the local DNB score for every gene in the data set, and (3) identification of the DNB module for the single sample (see Figure 3). Here, we briefly outline each step. For a more comprehensive derivation, see the Methods Section in [14].
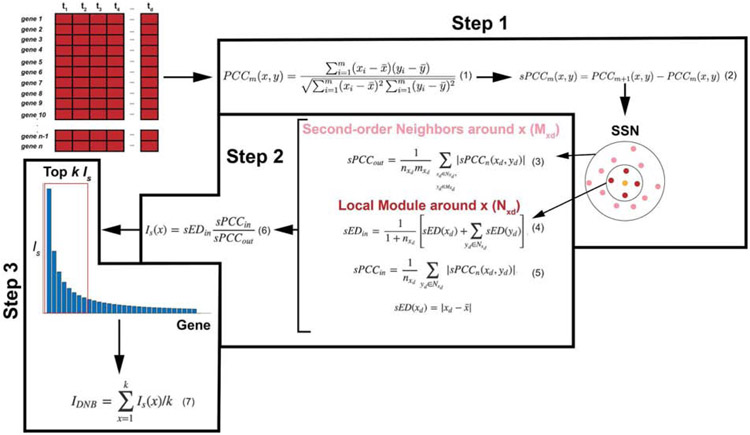
Figure 3: Outline of the steps to the landscape DNB method.
The l-DNB ethod takes as input a gene-expression data set. Step 1 calculates Pearson (PCCm) and differential Pearson correlation coefficients (sPCCm) to construct a single sample network (SSN) around gene x. The SSN is separated into first-order neighbors of gene x, Nxd (with nxd genes), and second-order neighbors, Mxd (with mxd genes). Next, based on the SSN found in Step 1, Step 2 computes local DNB values for each gene. sPCCin is the average absolute sPCCm value between gene x and its first-order neighbors. sPCCout is proportional to the average absolute value of sPCCm between gene x and its first-order neighbors and the second-order neighbors. sEDin is the average deviation in expression of gene x and all its first-order neighbors. Is(x) is the local DNB score of gene x. Finally, Step 3 ranks all local DNB scores and takes the average of the top k values to compute a global DNB score, IDNB.
Construction of a single sample network
At each time point of the data set, a single sample network (SSN) is computed to determine first- and second-modules for each gene (see Figure 3, Step 1). First, for each pair of genes in the sample, Pearson correlation coefficients (PCCs) are computed (see Eqn. (1), Figure 3). Then, with the PCCs, a differential Pearson correlation coefficient, sPCC, is computed (see Eqn. (2), Figure 3) that quantifies the correlation between the current sample and the next sample. The SSN is then constructed using significant sPCCs among all pairs of genes or molecules [14]. These significant sPCCs can be quantified in various ways. For example, by assuming a Gaussian distribution with a sufficiently large n, a Z-score is calculated. Then, the p-value is estimated from the standard normal cumulative distribution [46,47].
Calculation of local DNB scores
Next, at each time point, local DNB scores, IS, are computed for each gene or biomarker in the data set (see Figure 3, Step 2). First, using the SSN, a sPCCin score is calculated for a gene x and its first-order neighbors (Eqn. (4) in Figure 3). The sPCCin value quantifies how the correlation among a molecule and its first-order neighbors changes from one sample to the following, with the assumption that molecules linked will move together. Next, a sPCCout score is computed (Eqn. (3) in Figure 3), and, similar to the sPCCin value, the sPCCout value measures how moving from one sample to the next affects the correlation among a gene and its second-order neighbors; here, the assumption is that correlations among second-order neighbors will decrease. Finally, the sEDin value (Eqn. (4) in Figure 3) represents the average deviation in the expression of all of the genes in the local module of x. With these three values, the local DNB score for a gene x is calculated as in Eqn. (6) in Figure 3. Essentially, a large IS value signifies that the expression of gene x is deviating rapidly, the correlation among the gene and its first-order neighbors is increasing, and the correlation among the gene and its second-order neighbors is decreasing [14].
Identification of the DNB module and global DNB score
The IS values, for each gene at one time point, are then used to form a “landscape”, from which the global DNB for that time point is calculated (see Figure 3, Step 3). In particular, for each time point, the top-k genes are averaged to find the global DNB statistic, IDNB (see Eqn. (7) in Figure 3). The parameter k may vary in the application, so it is up to the user to determine the most appropriate value. The global DNB scores are then compared across all time points, with the highest one corresponding to the tipping point [14].
