Supplemental Digital Content is available in the text
Keywords: Abaloparatide, Bone mineral density, Osteoporosis, Fracture risk
Abstract
Objective:
Fracture risk increases with age, but few studies focus on persons ≥80 years. In the ACTIVE trial, treatment with abaloparatide for 18 months reduced osteoporotic fracture risk and increased bone mineral density. These effects were maintained with 24 months alendronate treatment in ACTIVExtend. We postulated that similar improvements in bone mineral density and safety would be demonstrated in women ≥80 years.
Methods:
Post hoc analyses of bone mineral density and fracture incidence in women with osteoporosis at high risk of fracture ≥80 years from ACTIVExtend.
Results:
In total, 56 women aged ≥80 years at ACTIVE baseline entered the ACTIVExtend study; 46 of these completed the study. Mean age was 83.3 years; other baseline characteristics were similar. At the end of ACTIVE, bone mineral density increased at all sites for abaloparatide versus placebo. Bone mineral density increased in parallel in both groups during alendronate therapy (19 to 43 months) in ACTIVExtend. At month 43, mean percent change in bone mineral density from baseline was 17.2% abaloparatide/alendronate versus 8.6% placebo/alendronate (P < 0.0001) at the lumbar spine, 5.3% abaloparatide/alendronate versus 3.0% placebo/alendronate (P = 0.024) at the total hip, and 4.6% abaloparatide/alendronate versus 3.1% placebo/alendronate (P = 0.044) at the femoral neck. Fracture incidence was low and did not differ significantly between groups. Sequential treatment with abaloparatide followed by alendronate was well tolerated; the proportion of participants reporting adverse events was similar between groups.
Conclusions:
Sequential treatment with abaloparatide followed by alendronate (43 months follow-up) in this small subgroup of ACTIVExtend participants suggests abaloparatide is well tolerated and effective in women aged ≥80 years.
Video Summary:
The prevalence of osteoporosis and the risk of fracture increase with age. The clinical consequences of fracture, including mortality, are also greater in older women with osteoporosis.1,2 For example, hip fractures represent one of the most serious types of osteoporotic fracture (including a high risk of mortality), and more than 50% of women hospitalized with a hip fracture are >80 years of age.3,4 As such, effective strategies to prevent fracture in older populations are essential. Management of osteoporosis in older women involves a multifaceted approach including adequate nutrition (ie, calcium and vitamin D), fall prevention strategies, and pharmacologic therapies.4 However, efficacy and safety information on pharmacologic treatments for osteoporosis in this population remain limited.4
Abaloparatide (ABL) is a selective activator of the parathyroid hormone 1 receptor signaling pathway that favors the stimulation of bone formation.5 The ABL Comparator Trial In Vertebral Endpoints (ACTIVE) study showed that 18 months of ABL treatment significantly increased bone mineral density (BMD) and reduced the risk of new vertebral, nonvertebral, clinical, and major osteoporotic fractures compared with placebo (PBO).6 A post hoc analysis of 94 participants (ABL, n = 51; PBO, n = 43) aged ≥80 years old in ACTIVE found similar effectiveness on BMD accrual in spine and hip and safety in this older subpopulation as in the parent study.7
Women treated with anabolic agents require antiresorptive treatment to preserve therapeutic benefits following cessation of anabolic therapy. Sequential anabolic therapy followed by antiresorptive treatment may help achieve the therapeutic goal of increasing and maintaining BMD, thus decreasing the risk of future fractures in women with osteoporosis at high risk for fracture.8-10 Women receiving either ABL or PBO in the ACTIVE study were offered enrollment in the ACTIVExtend study—a 24-month extension of ACTIVE in which both groups received open-label alendronate (ALN).8,11 In ACTIVExtend, participants treated with ABL in ACTIVE who switched to open-label ALN for 24 months (ABL/ALN) had sustained fracture risk reduction and greater BMD compared with PBO followed by ALN (PBO/ALN), demonstrating that treatment with ABL followed by ALN is an effective long-term treatment option for postmenopausal women at high risk for osteoporosis-related fractures. We postulated that similar improvements in BMD and safety would be demonstrated in women aged ≥80 years in the ACTIVExtend trial. Here, we describe the results of a post hoc analysis of the subgroup of women aged ≥80 years who participated in ACTIVExtend.