Previous applications of the l-DNB theory
The l-DNB theory has been applied successfully to the detection of critical transition points in cancer [14,18]. For example, the method was applied to tumor-associated gene-expression data sets for lung adenocarcinoma (LUAD), kidney renal clear cell carcinoma (KIRC), and thyroid carcinoma (THCA) [14]. The tipping point in LUAD was identified as stage IIB, after which the tumor state significantly decreased in patients. For KIRC, the tipping point was found at stage II. Survival curves of patients before and after the tipping point for KIRC were significantly different from each other. Finally, the l-DNB method identified the tipping point of THCA at stage III, which again delineates a significant decrease in the survival curve [14].
Coupling the l-DNB theory with physiological data in aGVHD patients
In previous applications of the l-DNB method, the tipping points were computed using gene-expression data for each time point [14,18]. The efficacy of the method potentially could be improved with more continuous biomarker data collection as well as continuous physiological data from wearables. In Figure 4, we outline a method to couple physiological data and biomarker data to aid in the early detection of aGVHD. As mentioned in the previous section, HCT patients are at high risk of developing aGVHD in the first 100 days. Therefore, for those 100 days, physiological data from wearables such as continuous temperature, heart rate, heart rate variability, respiration rate, and more could be collected from the patient.
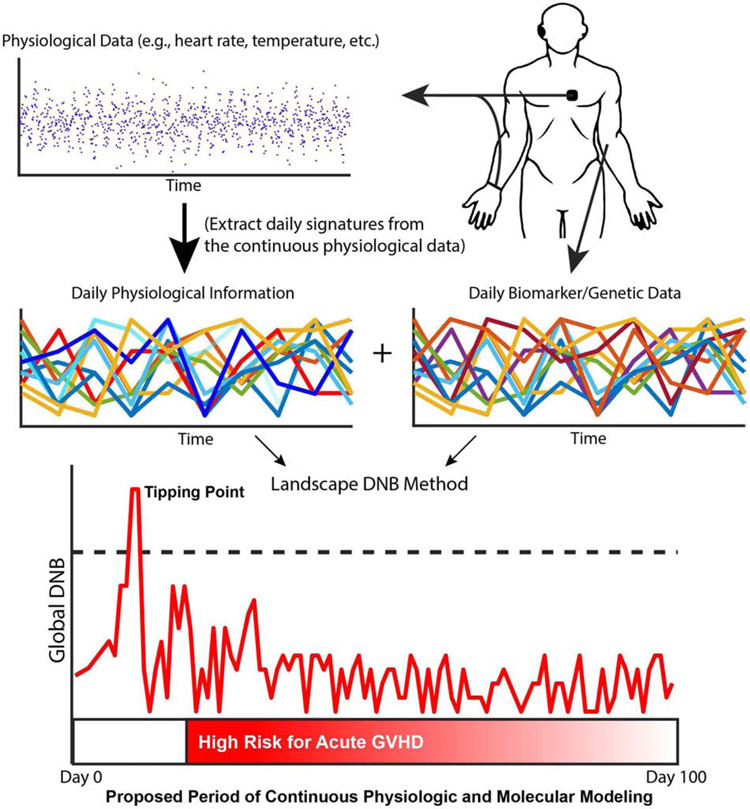
Figure 4: An example application of the l-DNB method to HCT patients using biomarker and physiological data.
Wearable technologies continuously record physiological data, and blood samples are processed for biomarker and genetic data. Next, daily signatures are extracted from the continuous physiological data to align the time points of the biomarker and physiological data sets. Finally, the l-DNB method takes as input both data streams to compute global DNB scores to find tipping points for GVHD in the first 100 days after treatment, which represents the high-risk period for aGVHD onset.
The continuous wearable data could then be incorporated into the l-DNB method, along with the biospecimen (e.g., blood)-based biomarker and genetic data. Since wearables collect data at a fine time resolution compared with biomarker collection, to apply I-DNB it will be important to identify and use methods to extract meaningful information from the continuous physiological data at a time resolution equivalent to that of the biomarker and genetic data. Once the time points of the data sets are aligned, nothing about the l-DNB method precludes its application to several data streams at once. In fact, the SSN identified in Step 1 may uncover novel groups of genes and biomarkers that relate to the dynamic variation of physiological parameters. Moreover, incorporating more physiological parameters may reveal that physiological data act as reliable surrogates for genetic and biomarker measurements. In this case, physiological parameters could replace genetic and biomarker data, reducing invasive data collection, saving money and time, and ultimately improving patient comfort and improving patient access to monitoring.
Conclusion
The current snapshot paradigm for biomarkers and disease prediction is ripe for transition to a next-generation, dynamic forecasting prediction paradigm where multi-parameter measurements are made in real-time to enable early detection and rapid intervention. Operationally, the new paradigm involves the collection of frequent, multi-parameter biomarker and systems-level physiological data measurements such as heart and respiration rate, heart rate variability, ECG and EEG monitoring, body temperature, blood pressure, the release of certain biochemicals, and many others, chosen based on their relevance to the specific disease of interest. Data would then be integrated over time and reported to clinicians and researchers remotely to provide real-time awareness of disease status and the need for intervention. Ultimately, the noninvasive wearable sensors data may prove to be correlated with biospecimen-based biomarker data, in such cases enabling noninvasive wearable devices to be the primary mode of monitoring.
Several emerging trends in technology make now an opportune time to develop these approaches. First, technological advances have resulted in validated wearable technologies that measure a variety of physiological parameters at a low cost. Now that the technology is available, we need collaborations among various specialists such as clinical researchers, biologists, computer and data scientists, applied mathematicians, software developers, and engineers to establish the relevant parameters to measure, the ideal frequency of collection, and the computational techniques to optimize the accumulating data. With more such collaborations, validated wearable technologies, and robust computational techniques, we may finally gain a deeper appreciation and understanding of the dynamic nature of disease onset and course, and ultimately realize the full potential of personalized and real-time medicine.
Acknowledgements.
We thank Dave Warner for helpful information and discussions and Mary Olesnavich for compiling information on current wearable sensor technologies. The A. Alfred Taubman Medical Research Institute has supported the GVHD work of Muneesh Tewari and Sung Won Choi proposed herein. An NIH/NHBLI grant (1R01HL146354) and the Edith S. Briskin and Shirley K. Schlafer Foundation also supports the work of Sung Won Choi. JT was supported by a National Institutes of Health Training Grant (T32 HL007622).
Footnotes
Disclosure of Conflicts of Interest. The authors have no conflicts-of-interest to disclose.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Harrap SB, Zammit KS, Wong ZYH, Williams FM, Bahlo M, Tonkin AM, Anderson ST, Genome-wide linkage analysis of the acute coronary syndrome suggests a locus on chromosome 2, Arterioscler. Thromb. Vasc. Biol 22 (2002) 874–878. [DOI] [PubMed] [Google Scholar]
- [2].Ozaki K, Ohnishi Y, lida A, Sekine A, Yamada R, Tsunoda T, Sato H, Sato H, Hori M, Nakamura Y, Tanaka T, Functional SNPs in the lymphotoxin-alpha gene that are associated with susceptibility to myocardial infarction, Nat. Genet 32 (2002) 650–654. [DOI] [PubMed] [Google Scholar]
- [3].Yamada Y, Izawa H, Ichihara S, Takatsu F, Ishihara H, Hirayama H, Sone T, Tanaka M, Yokota M, Prediction of the risk of myocardial infarction from polymorphisms in candidate genes, N. Engl. J. Med 347 (2002) 1916–1923. [DOI] [PubMed] [Google Scholar]
- [4].Bown MJ, Horsburgh T, Nicholson ML, Bell PRF, Sayers RD, Cytokines, their genetic polymorphisms, and outcome after abdominal aortic aneurysm repair, Eur. J. Vasc. Endovasc. Surg 28 (2004) 274–280. [DOI] [PubMed] [Google Scholar]
- [5].Hakonarson H, Thorvaldsson S, Helgadottir A, Gudbjartsson D, Zink F, Andresdottir M, Manolescu A, Arnar DO, Andersen K, Sigurdsson A, Thorgeirsson G, Jonsson A, Agnarsson U, Bjornsdottir H, Gottskalksson G, Einarsson A, Gudmundsdottir H, Adalsteinsdottir AE, Gudmundsson K, Kristjansson K, Hardarson T, Kristinsson A, Topol EJ, Gulcher J, Kong A, Gurney M, Thorgeirsson G, Stefansson K, Effects of a 5-lipoxygenase-activating protein inhibitor on biomarkers associated with risk of myocardial infarction: a randomized trial, JAMA. 293 (2005) 2245–2256. [DOI] [PubMed] [Google Scholar]