METHODS
Study design
Women aged ≥80 years from the open-label extension of the ACTIVE study (ACTIVExtend) were included in this post hoc analysis. The study design for ACTIVE (NCT01343004) and ACTIVExtend (NCT01657162) have been described in detail previously.6,8,11
Briefly, ACTIVE enrolled 2,463 postmenopausal women with osteoporosis aged 49 to 86 years at high risk of fracture. Eligible participants included women ≤65 years of age who had a BMD T-score of ≤ −2.5 at the lumbar spine or femoral neck and radiologic evidence of prior vertebral fractures or a nonvertebral fracture within the past 5 years. Women > 65 years who met fracture criteria but had a T-score of ≤ −2.0 were also eligible. Women > 65 years who did not meet the fracture criteria could enroll if their lumbar spine or femoral neck BMD T-score was ≤ −3.0. All participants were required to have T-scores above −5.0 at both the spine and femoral neck (since this was a PBO-controlled trial).
In ACTIVE, participants were randomized 1:1:1 to 18 months of blinded treatment with ABL 80 μg subcutaneously once daily or matching PBO, or open-label teriparatide 20 μg subcutaneously once daily. Following completion of the ACTIVE study, there was an interval of approximately 1 month during which eligible participants were recruited and provided written consent to participate in ACTIVExtend. Participants eligible for ACTIVExtend included those who completed 18 months of blinded treatment with ABL or PBO in ACTIVE and did not experience any treatment-related serious adverse events (SAEs). All who participated in the extension study received open-label ALN monotherapy 70 mg orally once per week for up to 24 months.
The study was approved by the ethics committees at all participating institutions and was conducted according to the recommendations of Good Clinical Practice and all other applicable local regulatory and ethical requirements and the Declaration of Helsinki (revised edition, Tokyo 2004). Written informed consent was obtained from all participants before any study-related procedures were performed.
Endpoints
The prespecified endpoints for this post hoc analysis, assessed over 43 months, included BMD (lumbar spine, total hip, and femoral neck) assessed at baseline for ACTIVExtend (cumulative month 19), and months 25, 31, 37, and 43. Vertebral fractures were also assessed on spine radiographs taken at ACTIVExtend baseline, and months 25 and 43; nonvertebral fractures were determined clinically and confirmed with source documents.6,11 Safety evaluations were summarized by treatment group based on their original randomization in the ACTIVE study, and included assessment of adverse events (AEs) and serious adverse events from ACTIVExtend. Safety data for women ≥80 years in ACTIVExtend are reported here. Previously published results for the overall population in ACTIVExtend are included in the text for comparison.8
Statistical analyses
The intent-to-treat population (primary population for all efficacy analyses except vertebral fractures) included all participants ≥80 years from ACTIVE who enrolled in ACTIVExtend. The percentage change from ACTIVE baseline in BMD for lumbar spine, total hip, and femoral neck was analyzed at months 25, 31, 37, and 43 using an analysis of covariance model, with missing data imputation by last observation carried forward. Fracture rates were low and are described descriptively.
The safety population included all participants from the ACTIVE intent-to-treat population who were enrolled in the ACTIVExtend study and received at least one dose of ALN. AEs were assessed for 24 months after ALN treatment.
RESULTS
Participant demographic and clinical characteristics
There were 94 women (≥80 y) who enrolled in the ACTIVE study (ABL, n = 51; PBO, n = 43).6 Of these, 34 (67%) from the ABL group and 31 (72%) from the PBO group completed the ACTIVE trial (Fig. 1). Of the 65 completers from ACTIVE who were aged ≥80 years, 9 did not enter the extension study for reasons including more than 33 days from last study drug administration (two ABL; two PBO); participant not willing to continue study (two ABL; one PBO); and other (one ABL; one PBO).
FIG. 1.
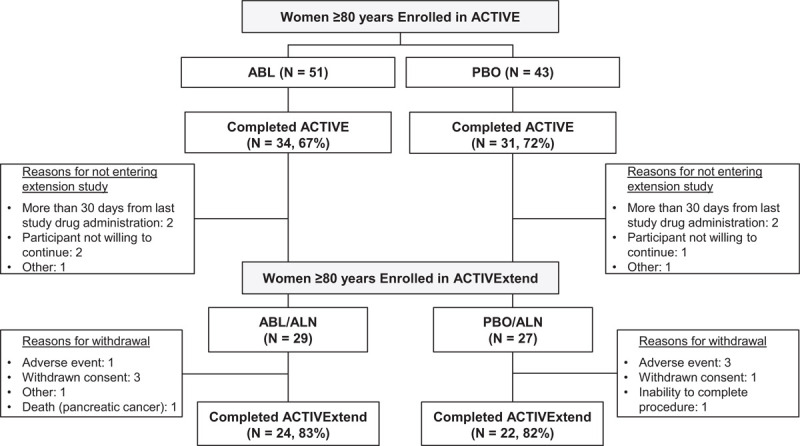
Participant disposition.
The current analysis included 56 women (ABL/ALN, n = 29; PBO/ALN, n = 27) who were aged ≥80 year at baseline in the ACTIVE trial and who entered the extension study. In total, 10 older women of more than 80 years (five in each arm) did not complete the ACTIVExtend study. The reasons for noncompletion of the study included: AE (three PBO/ALN; one ABL/ALN), withdrawn consent (one PBO/ALN; three ABL/ALN), inability to complete procedure (one PBO/ALN), and participant's death from pancreatic cancer (one ABL/ALN).
In general, baseline characteristics at ACTIVExtend baseline were well balanced between treatment groups (Table 1). The mean age of both treatment groups was 83.3 years, which is approximately 13 years older than the mean age of the overall ACTIVExtend population.8 Among the older women (≥ 80 y) included in the ACTIVExtend study, average baseline lumbar spine T-scores were −1.8 for the ABL/ALN group and −3.0 for the PBO/ALN group versus −2.1 (ABL/ALN) and −2.9 (PBO/ALN) for the overall ACTIVExtend population. Mean BMD T-scores in our older subgroup were −1.9 (ABL/ALN) and −2.4 (PBO/ALN) at total hip and −2.2 (ABL/ALN) and −2.5 (PBO/ALN) at femoral neck compared with total hip −1.6 (ABL/ALN) and −1.9 (PBO/ALN) and femoral neck −2.0 (ABL/ALN) and −2.2 (PBO/ALN) in the overall ACTIVExtend population.
TABLE 1.
Characteristics of ACTIVExtend participants aged ≥80 years at ACTIVExtend baseline (ITT population)
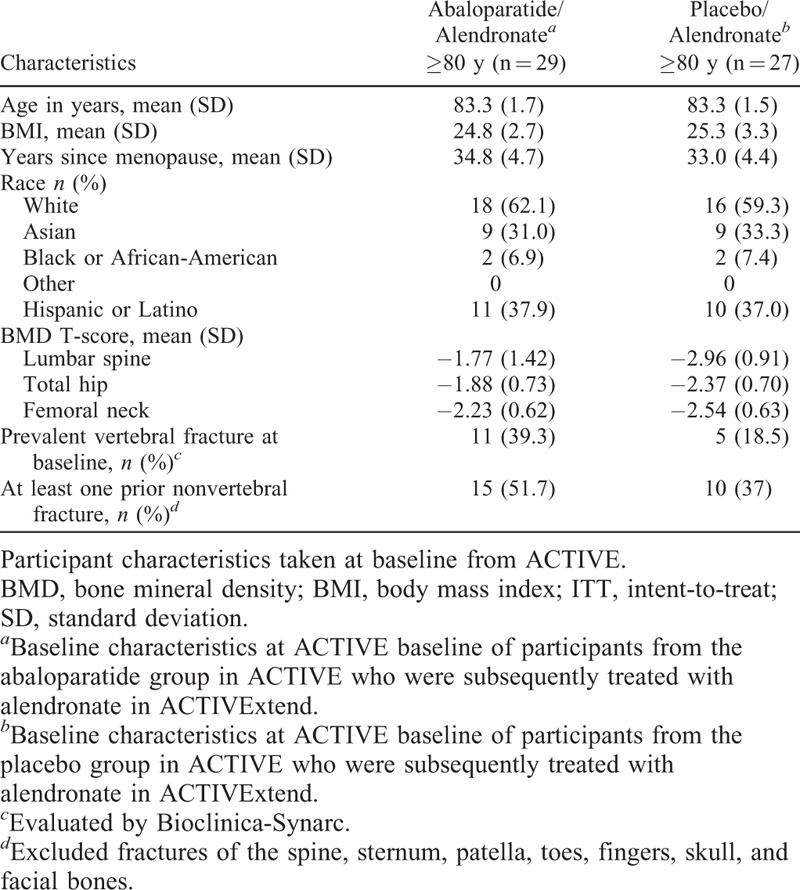
In the cohort of older participants, 15 (51.7%) in the ABL/ALN group and 10 (37%) in the PBO/ALN group had at least one prior nonvertebral fracture at ACTIVE baseline. The prior nonvertebral fracture prevalence in the overall ACTIVExtend population was 50% in both treatment groups. Among participants aged ≥80 years, 11 (39.3%) in the ABL/ALN group and 5 (18.5%) in the PBO/ALN group had a prevalent vertebral fracture at ACTIVExtend baseline compared with 22.3% in the ABL/ALN group and 24.1% in the PBO/ALN groups in the overall ACTIVExtend population. Baseline characteristics at ACTIVE baseline are shown in Supplemental Table 1.