- [6].Cohen JC, Boerwinkle E, Mosley TH Jr, Hobbs HH, Sequence variations in PCSK9, low LDL, and protection against coronary heart disease, N. Engl. J. Med 354 (2006) 1264–1272. [DOI] [PubMed] [Google Scholar]
- [7].Liu R, Li M, Liu Z-P, Wu J, Chen L, Aihara K, Identifying critical transitions and their leading biomolecular networks in complex diseases, Sci. Rep 2 (2012) 813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen L, Liu R, Liu Z-P, Li M, Aihara K, Detecting early-warning signals for sudden deterioration of complex diseases by dynamical network biomarkers, Sci. Rep 2 (2012) 342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ziegler A, Koch A, Krockenberger K, Grosshennig A, Personalized medicine using DNA biomarkers: a review, Hum. Genet 131 (2012) 1627–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu R, Wang X, Aihara K, Chen L, Early diagnosis of complex diseases by molecular biomarkers, network biomarkers, and dynamical network biomarkers, Med. Res. Rev 34 (2014) 455–478. [DOI] [PubMed] [Google Scholar]
- [11].Looker HC, Colombo M, Agakov F, Zeller T, Groop L, Thorand B, Palmer CN, Hamsten A, de Faire U, Nogoceke E, Livingstone SJ, Salomaa V, Leander K, Barbarini N, Bellazzi R, van Zuydam N, McKeigue PM, Colhoun HM, SUMMIT Investigators, Protein biomarkers for the prediction of cardiovascular disease in type 2 diabetes, Diabetologia. 58 (2015) 1363–1371. [DOI] [PubMed] [Google Scholar]
- [12].Soe HJ, Yong YK, Al-Obaidi MMJ, Raju CS, Gudimella R, Manikam R, Sekaran SD, Identifying protein biomarkers in predicting disease severity of dengue virus infection using immune-related protein microarray, Medicine . 97 (2018) e9713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Yao F, Zhang K, Zhang Y, Guo Y, Li A, Xiao S, Liu Q, Shen L, Ni J, Identification of Blood Biomarkers for Alzheimer’s Disease Through Computational Prediction and Experimental Validation, Front. Neurol 9 (2018) 1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Liu X, Chang X, Leng S, Tang H, Aihara K, Chen L, Detection for disease tipping points by landscape dynamic network biomarkers, Natl Sci Rev. 6 (2019) 775–785.** The authors present a novel way to use biomarker and genetic data to predict tipping points of disease. They apply their new method to an influenza data set to show that the theory can predict disease onset before symptoms appear. They also apply their method to genetic data sets of cancer stages and show that the tipping point coincides with important transitions between critical cancer stages.
- [15].Liu R, Zhong J, Yu X, Li Y, Chen P, Identifying Critical State of Complex Diseases by Single-Sample-Based Hidden Markov Model, Front. Genet 10 (2019) 285.* The authors develop a single-sample-based hidden Markov model approach to investigate the differences between normal and pre-disease states. They demonstrate the effectiveness of their method with two real datasets from stomach adenocarcinoma and influenza infections.
- [16].Younes L, Albert M, Moghekar A, Soldan A, Pettigrew C, Miller MI, Identifying Changepoints in Biomarkers During the Preclinical Phase of Alzheimer’s Disease, Front. Aging Neurosci 11 (2019) 74.** The authors used a changepoint model to determine if certain measures had a significant change in slope in relation to clinical symptom onset. They considered nine measurements based on cerebrospinal fluid, magnetic resonance imaging, and cognitive testing. They found that a single measure, CSF t-tau, had an early changepoint (34 years prior to symptom onset). The remaining CSF measures and all cognitive tests had changepoints around 10-15 years prior to clinical symptom onset. Their results highlight the long period of time that AD pathology is accumulating in the brain.
- [17].Ong KL, Chung RWS, Hui N, Festin K, Lundberg AK, Rye K-A, Jonasson L, Kristenson M, Usefulness of Certain Protein Biomarkers for Prediction of Coronary Heart Disease, Am. J. Cardiol 125 (2020) 542–548. [DOI] [PubMed] [Google Scholar]
- [18].Sun Y, Zhao H, Wu M, Xu J, Zhu S, Gao J, Identifying critical states of hepatocellular carcinoma based on landscape dynamic network biomarkers, Comput. Biol. Chem 85 (2020) 107202.* The authors improve on the landscape dynamic network biomarker theory to increase clinical applicability, particularly to identifying tipping points of hepatocellular carcinoma.