BMD and fracture rates
In our cohort of women aged ≥80 years, mean (± standard deviation, SD) percent changes in BMD from ACTIVE baseline during the ACTIVE trial in the ABL group at lumbar spine, total hip, and femoral neck were 11.9% (±7.9), 3.4% (±3.5), and 3.3% (±4.0), respectively, at 18 months compared with 1.1% (±3.6), 0.8% (±3.3), and 1.3% (±5.3) in the PBO group. During the ALN treatment period in women aged ≥80 years, BMD increased at all three skeletal sites in both groups to a similar degree (Fig. 2). Mean (± SD) percent changes from ACTIVE baseline in lumbar spine, total hip, and femoral neck BMD were 14.9% (±9.6), 5.1% (±4.4), and 4.1% (±5.0), respectively, in the ABL/ALN group compared with 5.6% (±4.3), 2.3% (±3.3), and 2.4% (±4.9) in the PBO/ALN group at 31 months; and 15.8% (±10.3), 5.5% (±4.5), and 4.7% (±5.0) (ABL/ALN) compared with 6.2% (±5.5), 2.2% (±3.8) and 1.6% (±5.1) (PBO/ALN) at 37 months; (P < 0.05 at both time points for all three skeletal sites). At month 43, BMD mean (± SD) percent change from ACTIVE baseline was 17.2% (±10.2) for ABL/ALN versus 8.6% (±6.2) for PBO/ALN at the lumbar spine, 5.3% (±4.1) for ABL/ALN versus 3.0% (±3.5) for PBO/ALN at the total hip, and 4.6% (±4.2) for ABL/ALN versus 3.1% (±5.1) for PBO/ALN at the femoral neck. The difference in least square mean percent change from ACTIVE baseline in BMD in the ABL/ALN arm versus the PBO/ALN arm was 10.5% (95% CI, 6.1% to 14.8%, P < 0.0001) at the lumbar spine; 2.5% (95% CI, 0.3% to 4.6%, P = 0.024) at the total hip; and 2.4% (95% CI, 0.1-4.7%, P = 0.044) at the femoral neck. The increases in BMD in women aged ≥80 years were similar to those observed in the overall ACTIVExtend population.8
FIG. 2.
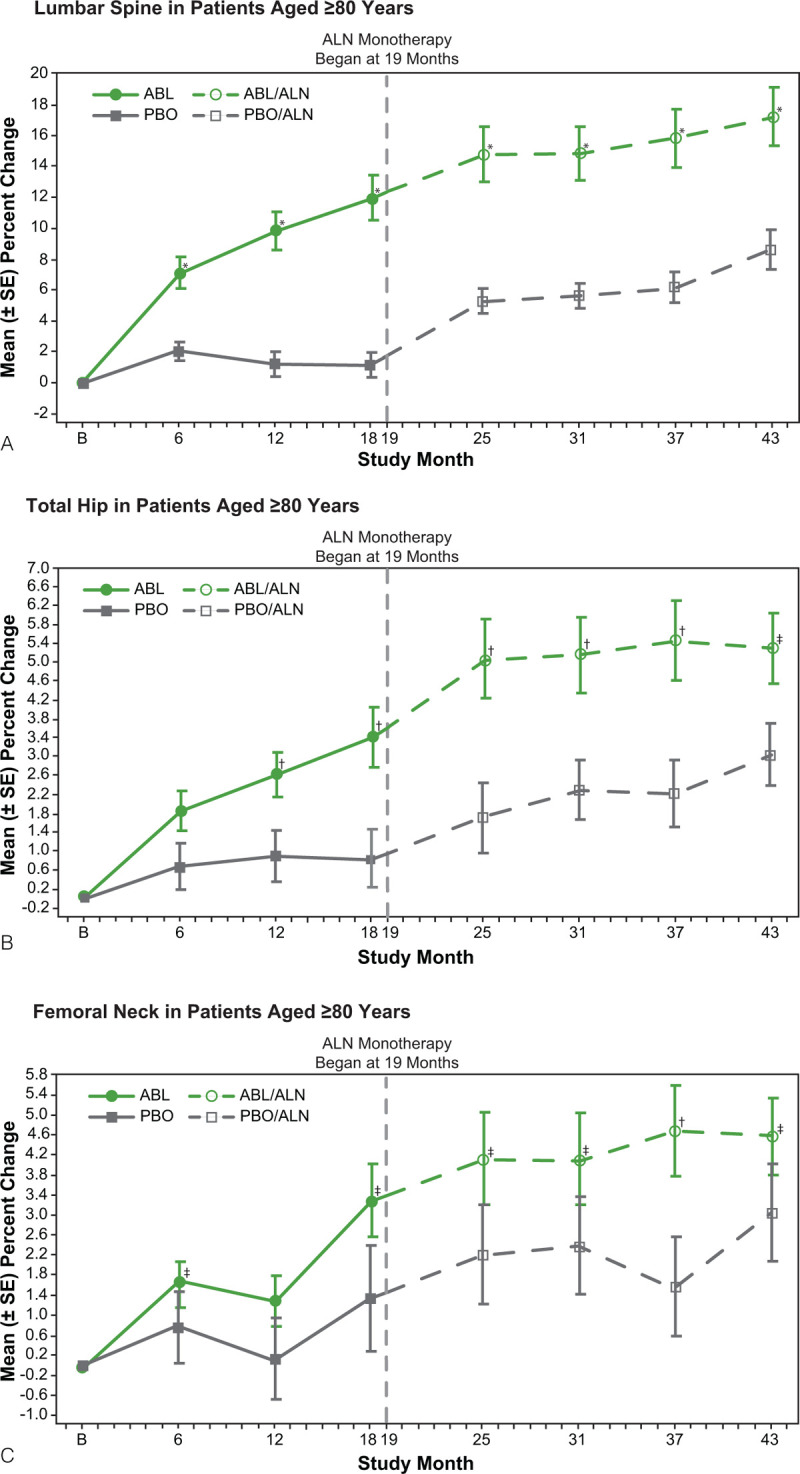
Percent change in bone mineral density from ACTIVE baseline to 43 months of ACTIVExtend in participants ≥80 years (intent-to-treat population). (A) Lumbar spine, (B) Total hip, and (C) Femoral neck. The ANCOVA model with missing data imputation by last observation carried forward was used for bone mineral density at each anatomical site. ∗P < 0.0001; †P < 0.01; ‡P < 0.05. ABL, abaloparatide; ALN, alendronate; PBO, placebo; SE, standard error.