- [19].Wilson BJ, Qureshi N, Santaguida P, Little J, Carroll JC, Allanson J, Raina P, Systematic review: family history in risk assessment for common diseases, Ann. Intern. Med 151 (2009) 878–885. [DOI] [PubMed] [Google Scholar]
- [20].Brentnall AR, Cuzick J, Buist DSM, Bowles EJA, Long-term Accuracy of Breast Cancer Risk Assessment Combining Classic Risk Factors and Breast Density, JAMA Oncol. 4 (2018) e180174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li BY, Oh J, Young VB, Rao K, Wiens J, Using Machine Learning and the Electronic Health Record to Predict Complicated Clostridium difficile Infection, Open Forum Infect Dis. 6 (2019) ofz186.* The authors use electronic health data in a machine learning approach for patient risk stratification of developing complications from Clostridium difficile infections. They found that, with the novel machine learning approach, electronic health data can accurately stratify CDI cases according to their risk of complications.
- [22].Mars N, Koskela JT, Ripatti P, Kiiskinen TTJ, Havulinna AS, Lindbohm JV, Ahola-Olli A, Kurki M, Karjalainen J, Palta P, FinnGen, Neale BM, Daly M, Salomaa V, Palotie A, Widen E, Ripatti S, Polygenic and clinical risk scores and their impact on age at onset and prediction of cardiometabolic diseases and common cancers, Nat. Med 26 (2020) 549–557.* The authors used polygenic risk scores to increase the clinical risk prediction of five common diseases: coronary heart disease, type 2 diabetes, atrial fibrillation, breast cancer, and prostate cancer. The PRSs improved model discrimination over age and sex in type 2 diabetes, atrial fibrillation, and breast and prostate cancer. In all five diseases, PRSs improved classifications over clinical methods.
- [23].Yang C-C, Hsu Y-L, A review of accelerometry-based wearable motion detectors for physical activity monitoring, Sensors . 10 (2010) 7772–7788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rhee H, Belyea MJ, Sterling M, Bocko MF, Evaluating the Validity of an Automated Device for Asthma Monitoring for Adolescents: Correlational Design, J. Med. Internet Res 17 (2015) e234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li X, Dunn J, Salins D, Zhou G, Zhou W, Schüssler-Fiorenza Rose SM, Perelman D, Colbert E, Runge R, Rego S, Sonecha R, Datta S, McLaughlin T, Snyder MP, Digital Health: Tracking Physiomes and Activity Using Wearable Biosensors Reveals Useful Health-Related Information, PLoS Biol. 15 (2017) e2001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Ohri N, Kabarriti R, Bodner WR, Mehta KJ, Shankar V, Halmos B, Haigentz M Jr, Rapkin B, Guha C, Kalnicki S, Garg M, Continuous Activity Monitoring During Concurrent Chemoradiotherapy, Int. J. Radiat. Oncol. Biol. Phys 97 (2017) 1061–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Guk K, Han G, Lim J, Jeong K, Kang T, Lim E-K, Jung J, Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare, Nanomaterials (Basel). 9 (2019). 10.3390/nano9060813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].He K, Wu Z, Fujiwara H, Whitesall S, Zajac CK, Choi SW, Reddy P, Tewari M, Computational analysis of continuous body temperature provides early discrimination of graft-versus-host disease in mice, Blood Adv. 3 (2019) 3977–3981.* The authors showed that continuous temperature monitoring holds promise for earlier diagnosis of GVHD in mouse models.
- [29].Pavic M, Klaas V, Theile G, Kraft J, Tröster G, Blum D, Guckenberger M, Mobile Health Technologies for Continuous Monitoring of Cancer Patients in Palliative Care Aiming to Predict Health Status Deterioration: A Feasibility Study, J. Palliat. Med (2019). 10.1089/jpm.2019.0342.* The authors recorded continuous physiological data from wearables and found that increase in heart rate, heart rate variability, and increased step speed correlated with hospital readmissions and emergency visits. This work shows that monitoring with wearables in a vulnerable population of palliative cancer patients is feasible. Also, data from wearables may help in predicting emergency visits and hospital readmissions, which in many cases can be avoided.