There were very few fractures in either treatment group in this cohort of women ≥80 years. Overall, during the full 43 months, there were no new vertebral fractures in the ABL/ALN group and one in the PBO/ALN group. There was one nonvertebral fracture in the ABL/ALN group and two in the PBO/ALN group.
Safety analyses
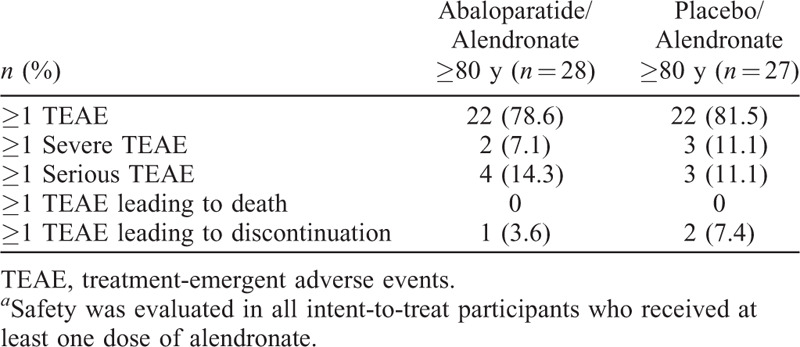
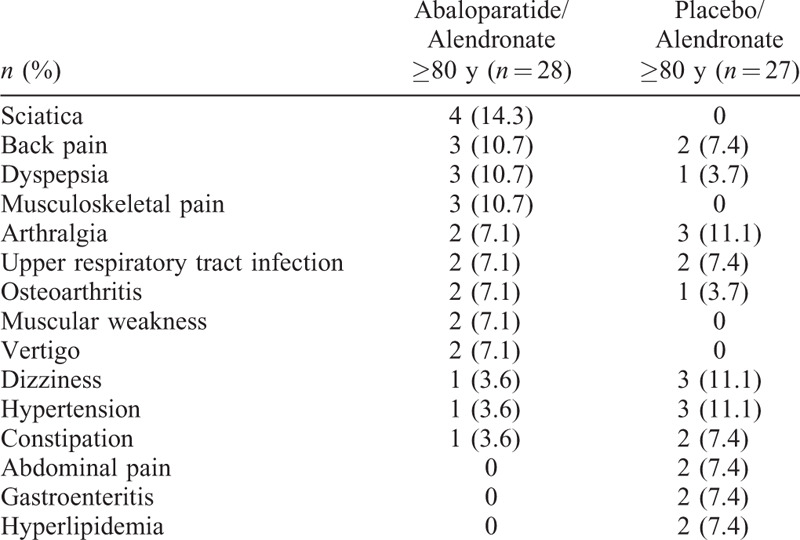
AE rates in the ACTIVExtend study, during which both groups received open-label ALN, were similar between treatment arms in the older subgroup described here and were consistent with the overall ACTIVExtend population and the recognized AE profile for ALN.8 (Table 2).
TABLE 2.
Summary of treatment-emergent adverse events in participants aged ≥80 years from ACTIVExtend (safety population)a
Treatment-emergent adverse events occurring in >5% of participants in either treatment group in women aged ≥80 years are listed in Table 3 and were similar to those observed in the overall ACTIVExtend population.8 Dizziness, hypertension, and arthralgia occurred more frequently in the PBO/ALN group, whereas dyspepsia, musculoskeletal pain, back pain, and sciatica occurred more frequently in the ABL/ALN group.
TABLE 3.
Treatment-emergent adverse events occurring in >5% of participants ≥80 years
DISCUSSION
The ACTIVE and ACTIVExtend studies demonstrated that treatment with ABL for 18 months in ACTIVE followed by continuation of treatment with ALN for up to two additional years in ACTIVExtend is effective and well tolerated in postmenopausal women with osteoporosis at high risk for fracture.6,8 In the full ACTIVExtend population, participants treated with ABL followed by ALN had sustained fracture risk reduction and larger BMD increments compared with PBO followed by ALN.8 A recent post hoc analysis of ACTIVE that included women aged ≥80 years demonstrated similar results in this clinically important subgroup as in the parent study.6 Here, we show the increases in BMD during the ACTIVExtend trial, in which ABL treatment was followed by ALN treatment, were similar in the subset of women ≥80 years compared to the overall study population.
Older women are at increased risk of having low BMD, which is an independent risk factor for fractures.12 Thus, effective measures to increase BMD in this population are paramount. Our results highlight sequential therapy of ABL followed by ALN as an effective therapy to prevent bone loss in older women, a population that is at increased risk for osteoporosis and the clinical consequences associated with osteoporosis. Furthermore, studies have demonstrated that sequential ABL/ALN is cost-effective versus generic ALN monotherapy in women ≥60 years with a BMD T-score ≤ −3.5 and in women with BMD T-score between −2.5 and −3.5 and history of osteoporotic fracture.13 Long-term cost-effectiveness models have also illustrated that sequential therapy with ABL/ALN is more cost-effective versus sequential treatment with teriparatide/ALN and, in high risk patients, sequential treatment with ABL/ALN is cost saving compared to no treatment.14
Given the small number of participants in this subgroup analysis, the number of AEs reported during the extension study was too low for any meaningful comparison between treatment groups; however, AE types and rates were comparable to those observed in the overall population and no new or unexpected safety signals were observed in either group.
Limitations of this post hoc subgroup analysis are those common to subgroup analyses, including a small number of participants and lack of adjustments for multiple comparisons. The small number of fractures observed precludes making any definitive conclusions regarding fracture incidence in this population. Additionally, of the 94 women aged ≥80 years from ACTIVE, only 65 completed the ACTIVE study (31% attrition) compared with a 23% attrition rate for all participants in the ACTIVE trial. Of these 65 participants, 56 (86%) entered ACTIVExtend and 46 completed the study (18% attrition) compared with 12% attrition for the full cohort of participants who enrolled in ACTIVExtend. Finally, degenerative changes in the spine, which are common with advanced age, could potentially have contributed to increased lumbar spine BMD in the older cohort compared with the full ACTIVExtend population. However, this would be expected to affect both the ABL/ALN and PBO/ALN groups equally. Furthermore, differences in BMD between treatment groups were also seen at total hip and femoral neck, sites less affected by degenerative change.
The strength of this study includes the focus on an older cohort of women, often excluded from clinical trials, but who are at the highest risk of fracture. In addition, this analysis provides a reasonable strategy to prevent bone loss after completion of ABL therapy.
CONCLUSION
The efficacy and safety demonstrated in ACTIVE and ACTIVExtend support the use of sequential therapy with 18 months of ABL followed by ALN. This approach represents a real-world therapeutic strategy for reducing fracture risk in older postmenopausal women with osteoporosis and a high risk of fracture. The results of this post hoc analysis provide important preliminary long-term safety and efficacy data in a small group of women ≥80 years and provides information that can help guide future studies in this important subgroup of women
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgments
Medical writing and editorial support (Sarah Hummasti, PhD) and graphic services were provided by AOIC, LLC, and were funded by Radius Health, Inc. All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors.
Footnotes
Funding/support: Funding for this study (NCT01657162) was provided by Radius Health, Inc.
Financial disclosure/conflicts of interest: SLG has received research funding from Amgen, Eli Lilly, NIH, and PCORI and participates in the advisory board for Amgen and Radius Health, Inc. LAF, BM, and YW are employees of and own company stock in Radius Health, Inc. NCH reports personal fees, consultancy, lecture fees, and honoraria from Alliance for Better Bone Health, Amgen, MSD, Eli Lilly, Servier, Shire, UCB, Radius Health, Inc., Consilient Healthcare, and Internis Pharma, outside the submitted work. CD has participated in advisory boards and is a speaker for Amgen, and Eli Lilly. He is a speaker for Radius Health, Inc. and his institution has received grant support from Radius Health, Inc. for conducting clinical trials. FC is a consultant, advisor, and speaker for Radius Health, Inc.; a consultant, advisor, and speaker for Amgen. MM has received consulting fees and honoraria from Amgen.