- [30].Schüssler-Fiorenza Rose SM, Contrepois K, Moneghetti KJ, Zhou W, Mishra T, Mataraso S, Dagan-Rosenfeld O, Ganz AB, Dunn J, Hornburg D, Rego S, Perelman D, Ahadi S, Sailani MR, Zhou Y, Leopold SR, Chen J, Ashland M, Christle JW, Avina M, Limcaoco P, Ruiz C, Tan M, Butte AJ, Weinstock GM, Slavich GM, Sodergren E, McLaughlin TL, Haddad F, Snyder MP, A longitudinal big data approach for precision health, Nat. Med 25 (2019) 792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zahiri M, Chen KM, Zhou H, Nguyen H, Workeneh BT, Yellapragada SV, Sada YH, Schwenk M, Najafi B, Using wearables to screen motor performance deterioration because of cancer and chemotherapy-induced peripheral neuropathy (CIPN) in adults - Toward an early diagnosis of CIPN, J. Geriatr. Oncol 10 (2019) 960–967.* The study examined how motor deterioration correlated to physiological parameters from wearables. In particular, they found that the Vibration Perception Threshold (VPT) is a predictor of motor deterioration and may be used to determine onset/severity of chemotherapy-induced peripheral neuropathy.
- [32].Radin JM, Wineinger NE, Topol EJ, Steinhubl SR, Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: a populationbased study, The Lancet Digital Health. 2 (2020) e85–e93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM, Diabetes Prevention Program Research Group, Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin, N. Engl. J. Med 346 (2002) 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tabak AG, Herder C, Rathmann W, Brunner EJ, Kivimaki M, Prediabetes: a high-risk state for diabetes development, Lancet. 379 (2012) 2279–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Guzick DS, Efficacy of screening for cervical cancer: a review, Am. J. Public Health 68 (1978) 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Strogatz SH, Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry, and Engineering, CRC Press, 2018. [Google Scholar]
- [37].Scheffer M, Bascompte J, Brock WA, Brovkin V, Carpenter SR, Dakos V, Held H, van Nes DH, Rietkerk M, Sugihara G, Early-warning signals for critical transitions, Nature. 461 (2009) 53–59. [DOI] [PubMed] [Google Scholar]
- [38].Cox SM, Lane A, Volchenboum SL, Use of Wearable, Mobile, and Sensor Technology in Cancer Clinical Trials, JCO Clin Cancer Inform. 2 (2018) 1–11. [DOI] [PubMed] [Google Scholar]
- [39].Piwek L, Ellis DA, Andrews S, Joinson A, The Rise of Consumer Health Wearables: Promises and Barriers, PLoS Med. 13 (2016) e1001953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Fisch MJ, Chung AE, Accordino MK, Using Technology to Improve Cancer Care: Social Media, Wearables, and Electronic Health Records, Am Soc Clin Oncol Educ Book. 35 (2016) 200–208. [DOI] [PubMed] [Google Scholar]
- [41].Tabbara IA, Zimmerman K, Morgan C, Nahleh Z, Allogeneic hematopoietic stem cell transplantation: complications and results, Arch. Intern. Med 162 (2002) 1558–1566. [DOI] [PubMed] [Google Scholar]
- [42].Gyurkocza B, Rezvani A, Storb RF, Allogeneic hematopoietic cell transplantation: the state of the art, Expert Rev. Hematol 3 (2010) 285–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ferrara JL, Deeg HJ, Graft-versus-host disease, N. Engl. J. Med 324 (1991) 667–674. [DOI] [PubMed] [Google Scholar]
- [44].He FC, Holtan SG, Biomarkers in Graft-Versus-Host Disease: from Prediction and Diagnosis to Insights into Complex Graft/Host Interactions, Curr. Hematol. Malig. Rep 13 (2018) 44–52. [DOI] [PubMed] [Google Scholar]
- [45].Zhao X-S, Huang X-J, Seeking biomarkers for acute graft-versus-host disease: where we are and where we are heading?, Biomark Res. 7 (2019) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Sprinthall RC, Fisk ST, Basic statistical analysis, Prentice Hall Englewood Cliffs, NJ, 1990. [Google Scholar]
- [47].Liu X, Wang Y, Ji H, Aihara K, Chen L, Personalized characterization of diseases using sample-specific networks, Nucleic Acids Res. 44 (2016) e164. [DOI] [PMC free article] [PubMed] [Google Scholar]