Previous presentations: The results of this study were previously presented at the American Society for Bone Mineral Research 2018 held in Montréal, Québec, Canada, from September 28-October 1, 2018. The abstract (#0907) was published online at https://www.asbmr.org/education/2018-abstracts and available to the members of the society. Encore presentations were presented at the 2018 ACR/ARHP Annual Meeting held in Chicago, IL, from October 19-25, 2018; the American Geriatrics Society Annual Scientific Meeting held in Portland, OR, from May 2 to May 4, 2019; and the National Conference for Nurse Practitioners, Chicago, IL, from May 14 to May 17, 2019.
REFERENCES
- 1.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA 2009; 301:513–521. [DOI] [PubMed] [Google Scholar]
- 2.Curtis JR, Arora T, Matthews RS, et al. Is withholding osteoporosis medication after fracture sometimes rational? A comparison of the risk for second fracture versus death. J Am Med Dir Assoc 2010; 11:584–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chevalley T, Guilley E, Herrmann FR, Hoffmeyer P, Rapin CH, Rizzoli R. Incidence of hip fracture over a 10-year period (1991-2000): reversal of a secular trend. Bone 2007; 40:1284–1289. [DOI] [PubMed] [Google Scholar]
- 4.Rizzoli R, Branco J, Brandi ML, et al. Management of osteoporosis of the oldest old. Osteoporos Int 2014; 25:2507–2529. [DOI] [PubMed] [Google Scholar]
- 5.Hattersley G, Dean T, Corbin BA, Bahar H, Gardella TJ. Binding selectivity of abaloparatide for PTH-type-1-receptor conformations and effects on downstream signaling. Endocrinology 2016; 157:141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 2016; 316:722–733. [DOI] [PubMed] [Google Scholar]
- 7.McClung MR, Harvey NC, Fitzpatrick LA, et al. Effects of abaloparatide on bone mineral density and risk of fracture in postmenopausal women aged 80 years or older with osteoporosis. Menopause 2018; 25:767–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bone HG, Cosman F, Miller PD, et al. ACTIVExtend: 24 months of alendronate after 18 months of abaloparatide or placebo for postmenopausal osteoporosis. J Clin Endocrinol Metab 2018; 103:2949–2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leder BZ, Tsai JN, Jiang LA, Lee H. Importance of prompt antiresorptive therapy in postmenopausal women discontinuing teriparatide or denosumab: the denosumab and teriparatide follow-up study (DATA-Follow-up). Bone 2017; 98:54–58. [DOI] [PubMed] [Google Scholar]
- 10.Cosman F, Nieves JW, Dempster DW. Treatment sequence matters: anabolic and antiresorptive therapy for osteoporosis. J Bone Miner Res 2017; 32:198–202. [DOI] [PubMed] [Google Scholar]
- 11.Cosman F, Miller PD, Williams GC, et al. Eighteen months of treatment with subcutaneous abaloparatide followed by 6 months of treatment with alendronate in postmenopausal women with osteoporosis: results of the ACTIVExtend trial. Mayo Clin Proc 2017; 92:200–210. [DOI] [PubMed] [Google Scholar]
- 12.Cummings SR, Nevitt MC, Browner WS, et al. Risk factors for hip fracture in white women. Study of Osteoporotic Fractures Research Group. N Engl J Med 1995; 332:767–773. [DOI] [PubMed] [Google Scholar]
- 13.Hiligsmann M, Williams SA, Fitzpatrick LA, Silverman SS, Weiss R, Reginster JY. Cost-effectiveness of sequential treatment with abaloparatide followedby alendronate vs. alendronate monotherapy in women at increased riskof fracture: a US payer perspective. Semin Arthritis Rheum 2020; pii: S0049-0172(20)30023-8. doi: 10.1016/j.semarthrit.2020.02.004. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 14.Hiligsmann M, Williams SA, Fitzpatrick LA, Silverman SS, Weiss R, Reginster JY. Cost-effectiveness of sequential treatment with abaloparatide vs. teriparatide for United States women at increased risk of fracture. Semin Arthritis Rheum 2019; 49:184–196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


